Association of Receipt of Opioid Prescription for Acute Post-Delivery Pain Management with Buprenorphine Discontinuation among Postpartum People with Opioid Use Disorder
Abstract
1. Introduction
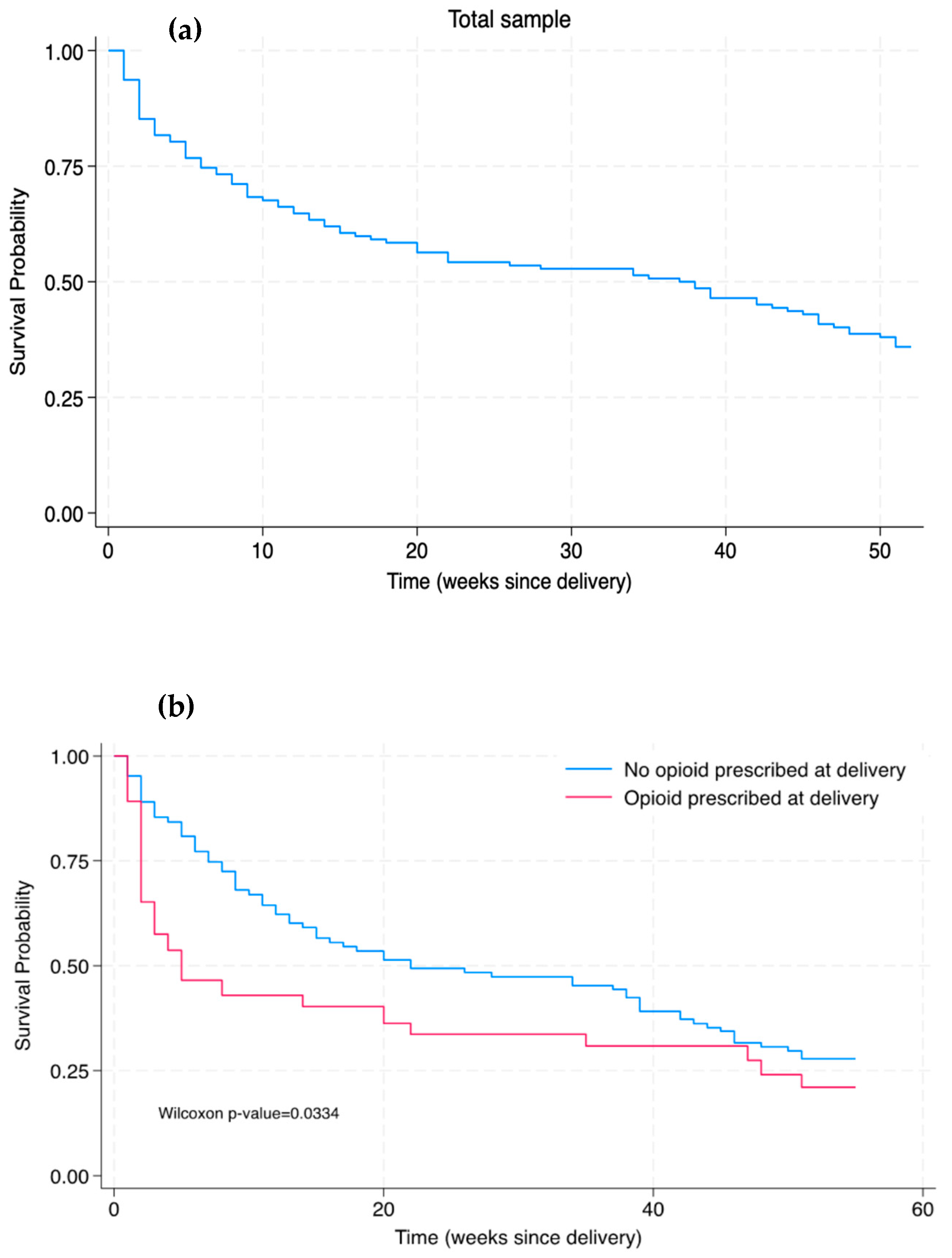
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Sample Criteria
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Committee on Obstetric Practice. Committee Opinion No. 711: Opioid Use and Opioid Use Disorder in Pregnancy. Obstet. Gynecol. 2017, 130, e81–e94. [Google Scholar] [CrossRef] [PubMed]
- Trost, S.; Beauregard, J.; Chandra, G.; Njie, F.; Berry, J.; Harvey, A.; Goodman, D. Pregnancy-Related Deaths: Data from Maternal Mortality Review Committees in 36 US States, 2017–2019. Education 2022, 45. Available online: https://www.cdc.gov/reproductivehealth/maternal-mortality/erase-mm/data-mmrc.html (accessed on 31 January 2024).
- Suarez, E.A.; Huybrechts, K.F.; Straub, L.; Hernández-Díaz, S.; Creanga, A.A.; Connery, H.S.; Gray, K.J.; Vine, S.M.; Jones, H.E.; Bateman, B.T. Postpartum Opioid-Related Mortality in Patients With Public Insurance. Obstet. Gynecol. 2023, 141, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Schiff, D.M.; Nielsen, T.C.; Hoeppner, B.B.; Terplan, M.; Hadland, S.E.; Bernson, D.; Greenfield, S.F.; Bernstein, J.; Bharel, M.; Reddy, J.; et al. Methadone and buprenorphine discontinuation among postpartum women with opioid use disorder. Am. J. Obstet. Gynecol. 2021, 225, 424.E1–424.E12. [Google Scholar] [CrossRef] [PubMed]
- Shadowen, H.; Violante, S.; Gataric, A.; Goulding, A.N.; Martin, C.E. Psychiatric comorbidities and their treatment predict buprenorphine continuation among postpartum people with opioid use disorder. Drug Alcohol Depend. Rep. 2022, 5, 100121. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Meisel, Z.; Kellom, K.; Whitaker, J.; Strane, D.; Chatterjee, A.; Rosenquist, R.; Matone, M. Receipt and duration of buprenorphine treatment during pregnancy and postpartum periods in a national privately-insured cohort. Drug Alcohol Depend. Rep. 2023, 9, 100206. [Google Scholar] [CrossRef] [PubMed]
- Osmundson, S.S.; Min, J.Y.; Wiese, A.D.; Hawley, R.E.; Mitchel, E.; Patrick, S.W.; Samuels, L.R.; Griffin, M.R.; Grijalva, C.G. Opioid Prescribing After Childbirth and Risk for Serious Opioid-Related Events: A Cohort Study. Ann. Intern. Med. 2020, 173, 412–414. [Google Scholar] [CrossRef] [PubMed]
- Peahl, A.F.; Keer, E.; Hallway, A.; Kenney, B.; Waljee, J.F.; Townsel, C. Postpartum Opioid Prescribing in Patients with Opioid Use Prior to Birth. Am. J. Perinatol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.S.; Kasper, Z.; Cicero, T. Assessment of Chronic Pain Management in the Treatment of Opioid Use Disorder: Gaps in Care and Implications for Treatment Outcomes. J. Pain 2021, 22, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Gopman, S. Prenatal and postpartum care of women with substance use disorders. Obstet. Gynecol. Clin. N. Am. 2014, 41, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Holland, E.; Gibbs, L.; Spence, N.Z.; Young, M.; Werler, M.M.; Guang, Z.; Saia, K.; Bateman, B.T.; Achu, R.; Wachman, E.M. A comparison of postpartum opioid consumption and opioid discharge prescriptions among opioid-naïve patients and those with opioid use disorder. Am. J. Obstet. Gynecol. MFM 2023, 5, 101025. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, E.; Dayananda, S.; Morgan, M.; Jarvis, O.; Altamirano, V.; LaSorda, K.R.; Krans, E.; Lim, G. Obstetric pain management for pregnant women with opioid use disorder: A qualitative and quantitative comparison of patient and provider perspectives (QUEST study). Addiction 2023, 118, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.; Soens, M.; Wanaselja, A.; Chyan, A.; Carvalho, B.; Landau, R.; George, R.B.; Klem, M.L.; Osmundson, S.S.; Krans, E.E.; et al. A Systematic Scoping Review of Peridelivery Pain Management for Pregnant People With Opioid Use Disorder: From the Society for Obstetric Anesthesia and Perinatology and Society for Maternal Fetal Medicine. Anesth. Analg. 2022, 135, 912–925. [Google Scholar] [CrossRef] [PubMed]
- Townsel, C.; Irani, S.; Nguyen, B.H.; Hallway, A.; Shuman, C.J.; Waljee, J.; Jaffe, K.; Peahl, A.F. Use of Opioid-Sparing Protocols and Perceived Postpartum Pain in Patients with Opioid Use Disorder and Chronic Prenatal Opioid Exposure. Matern. Child Health J. 2023, 27, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.P.; Parlier-Ahmad, A.B.; Scheikl, M.; Martin, C.E. An Integrated Care Model for Pregnant and Postpartum Individuals Receiving Medication for Opioid Use Disorder. J. Addict. Med. 2022, 17, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Lo-Ciganic, W.; Donohue, J.M.; Kim, J.Y.; Krans, E.E.; Jones, B.L.; Kelley, D.; James, A.E.; Jarlenski, M.P. Adherence trajectories of buprenorphine therapy among pregnant women in a large state Medicaid program in the United States. Pharmacoepidemiol. Drug Saf. 2019, 28, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Shadowen, H.; Shadowen, C.; Thakkar, B.; Knittel, A.K.; Martin, C.E. Incarceration status at buprenorphine initiation and OUD treatment outcomes during pregnancy. Front. Psychiatry 2023, 14, 1157611. [Google Scholar] [CrossRef] [PubMed]
- StataCorp. Stata, Release 18. Statistical Software; StataCorp LLC: College Station, TX, USA, 2023. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]

| Total Sample N(%) N = 142 | Continued Buprenorphine for All 52 Weeks N(%) N = 51 | Discontinued Buprenorphine before 52 Weeks N(%) N = 91 | p-Value | |
|---|---|---|---|---|
| Age (mean years ± SD) 1 | 28.9 ± 4.8 | 28.4 ± 7.3 | 29.1 ± 6.2 | 0.424 |
| Race 2,3 | <0.001 * | |||
| Black | 41 (28.9) | 24 (47.1) | 17 (18.7) | |
| White | 99 (69.7) | 26 (50.9) | 73 (80.2) | |
| Not reported | 2 (1.4) | 1 (2.0) | 1 (1.1) | |
| Delivery type 2 | 0.173 | |||
| Vaginal | 85 (59.9) | 32 (62.8) | 53 (58.2) | |
| Cesarean section | 51 (35.9) | 19 (37.2) | 32 (35.2) | |
| Not reported | 6 (4.2) | 0 (0.0) | 6 (6.6) | |
| Education 2 | 0.446 | |||
| Less than high school | 28 (19.7) | 8 (15.7) | 20 (22.0) | |
| High school completed | 48 (33.8) | 20 (39.2) | 28 (30.8) | |
| Some college or more | 20 (14.1) | 9 (17.7) | 11 (12.1) | |
| Not reported | 46 (32.4) | 14 (27.5) | 32 (35.2) | |
| Insurance status 2 | 0.042 * | |||
| No | 21 (14.8) | 3 (5.9) | 18 (19.8) | |
| Yes | 120 (84.5) | 47 (92.2) | 73 (80.2) | |
| Not reported | 1 (0.7) | 1 (2.0) | 0 (0.0) | |
| Incarcerated at time of delivery 2 | 0.046 * | |||
| No | 115 (81.0) | 46 (90.2) | 69 (75.8) | |
| Yes | 19 (13.4) | 2 (3.9) | 17 (18.7) | |
| Not reported | 8 (5.6) | 3 (5.9) | 5 (5.5) | |
| Opioid prescribed at delivery 2 | 0.608 | |||
| No | 109 (76.8) | 42 (82.4) | 67 (73.6) | |
| Yes | 33 (23.2) | 9 (17.7) | 24 (26.4) | |
| Duration of buprenrophine receipt before delivery (mean weeks ± SD) 1 | 16.5 ± 9.3 | 17.8 ± 8.2 | 15.8 ± 9.8 | 0.223 |
| Psychiatric comorbidity (any) 2 | 0.090 | |||
| No | 40 (28.2) | 10 (19.6) | 30 (33.0) | |
| Yes | 102 (71.8) | 41 (80.4) | 61 (67.0) | |
| Type of psychiatric comorbidity 2,4 | ||||
| ADD/ADHD | 8 (5.6) | 1 (2.0) | 7 (7.7) | 0.155 |
| Anxiety | 78 (54.9) | 31 (60.8) | 47 (51.7) | 0.294 |
| Bipolar disorder/mania | 26 (18.3) | 13 (25.5) | 13 (14.3) | 0.098 |
| Depression | 74 (52.1) | 31 (60.8) | 43 (47.3) | 0.121 |
| Schizophrenia | 5 (3.5) | 3 (5.9) | 2 (2.2) | 0.253 |
| PTSD | 15 (10.6) | 6 (11.8) | 9 (9.9) | 0.727 |
| Types of other substance use disorder comorbidity 2,4 | ||||
| Cocaine-use disorder | 39 (27.5) | 15 (62.8) | 24 (26.4) | 0.920 |
| Cannabis-use disorder | 22 (15.5) | 9 (17.7) | 13 (14.3) | 0.847 |
| Benzodiazepine-use disorder | 14 (9.9) | 4 (7.8) | 10 (11.0) | 0.301 |
| Amphetamine-use Disorder | 5 (3.5) | 1 (2.0) | 4 (4.4) | 0.160 |
| None of the above | 86 (60.1) | 30 (58.8) | 56 (61.5) | 0.761 |
| Chronic pain 2 | 0.137 | |||
| No | 127 (89.4) | 43 (84.3) | 84 (92.3) | |
| Yes | 15 (10.6) | 8 (15.7) | 7 (7.7) | |
| Buprenorphine daily dose range (mg) 2 | 0.151 | |||
| (0–4) | 24 (16.9) | 14 (27.5) | 14 (15.4) | |
| (4–8) | 46 (32.4) | 14 (27.5) | 30 (33.0) | |
| (8–12) | 20 (14.1) | 6 (11.8) | 14 (15.4) | |
| (12–16) | 40 (28.2) | 18 (35.3) | 16 (17.6) | |
| (16–20) | 5 (3.5) | 0 (0.0) | 5 (5.5) | |
| (20–24) | 7 (5.0) | 0 (0.0) | 7 (7.7) | |
| Current tobacco smoking status 2 | 0.520 | |||
| No | 34 (23.9) | 15 (29.4) | 19 (20.9) | |
| Yes | 105 (73.9) | 35 (68.6) | 70 (76.9) | |
| Not reported | 3 (2.2) | 1 (2.0) | 2 (2.2) |
| Unadjusted HR (95% CI) | Adjusted HR (95% CI) 1 | |
|---|---|---|
| Opioid prescribed at delivery | ||
| No | Ref | Ref |
| Yes | 1.40 (0.88, 2.24) | 1.44 (0.90, 2.31) |
| Incarcerated at time of delivery | ||
| No | — | Ref |
| Yes | — | 0.96 (0.91, 1.01) |
| Duration of buprenorphine receipt before delivery (weeks) | — | 1.02 (0.99, 1.06) |
| Psychiatric comorbidity (any) | ||
| No | — | Ref |
| Yes | — | 0.51 (0.32, 0.80) |
| Chronic pain | ||
| No | — | Ref |
| Yes | — | 0.52 (0.24, 1.14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hallet, T.N.; Zhu, D.T.; Shadowen, H.; Thumma, L.; Marcus, M.M.; Salisbury, A.; Martin, C.E. Association of Receipt of Opioid Prescription for Acute Post-Delivery Pain Management with Buprenorphine Discontinuation among Postpartum People with Opioid Use Disorder. Pharmacoepidemiology 2024, 3, 198-207. https://doi.org/10.3390/pharma3020012
Hallet TN, Zhu DT, Shadowen H, Thumma L, Marcus MM, Salisbury A, Martin CE. Association of Receipt of Opioid Prescription for Acute Post-Delivery Pain Management with Buprenorphine Discontinuation among Postpartum People with Opioid Use Disorder. Pharmacoepidemiology. 2024; 3(2):198-207. https://doi.org/10.3390/pharma3020012
Chicago/Turabian StyleHallet, Taylor N., David T. Zhu, Hannah Shadowen, Lillia Thumma, Madison M. Marcus, Amy Salisbury, and Caitlin E. Martin. 2024. "Association of Receipt of Opioid Prescription for Acute Post-Delivery Pain Management with Buprenorphine Discontinuation among Postpartum People with Opioid Use Disorder" Pharmacoepidemiology 3, no. 2: 198-207. https://doi.org/10.3390/pharma3020012
APA StyleHallet, T. N., Zhu, D. T., Shadowen, H., Thumma, L., Marcus, M. M., Salisbury, A., & Martin, C. E. (2024). Association of Receipt of Opioid Prescription for Acute Post-Delivery Pain Management with Buprenorphine Discontinuation among Postpartum People with Opioid Use Disorder. Pharmacoepidemiology, 3(2), 198-207. https://doi.org/10.3390/pharma3020012









