Drug Prescriptions during Pregnancy in Lombardy: Temporal Trends and the Impact of the Onset of the COVID-19 Pandemic
Abstract
1. Introduction
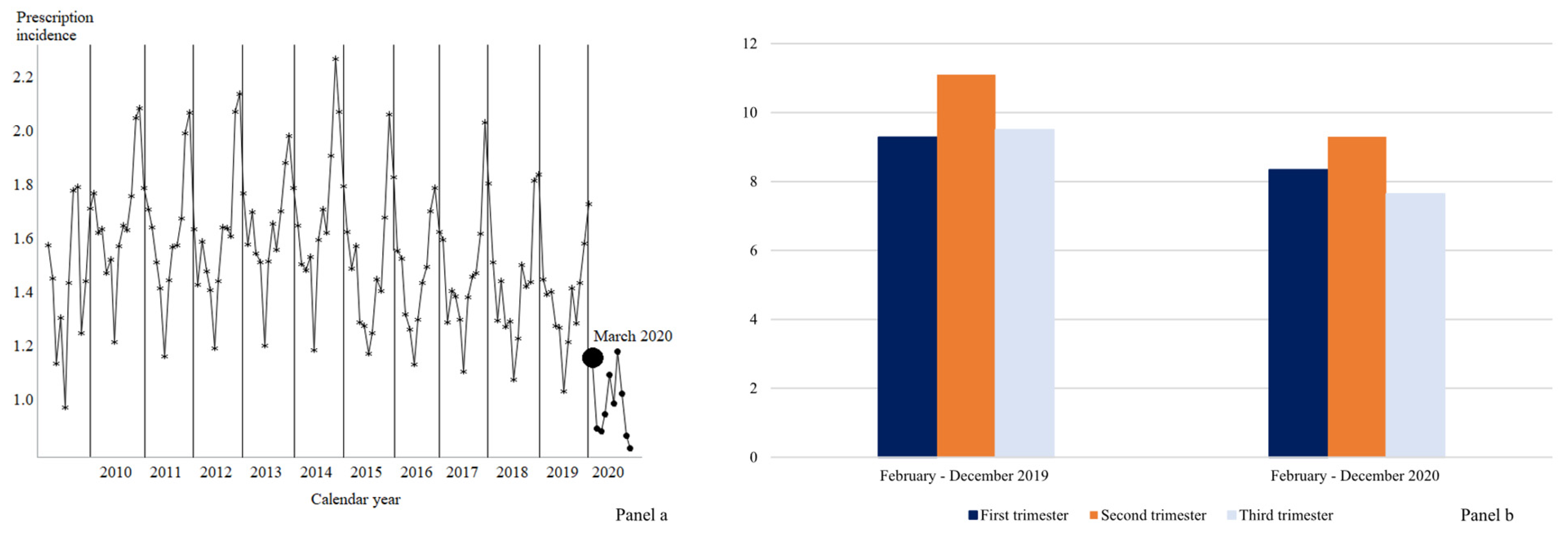
2. Results
3. Discussion
4. Materials and Methods
4.1. Drugs Utilization Patterns
4.2. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ayad, M.; Costantine, M.M. Epidemiology of medications use in pregnancy. Semin. Perinatol. 2015, 39, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Bookstaver, P.B.; Bland, C.M.; Griffin, B.; Stover, K.R.; Eiland, L.S.; McLaughlin, M. A review of antibiotic use in pregnancy. Pharmacotherapy 2015, 35, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Belay, M.; Kahaliw, W.; Ergetie, Z. Assessment of drug utilization pattern during pregnancy in adama riferral hospital, oromia region, ethiopia. Int. J. Pharm. Sci. Res. 2013, 4, 1905–1911. [Google Scholar]
- Yates, L.M.; Thomas, S.H. Prescribing medicines in pregnancy. Medicine 2016, 44, 438–443. [Google Scholar] [CrossRef]
- Daw, J.R.; Hanley, G.E.; Greyson, D.L.; Morgan, S.G. Prescription drug use during pregnancy in developed countries: A systematic review. Pharmacoepidem. Drug Saf. 2011, 20, 895–902. [Google Scholar] [CrossRef]
- Gagne, J.J.; Maio, V.; Berghella, V.; Louis, D.Z.; Gonnella, J.S. Prescription drug use during pregnancy: A population-based study in regione emilia-romagna, italy. Eur. J. Clin. Pharmacol. 2008, 64, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Fortinguerra, F.; Belleudi, V.; Poggi, F.R.; Bortolus, R.; Puccini, A.; Solfrini, V.; Stella, P.; Trotta, F. Medication prescriptions before, during and after pregnancy in italy: A population-based study. Ann. Ist. Super. Sanita 2021, 57, 249–258. [Google Scholar]
- D’Aloja, P.; Da Cas, R.; Belleudi, V.; Fortinguerra, F.; Poggi, F.R.; Perna, S.; Trotta, F.; Donati, S.; Mo, M.N.G. Drug prescriptions among italian and immigrant pregnant women resident in italy: A cross-sectional population-based study. Int. J. Environ. Res. Public Health 2022, 19, 4186. [Google Scholar] [CrossRef]
- Ventura, M.; Maraschini, A.; D’Aloja, P.; Kirchmayer, U.; Lega, I.; Davoli, M.; Donati, S. Drug prescribing during pregnancy in a central region of italy, 2008-2012. BMC Public Health 2018, 18, 623. [Google Scholar] [CrossRef]
- Belleudi, V.; Fortinguerra, F.; Poggi, F.R.; Perna, S.; Bortolus, R.; Donati, S.; Clavenna, A.; Locatelli, A.; Davoli, M.; Addis, A.; et al. The italian network for monitoring medication use during pregnancy (mom-net): Experience and perspectives. Front. Pharmacol. 2021, 12, 699062. [Google Scholar] [CrossRef]
- Available online: https://www.aifa.gov.it/documents/20142/1228539/osmed_uso_farmaci_in_gravidanza.pdf (accessed on 12 January 2023).
- Chianale, M.P.; Gho, E.; Rovere, F.; Ostino, G.; Borga, A.D.; Maggiorotti, P. La gravidanza: La prescrizione e il ricorso ai servizi sanitari studio epidemiologico nel territorio delle UU.SS.LL. di Torino. G. Ital. Farm. Clin. 1990, 4, 5–17. [Google Scholar]
- De Vigan, C.; De Walle, H.E.K.; Cordier, S.; Goujard, J.; Knill-Jones, R.; Ayme, S.; Calzolari, E.; Bianchi, F.; Grp, O.W. Therapeutic drug use during pregnancy: A comparison in four european countries. J. Clin. Epidemiol. 1999, 52, 977–982. [Google Scholar] [CrossRef]
- Donati, S.; Baglio, G.; Spinelli, A.; Grandolfo, M.E. Drug use in pregnancy among italian women. Eur. J. Clin. Pharmacol. 2000, 56, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Maraschini, A.; D’Aloja, P.; Lega, I.; Buoncristiano, M.; Kirchmayer, U.; Ventura, M.; Donati, S. Do italian pregnant women use periconceptional folate supplementation? Ann. Ist. Super. Sanita 2017, 53, 118–124. [Google Scholar] [PubMed]
- U.S. Preventive Services Task Force. Folic acid supplementation for the prevention of neural tube defects: Recommendation statement. Am. Fam. Physician 2017, 95. [Google Scholar]
- Bortolus, R.; Parazzini, F.; Addis, A. Folic acid for the prevention of neural tube defects. JAMA Pediatr. 2017, 171, 709–710. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, R.M.; Leoncini, E.; Gastaldi, P.; Allegri, V.; Agostino, R.; Faravelli, F.; Ferrazzoli, F.; Finale, E.; Ghirri, P.; Scarano, G.; et al. Prevalence and determinants of preconception folic acid use: An italian multicenter survey. Ital. J. Pediatr. 2016, 42, 65. [Google Scholar] [CrossRef]
- De Santis, M.; Quattrocchi, T.; Mappa, I.; Spagnuolo, T.; Licameli, A.; Chiaradia, G.; De Luca, C. Folic acid use in planned pregnancy: An italian survey. Matern. Child Health J. 2013, 17, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, G.C.; Tosto, V.; Tsibizova, V. Progesterone: History, facts, and artifacts. Best. Pract. Res. Clin. Obstet. Gynaecol. 2020, 69, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Engeland, A.; Bjorge, T.; Klungsoyr, K.; Hjellvik, V.; Skurtveit, S.; Furu, K. Trends in prescription drug use during pregnancy and postpartum in norway, 2005 to 2015. Pharmacoepidemiol. Drug Saf. 2018, 27, 995–1004. [Google Scholar] [CrossRef]
- Demailly, R.; Escolano, S.; Quantin, C.; Tubert-Bitter, P.; Ahmed, I. Prescription drug use during pregnancy in france: A study from the national health insurance permanent sample. Pharmacoepidemiol. Drug Saf. 2017, 26, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, M.; Buckingham, K.; Farquhar, C.; Kremer, J.A.M.; Metwally, M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst. Rev. 2015, 2015, CD009154. [Google Scholar] [CrossRef] [PubMed]
- Devall, A.J.; Papadopoulou, A.; Podesek, M.; Haas, D.M.; Price, M.J.; Coomarasamy, A.; Gallos, I.D. Progestogens for preventing miscarriage: A network meta-analysis. Cochrane Database Syst. Rev. 2021, 4, CD013792. [Google Scholar] [PubMed]
- Yan, Y.; Chen, Z.; Yang, Y.; Zheng, X.; Zou, M.; Cheng, G.; Yuan, Z. Efficacy of progesterone on threatened miscarriage: An updated meta-analysis of randomized trials. Arch. Gynecol. Obstet. 2021, 303, 27–36. [Google Scholar] [CrossRef]
- Stokholm, J.; Schjorring, S.; Pedersen, L.; Bischoff, A.L.; Folsgaard, N.; Carson, C.G.; Chawes, B.L.; Bonnelykke, K.; Molgaard, A.; Krogfelt, K.A.; et al. Prevalence and predictors of antibiotic administration during pregnancy and birth. PLoS ONE 2013, 8, e82932. [Google Scholar] [CrossRef]
- Broe, A.; Pottegard, A.; Lamont, R.F.; Jorgensen, J.S.; Damkier, P. Increasing use of antibiotics in pregnancy during the period 2000-2010: Prevalence, timing, category, and demographics. BJOG 2014, 121, 988–996. [Google Scholar] [CrossRef]
- Cantarutti, A.; Rea, F.; Franchi, M.; Beccalli, B.; Locatelli, A.; Corrao, G. Use of antibiotic treatment in pregnancy and the risk of several neonatal outcomes: A population-based study. Int. J. Environ. Res. Public. Health 2021, 18, 12621. [Google Scholar] [CrossRef]
- Fukushige, M.; Ngo, N.H.; Lukmanto, D.; Fukuda, S.; Ohneda, O. Effect of the COVID-19 pandemic on antibiotic consumption: A systematic review comparing 2019 and 2020 data. Front Public Health 2022, 10, 946077. [Google Scholar] [CrossRef]
- Corrao, G.; Cantarutti, A.; Monzio Compagnoni, M.; Franchi, M.; Rea, F. Change in healthcare during COVID-19 pandemic was assessed through observational designs. J. Clin. Epidemiol. 2022, 142, 45–53. [Google Scholar] [CrossRef]
- Esposito, G.; Rossi, M.; Favilli, A.; Franchi, M.; Corrao, G.; Parazzini, F.; La Vecchia, C. Impact of the First and Second Lockdown for COVID-19 Pandemic on Preterm Birth, Low Birth Weight, Stillbirth, Mode of Labor, and of Delivery in Lombardy, Italy. J. Pers. Med. 2023, 13, 499. [Google Scholar] [CrossRef]

| ATC | Drug | 2010–2012 | 2013–2015 | 2016–2018 | Data from National Report from AIFA | 2019–2020 |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |||
| B03BB01 | folic acid | 41,804 (18.0) | 52,527 (23.6) | 54,419 (26.6) | 26.8 | 30,741 (25.2) |
| B03AA07 | ferrous solfate | 40,590 (17.5) | 42,874 (19.3) | 40,473 (19.8) | 20.1 | 24,370 (20.0) |
| G03DA04 | progestogen | 21,353 (9.2) | 28,386 (12.8) | 29,848 (14.6) | 14.5 | 17,532 (14.4) |
| J01CR02 | amoxicillin/clavulanic acid | 26,411 (11.4) | 26,646 (12.0) | 23,257 (11.4) | 11.4 | 12,953 (10.6) |
| J01CA04 | amoxicillin | 17,219 (7.4) | 15,878 (7.1) | 12,384 (6.1) | 6.2 | 6144 (5.0) |
| J01XX01 | fosfomycin | 11,155 (4.8) | 11,674 (5.3) | 11,010 (5.4) | 5.4 | 6397 (5.2) |
| H03AA01 | levothyroxine sodium | 7961 (3.4) | 8817 (4.0) | 8030 (3.9) | 3.9 | 5027 (4.1) |
| J01FA10 | azithromycin | 7730 (3.3) | 8775 (4.0) | 5927 (2.9) | 3.0 | 2712 (2.2) |
| R03BA01 | beclometasone | 5996 (2.6) | 6615 (3.0) | 5543 (2.7) | 2.7 | 2490 (2.0) |
| B01AB05 | enoxaparin | 3697 (1.6) | 4109 (1.8) | 4011 (2.0) | 2720 (2.2) | |
| A11CC05 | colecalciferol | 231 (0.1) | 1660 (0.7) | 5723 (2.8) | 2.4 | 6444 (5.3) |
| A02BX13 | alginic acid | 4017 (1.7) | 4441 (2.0) | 3651 (1.8) | 1858 (1.5) | |
| B03AA01 | ferrous glycine sulfate | 3897 (1.7) | 2717 (1.2) | 2475 (1.2) | 1720 (1.4) | |
| G03CA03 | estradiol | 1681 (0.7) | 2707 (1.2) | 3298 (1.6) | 1704 (1.4) | |
| R03AC02 | salbutamol | 2908 (1.3) | 2808 (1.3) | 2388 (1.2) | 1196 (1.0) | |
| J01FA09 | clarithromycin | 2724 (1.2) | 2605 (1.2) | 2292 (1.1) | 1204 (1.0) | |
| J01DD08 | cefixime | 1866 (0.8) | 2201 (1.0) | 2525 (1.2) | 1883 (1.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, G.; Cantarutti, A.; Franchi, M.; Corrao, G.; Parazzini, F. Drug Prescriptions during Pregnancy in Lombardy: Temporal Trends and the Impact of the Onset of the COVID-19 Pandemic. Pharmacoepidemiology 2023, 2, 249-256. https://doi.org/10.3390/pharma2030021
Esposito G, Cantarutti A, Franchi M, Corrao G, Parazzini F. Drug Prescriptions during Pregnancy in Lombardy: Temporal Trends and the Impact of the Onset of the COVID-19 Pandemic. Pharmacoepidemiology. 2023; 2(3):249-256. https://doi.org/10.3390/pharma2030021
Chicago/Turabian StyleEsposito, Giovanna, Anna Cantarutti, Matteo Franchi, Giovanni Corrao, and Fabio Parazzini. 2023. "Drug Prescriptions during Pregnancy in Lombardy: Temporal Trends and the Impact of the Onset of the COVID-19 Pandemic" Pharmacoepidemiology 2, no. 3: 249-256. https://doi.org/10.3390/pharma2030021
APA StyleEsposito, G., Cantarutti, A., Franchi, M., Corrao, G., & Parazzini, F. (2023). Drug Prescriptions during Pregnancy in Lombardy: Temporal Trends and the Impact of the Onset of the COVID-19 Pandemic. Pharmacoepidemiology, 2(3), 249-256. https://doi.org/10.3390/pharma2030021









