Impact of Antibiotic De-Escalation on Antibiotic Consumption, Length of Hospitalization, Mortality, and Cost: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
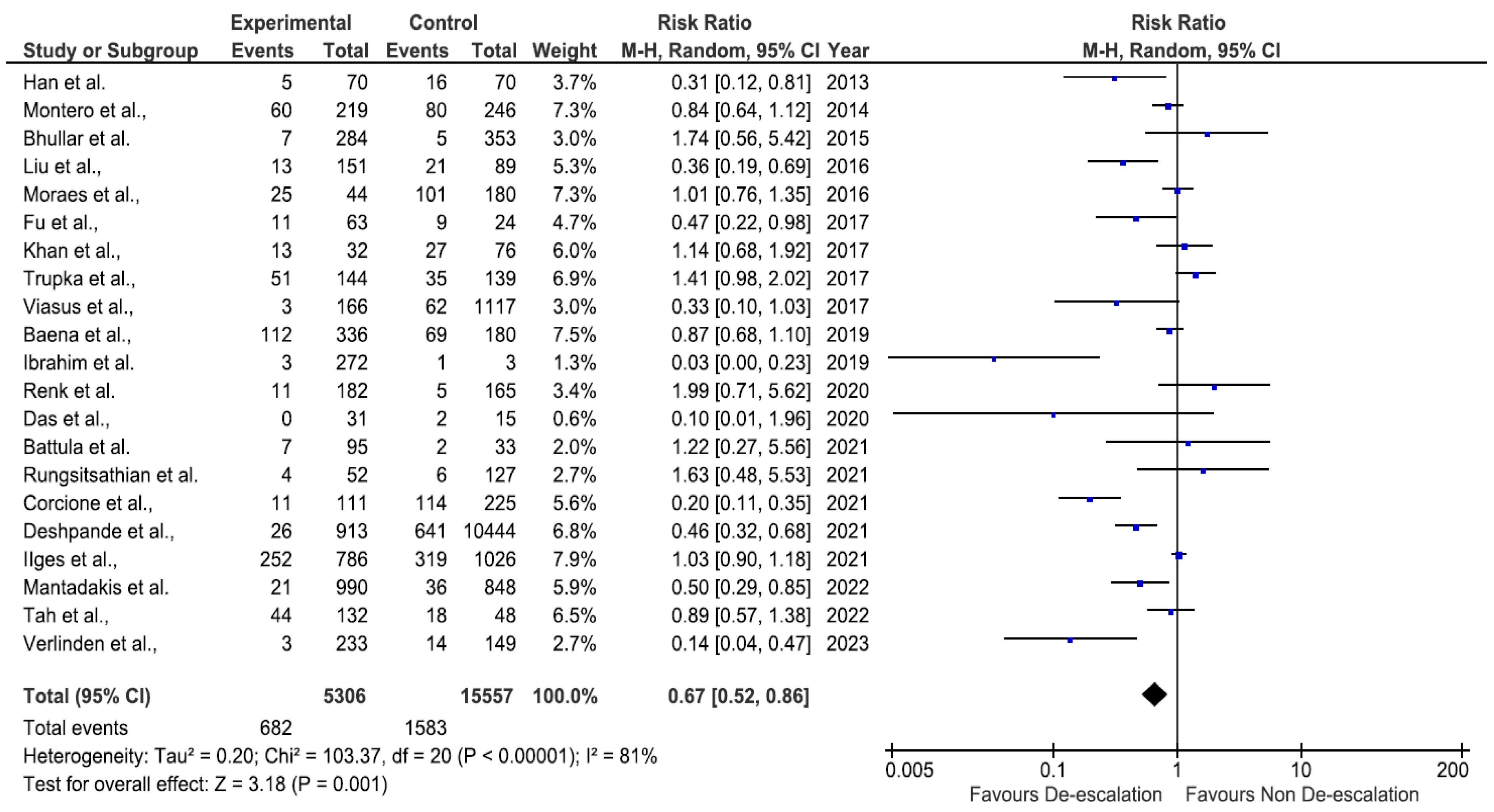
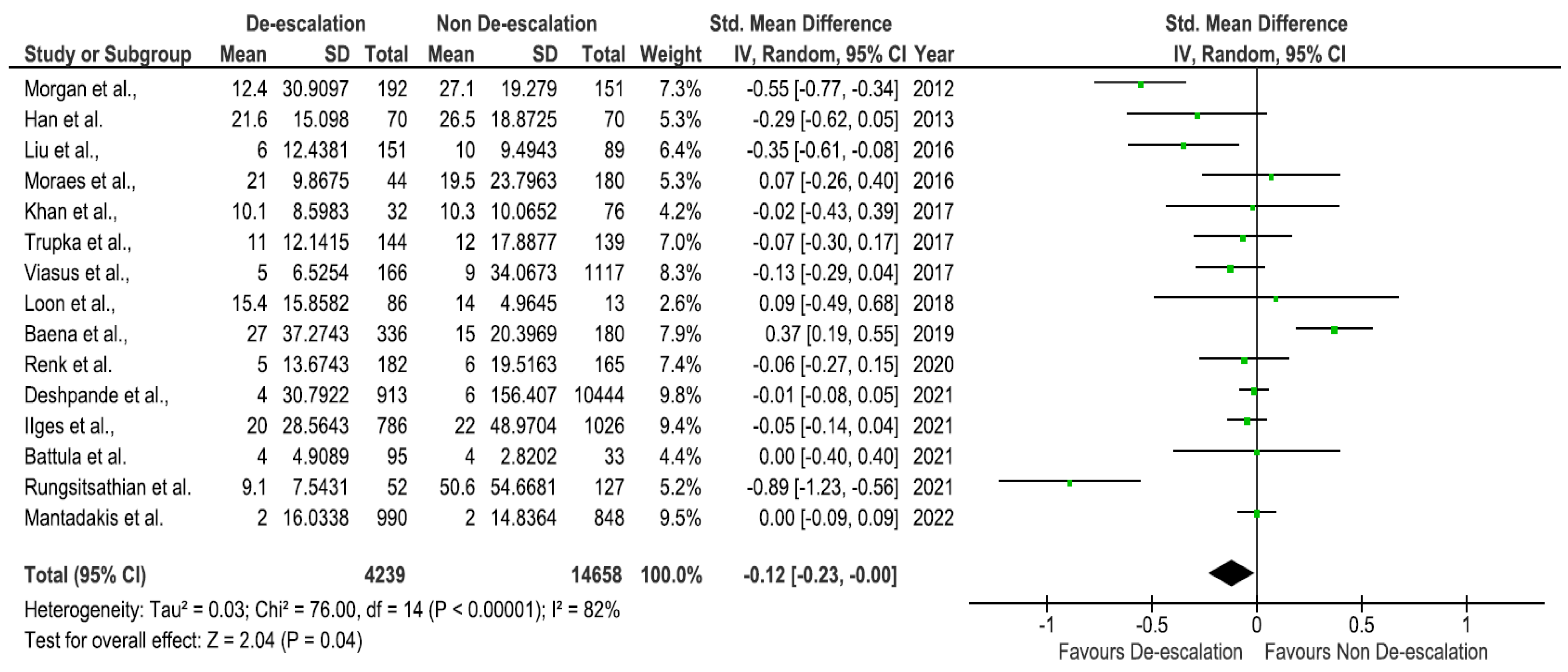
2. Results
| Author and Year | Country | Study Design | Study Duration | Settings | Sample Size | Condition of Patients | De-Escalation Definition | Type of Empirical Antibiotics Used | Endpoints Measured | Reported De-Escalation Rate * |
|---|---|---|---|---|---|---|---|---|---|---|
| Renk et al., 2020 [26] | Germany | Prospective study | 2017–2018 | PICU | 347 | Mixed | Not specified | Cefazolin Meropenem Vancomycin | Antimicrobial utilization Length of stay Infection related mortality | 28.0% |
| Battula et al., 2021 [33] | India | Prospective study | January 2019–June 2019 | PICU | 247 | Sepsis | Specified | Cephalosporins Carbapenems | Antimicrobial utilization Length of stay Infection related mortality | 38.4% |
| Bhullar et al., 2015 [34] | India | Prospective study | June 2013–March 2014 | PICU | 637 | Mixed | Not specified | Piperacillin Meropenem linezolid | Duration of antibiotic used | 34.6% |
| Han et al., 2013 [25] | China | Prospective study | February 2012–February 2017 | PICU | 140 | Pneumonia | Not specified | Not stated | Therapy efficacy Length of stay Duration of antibiotic used | 50.0% |
| Rungsitsathian et al., 2021 [35] | Thailand | Prospective study | March–December 2019 | General Units, Oncology Unit and ICU | 225 | Mixed | Specified | Meropenem | Clinical success rate. Prevalence of acquisition of CR-GNB | 57.8% |
| Mantadakis et al., 2022 [36] | Greece | Prospective study | June 2016–November 2017 | Oncology and BMT units | 1838 | Febrile neutropenia | Not specified | Amikacin/Gentamicin Cefepime Ceftriaxone/cefotaxime | Clinical success rate Mortality rate Length of ICU stay | 53.5% |
| Ibrahim et al., 2019 [37] | Malaysia | Prospective study | September 2017–December 2017 | NICU | 276 | EOS | Specified | Penicillin/gentamicin Penicillin/amikacin Ampicillin/gentamicin | Neonatal risk factors Maternal risk factors Length of ICU stay | 98.5% |
| Study and Year | Days of Antibiotic Therapy DOT/1000 Patients | Mortality Rates | Mean Length of Stay | Overall Costs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-De-Escalation Group (Days) | De-Escalation Group (Days) | Differences in Percentage Points (Days) | Non-De-Escalation Group N (%) | De-Escalation Group N (%) | Differences in Percentage Points (%) | Non-De-Escalation Group (Days) | De-Escalation Group (Days) | Differences in Days | Non-De-Escalation Group (in USD) | De-Escalation Group (in USD) | Differences in Costs | |
| Renk et al., 2020 [26] | 1569 | 1333 | −236 | 5 (3.0%) | 11 (6.0%) | 3.0% | 6 | 5 | −1 | 4688 | 3463 | −1225 |
| Battula et al., 2021 [33] | Not stated | Not stated | - | 2 (6.0%) | 7 (7.3%) | 1.3% | 4 | 4 | 0 | Not stated | Not stated | - |
| Bhullar et al., 2015 [34] | 7.4 | 6.3 | −1.1 | 5 (1.4%) | 7 (2.4%) | 1.0% | Not stated | Not stated | - | Not stated | Not stated | - |
| Han et al., 2013 [25] | 18.8 | 14.6 | −4.2 | 16 (22.8%) | 5 (7.1%) | −15.7% | 26.5 | 21.9 | −4.6 | 2193 | 1297 | −896 |
| Rungsitsathian et al., 2021 [35] | 50.6 | 11 | −39.6 | 6 (4.7%) | 4 (7.6%) | 2.9% | 50.6 | 9.1 | −41.5 | Not stated | Not stated | - |
| Mantadakis et al., 2022 [36] | 517 | 501 | −16 | 36 (4.2%) | 21 (2.1%) | −2.1% | 2 | 2 | 0 | Not stated | Not stated | - |
| Ibrahim et al., 2019 [37] | 3.9 | 2.2 | −1.7 | 1 (33.3%) | 3 (1.1%) | −32.2% | Not stated | Not stated | - | Not stated | Not stated | - |
| Author and Year | Country | Study Design | Study Duration | Settings | Sample Size | Condition of Patients | De-Escalation Definition | Type of Antibiotics Used | Endpoints Measured | Reported De-Escalation Rate |
|---|---|---|---|---|---|---|---|---|---|---|
| Viasus et al., 2017 [27] | Spain | Retrospective study | February 1995–December 2014 | Emergency department | 1283 | CAP | Specified | Beta-lactams | Mortality rate Length of stay Antibiotics utilization | 12.9% |
| Tah et al., 2022 [29] | Malaysia | Retrospective study | January 2016–July 2019 | Medical wards | 180 | CAP and HAP | Specified | Carbapenems, colistin, and vancomycin | Mortality rate Survival rate | 73.3% |
| Fu et al., 2017 [38] | China | Retrospective study | 2006–2015 | Tertiary care hospital | 87 | Severe Aplastic anemia | Specified | Not stated | Mortality rate Survival rate | 72.41% |
| Verlinden et al., 2023 [39] | Belgium | Retrospective study | November 2011–February 2021 | Hematology ward | 958 | Mixed | Specified | Amikacin, meropenem, and piperacillin/tazobactam | Infection related ICU admission Mortality rate Antibiotics utilization | - |
| Morgan et al., 2012 [32] | USA | Retrospective study | September 2009–October 2010 | 6 Hospitals | 631 | Mixed | Not specified | Cephalosporins, fluoroquinolones, and penicillin | Antibiotics utilization | 30.43% |
| Deshpande et al., 2021 [31] | USA | Retrospective study | 2010–2015 | 164 Hospitals | 14,170 | Pneumonia | Specified | Not stated | Length of stay Healthcare costs Antibiotic utilization | <50% |
| Loon et al. 2018 [30] | Malaysia | Prospective study | July 2017–September 2017 | Medical wards | 99 | Mixed | Not specified | Cephalosporins, piperacillin/tazobactam, and carbapenems | Length of stay Antibiotic utilization | 86.9% |
| Liu et al., 2016 [40] | USA | Retrospective study | January 2011–December 2011 | Medical center | 240 | Mixed | Specified | Vancomycin and piperacillin/tazobactam | Length of stay Antibiotic utilization | 63.0% |
| Lim et al., 2021 [28] | Malaysia | Retrospective study | November 2018–November 2019 | ICU | 382 | Mixed | Not specified | Carbapenems and vancomycin | Antibiotic utilization Isolation of pathogens in ICU | 96.2% |
| Corcione et al., 2021 [41] | Italy | Retrospective study | January 2016–November 2017 | Emergency department | 336 | Mixed | Not specified | Not stated | Frequency of ADE Length of stay In-hospital mortality | 33.03% |
| Khan et al., 2017 [13] | Malaysia | Retrospective study | January 2012–December 2014 | ICU | 108 | VAP | Specified | Carbapenems, colistin, and cefepime | Mortality rate Length of ICU stay | 42.1% |
| Singh et al., 2019 [42] | India | Prospective study | June 2017–December 2017 | ICU | 75 | Mixed | Specified | Colistin, carbapenems, and piperacillin/tazobactam | Adequacy of antibiotic therapy Culture positivity rates | 24% |
| Trupka et al., 2017 [43] | USA | Prospective study | January 2016–December 2016 | ICU | 283 | Pneumonia | Specified | Carbapenems, quinolones and cephalosporins | Mortality rate Length of ICU stay Antibiotic utilization | 50.9% |
| Ilges et al., 2021 [44] | USA | Retrospective study | 2016–2019 | Medical center | 1812 | Pneumonia | Specified | Not stated | Mortality rate Length of ICU stay Onset of infection | 43.37% |
| Das et al., 2020 [45] | India | Retrospective study | July 2018–September 2018 | ICU | 83 | Mixed | Not specified | Carbapenem, glycopeptides, and monobactam | Clinical success rate Length of hospital stay | 55.4% |
| Montero et al., 2014 [46] | Spain | Prospective study | January 2008–May 2012 | ICU | 712 | Sepsis and septic shock | Specified | Not stated | Length of hospital stay Mortality rate | 34.9% |
| Baena et al., 2019 [47] | Spain | Prospective study | January 2012–December 2013 | 13 hospitals | 516 | Bacteremia | Specified | Piperacillin/tazobactam, carbapenems, and cephalosporins | Length of hospital stay Mortality rate Clinical success rate | 65.1% |
| Moraes et al., 2016 [48] | Brazil | Prospective study | April 2013–September 2013 | Tertiary care hospital | 224 | Severe sepsis | Specified | Not stated | Antibiotic adequacy Culture positivity Mortality rate Length of hospital stay | 19.6% |
| Study and Year | Day of Antibiotic Therapy DOT/1000 Patients | Mortality Rates | Mean Length of Stay | Overall Costs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-De-Escalation Group (Days) | De-Escalation Group (Days) | Differences in Percentage Points (Days) | Non-De-Escalation Group N (%) | De-Escalation Group N (%) | Differences in Percentage Points (%) | Non-De-Escalation Group (Days) | De-Escalation Group (Days) | Differences in Days | Non-De-Escalation Group (in USD) | De-Escalation Group (in USD) | Differences in Costs | |
| Viasus et al., 2017 [27] | 5 | 3 | −2 | 62 (5.5%) | 3 (1.8%) | −3.7 | 9 | 5 | −4 | Not stated | Not stated | - |
| Tah et al., 2022 [29] | Not stated | Not stated | Not stated | 18 (37.5%) | 44 (33.3%) | −4.2 | Not stated | Not stated | - | Not stated | Not stated | - |
| Fu et al., 2017 [38] | Not stated | Not stated | Not stated | 9 (37.5%) | 11 (17.4%) | −20.1 | Not stated | Not stated | - | Not stated | Not stated | - |
| Verlinden et al., 2023 [39] | 14 | 12 | −2 | 14 (9.3%) | 3 (1.2%) | −8.1 | Not stated | Not stated | - | Not stated | Not stated | - |
| Morgan et al., 2012 [32] | Not stated | Not stated | Not stated | Not stated | Not stated | - | 27.1 | 12.4 | −14.7 | Not stated | Not stated | - |
| Deshpande et al., 2021 [31] | 7 | 5 | −2 | 641 (6.1%) | 26 (2.8%) | −3.3 | 6 | 4 | −2 | 10,869 | 7855 | −3014 |
| Loon et al. 2018 [30] | Not stated | Not stated | Not stated | Not stated | Not stated | - | 14 | 15.4 | −1.4 | Not stated | Not stated | - |
| Liu et al., 2016 [40] | Not stated | Not stated | Not stated | 21 (23.5%) | 13 (8.6%) | −14.9 | 10 | 6 | −4 | Not stated | Not stated | - |
| Lim et al., 2021 [28] | Not stated | Not stated | Not stated | Not stated | Not stated | - | Not stated | Not stated | - | Not stated | Not stated | - |
| Corcione et al., 2021 [41] | Not stated | Not stated | Not stated | 114 (50.6%) | 11 (9.9%) | −40.7 | Not stated | Not stated | - | Not stated | Not stated | - |
| Khan et al., 2017 [13] | Not stated | Not stated | Not stated | 27 (35.5%) | 13 (40.6%) | 5.1 | 10.3 | 10.1 | −0.2 | Not stated | Not stated | - |
| Singh et al., 2019 [42] | Not stated | Not stated | Not stated | Not stated | Not stated | - | Not stated | Not stated | - | Not stated | Not stated | - |
| Trupka et al., 2017 [43] | 7 | 7 | 0 | 35 (25.1%) | 51 (35.4%) | 10.3 | 12 | 11 | 1 | Not stated | Not stated | - |
| Ilges et al., 2021 [44] | 11 | 9 | −2 | 319 (31.0%) | 252 (32.0%) | 1 | 22 | 20 | −2 | Not stated | Not stated | - |
| Das et al., 2020 [45] | Not stated | Not stated | Not stated | 2 (13.3%) | 0 (0%) | −13.3 | Not stated | Not stated | - | Not stated | Not stated | - |
| Montero et al., 2014 [46] | Not stated | Not stated | Not stated | 80 (32.5%) | 60 (27.3%) | −5.2 | Not stated | Not stated | - | Not stated | Not stated | - |
| Baena et al., 2019 [47] | 15 | 27 | 12 | 69 (38.3%) | 112 (33.3%) | −5 | 15 | 27 | 12 | Not stated | Not stated | - |
| Moraes et al., 2016 [48] | 19.5 | 21 | 1.5 | 101 (56.1%) | 25 (56.8%) | 0.7 | 19.5 | 21 | 1.5 | Not stated | Not stated | - |
| Selection | Comparability | Outcomes | ||||||
|---|---|---|---|---|---|---|---|---|
| Reference | Representative of Sample A | Sample Size B | Non-Respondents C | Ascertainment of Exposure D | Comparability of Cohort Studies on Basis of Design E | Assessment of Outcomes F | Statistical Analysis G | Quality Score |
| Renk et al., 2020 [26] | * | * | - | * | * | ** | * | 7 |
| Battula et al., 2021 [33] | * | * | - | - | * | ** | * | 6 |
| Bhullar et al., 2015 [34] | * | * | - | - | * | ** | * | 6 |
| Han et al., 2013 [25] | * | * | - | - | * | ** | * | 6 |
| Rungsitsathian et al., 2021 [35] | * | * | - | * | * | ** | * | 7 |
| Mantadakis et al., 2022 [36] | * | * | - | * | * | ** | * | 7 |
| Ibrahim et al., 2019 [37] | * | * | - | * | * | ** | * | 7 |
| Viasus et al., 2017 [27] | * | * | - | * | * | ** | * | 7 |
| Tah et al., 2022 [29] | * | * | - | * | * | ** | * | 7 |
| Fu et al., 2017 [38] | * | * | - | * | * | ** | * | 7 |
| Verlinden et al., 2023 [39] | * | * | - | * | * | ** | * | 7 |
| Morgan et al., 2012 [32] | * | * | - | - | * | ** | - | 5 |
| Deshpande et al., 2021 [31] | * | * | - | - | * | ** | * | 6 |
| Loon et al. 2018 [30] | * | * | - | - | * | ** | * | 6 |
| Liu et al., 2016 [40] | * | * | - | * | * | ** | * | 7 |
| Lim et al., 2021 [28] | * | * | - | - | * | ** | * | 6 |
| Corcione et al., 2021 [41] | * | * | - | * | * | ** | * | 7 |
| Khan et al., 2017 [13] | * | * | - | - | * | ** | * | 6 |
| Singh et al., 2019 [42] | * | * | - | - | * | ** | - | 5 |
| Trupka et al., 2017 [43] | * | * | - | * | * | ** | * | 7 |
| Ilges et al., 2021 [44] | * | * | - | - | * | ** | * | 6 |
| Das et al., 2020 [45] | * | * | * | * | ** | * | 7 | |
| Montero et al., 2014 [46] | * | * | - | * | * | ** | * | 7 |
| Baena et al., 2019 [47] | * | * | - | - | * | ** | * | 6 |
| Moraes et al., 2016 [48] | * | * | - | - | * | ** | * | 6 |
3. Discussion
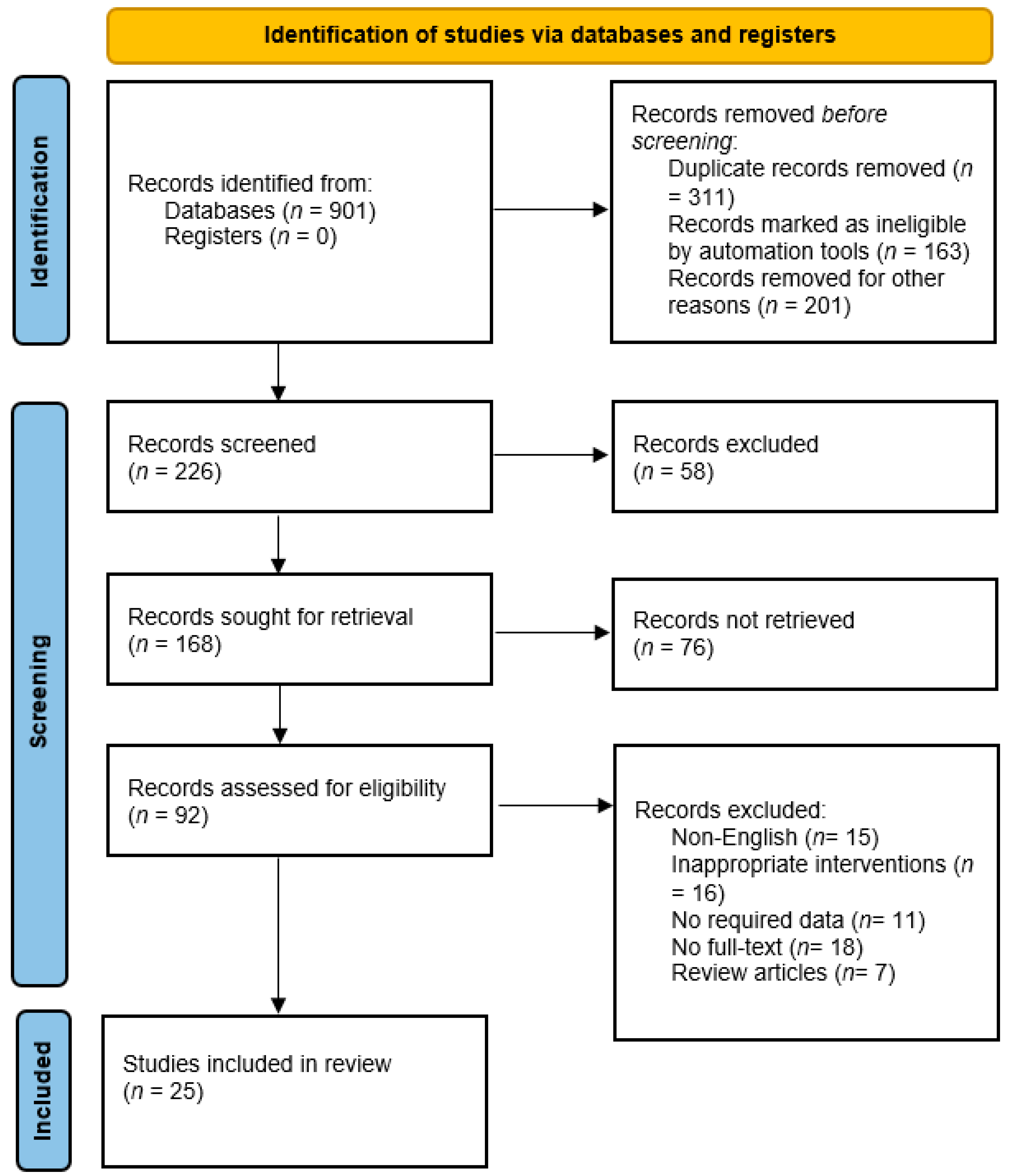
4. Materials and Methods
4.1. Search Strategy
4.2. Eligibility Criteria
4.3. Data Extraction
4.4. Quality Assessment
4.5. Meta-Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamilton, K.W. Miracle Cure: The Creation of Antibiotics and the Birth of Modern Medicine. Emerg. Infect. Dis. 2019, 25, 196. [Google Scholar] [CrossRef]
- Zou, X.-X.; Fang, Z.; Min, R.; Bai, X.; Zhang, Y.; Xu, D.; Fang, P.-Q. Is nationwide special campaign on antibiotic stewardship program effective on ameliorating irrational antibiotic use in China? Study on the antibiotic use of specialized hospitals in China in 2011–2012. J. Huazhong Univ. Sci. Technol. Med. Sci. 2014, 34, 456–463. [Google Scholar]
- Karakonstantis, S.; Kalemaki, D. Antimicrobial overuse and misuse in the community in Greece and link to antimicrobial resistance using methicillin-resistant S. aureus as an example. J. Infect. Public Health 2019, 12, 460–464. [Google Scholar] [PubMed]
- Carey, B.; Cryan, B. Antibiotic misuse in the community—A contributor to resistance? Ir. Med. J. 2003, 96, 43–44, 46. [Google Scholar] [PubMed]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar]
- Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar]
- Lorgelly, P.K.; Atkinson, M.; Lakhanpaul, M.; Smyth, A.R.; Vyas, H.; Weston, V.; Stephenson, T. Oral versus iv antibiotics for community-acquired pneumonia in children: A cost-minimisation analysis. Eur. Respir. J. 2010, 35, 858–864. [Google Scholar] [CrossRef]
- Knaak, E.; Cavalieri, S.J.; Elsasser, G.N.; Preheim, L.C.; Gonitzke, A.; Destache, C.J. Does antibiotic de-escalation for nosocomial pneumonia impact intensive care unit length of stay? Infect. Dis. Clin. Pract. 2013, 21, 172–176. [Google Scholar]
- Kalungia, A.C.; Mwambula, H.; Munkombwe, D.; Marshall, S.; Schellack, N.; May, C.; Jones, A.S.C.; Godman, B. Antimicrobial stewardship knowledge and perception among physicians and pharmacists at leading tertiary teaching hospitals in Zambia: Implications for future policy and practice. J. Chemother. 2019, 31, 378–387. [Google Scholar]
- Wei, X.; Zhang, Z.; Walley, J.D.; Hicks, J.P.; Zeng, J.; Deng, S.; Zhou, Y.; Yin, J.; Newell, J.N.; Sun, Q. Effect of a training and educational intervention for physicians and caregivers on antibiotic prescribing for upper respiratory tract infections in children at primary care facilities in rural China: A cluster-randomised controlled trial. Lancet Glob. Health 2017, 5, e1258–e1267. [Google Scholar] [CrossRef]
- Tabah, A.; Cotta, M.O.; Garnacho-Montero, J.; Schouten, J.; Roberts, J.A.; Lipman, J.; Tacey, M.; Timsit, J.-F.; Leone, M.; Zahar, J.R. A systematic review of the definitions, determinants, and clinical outcomes of antimicrobial de-escalation in the intensive care unit. Clin. Infect. Dis. 2016, 62, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.; Aziz, Z. A retrospective study of antibiotic de-escalation in patients with ventilator-associated pneumonia in Malaysia. Int. J. Clin. Pharm. 2017, 39, 906–912. [Google Scholar] [CrossRef]
- Alshareef, H.; Alfahad, W.; Albaadani, A.; Alyazid, H.; Talib, R.B. Impact of antibiotic de-escalation on hospitalized patients with urinary tract infections: A retrospective cohort single center study. J. Infect. Public Health 2020, 13, 985–990. [Google Scholar] [CrossRef]
- Evodia, E.; Myrianthefs, P.; Prezerakos, P.; Baltopoulos, G. Antibiotic costs in bacteremic and nonbacteremic patients treated with the de-escalation approach. Crit. Care 2008, 12, P15. [Google Scholar] [CrossRef][Green Version]
- Hajiabdolbaghi, M.; Makarem, J.; Salehi, M.; Manshadi, S.A.D.; Mohammadnejad, E.; Mazaherpoor, H.; Seifi, A. Does an antimicrobial stewardship program for Carbapenem use reduce Costs? An observation in Tehran, Iran. Casp. J. Intern. Med. 2020, 11, 329. [Google Scholar]
- Durojaiye, O.C.; Bell, H.; Andrews, D.; Ntziora, F.; Cartwright, K. Clinical efficacy, cost analysis and patient acceptability of outpatient parenteral antibiotic therapy (OPAT): A decade of Sheffield (UK) OPAT service. Int. J. Antimicrob. Agents 2018, 51, 26–32. [Google Scholar] [CrossRef]
- Paul, M.; Dickstein, Y.; Raz-Pasteur, A. Antibiotic de-escalation for bloodstream infections and pneumonia: Systematic review and meta-analysis. Clin. Microbiol. Infect. 2016, 22, 960–967. [Google Scholar] [CrossRef]
- Masterton, R.G. Antibiotic de-escalation. Crit. Care Clin. 2011, 27, 149–162. [Google Scholar] [CrossRef]
- Woerther, P.-L.; Barbier, F.; Lepeule, R.; Fihman, V.; Ruppé, É. Assessing the ecological benefit of antibiotic de-escalation strategies to elaborate evidence-based recommendations. Clin. Infect. Dis. 2020, 71, 1128–1129. [Google Scholar] [CrossRef] [PubMed]
- Campion, M.; Scully, G. Antibiotic use in the intensive care unit: Optimization and de-escalation. J. Intensive Care Med. 2018, 33, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.F.; Cowling, B.J.; Feng, S.; Aso, H.; Wu, P.; Fukuda, K.; Seto, W.H. Impact of antibiotic stewardship programmes in Asia: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2018, 73, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, S.; Padzil, F.; Shamsudin, S.H.; Bux, S.H.; Jamaluddin, A.A.; Bhagavathula, A.S. Antibiotic Stewardship in Community Pharmacies: A Scoping Review. Pharmacy 2018, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Boyles, T.H.; Whitelaw, A.; Bamford, C.; Moodley, M.; Bonorchis, K.; Morris, V.; Rawoot, N.; Naicker, V.; Lusakiewicz, I.; Black, J. Antibiotic stewardship ward rounds and a dedicated prescription chart reduce antibiotic consumption and pharmacy costs without affecting inpatient mortality or re-admission rates. PLoS ONE 2013, 8, e79747. [Google Scholar] [CrossRef]
- Han, L.; Feng, R.; Meng, J. Treatment of severe pediatric pneumonia by antibiotic de-escalation therapy. Int. J. Clin. Exp. Med. 2018, 11, 2610–2616. [Google Scholar]
- Renk, H.; Sarmisak, E.; Spott, C.; Kumpf, M.; Hofbeck, M.; Hölzl, F. Antibiotic stewardship in the PICU: Impact of ward rounds led by paediatric infectious diseases specialists on antibiotic consumption. Sci. Rep. 2020, 10, 8826. [Google Scholar] [CrossRef]
- Viasus, D.; Simonetti, A.F.; Garcia-Vidal, C.; Niubó, J.; Dorca, J.; Carratalà, J. Impact of antibiotic de-escalation on clinical outcomes in community-acquired pneumococcal pneumonia. J. Antimicrob. Chemother. 2017, 72, 547–553. [Google Scholar] [CrossRef]
- Lim, S.H.; Kuai, C.C.; Lim, C.H. Antibiotic De-escalation Practice in General Intensive Care Unit Penang General Hospital. J. Infect. Dis. Epidemiol. 2021, 7, 185. [Google Scholar]
- Teh, H.L.; Abdullah, S.; Ghazali, A.K.; Khan, R.A.; Ramadas, A.; Leong, C.L. Impact of extended and restricted antibiotic deescalation on mortality. Antibiotics 2021, 11, 22. [Google Scholar] [CrossRef]
- Loon, W.K.; Ling, W.M.; Kai, C.Z.; Ganasen, S.M.; Hasbullah, W.S.W.; Hui, C.S.; Soo, C.T.; Nim, L.K.; Shyan, W.P. A Prospective Study on Antibiotic De-escalation Practice in the Medical Wards of Penang General Hospital. Pharm. Res. Rep. 2018, 1, 24. [Google Scholar]
- Deshpande, A.; Richter, S.S.; Haessler, S.; Lindenauer, P.K.; Yu, P.-C.; Zilberberg, M.D.; Imrey, P.B.; Higgins, T.; Rothberg, M.B. De-escalation of empiric antibiotics following negative cultures in hospitalized patients with pneumonia: Rates and outcomes. Clin. Infect. Dis. 2021, 72, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.J.; Schweizer, M.L.; Braykov, N.B.; Weisenberg, S.A.; Uslan, D.Z.; Kelesidis, T.; Young, H.; Cantey, J.B.; Septimus, E.J.; Srinivasan, A.; et al. The Frequency of Antibiotic De-Escalation Over Six US Hospitals: Results from a Multicenter Cross-Sectional Study. OneHealth Trust 2012, 1, 1. [Google Scholar]
- Battula, V.; Krupanandan, R.K.; Nambi, P.S.; Ramachandran, B. Safety and feasibility of antibiotic de-escalation in critically ill children with sepsis—A prospective analytical study from a pediatric ICU. Front. Pediatr. 2021, 9, 640857. [Google Scholar] [CrossRef]
- Bhullar, H.S.; Shaikh, F.A.R.; Deepak, R.; Poddutoor, P.K.; Chirla, D. Antimicrobial justification form for restricting antibiotic use in a pediatric intensive care unit. Indian Pediatr. 2016, 53, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Rungsitsathian, K.; Wacharachaisurapol, N.; Nakaranurack, C.; Usayaporn, S.; Sakares, W.; Kawichai, S.; Jantarabenjakul, W.; Puthanakit, T.; Anugulruengkitt, S. Acceptance and outcome of interventions in a meropenem de-escalation antimicrobial stewardship program in pediatrics. Pediatr. Int. 2021, 63, 1458–1465. [Google Scholar] [CrossRef]
- Mantadakis, E.; Kopsidas, I.; Coffin, S.; Dimitriou, G.; Gkentzi, D.; Hatzipantelis, E.; Kaisari, A.; Kattamis, A.; Kourkouni, E.; Papachristidou, S. A national study of antibiotic use in Greek pediatric hematology oncology and bone marrow transplant units. Antimicrob. Steward. Healthc. Epidemiol. 2022, 2, e71. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Bakry, M.M.; See, K.C.; Tahir, N.A.M.; Shah, N.M. Early Empiric Antibiotic De-escalation in Suspected Early Onset Neonatal Sepsis. Indian J. Pharm. Sci. 2019, 81, 913–921. [Google Scholar] [CrossRef]
- Fu, R.; Chen, T.; Song, J.; Wang, G.; Li, L.; Ruan, E.; Liu, H.; Wang, Y.; Wang, H.; Xing, L. De-escalation empirical antibiotic therapy improved survival for patients with severe aplastic anemia treated with antithymocyte globulin. Medicine 2017, 96, e5905. [Google Scholar] [CrossRef]
- Verlinden, A.; Jansens, H.; Goossens, H.; Anguille, S.; Berneman, Z.N.; Schroyens, W.A.; Gadisseur, A.P. Safety and efficacy of antibiotic de-escalation and discontinuation in high-risk hematological patients with febrile neutropenia: A single-center experience. Open Forum Infect. Dis. 2022, 9, 624. [Google Scholar] [CrossRef]
- Liu, P.; Ohl, C.; Johnson, J.; Williamson, J.; Beardsley, J.; Luther, V. Frequency of empiric antibiotic de-escalation in an acute care hospital with an established antimicrobial stewardship program. BMC Infect. Dis. 2016, 16, 751. [Google Scholar] [CrossRef]
- Corcione, S.; Mornese Pinna, S.; Lupia, T.; Trentalange, A.; Germanò, E.; Cavallo, R.; Lupia, E.; De Rosa, F.G. Antibiotic De-escalation experience in the setting of emergency department: A retrospective, observational study. J. Clin. Med. 2021, 10, 3285. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Azim, A.; Gurjar, M.; Poddar, B.; Baronia, A.K. Audit of antibiotic practices: An experience from a tertiary referral center. Indian J. Crit. Care Med. 2019, 23, 7. [Google Scholar] [PubMed]
- Trupka, T.; Fisher, K.; Micek, S.T.; Juang, P.; Kollef, M.H. Enhanced antimicrobial de-escalation for pneumonia in mechanically ventilated patients: A cross-over study. Crit. Care 2017, 21, 180. [Google Scholar] [CrossRef] [PubMed]
- Ilges, D.; Ritchie, D.J.; Krekel, T.; Neuner, E.A.; Hampton, N.; Kollef, M.H.; Micek, S. Assessment of antibiotic de-escalation by spectrum score in patients with nosocomial pneumonia: A single-center, retrospective cohort study. Open Forum Infect. Dis. 2021, 8, 508. [Google Scholar] [CrossRef]
- Das, S.; Haque, I.; Gaur, A.; Mustafa, M.S. De-Escalation Pattern of Antibiotics in the Medical Intensive Care Unit of a Tertiary Care Hospital in North India. World J. Pharm. Pharm. Sci. 2020, 9, 1451–1460. [Google Scholar]
- Garnacho-Montero, J.; Gutiérrez-Pizarraya, A.; Escoresca-Ortega, A.; Corcia-Palomo, Y.; Fernandez-Delgado, E.; Herrera-Melero, I.; Ortiz-Leyba, C.; Márquez-Vácaro, J.A. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014, 40, 32–40. [Google Scholar] [CrossRef]
- Palacios-Baena, Z.R.; Delgado-Valverde, M.; Valiente Méndez, A.; Almirante, B.; Gómez-Zorrilla, S.; Borrell, N.; Corzo, J.E.; Gurguí, M.; De la Calle, C.; García-Álvarez, L. Impact of de-escalation on prognosis of patients with bacteremia due to Enterobacteriaceae: A post hoc analysis from a multicenter prospective cohort. Clin. Infect. Dis. 2019, 69, 956–962. [Google Scholar] [CrossRef]
- Moraes, R.B.; Guillén, J.A.V.; Zabaleta, W.J.C.; Borges, F.K. De-escalation, adequacy of antibiotic therapy and culture positivity in septic patients: An observational study. Rev. Bras. Ter. Intensiva 2016, 28, 315–322. [Google Scholar] [CrossRef]
- Zhang, D.; Cui, K.; Wang, T.; Dong, H.; Feng, W.; Ma, C.; Dong, Y. Trends in and correlations between antibiotic consumption and resistance of Staphylococcus aureus at a tertiary hospital in China before and after introduction of an antimicrobial stewardship programme. Epidemiol. Infect. 2019, 147, 315–322. [Google Scholar] [CrossRef]
- Sharland, M.; Gandra, S.; Huttner, B.; Moja, L.; Pulcini, C.; Zeng, M.; Mendelson, M.; Cappello, B.; Cooke, G.; Magrini, N. Encouraging AWaRe-ness and discouraging inappropriate antibiotic use—The new 2019 Essential Medicines List becomes a global antibiotic stewardship tool. Lancet Infect. Dis. 2019, 19, 1278–1280. [Google Scholar] [CrossRef]
- Pauwels, I.; Versporten, A.; Drapier, N.; Vlieghe, E.; Goossens, H. Hospital antibiotic prescribing patterns in adult patients according to the WHO Access, Watch and Reserve classification (AWaRe): Results from a worldwide point prevalence survey in 69 countries. J. Antimicrob. Chemother. 2021, 76, 1614–1624. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Micek, S.T. Rational use of antibiotics in the ICU: Balancing stewardship and clinical outcomes. JAMA 2014, 312, 1403–1404. [Google Scholar] [CrossRef]
- Nausheen, S.; Hammad, R.; Khan, A. Rational use of antibiotics—A quality improvement initiative in hospital setting. J. Pak. Med. Assoc. 2013, 63, 60. [Google Scholar] [PubMed]
- Slama, T.G.; Amin, A.; Brunton, S.A.; File, T.M.; Milkovich, G.; Rodvold, K.A.; Sahm, D.F.; Varon, J.; Weiland, D. A clinician’s guide to the appropriate and accurate use of antibiotics: The Council for Appropriate and Rational Antibiotic Therapy (CARAT) criteria. Am. J. Med. 2005, 118 (Suppl. S7), 1S–6S. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, M.; Hussain, M. Need and duration of antibiotic therapy in clean and clean contaminated operations. J. Pak. Med. Assoc. 2002, 52, 284–287. [Google Scholar] [PubMed]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control. 2017, 6, 47. [Google Scholar] [CrossRef]
- Hofer, U. The cost of antimicrobial resistance. Nat. Rev. Microbiol. 2019, 17, 3. [Google Scholar] [CrossRef]
- Tillotson, G.S.; Zinner, S.H. Burden of antimicrobial resistance in an era of decreasing susceptibility. Expert Rev. Anti. Infect. Ther. 2017, 15, 663–676. [Google Scholar] [CrossRef]
- Huang, V.; Ruhe, J.J.; Lerner, P.; Fedorenko, M. Risk factors for readmission in patients discharged with outpatient parenteral antimicrobial therapy: A retrospective cohort study. BMC Pharmacol. Toxicol. 2018, 19, 50. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, S.; Yin, X.; Bai, J.; Gong, Y.; Lu, Z. Factors associated with doctors’ knowledge on antibiotic use in China. Sci. Rep. 2016, 6, 23429. [Google Scholar] [CrossRef]
- Magedanz, L.; Silliprandi, E.M.; dos Santos, R.P. Impact of the pharmacist on a multidisciplinary team in an antimicrobial stewardship program: A quasi-experimental study. Int. J. Clin. Pharm. 2012, 34, 290–294. [Google Scholar] [CrossRef]
- MacDougall, C.; Polk, R.E. Antimicrobial stewardship programs in health care systems. Clin. Microbiol. Rev. 2005, 18, 638–656. [Google Scholar] [CrossRef]
- Paul, M.; Daikos, G.L.; Durante-Mangoni, E.; Yahav, D.; Carmeli, Y.; Benattar, Y.D.; Skiada, A.; Andini, R.; Eliakim-Raz, N.; Nutman, A. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: An open-label, randomised controlled trial. Lancet Infect. Dis. 2018, 18, 391–400. [Google Scholar] [CrossRef]
- Giacobbe, D.; Del Bono, V.; Trecarichi, E.; De Rosa, F.G.; Giannella, M.; Bassetti, M.; Bartoloni, A.; Losito, A.; Corcione, S.; Bartoletti, M. Risk factors for bloodstream infections due to colistin-resistant KPC-producing Klebsiella pneumoniae: Results from a multicenter case-control-control study. Clin. Microbiol. Infect. 2015, 21, e1–e1106. [Google Scholar] [CrossRef]
- Li, D.X.; Cosgrove, S.E. Antimicrobial Stewardship: Efficacy and Implementation of Strategies to Address Antimicrobial Overuse and Resistance. Antimicrob. Steward. 2017, 2, 13. [Google Scholar]
- Süzük Yıldız, S.; Kaşkatepe, B.; Şimşek, H.; Sarıgüzel, F.M. High rate of colistin and fosfomycin resistance among carbapenemase-producing Enterobacteriaceae in Turkey. Acta Microbiol. Immunol. Hung. 2019, 66, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Naylor, N.R.; Atun, R.; Zhu, N.; Kulasabanathan, K.; Silva, S.; Chatterjee, A.; Knight, G.M.; Robotham, J.V. Estimating the burden of antimicrobial resistance: A systematic literature review. Antimicrob. Resist. Infect. Control. 2018, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Velasco, E.; Ziegelmann, A.; Eckmanns, T.; Krause, G. Eliciting views on antibiotic prescribing and resistance among hospital and outpatient care physicians in Berlin, Germany: Results of a qualitative study. BMJ Open 2012, 2, e000398. [Google Scholar] [CrossRef] [PubMed]
- Easterbrook, P.J.; Gopalan, R.; Berlin, J.A.; Matthews, D.R. Publication bias in clinical research. Lancet 1991, 337, 867–872. [Google Scholar] [CrossRef]
- Tariq, R.; Cho, J.; Kapoor, S.; Orenstein, R.; Singh, S.; Pardi, D.S.; Khanna, S. Low risk of primary Clostridium difficile infection with tetracyclines: A systematic review and metaanalysis. Clin. Infect. Dis. 2018, 66, 514–522. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alanazi, A.; Almuhaya, R.; Almohaimeed, M.; Alahmari, N.; Abdulrahim, N.; Basyouni, M.; Althikrallah, F.; Al Badwyi, J.; Khallaf, A.; Albalawi, K.; et al. Impact of Antibiotic De-Escalation on Antibiotic Consumption, Length of Hospitalization, Mortality, and Cost: A Systematic Review and Meta-Analysis. Pharmacoepidemiology 2023, 2, 289-306. https://doi.org/10.3390/pharma2040025
Alanazi A, Almuhaya R, Almohaimeed M, Alahmari N, Abdulrahim N, Basyouni M, Althikrallah F, Al Badwyi J, Khallaf A, Albalawi K, et al. Impact of Antibiotic De-Escalation on Antibiotic Consumption, Length of Hospitalization, Mortality, and Cost: A Systematic Review and Meta-Analysis. Pharmacoepidemiology. 2023; 2(4):289-306. https://doi.org/10.3390/pharma2040025
Chicago/Turabian StyleAlanazi, Abeer, Reem Almuhaya, Mohammad Almohaimeed, Nada Alahmari, Noor Abdulrahim, Marouj Basyouni, Farah Althikrallah, Jumanah Al Badwyi, Abdulrahman Khallaf, Khalid Albalawi, and et al. 2023. "Impact of Antibiotic De-Escalation on Antibiotic Consumption, Length of Hospitalization, Mortality, and Cost: A Systematic Review and Meta-Analysis" Pharmacoepidemiology 2, no. 4: 289-306. https://doi.org/10.3390/pharma2040025
APA StyleAlanazi, A., Almuhaya, R., Almohaimeed, M., Alahmari, N., Abdulrahim, N., Basyouni, M., Althikrallah, F., Al Badwyi, J., Khallaf, A., Albalawi, K., Almalki, A., Alsaedi, K., Bakarman, F., Alotaibi, F., & Kanan, M. (2023). Impact of Antibiotic De-Escalation on Antibiotic Consumption, Length of Hospitalization, Mortality, and Cost: A Systematic Review and Meta-Analysis. Pharmacoepidemiology, 2(4), 289-306. https://doi.org/10.3390/pharma2040025