Abstract
The state of knowledge regarding the teratogenic effects of maternal use of medications during pregnancy is constantly evolving and is often uncertain. Timely access to high-quality information may reduce prolonged harmful exposures, decrease the number of preventable birth defects, empower patients with accurate information about the risks of exposure, and prevent unnecessary patient anxiety and pregnancy termination. In this narrative review, we describe the process by which the teratogenic risk of medications is assessed by experts in medicine, genetics, and epidemiology and how identifiable risks can be effectively communicated to patients. Risk assessment of birth defects in human pregnancy involves collecting and synthesizing available data through a proper and rule-driven evaluation of scientific literature. Expert consensus is a practical approach to determine whether a given exposure produces damage after careful consideration of gestational timing, dose and route of the exposure, maternal and fetal genetic susceptibility, as well as evidence for biological plausibility. The provision of teratogen risk counseling through appropriate interpretation of information and effective knowledge translation to the patient is critical for the prevention of birth defects and maximizing healthy pregnancies.
1. Introduction
Birth defects are structural or functional abnormalities that occur during gestation and are identified prenatally or after birth. They are typically seen in about 3–6% of infants [1,2]. Globally, an estimated 8.52 million infants are born with a major malformation every year [3]. Birth defects are considered the second most common cause of infant mortality, following prematurity, and account for up to 25% of all perinatal deaths [4]. Structural anomalies (typically referred to as malformations) are inherent defects in the development of an organ or part of an organ, such as congenital heart defects or neural tube defects. Most structural anomalies arise during the first trimester of pregnancy when organogenesis is taking place [5]. At the severe end, malformations can be incompatible with survival, and their prevalence would be higher in spontaneous abortions than in live-born infants. Functional birth defects, which are not usually apparent at birth, are abnormalities that occur in certain body systems, leading to neurodevelopmental, sensory, metabolic, and immune disorders. The underlying etiology of birth defects is only identifiable in about half of all cases, including chromosomal and single gene conditions, environmental factors, and in those with an established multifactorial origin [2]. Environmental factors that cause birth defects are known as teratogenic exposures, which account for about 5–10% of all cases [6] and are especially important because they are potentially preventable.
The field of clinical teratology involves recognizing and counseling patients and their families about the potential risks of various maternal exposures, including medications and drugs of abuse, infections (e.g., viruses), physical factors (e.g., ionizing radiation), metabolic factors (e.g., obesity), and environmental chemicals, that can cause abnormalities of form, function, or both, in an exposed embryo or fetus. The effects of medication use during pregnancy are probably of most concern to healthcare providers and their patients, as studies show that women take an average of three medications during their entire pregnancy [7,8,9]. However, both prescription and non-prescription medications are not tested for safety in human pregnancy before they are approved for marketing, and the passive adverse event reporting strategies required after approval have not proven sufficient in recognizing drug treatments that are harmful. As a consequence, the average time required to recognize the potential of a teratogenic effect of a newly marketed medication is more than 11 years [10]. Experimental studies with animal models performed by the manufacturer often provide the only data available on maternal medication exposures during pregnancy and embryo–fetal toxicity, but those are seldom published in the peer-reviewed literature. It is also important to recognize the possible teratogenic effects of the mother’s underlying illness, particularly if left untreated. Some maternal conditions, such as diabetes, epilepsy, and depression, have been associated with higher rates of congenital anomalies and other adverse pregnancy and neonatal outcomes in humans [11,12,13,14,15] and adverse effects on embryonic and fetal development in animals [16,17,18]. Epidemiological studies of malformations among infants born to women with chronic medical conditions are often difficult to interpret because the effects of pharmacological treatment cannot be separated from the effects of maternal disease itself. Other confounders, including severity of illness and/or related co-morbidities, make studies such as these challenging to conduct. Healthcare providers and their patients require accessible and timely evidence-based information about the safety of medication use in pregnancy so they are empowered to make informed choices. Clinical assessment of the teratogenic potential of medication exposures requires careful interpretation of the available literature from several kinds of studies [19]. The advent of online clinical teratology knowledge databases, such as TERIS (https://deohs.washington.edu/teris/ (accessed on 1 August 2024)) and Reprotox (https://reprotox.org (accessed on 1 August 2024)), has greatly simplified the process of analyzing epidemiological, clinical, and experimental data, providing clinicians with teratogenic risk ratings and/or narrative guidelines to facilitate communications with their patients. Furthermore, pharmaceutical labels developed in accordance with the revised United States Food and Drug Administration (FDA) Pregnancy and Lactation Labeling Rule [20] and the European Medicines Agency (EMA) [21] have also been of some use to inform prescribing practices, and an opportunity for alignment in labeling across regions is being actively discussed [22].
In addition to the resources described above, Teratology Information Services (TIS) are available for pregnant individuals and the public throughout North America (https://mothertobaby.org (accessed on 1 August 2024)) and Europe (https://www.entis-org.eu (accessed on 1 August 2024)) and provide comprehensive, up-to-date factsheet summaries as well as telephone, texting, and chat services on the reproductive effects of exposures during pregnancy and while breastfeeding [23,24].
2. What Are the Different Measures of “Risk”?
In assessing the teratogenic potential of medications or drugs of abuse in pregnancy, different kinds of risk estimates are usually encountered in the medical literature. In epidemiological studies, two types of controlled methodological approaches are used to assess risks associated with medication exposure in pregnancy. The cohort approach can be used to calculate the risk or odds of events by comparing these outcomes between exposed and unexposed groups, and a case-control approach which compares the odds of exposure between affected and unaffected groups. Relative risk is calculated in cohort studies to estimate how much more likely women who have been treated with a particular medication during pregnancy are to have an affected child compared to women who have not been treated with this medication. Similarly, an odds ratio, which is often used as a numerical equivalent to a relative risk where the event under analysis is rare (such as a malformation), measures the statistical strength of an association using the ratio of the odds of maternal treatment with a particular agent during pregnancy among infants born with birth defects to the odds of it occurring among controls or infants born without birth defects. Relative risks and/or odds ratios are generally simple for epidemiologists to calculate and interpret; however, these measures are only able to establish correlations between exposures and outcomes and not causation. Therefore, confirmatory findings produced from alternative data sources and epidemiological techniques can help to improve confidence that a true association may exist.
An absolute risk is defined as the probability that a woman with a particular drug exposure during pregnancy will deliver a neonate with a birth defect. Health care providers and genetic counselors would typically use an absolute risk measure to communicate risk estimates to their patients as it can be compared directly to other quantifiable and familiar risks, such as the risk of miscarriage, or alternatively could be contrasted against the risk of not treating the mother’s underlying illness. When counseling patients, it can be helpful to use estimates of absolute risk with percentages replaced by expected frequencies (e.g., 1% can be replaced by 1 out of every 100). It is also useful to employ infographics where resources allow one to be mindful of uncertainties in the data (e.g., factoring in the confidence limits) and highlight the importance of contrasting these risks against any perceived benefits of use.
Relative risks and odds ratios can be converted to absolute risks when counseling patients by using the incidence of birth defect(s) of interest in the population studied. For example, a case-control study of maternal use of fluconazole during the first trimester of pregnancy was significantly more frequent than expected among infants with cleft lip with or without cleft palate (odds ratio = 5.53, 95% confidence interval 1.68–18.24) and among infants with d-transposition of the great arteries (odds ratio = 7.56, 95% confidence interval 1.22–35.45) [25]. When given the prevalence of these two birth defects in the general population, these estimates would translate to an absolute risk of 0.4% for cleft lip with or without cleft palate and 0.2% for d-transposition of the great arteries among the infants of women treated with fluconazole during the first trimester.
A third kind of calculated risk commonly reported in the literature is the population-attributable risk. This is the proportion of birth defects (or an adverse outcome of a given type) in the population as a whole that is actually caused by the particular drug treatment during pregnancy. A population-attributable risk is generally used by public health officials when they estimate the amount by which the overall rate of a particular birth defect can be reduced by the prevention of teratogenic exposure in a specific group of patients. For example, the crude population attributable risk for neural tube defects from maternal use of an anticonvulsant agent during pregnancy has been estimated to be 1.02%, whereas it is 9% from maternal obesity, using data from the United States National Birth Defects Prevention Study [26]. These findings suggest that about 1% of cases of neural tube defects are caused by maternal treatment with an anticonvulsant medication in early pregnancy, while 9% are associated with obesity during pregnancy, both of which can potentially be avoided.
When it comes to pregnancy counseling and providing risk estimates to the inquiring patient, it is important to note that the term “high risk” may have different meanings to patients. A teratogenic risk could be considered high if it produces a severe adverse effect, even if the absolute risk of the malformation is relatively low. For example, maternal use of lithium during pregnancy is associated with a rare but severe congenital heart defect called “Epstein’s anomaly”, which has an approximate background prevalence of approximately 1 in 20,000 live-born infants [27,28,29]. In other instances, a risk that is considered numerically large could be interpreted as high even if the resulting effect is mild. An example of this would be maternal use of tetracycline in the second or third trimester of pregnancy and staining of the primary dentition in infants, which is primarily of cosmetic significance [30]. The greatest risk would occur when both the severity and frequency of the birth defect are associated with maternal exposure during critical gestational times. Examples of medications that fall into this category are thalidomide or isotretinoin, which produce major malformations with syndromic embryopathies [31,32].
3. Evaluation and Assessment of the Scientific Literature
In order to establish that a specific exposure is teratogenic in humans, a causal relationship between the occurrence of birth defects and maternal exposure must be demonstrated [19]. The evidence to support or refute such an association can be gleaned from a careful review of the epidemiological, clinical, and experimental literature. Studies that contribute to the determination of teratogenic risk vary in scope and design, and the details of individual study types are discussed below.
3.1. Animal Experimental Studies
The design of human clinical studies that aim to evaluate the teratogenicity of pharmacological drug treatments in pregnancy is often observational because of the ethical complexity of conducting randomized controlled trials in pregnant women. The use of animal models is, therefore, important, as they provide an opportunity to identify exposures that can be teratogenic before humans are harmed. Animal experimental studies also allow precise experimental control over the dose, route, and timing of maternal exposures as well as other important factors (e.g., nutrition, veterinary care, and housing environment), minimizing the influence of these variables on study results. Preclinical testing of prescription medications is carried out by manufacturers using standardized protocols in species such as mice, rats, and rabbits [33]. Nonhuman primates are used in some specialized cases. For many medications, the only data available on the effects of maternal exposure during pregnancy would come from animal teratology studies. However, extrapolating data from these experiments to humans can be challenging because of basic differences in reproduction and development [34]. Most animal teratology studies use genetically inbred strains of rodents or rabbits to reduce genetic variability and maximize the probability that study results can be replicated. This does not, however, model real-world exposures in human populations where differences in genetic susceptibility may play an important role in maternal–fetal outcomes. Another important consideration is that animal experimental studies often administer high doses of medication, which can result in maternal toxicity and reduce specificity and sensitivity for predicting risk in humans. Nevertheless, animal modeling work continues to provide insights into the teratogenicity of agents during pregnancy, but results must be interpreted within the context of species and pharmacokinetic differences.
3.2. Case Reports and Clinical Series
Case reports provide descriptions of individual cases of infants with birth defects born to mothers who have been treated with a specific medication during pregnancy. However, such co-occurrences are common and can be coincidental. Although case reports cannot provide reliable quantitative estimates of the risk of birth defects in an exposed pregnancy, they are important in raising causal hypotheses that can be tested using other types of studies. Clinical series are similar to case reports, but instead of describing only a few cases, they track higher numbers of infants (usually more than five) with a common medication exposure and sometimes a characteristic malformation pattern. Many medications that are currently known to be teratogenic were discovered through publications of clinical series based on observations of distinctive patterns of congenital anomalies, such as isotretinoin [32], mycophenolate mofetil [35], aminopterin, and methotrexate [36]. Clinical series typically include a very thorough assessment of the circumstances of maternal exposure and the clinical manifestations of each affected child, making them well-suited for the recognition of such syndromes. However, a quantitative risk estimate cannot be provided from the case series either, as they lack comparison to controls along with other biases in ascertainment and confounding issues.
3.3. Pregnancy Registries
Pregnancy exposure registry studies are observational studies that are specifically designed to collect information on outcomes among women who have taken a particular medication or a group of medications for a specific indication during pregnancy. They can be regarded as a form of post-marketing surveillance studies that aim to detect signals of teratogenic potential in newly marketed drugs and depend on the voluntary reporting of the exposure either by pregnant women themselves or their health care provider. Pregnancy registries are most useful if such reporting happens in a prospective manner. In a well-designed prospective pregnancy registry, participants typically enroll at conception or in early gestation and are followed through pregnancy or for a defined period afterward. This allows information on study outcomes to be fully collected and evaluated before pregnancy outcomes are known and avoid the potential biases associated with retrospective reporting. Pregnancy registries can also be disease-specific and aim to evaluate the potential for teratogenic risks associated with the disease and its associated therapies. A prototype example is the ongoing North American Antiepileptic Drug Pregnancy Registry, which has provided valuable information to clinicians who counsel women with epilepsy on the teratogenic risks of newer and older antiepileptic medications [37].
There are, however, some inherent limitations in studies based on pregnancy registry design. These include the lack of an appropriate control group, whereby comparisons of the observed rates of congenital anomalies are usually made to expected rates obtained from population-based surveillance systems, such as the Metropolitan Atlanta Congenital Defects Program [38], a population-based surveillance system that uses active methods to identify and classify birth defects. Another limitation of pregnancy registries is the often incomplete reporting of outcomes. This can occur when prenatal care providers only report information on congenital anomalies diagnosed within a short period of time after birth, lacking a comprehensive view of the first post-natal year. In addition, the reliability and consistency of these assessments varies between examiners. Furthermore, a commonly encountered problem with pregnancy registries is the lengthy period of time (and associated high costs) required to enroll an adequate number of subjects so that accurate estimates of risk can be calculated. Because pregnancy registries are often not population-based, and some depend on voluntary recruiting, selection bias can be an issue. It is important to note, however, that when exposure and outcome data are collected in a standardized and rigorous manner, pregnancy registries become an important tool to ascertain data for high-quality exposure cohort studies such as those conducted by OTIS/MotherToBaby [39].
3.4. Cohort Studies
Cohort studies can be prospective (Figure 1) or retrospective and are designed to compare the frequency of birth defects among children born to women treated with a particular agent during pregnancy to the frequency of the same outcome among children whose mothers were untreated. Untreated women in comparator groups can be healthy or matched for maternal disease. A relative risk is estimated by comparing the frequency of a specific adverse outcome between exposed and unexposed infants. Insufficient statistical power can be encountered in cohort studies, especially if the exposures and the outcomes being assessed are both rare.
In population-based prospective cohort studies, medication exposures are recorded during pregnancy before birth outcomes are known, and this information is recorded for all pregnancies in a specific population or country. These studies often continue after the birth of the infant and provide an opportunity for follow-up research on child health and development. The main advantage of these studies is that the number of affected births accumulates over time, allowing for increased power to test associations of rare outcomes without the need for the high costs entailed by project-specific data collection. At the same time, maintaining a long follow-up for these studies can be costly and challenging, and only a few of these types of studies have been successful in assessing the teratogenic risks of maternal medication use during pregnancy. The oldest existing study of this type that continues to assess the risk of several types of medications during pregnancy is the Swedish Medical Birth Register [40]. Data from this study are collected through maternal interviews in early and late pregnancy exposures and postpartum on nearly all births in Sweden, while information on the outcomes is based on standardized medical records and physical examinations by qualified pediatricians. The teratogenic risk of medication use during pregnancy has also been investigated using similar and larger studies by combining data from all Nordic countries (Denmark, Norway, Finland, Iceland, and Sweden) that adopted the same approach of data collection and follow-up [41,42,43,44].
Prospective project-specific cohorts, which are often called exposure cohort studies, identify women when they request counseling about the teratogenic potential of a medication of interest through the Teratogen Information Service (TIS) provision. Women are then prospectively followed through maternal interviews for the course of their pregnancy to determine the frequency of the outcome under study as compared to that in an unexposed group of women. In the strongest of these exposure cohort studies, meticulous, blinded physical examinations of the children born to exposed and unexposed women would be performed by a dysmorphologist rather than relying on information on the birth outcomes from mothers, physicians, or medical records [45]. One disadvantage of these studies, however, is the relatively small sample sizes, which can only detect very strong teratogenic effects. Another limitation is the voluntary enrollment of women who call a TIS, which may not be representative of the population as a whole, therefore presenting a selection bias to the data reported from these studies. In retrospective (historical) cohort studies, the same methodology described above is utilized, but investigators often use pre-existing data to identify exposed and unexposed mothers and their birth outcomes. In both prospective and retrospective cohort investigations, the effect of medication exposure is tested for an association with an increase in the overall frequency of congenital anomalies or anatomical classes of major malformations. This approach, however, may not reflect the contemporary understanding of teratogenic and developmental mechanisms [46]. Therefore, these studies are sometimes not able to identify recurrent patterns of minor or major congenital anomalies that constitute the characteristic syndromes associated with most human teratogenic exposures.
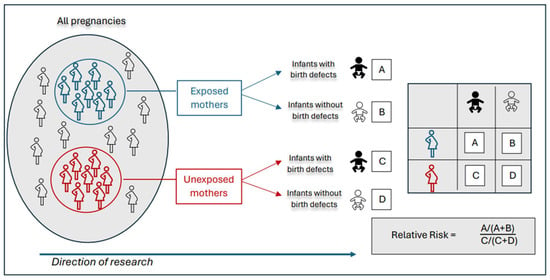
Figure 1.
In a prospective cohort study, exposed and unexposed pregnant women are identified in a population and followed over time. Outcome assessments occur after birth to determine whether infants are born with or without birth defects. Relative risk is calculated as the proportion of affected infants among exposed pregnancies divided by the proportion of affected infants among unexposed pregnancies. Adapted from Hales et al. [47].
3.5. Case-Control Studies
In contrast to cohort studies, case-control studies are used to compare the frequency of maternal medication use during pregnancy between mothers of children with birth defects (cases) and those without birth defects (controls) (Figure 2). Exposure information in case-control studies is usually collected retrospectively through interviews or questionnaires given to the mothers after they have had their babies, which may present bias of recall. However, given that most of these studies are population-based, many kinds of ascertainment bias can also be avoided, and they become very useful for studying rare outcomes, such as specific anomalies. One of the limitations of these investigations is that they only provide information regarding the outcome or outcomes of interest to the study, and large datasets are required to obtain sufficient statistical power.
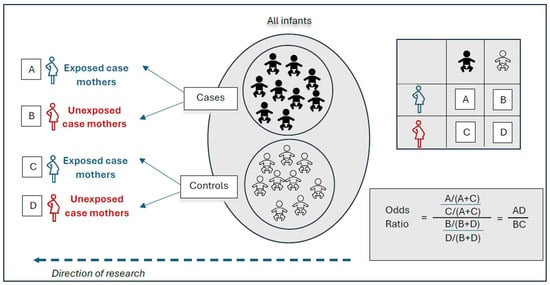
Figure 2.
In a retrospective case-control study, cases (infants with birth defects) and controls (infants without birth defects) are identified from the same population. Maternal exposure assessments are made after birth. The odds ratio is calculated by dividing the odds of maternal exposure among case infants by the odds of maternal exposure among the control infants. Adapted from Hales et al. [47].
One of the largest case-control studies that had the power to assess the teratogenic risk of many drug exposures during pregnancy was the National Birth Defects Prevention Study in the United States (NBDPS). In its original design, the NBDPS was a large, multi-site study of environmental and genetic risk factors for 34 selected categories of major birth defects [48]. Case infants were live-born, stillborn, or induced terminations that were ascertained through population-based birth defects surveillance systems at each participating site. Controls were live-born infants without a diagnosis of major birth defect identified from the same geographical areas and time periods as case infants through hospital state birth records. Hundreds of studies have been published using data from the NBDPS, adding substantially to our understanding of the relationship between commonly used medications during pregnancy and the risk of birth defects [49]. These include, but are not limited to, studies on maternal use of common antidepressant medications, specifically with regard to selective serotonin reuptake inhibitors (SSRIs) [50,51], opioid analgesics [52], and antibacterial medications [53]. Another example is the Slone Epidemiology Center Birth Defects Study (also known as the Pregnancy Health Interview Study), an older, multi-site case-control surveillance program based at Boston University, similar in design to the NBDPS, but not overlapping as catchment and eligibility criteria prevented participants from enrolling in both studies [54,55].
An important factor of concern in case-control studies, such as the NBPDS and the Pregnancy Health Interview Study, is that many case groups of different kinds of birth defects will be analyzed for associations with several types of medication exposures (such as separate medications within one class) simultaneously. This creates a problem called “multiple comparisons”, whereby some of the associations that are observed in the study will probably not be causal but instead are due to chance. Another problem that can occur in such studies is when a statistically significant risk is identified that is real but clinically irrelevant. For example, an exposure that doubles the risk of a rare (and not very severe) birth defect from 1 in 100,000 in unexposed pregnancies to 2 in 100,000 in exposed pregnancies but does not affect the risk of other congenital anomalies would have little clinical significance to the individual pregnant woman who seeks counseling, even if it could add ample scientific evidence to the pathogenesis of this defect.
3.6. Record Linkage Studies
In record linkage studies, data are collected for administrative purposes (e.g., prescription records or hospital discharge summaries) at the time a medical service is provided and are electronically linked to birth outcomes on a case-by-case basis. Record linkage studies are population-based and provide a way of performing studies in a cost-effective manner. Cohort studies are often conducted using data extracted from linked administrative databases, but a nested case-control analysis can also be performed through record linkage studies. The main advantage of linked administrative record studies is their relative cost-effectiveness as they use existing databases to identify exposed pregnancies and adverse outcomes, so it is not necessary to collect such data specifically for the study. This can also be the greatest limitation for these studies because data collected for other purposes are not ideal for identifying associations between maternal exposures during pregnancy and birth defects in the offspring. As an example, a record showing that a particular medication was dispensed does not always indicate the timing of exposure or if the medication was actually taken. In addition, information on important covariates is usually limited and cannot be managed when analyzing the data. Nevertheless, population-based record linkage studies have been performed to estimate the risk and/or safety of medication exposures in different regions of the world, but the largest of these is the European Surveillance of Congenital Anomalies (EUROCAT) network, which consists of 43 population-based registries (covering 29% of all births in Europe) set up for the epidemiological surveillance of congenital anomalies [56].
3.7. Meta-Analyses
Meta-analytic reviews provide a quantitative approach to identifying, evaluating, and synthesizing data across multiple epidemiology studies. Conclusions on teratogenic risk drawn from meta-analyses benefit from larger sample sizes and increased statistical power to detect adverse birth outcomes. However, meta-analyses are often subject to the limitation of combining data from studies with different methodologies and/or definitions of exposure or outcome, which could result in an inaccurate interpretation of the available data. An important bias that often occurs in meta-analyses is publication bias, which is when studies that show a positive effect, especially a large one, are more likely to be published in comparison to those that have negative findings [57,58].
4. How Do Clinical Teratology Experts Determine the Teratogenicity of Medication Exposures?
Key attributes of results from different types of studies are carefully extracted and used to generate data-driven estimates of the danger an agent may pose to the embryo or fetus after gestational exposure. Consensus across a team of experts on an agent’s teratogenic risk rating can be a particularly valuable qualitative approach because specialists from different fields of medicine make unique contributions to the risk determination. These experts are generally guided by the Six Principles of Teratology (summarized in Table 1) set forth by the former teratology pioneer, Dr. James G. Wilson [59]. Many clinical teratology authorities that followed James Wilson adopted these principles in their research and practice, including Drs. Thomas Shepard [60], Robert Brent [61], and Lewis Holmes [62]. Some of the currently established teratogenic agents were brought to light using evidence from those principles, such as warfarin [63], aminopterin [36], and isotretinoin [32] as prescription medications, alcohol as a recreational drug [64], and rubella and varicella as viruses [65,66].
In order to facilitate decision-making, Thomas Shepard provided an updated framework of seven criteria to “proof” teratogenicity based on his review of the collective work of former colleagues [60]. Shepard’s Criteria (see Table 2), also referred to as the Astute Clinician Model, remains a valuable asset for expert consensus on the causality of an association that has been observed between maternal exposure during pregnancy and the occurrence of birth defects in exposed infants [67]. In fact, Shepard’s criteria played a crucial role in discovering the teratogenicity of the Zika virus by scientists at the United States Centers for Disease Control and Prevention [68] and provided valuable insights into clinical practice and public health measures. In this viral outbreak, causality was established through expert consensus using the “rare exposure rare defect approach”, when criteria 1, 3, and 4 were fulfilled. Alternatively, using the “epidemiological approach”, the causality of the virus was confirmed when criteria 1, 2, and 3 were fulfilled. In this example, the Shepard framework, along with clinical research and an exhaustive literature review, were vital components of the risk determination process. In large teratology databases, such as TERIS, clinical experts from the fields of medicine, genetics, and epidemiology use these guiding principles to generate consensus-driven teratogenic risk ratings for thousands of medications.

Table 1.
Several factors that determine the teratogenicity of an exposure have been set forth in the Six Principles of Teratology by James G. Wilson [69].
Table 1.
Several factors that determine the teratogenicity of an exposure have been set forth in the Six Principles of Teratology by James G. Wilson [69].
|
|
|
|
|
|

Table 2.
Criteria for establishing teratogenicity of environmental exposures as proposed by Thomas H. Shepard [60].
Table 2.
Criteria for establishing teratogenicity of environmental exposures as proposed by Thomas H. Shepard [60].
|
|
|
|
|
|
|
5. Knowledge Translation and Teratogenic Risk Counseling
Providing teratogen risk counseling involves knowing how to effectively communicate the estimated magnitude of risk to patients in a way that allows them to make informed decisions about the management of their pregnancy. This may be dependent on many factors, including the woman’s cultural and social background and her level of scientific knowledge, as well as her commitment to the pregnancy. Furthermore, counselors must be on the lookout for over-estimated fears from misinformation or the negatively biased information patients have seen on the internet or social media platforms [70,71,72,73]. The fact that there is insufficient available information to estimate the magnitude and severity of the risk associated with the majority of prescription medications [74] and that the teratogenic risk for many over-the-counter and herbal remedies remains undetermined further complicates counseling efforts when patients expect to be provided with precise, data-driven estimates of the safety or risk of their medication use. This uncertainty and limitation of knowledge from the perspective of health care providers or clinical counselors is important to be acknowledged and admitted to patients. While this may be unsatisfactory for the patient as well as the counselor, it is certainly more appropriate than assuming that lack of data means a lack of risk or that this exposure must be avoided or poses a significant risk to the developing baby.
Nevertheless, the existence of TIS centers across North America, Europe, and other parts of the world provide timely evidence-based information directly to patients and healthcare providers about exposures in pregnancy through a toll-free telephone line and/or chat-based online service hosted on informative websites [45,75,76]. These services consolidate multidisciplinary expertise representing teratology, toxicology, pharmacology, epidemiology, obstetrics, perinatology, clinical genetics, psychology, infectious disease, and occupational health to address questions regarding the potential of a specific maternal exposure during pregnancy to interfere with normal embryonic or fetal development [45]. Key features of an ideal TIS should include immediacy, where information is provided directly and effectively to patients in real-time, and scalability that allows the service to be delivered through a virtual workspace. Another important feature of TIS is accessibility, whereby women living in remote and rural areas can have equal access to medical expertise. Some patients who have had previous negative experiences in accessing health services may feel vulnerable or judged around their potential exposures, such as having used recreational drugs, and may feel hesitant about seeking help in person or even over the phone. Online accessibility and anonymity are important features of some TIS centers that have bridged this barrier by increasing service uptake and improving fetal outcomes for this population [77]. In general, TIS centers have demonstrated high levels of clinical effectiveness and satisfaction among users and have shown enormous benefits in public health measures of improving maternal and neonatal health outcomes [78,79].
6. Conclusions
The majority of birth defects cannot be prevented, but those associated with maternal use of prescribed medications during pregnancy can potentially be avoided through informed clinical decision-making and patient education. Determining the magnitude of the embryonic or fetal developmental risk from maternal medication use is, however, a challenging task and one that requires the distillation and analysis of complex scientific literature. Both observational (clinical and epidemiological) studies using human data and experimental studies with animal models contribute to identifying the teratogenic potential of medications and other exposures in pregnancy. The benefit of human epidemiological studies is obtaining quantitative estimates regarding the strength and statistical significance of associations between medication or drug exposures during pregnancy and congenital anomalies, but they are only informative after damage has already taken place. Experimental animal studies, on the other hand, are useful in providing a means of identifying teratogenic agents before being marketed, but it is not always possible to translate findings from animal teratology studies to human clinical cases. On the other hand, one should never underestimate the impact of the underlying maternal illness on fetal development if left untreated. There are plenty of examples when untreated maternal disease poses a much greater risk to the embryo or fetus than does exposure to the medication [80,81].
To assist healthcare professionals and maximize the chance of healthy births, clinical teratology experts employ a variety of assessment methods to generate quantitative and/or qualitative estimates of teratogenic risk from maternal exposures. These data-driven risk estimates should be made on the basis of the reproducibility, biological plausibility, and consistency of the available literature. The utilization of established teratogenic risk criteria and the adoption of consensus-based decision-making provide a globally consistent framework for standardized teratogen risk ratings to guide patient counseling.
Funding
This review received no external funding.
Acknowledgments
The authors would like to thank Thomas M. Burbacher, for his review and constructive criticism of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Centers for Disease Control and Prevention. Update on Overall Prevalence of Major Birth Defects–Atlanta, Georgia, 1978–2005. MMWR Morb. Mortal Wkly. Rep. 2008, 57, 1–5. [Google Scholar]
- Lobo, I.; Zhaurova, K. Birth defects: Causes and statistics. Nat. Educ. 2008, 1, 18. [Google Scholar]
- Kang, L.; Cao, G.; Jing, W.; Liu, J.; Liu, M. Global, regional, and national incidence and mortality of congenital birth defects from 1990 to 2019. Eur. J. Pediatr. 2023, 182, 1781–1792. [Google Scholar] [CrossRef] [PubMed]
- National Center for Health Statistics (U.S.). Infant Mortality in the United States, 2020: Data From the Period Linked Birth/Infant Death File. 2022. Available online: https://stacks.cdc.gov/view/cdc/120700 (accessed on 4 October 2024).
- Sadler, T.W. Langman’s Medical Embryology, 15th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2024. [Google Scholar]
- Brent, R.L. The role of the pediatrician in preventing congenital malformations. Pediatr. Rev. 2011, 32, 411–421; quiz 422. [Google Scholar] [CrossRef] [PubMed]
- Werler, M.M.; Kerr, S.M.; Ailes, E.C.; Reefhuis, J.; Gilboa, S.M.; Browne, M.L.; Kelley, K.E.; Hernandez-Diaz, S.; Smith-Webb, R.S.; Garcia, M.H.; et al. Patterns of Prescription Medication Use during the First Trimester of Pregnancy in the United States, 1997–2018. Clin. Pharmacol. Ther. 2023, 114, 836–844. [Google Scholar] [CrossRef]
- Subramanian, A.; Azcoaga-Lorenzo, A.; Anand, A.; Phillips, K.; Lee, S.I.; Cockburn, N.; Fagbamigbe, A.F.; Damase-Michel, C.; Yau, C.; McCowan, C.; et al. Polypharmacy during pregnancy and associated risk factors: A retrospective analysis of 577 medication exposures among 1.5 million pregnancies in the UK, 2000–2019. BMC Med. 2023, 21, 21. [Google Scholar] [CrossRef]
- Mitchell, A.A.; Gilboa, S.M.; Werler, M.M.; Kelley, K.E.; Louik, C.; Hernandez-Diaz, S. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am. J. Obstet. Gynecol. 2011, 205, e51–e58. [Google Scholar] [CrossRef]
- Adam, M.P.; Polifka, J.E.; Friedman, J.M. Evolving knowledge of the teratogenicity of medications in human pregnancy. Am. J. Med. Genet. C Semin. Med. Genet. 2011, 157c, 175–182. [Google Scholar] [CrossRef]
- Nimby, G.T.; Lundberg, L.; Sveger, T.; McNeil, T.F. Maternal distress and congenital malformations: Do mothers of malformed fetuses have more problems? J. Psychiatr. Res. 1999, 33, 291–301. [Google Scholar] [CrossRef]
- Carmichael, S.L.; Shaw, G.M. Maternal life event stress and congenital anomalies. Epidemiology 2000, 11, 30–35. [Google Scholar] [CrossRef]
- Chan, J.; Natekar, A.; Einarson, A.; Koren, G. Risks of untreated depression in pregnancy. Can. Fam. Physician 2014, 60, 242–243. [Google Scholar] [PubMed]
- Kokhanov, A. Congenital Abnormalities in the Infant of a Diabetic Mother. Neoreviews 2022, 23, e319–e327. [Google Scholar] [CrossRef] [PubMed]
- Yerby, M.S. Pregnancy, teratogenesis, and epilepsy. Neurol. Clin. 1994, 12, 749–771. [Google Scholar] [CrossRef] [PubMed]
- Zabihi, S.; Loeken, M.R. Understanding diabetic teratogenesis: Where are we now and where are we going? Birth Defects Res. A Clin. Mol. Teratol. 2010, 88, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, P.; Yan, F.; Luo, Y.; Zhao, G. Animal Models of Epilepsy: A Phenotype-oriented Review. Aging Dis. 2022, 13, 215–231. [Google Scholar] [CrossRef]
- Cottrell, E.C.; Seckl, J.R. Prenatal stress, glucocorticoids and the programming of adult disease. Front. Behav. Neurosci. 2009, 3, 19. [Google Scholar] [CrossRef]
- Friedman, J.M. Editorial In Bed with The Devil: Recognizing Human Teratogenic Exposures. Birth Defects Res. 2017, 109, 1407–1413. [Google Scholar] [CrossRef]
- FDA. Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. Final rule. Fed. Regist. 2014, 79, 72063–72103. [Google Scholar]
- European Medicines Agency. Risk Assessment of Medicinal Products on Human. Reproduction and Lactation: From Data to Labelling–European Medicines Agency. 2009. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-risk-assessment-medicinal-products-human-reproduction-and-lactation-data-labelling_en.pdf (accessed on 4 October 2024).
- Kappel, D.; Sahin, L.; Yao, L.; Thor, S.; Kweder, S. A Comparison of FDA and EMA Pregnancy and Lactation Labeling. Clin. Pharmacol. Ther. 2023, 113, 1251–1257. [Google Scholar] [CrossRef]
- Hancock, R.L.; Ungar, W.J.; Einarson, A.; Koren, G. International practices in the provision of teratology information: A survey of international teratogen information programmes and comparisons with the North American model. J. Eval. Clin. Pract. 2010, 16, 957–963. [Google Scholar] [CrossRef]
- Schaefer, C. Drug safety in pregnancy: Utopia or achievable prospect? Risk information, risk research and advocacy in Teratology Information Services. Congenit. Anom. 2011, 51, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Howley, M.M.; Carter, T.C.; Browne, M.L.; Romitti, P.A.; Cunniff, C.M.; Druschel, C.M.; National Birth Defects Prevention, S. Fluconazole use and birth defects in the National Birth Defects Prevention Study. Am. J. Obstet. Gynecol. 2016, 214, 657.e1–657.e9. [Google Scholar] [CrossRef] [PubMed]
- Agopian, A.J.; Tinker, S.C.; Lupo, P.J.; Canfield, M.A.; Mitchell, L.E. Proportion of neural tube defects attributable to known risk factors. Birth Defects Res. A Clin. Mol. Teratol. 2013, 97, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Giles, J.J.; Bannigan, J.G. Teratogenic and developmental effects of lithium. Curr. Pharm. Des. 2006, 12, 1531–1541. [Google Scholar] [CrossRef]
- Yacobi, S.; Ornoy, A. Is lithium a real teratogen? What can we conclude from the prospective versus retrospective studies? A review. Isr. J. Psychiatry Relat. Sci. 2008, 45, 95–106. [Google Scholar]
- Grandjean, E.M.; Aubry, J.M. Lithium: Updated human knowledge using an evidence-based approach: Part III: Clinical safety. CNS Drugs 2009, 23, 397–418. [Google Scholar] [CrossRef]
- Billings, R.J.; Berkowitz, R.J.; Watson, G. Teeth. Pediatrics 2004, 113, 1120–1127. [Google Scholar] [CrossRef]
- McBride, W.G. Thalidomide embryopathy. Teratology 1977, 16, 79–82. [Google Scholar] [CrossRef]
- Lammer, E.J.; Chen, D.T.; Hoar, R.M.; Agnish, N.D.; Benke, P.J.; Braun, J.T.; Curry, C.J.; Fernhoff, P.M.; Grix, A.W., Jr.; Lott, I.T.; et al. Retinoic acid embryopathy. N. Engl. J. Med. 1985, 313, 837–841. [Google Scholar] [CrossRef]
- Wise, L.D.; Buschmann, J.; Feuston, M.H.; Fisher, J.E.; Hew, K.W.; Hoberman, A.M.; Lerman, S.A.; Ooshima, Y.; Stump, D.G. Embryo-fetal developmental toxicity study design for pharmaceuticals. Birth Defects Res. B Dev. Reprod. Toxicol. 2009, 86, 418–428. [Google Scholar] [CrossRef]
- Daston, G.P. Laboratory models and their role in assessing teratogenesis. Am. J. Med. Genet. C Semin. Med. Genet. 2011, 157c, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Sifontis, N.M.; Coscia, L.A.; Constantinescu, S.; Lavelanet, A.F.; Moritz, M.J.; Armenti, V.T. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation 2006, 82, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Warkany, J. Aminopterin and methotrexate: Folic acid deficiency. Teratology 1978, 17, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Diaz, S.; Smith, C.R.; Shen, A.; Mittendorf, R.; Hauser, W.A.; Yerby, M.; Holmes, L.B.; North American, A.E.D.P.R.; North American, A.E.D.P.R. Comparative safety of antiepileptic drugs during pregnancy. Neurology 2012, 78, 1692–1699. [Google Scholar] [CrossRef]
- Correa, A.; Cragan, J.D.; Kucik, J.E.; Alverson, C.J.; Gilboa, S.M.; Balakrishnan, R.; Strickland, M.J.; Duke, C.W.; O’Leary, L.A.; Riehle-Colarusso, T.; et al. Reporting birth defects surveillance data 1968–2003. Birth Defects. Res. A Clin. Mol. Teratol. 2007, 79, 65–186. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.D.; Braddock, S.R.; Briggs, G.G.; Einarson, A.; Johnson, Y.R.; Miller, R.K.; Polifka, J.E.; Robinson, L.K.; Stepanuk, K.; Lyons Jones, K. Postmarketing surveillance for human teratogenicity: A model approach. Teratology 2001, 64, 252–261. [Google Scholar] [CrossRef]
- Cnattingius, S.; Källén, K.; Sandström, A.; Rydberg, H.; Månsson, H.; Stephansson, O.; Frisell, T.; Ludvigsson, J.F. The Swedish medical birth register during five decades: Documentation of the content and quality of the register. Eur. J. Epidemiol. 2023, 38, 109–120. [Google Scholar] [CrossRef]
- Laugesen, K.; Ludvigsson, J.F.; Schmidt, M.; Gissler, M.; Valdimarsdottir, U.A.; Lunde, A.; Sørensen, H.T. Nordic Health Registry-Based Research: A Review of Health Care Systems and Key Registries. Clin. Epidemiol. 2021, 13, 533–554. [Google Scholar] [CrossRef]
- Pedersen, L.; Petronis, K.R.; Nørgaard, M.; Mo, J.; Frøslev, T.; Stephansson, O.; Granath, F.; Kieler, H.; Sørensen, H.T. Risk of adverse birth outcomes after maternal varenicline use: A population-based observational study in Denmark and Sweden. Pharmacoepidemiol. Drug Saf. 2020, 29, 94–102. [Google Scholar] [CrossRef]
- Graner, S.; Svensson, T.; Beau, A.B.; Damase-Michel, C.; Engeland, A.; Furu, K.; Hviid, A.; Håberg, S.E.; Mølgaard-Nielsen, D.; Pasternak, B.; et al. Neuraminidase inhibitors during pregnancy and risk of adverse neonatal outcomes and congenital malformations: Population based European register study. Bmj 2017, 356, j629. [Google Scholar] [CrossRef]
- Pasternak, B.; Wintzell, V.; Furu, K.; Engeland, A.; Neovius, M.; Stephansson, O. Oral Fluconazole in Pregnancy and Risk of Stillbirth and Neonatal Death. Jama 2018, 319, 2333–2335. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C. The role of teratology information services in screening for teratogenic exposures: Challenges and opportunities. Am. J. Med. Genet. C Semin. Med. Genet. 2011, 157C, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M. Is it time to re-think how we look for teratogenic effects in exposure cohort studies? Paediatr. Perinat. Epidemiol. 2024, 38, 532–534. [Google Scholar] [CrossRef]
- Hales, B.; Scialli, A.; Tassinari, M. Teratology Primer, 3rd ed.; Society for Birth Defects Research and Prevention: Reston, VA, USA, 2018; Available online: https://www.birthdefectsresearch.org/primer/ (accessed on 4 October 2024).
- Reefhuis, J.; Gilboa, S.M.; Anderka, M.; Browne, M.L.; Feldkamp, M.L.; Hobbs, C.A.; Jenkins, M.M.; Langlois, P.H.; Newsome, K.B.; Olshan, A.F.; et al. The National Birth Defects Prevention Study: A review of the methods. Birth Defects Res. A Clin. Mol. Teratol. 2015, 103, 656–669. [Google Scholar] [CrossRef]
- Alwan, S.; Chambers, C.D. Findings from the National Birth Defects Prevention Study: Interpretation and translation for the clinician. Birth Defects Res. A Clin. Mol. Teratol. 2015, 103, 721–728. [Google Scholar] [CrossRef]
- Alwan, S.; Reefhuis, J.; Rasmussen, S.A.; Olney, R.S.; Friedman, J.M. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N. Engl. J. Med. 2007, 356, 2684–2692. [Google Scholar] [CrossRef]
- Anderson, K.N.; Lind, J.N.; Simeone, R.M.; Bobo, W.V.; Mitchell, A.A.; Riehle-Colarusso, T.; Polen, K.N.; Reefhuis, J. Maternal Use of Specific Antidepressant Medications During Early Pregnancy and the Risk of Selected Birth Defects. JAMA Psychiatry 2020, 77, 1246–1255. [Google Scholar] [CrossRef]
- Broussard, C.S.; Rasmussen, S.A.; Reefhuis, J.; Friedman, J.M.; Jann, M.W.; Riehle-Colarusso, T.; Honein, M.A. Maternal treatment with opioid analgesics and risk for birth defects. Am. J. Obstet. Gynecol. 2011, 204, e311–e314. [Google Scholar] [CrossRef] [PubMed]
- Crider, K.S.; Cleves, M.A.; Reefhuis, J.; Berry, R.J.; Hobbs, C.A.; Hu, D.J. Antibacterial medication use during pregnancy and risk of birth defects: National Birth Defects Prevention Study. Arch. Pediatr. Adolesc. Med. 2009, 163, 978–985. [Google Scholar] [CrossRef]
- Werler, M.M.; Shapiro, S.; Mitchell, A.A. Periconceptional folic acid exposure and risk of occurrent neural tube defects. JAMA 1993, 269, 1257–1261. [Google Scholar] [CrossRef]
- Louik, C.; Lin, A.E.; Werler, M.M.; Hernandez-Diaz, S.; Mitchell, A.A. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N. Engl. J. Med. 2007, 356, 2675–2683. [Google Scholar] [CrossRef] [PubMed]
- Greenlees, R.; Neville, A.; Addor, M.C.; Amar, E.; Arriola, L.; Bakker, M.; Barisic, I.; Boyd, P.A.; Calzolari, E.; Doray, B.; et al. Paper 6: EUROCAT member registries: Organization and activities. Birth Defects Res. A Clin. Mol. Teratol. 2011, 91 (Suppl. S1), S51–S100. [Google Scholar] [CrossRef] [PubMed]
- Koren, G.; Nickel, C. Perpetuating fears: Bias against the null hypothesis in fetal safety of drugs as expressed in scientific citations. J. Popul. Ther. Clin. Pharmacol. 2011, 18, e28–e32. [Google Scholar]
- Koren, G.; Graham, K.; Shear, H.; Einarson, T. Bias against the null hypothesis: The reproductive hazards of cocaine. Lancet 1989, 2, 1440–1442. [Google Scholar] [CrossRef]
- Wilson, J. Current status of teratology: General principles and mechanisms derived from animal studies. In Handbook of Teratalogy; Wilson, J., Fraser, F., Eds.; Plenum Press: New York, NY, USA, 1977; pp. 47–74. [Google Scholar]
- Shepard, T.H. “Proof” of human teratogenicity. Teratology 1994, 50, 97–98. [Google Scholar] [CrossRef]
- Brent, R.L. Evaluating the alleged teratogenicity of environmental agents. Clin. Perinatol. 1986, 13, 609–613. [Google Scholar] [CrossRef]
- Holmes, L.B. Human teratogens: Delineating the phenotypic effects, the period of greatest sensitivity, the dose-response relationship and mechanisms of action. Prog. Clin. Biol. Res. 1988, 281, 177–191. [Google Scholar] [PubMed]
- van Driel, D.; Wesseling, J.; Sauer, P.J.; Touwen, B.C.; van der Veer, E.; Heymans, H.S. Teratogen update: Fetal effects after in utero exposure to coumarins overview of cases, follow-up findings, and pathogenesis. Teratology 2002, 66, 127–140. [Google Scholar] [CrossRef]
- Jones, K.L.; Smith, D.W.; Ulleland, C.N.; Streissguth, P. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet 1973, 1, 1267–1271. [Google Scholar] [CrossRef]
- Miller, E.; Cradock-Watson, J.E.; Pollock, T.M. Consequences of confirmed maternal rubella at successive stages of pregnancy. Lancet 1982, 2, 781–784. [Google Scholar] [CrossRef]
- Enders, G.; Miller, E. Varicella and herpes zoster in pregnancy and the newborn. In Varicella-Zoster Virus: Virology and Clinical Management; Cambridge University Press: Cambridge, UK, 2000; pp. 317–348. [Google Scholar]
- Carey, J.C.; Martinez, L.; Balken, E.; Leen-Mitchell, M.; Robertson, J. Determination of human teratogenicity by the astute clinician method: Review of illustrative agents and a proposal of guidelines. Birth Defects Res. A Clin. Mol. Teratol. 2009, 85, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Jamieson, D.J.; Honein, M.A.; Petersen, L.R. Zika Virus and Birth Defects--Reviewing the Evidence for Causality. N. Engl. J. Med. 2016, 374, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.G. Experimental studies on congenital malformations. J. Chronic Dis. 1959, 10, 111–130. [Google Scholar] [CrossRef] [PubMed]
- Shahin, I.; Einarson, A. Knowledge transfer and translation: Examining how teratogen information is disseminated. Birth Defects Res. A Clin. Mol. Teratol. 2011, 91, 956–961. [Google Scholar] [CrossRef]
- Sayakhot, P.; Carolan-Olah, M. Internet use by pregnant women seeking pregnancy-related information: A systematic review. BMC Pregnancy Childbirth 2016, 16, 65. [Google Scholar] [CrossRef]
- Yakuwa, N.; Nakajima, K.; Koinuma, S.; Goto, M.; Suzuki, T.; Ito, N.; Watanabe, O.; Murashima, A. Perception of pregnant Japanese women regarding the teratogenic risk of medication exposure during pregnancy and the effect of counseling through the Japan drug information institute in pregnancy. Reprod. Toxicol. 2018, 79, 66–71. [Google Scholar] [CrossRef]
- Nörby, U.; Noël-Cuppers, B.; Hristoskova, S.; Desai, M.; Härmark, L.; Steel, M.; El-Haddad, C.; Douarin, L. Online information discrepancies regarding safety of medicine use during pregnancy and lactation: An IMI ConcePTION study. Expert. Opin. Drug Saf. 2021, 20, 1117–1124. [Google Scholar] [CrossRef]
- Lo, W.Y.; Friedman, J.M. Teratogenicity of Recently Intorduced Medications in Human Pregnancy. Obstet. Gynecol. 2002, 100, 465–473. [Google Scholar]
- Schaefer, C.; Hannemann, D.; Meister, R. Post-marketing surveillance system for drugs in pregnancy--15 years experience of ENTIS. Reprod. Toxicol. 2005, 20, 331–343. [Google Scholar] [CrossRef]
- Clementi, M.; Di Gianantonio, E.; Ornoy, A. Teratology information services in Europe and their contribution to the prevention of congenital anomalies. Community Genet. 2002, 5, 8–12. [Google Scholar] [CrossRef]
- De Santis, M.; De Luca, C.; Mappa, I.; Quattrocchi, T.; Angelo, L.; Cesari, E. Smoke, alcohol consumption and illicit drug use in an Italian population of pregnant women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 159, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.L.; Koren, G.; Einarson, A.; Ungar, W.J. The effectiveness of Teratology Information Services (TIS). Reprod. Toxicol. 2007, 23, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, Y.C.; Karadas, B.; Kucuksolak, G.; Ediz, B.; Demir, O.; Sozmen, K.; Nordeng, H. Counselling pregnant women at the crossroads of Europe and Asia: Effect of Teratology Information Service in Turkey. Int. J. Clin. Pharm. 2017, 39, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Eichler, J.; Schmidt, R.; Poulain, T.; Hiemisch, A.; Kiess, W.; Hilbert, A. Maternal depressive symptoms and stress during pregnancy as predictors of gestational age at birth and standardized body mass index from birth up to 2 years of age. BMC Pregnancy Childbirth 2021, 21, 635. [Google Scholar] [CrossRef]
- Labor, S.; Dalbello Tir, A.M.; Plavec, D.; Juric, I.; Roglic, M.; Pavkov Vukelic, J.; Labor, M. What is safe enough–asthma in pregnancy—A review of current literature and recommendations. Asthma Res. Pract. 2018, 4, 11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).