Suspected Suicide Attempt and Intentional Misuse Cases Aged 50+ Involving Amphetamine or Methylphenidate and Medical Outcomes: Associations with Co-Used Other Substances
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Measures
2.3. Analysis
3. Results
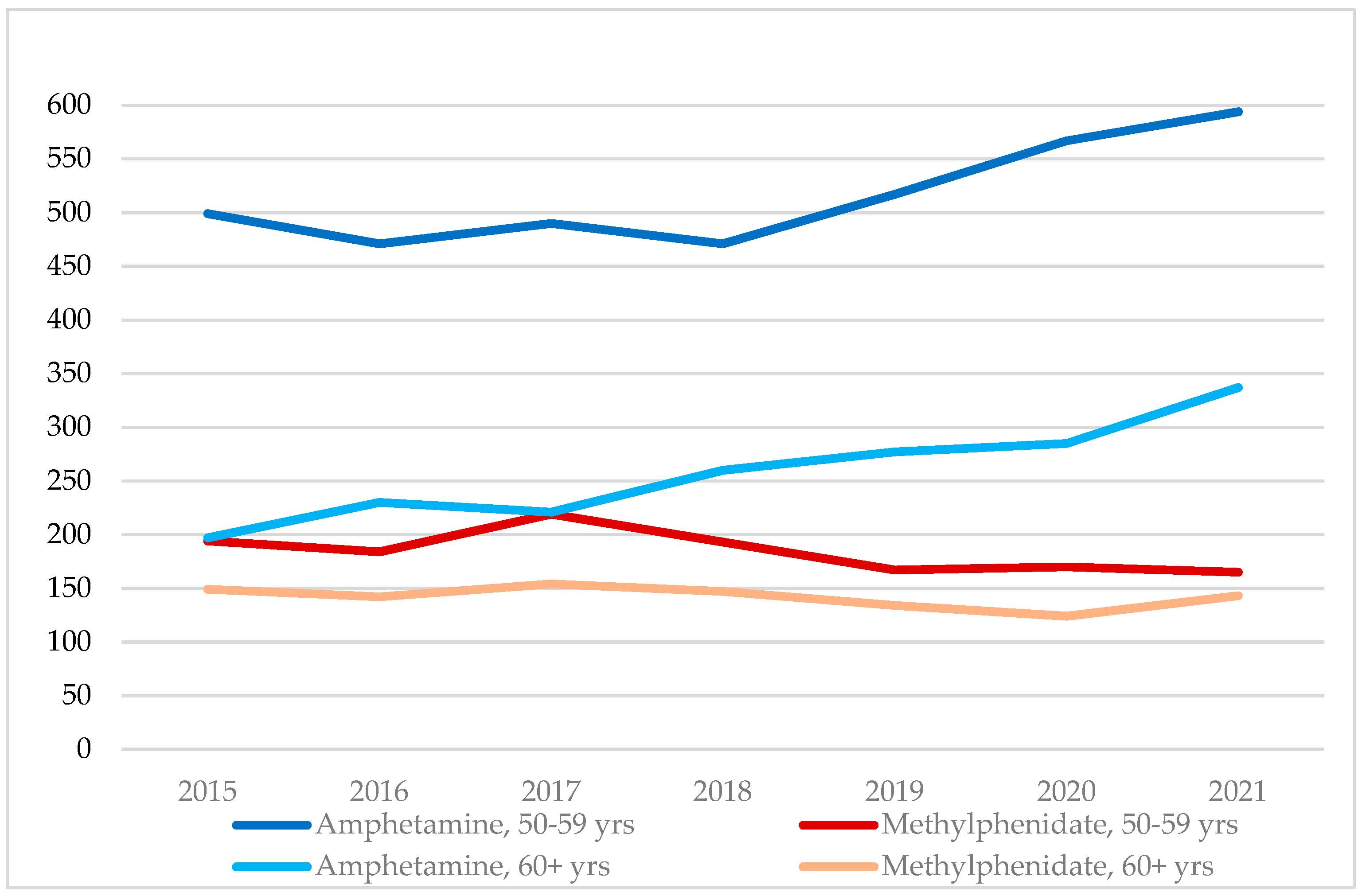
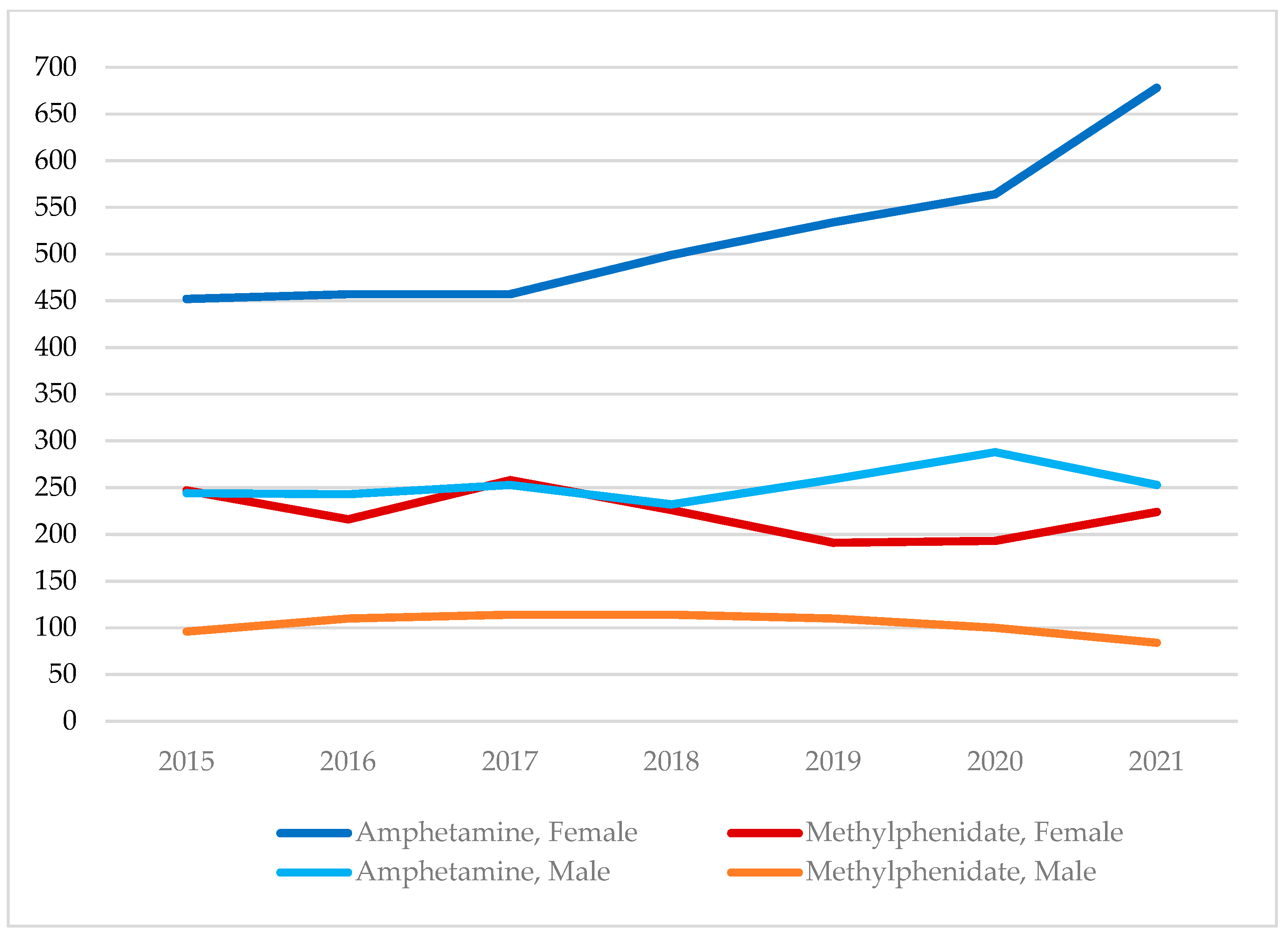
3.1. Amphetamine and Methylphenidate Cases, 2015–2021, by Age Group and Sex
3.2. Unintentional Exposure, Suspected Suicide Attempt, and Intentional Misuse
3.3. Associations between Co-Used Substances and Suspected Suicide Attempts vs. Intentional Misuses
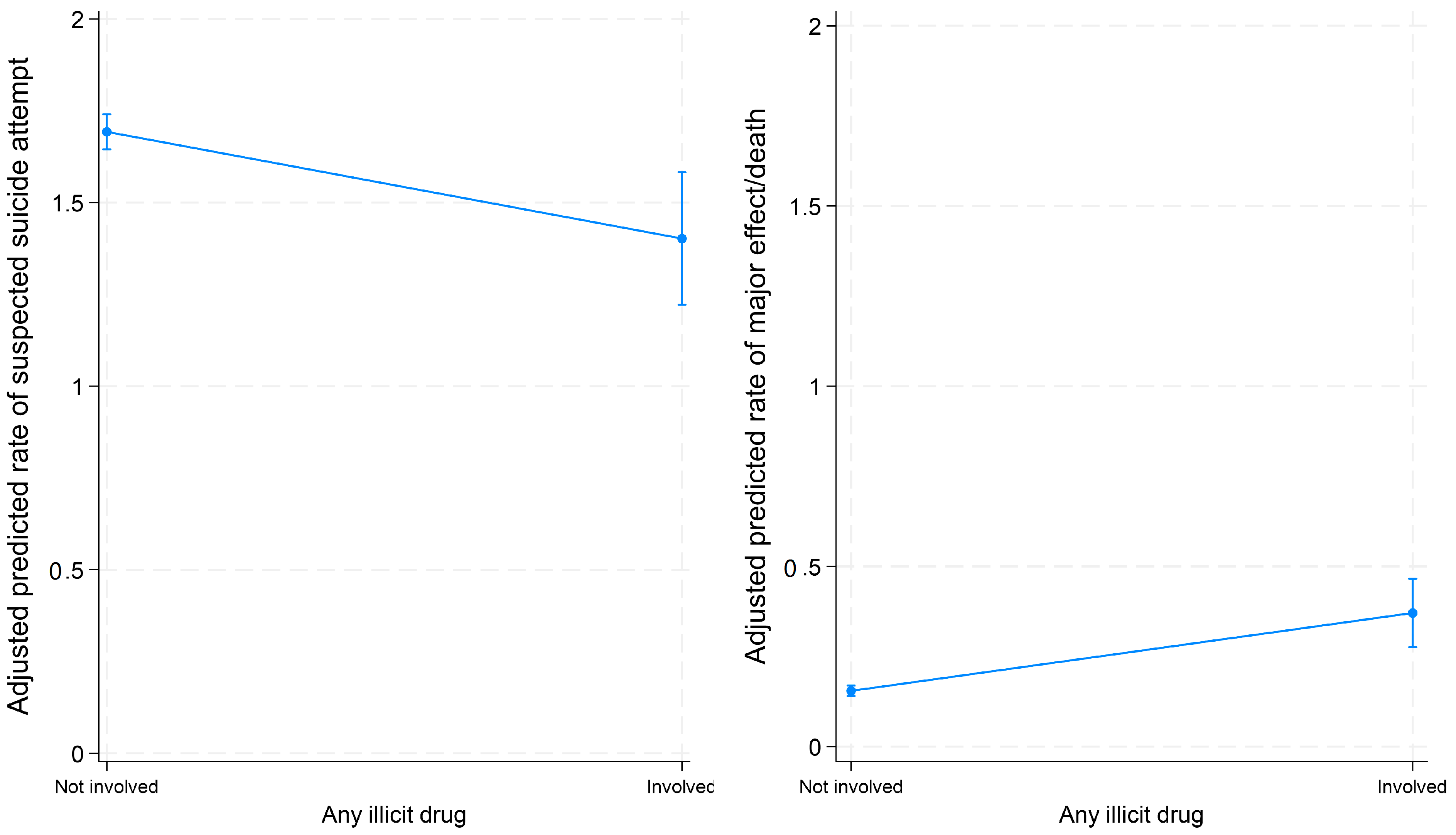
3.4. Associations between Co-Used Substances and Major Effect/Death among Suspected Suicide Attempt and Intentional Misuse Cases
4. Discussion
4.1. Summary and Contributions
4.2. Study Limitations
4.3. Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NPDS | The National Poison Data System |
| GLM | Generalized linear model |
| IRR | Incidence rate ratio |
| CIs | Confidence intervals |
References
- Moore, T.J.; Wirtz, P.W.; Kruszewski, S.P.; Alexander, G.C. Changes in medical use of central nervous system stimulants among US adults; 2013 and 2018: A cross-sectional study. BMJ Open 2021, 11, e048528. [Google Scholar] [CrossRef]
- Schepis, T.S.; McCabe, S.E. Trends in older adult nonmedical prescription drug use prevalence: Results from the 2002–2003 and 2012–2013 National Survey on Drug Use and Health. Addict. Behav. 2016, 60, 219–222. [Google Scholar] [CrossRef]
- Cortese, S.; Adamo, N.; Del Giovane, C.; Mohr-Jensen, C.; Hayes, A.J.; Carucci, S.; Cipriani, A. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. Lancet Psychiatry 2018, 5, 727–738. [Google Scholar] [CrossRef]
- Stuhec, M.; Lukić, P.; Locatelli, I. Efficacy, acceptability, and tolerability of lisdexamphetamine, mixed amphetamine salts, methylphenidate, and modafinil in the treatment of attention-deficit hyperactivity disorder in adults: A systematic review and meta-analysis. Ann. Pharmacother. 2019, 53, 121–133. [Google Scholar] [CrossRef]
- Thorpy, M.J.; Bogan, R.K. Update on the pharmacologic management of narcolepsy: Mechanisms of action and clinical implications. Sleep Med. 2020, 68, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.; Johnston, A.; Husereau, D.; Kelly, S.E.; Eagles, C.; Charach, A.; Hsieh, S.C.; Bai, Z.; Hossain, A.; Skidmore, B.; et al. Pharmacologic treatment of attention deficit hyperactivity disorder in adults: A systematic review and network meta-analysis. PLoS ONE 2020, 15, e0240584. [Google Scholar] [CrossRef] [PubMed]
- Mechler, K.; Banaschewski, T.; Hohmann, S.; Häge, A. Evidence-based pharmacological treatment options for attention deficit hyperactivity disorder in children and adolescents. Pharmacol. Ther. 2022, 230, 107940. [Google Scholar] [CrossRef] [PubMed]
- Sassi, K.L.M.; Rocha, N.P.; Colpo, G.D.; John, V.; Teixeira, A.L. Amphetamine use in the elderly: A systematic review of the literature. Curr. Neuropharmacol. 2020, 18, 126–135. [Google Scholar] [CrossRef]
- Castells, X.; Blanco-Silvente, L.; Cunill, R. Amphetamine for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst. Rev. 2018, 8, CD007813. [Google Scholar] [CrossRef]
- Cândido, R.C.F.; Menezes de Padua, C.A.; Golder, S.; Junqueira, D.R. Immediate-release methylphenidate for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst. Rev. 2021, 1, CD013011. [Google Scholar] [CrossRef] [PubMed]
- Steinkellner, T.; Freissmuth, M.; Sitte, H.H.; Montgomery, T. The ugly side of amphetamine: Short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’); methamphetamine and D-amphetamine. Biol. Chem. 2011, 392, 103–115. [Google Scholar] [CrossRef]
- Torres-Acosta, N.; O’Keefe, J.H.; O’Keefe, C.L.; Lavie, C.J. Cardiovascular effects of ADHD therapies: JACC review topic of the week. J. Am. Coll. Cardiol. 2020, 76, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Tadrous, M.; Shakeri, A.; Chu, C.; Watt, J.; Mamdani, M.M.; Juurlink, D.N.; Gomes, T. Assessment of stimulant use and cardiovascular event risks among older adults. JAMA Netw. Open 2021, 4, e2130795. [Google Scholar] [CrossRef]
- Liao, S. Why Are ADHD Medicines Controlled Substances? 2017. Available online: https://www.webmd.com/add-adhd/features/adhd-medicines-controlled-substances#:~:text=The%20majority%20of%20ADHD%20stimulant;risk%20of%20abuse%20and%20dependence (accessed on 10 April 2023).
- Shellenberg, T.P.; Stoops, W.W.; Lile, J.A.; Rush, C.R. An update on the clinical pharmacology of methylphenidate: Therapeutic efficacy, abuse potential and future considerations. Expert Rev. Clin. Pharmacol. 2020, 13, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Weyandt, L.L.; Oster, D.R.; Marraccini, M.E.; Gudmundsdottir, B.G.; Munro, B.A.; Rathkey, E.S.; McCallum, A. Prescription stimulant medication misuse: Where are we and where do we go from here? Exp. Clin. Psychopharmacol. 2016, 24, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Department of Justice/Drug Enforcement Agency. Drug Fact Sheet: Amphetamine. 2020. Available online: https://www.dea.gov/sites/default/files/2020-06/Amphetamine-2020_0.pdf (accessed on 1 April 2023).
- Thurn, D.; Riedner, A.; Wolstein, J. Use motives of patients with amphetamine-type stimulants use disorder and attention-deficit/hyperactivity disorder. Eur. Addict. Res. 2020, 26, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Clemow, D.B.; Walker, D.J. The potential for misuse and abuse of medications in ADHD: A review. Postgrad. Med. 2014, 126, 64–81. [Google Scholar] [CrossRef] [PubMed]
- Winkelman, T.N.A.; Admon, L.K.; Jennings, L.; Shippee, N.D.; Richardson, C.R.; Bart, G. Evaluation of amphetamine-related hospitalizations and associated clinical outcomes and costs in the United States. JAMA Netw. Open 2018, 1, e183758. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V.; Hess, J.; Wilens, T. Prevalence and consequences of the nonmedical use of amphetamine among persons calling poison control centers. J. Atten. Disord. 2019, 23, 1219–1228. [Google Scholar] [CrossRef]
- Wong, S.H.M.; Stevens, C.; Liu, C.H.; Chen, J.A. Prevalence and correlates of prescription stimulant misuse among US college students: Results from a national survey. J. Clin. Psychiatry 2022, 84, 22m14420. [Google Scholar] [CrossRef]
- Sepúlveda, D.R.; Thomas, L.M.; McCabe, S.E.; Cranford, J.A.; Boyd, C.J.; Teter, C.J. Misuse of prescribed stimulant medication for ADHD and associated patterns of substance use: Preliminary analysis among college students. J. Pharm. Pract. 2011, 24, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Clemow, D.B. Misuse of methylphenidate. Curr. Top. Behav. Neurosci. 2017, 34, 99–124. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Dertien, D.; Bentley, S.I. Prevalence of ADHD symptom malingering, nonmedical use, and drug diversion among college-enrolled adults with a prescription for stimulant medications. J. Addict. Dis. 2020, 38, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Chawarski, M.C.; Hawk, K.; Edelman, E.J.; O’Connor, P.; Owens, P.; Martel, S.; Coupet, E., Jr.; Whiteside, L.; Tsui, J.I.; Rothman, R.; et al. Use of amphetamine-type stimulants among emergency department patients with untreated opioid use disorder. Ann. Emerg. Med. 2020, 76, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.E. Emergency department visits involving attention deficit/hyperactivity disorder stimulant medications. In The CBHSQ Report; Substance Abuse and Mental Health Services Administration (US): Rockville, MD, USA, 2013; pp. 1–8. [Google Scholar]
- Compton, W.M.; Han, B.; Blanco, C.; Johnson, K.; Jones, C.M. Prevalence and correlates of prescription stimulant use, misuse, use disorders, and motivations for misuse among adults in the United States. Am. J. Psychiatry 2018, 175, 741–755. [Google Scholar] [CrossRef]
- Shearer, R.D.; Jones, A.; Howell, B.A.; Segel, J.E.; Winkelman, T.N.A. Associations between prescription and illicit stimulant and opioid use in the United States; 2015–2020. J. Subst. Abuse Treat. 2022, 143, 108894. [Google Scholar] [CrossRef]
- Crunelle, C.L.; van den Brink, W.; Moggi, F.; Konstenius, M.; Franck, J.; Levin, F.R.; Matthys, F. International consensus statement on screening, diagnosis and treatment of substance use disorder patients with comorbid attention deficit/hyperactivity disorder. Eur Addict. Res. 2018, 24, 43–51. [Google Scholar] [CrossRef]
- Westover, A.N.; Nakonezny, P.A.; Halm, E.A.; Adinoff, B. Risk of amphetamine use disorder and mortality among incident users of prescribed stimulant medications in the Veterans Administration. Addiction 2018, 113, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Vosburg, S.K.; Robbins, R.S.; Antshel, K.M.; Faraone, S.V.; Green, J.L. Characterizing prescription stimulant nonmedical use (NMU) among adults recruited from Reddit. Addict. Behav. Rep. 2021, 14, 100376. [Google Scholar] [CrossRef]
- Garnett, M.F.; Curtin, S.C.; Stone, D.M. Suicide mortality in the United States, 2000–2020. NCHS Data Brief 2022, 433, 1–8. [Google Scholar]
- Choi, N.G.; Marti, C.N.; Choi, B.Y. Three leading suicide methods in the United States, 2017–2019: Associations with decedents’ demographic and clinical characteristics. Front. Public Health 2022, 10, 955008. [Google Scholar] [CrossRef]
- Gummin, D.D.; Mowry, J.B.; Beuhler, M.C. 2020 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 38th annual report. Clin. Toxicol. 2021, 59, 1282–1501. [Google Scholar] [CrossRef]
- Grimes, D.A.; Schulz, K.F. Making sense of odds and odds ratios. Obstet. Gynecol. 2008, 111 Pt 1, 423–426. [Google Scholar] [CrossRef]
- Allison, P. When Can You Safely Ignore Multicollinearity? 2012. Available online: https://statisticalhorizons.com/multicollinearity/ (accessed on 30 March 2023).
- Cassidy, T.A.; McNaughton, E.C.; Varughese, S.; Russo, L.; Zulueta, M.; Butler, S.F. Nonmedical use of prescription ADHD stimulant medications among adults in a substance abuse treatment population: Early findings from the NAVIPPRO surveillance system. J. Atten. Disord. 2015, 19, 275–283. [Google Scholar] [CrossRef]
- Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results from the 2020 National Survey on Drug Use and Health; HHS Publication No. PEP21-07-01-003; NSDUH Series H-56; Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2021. Available online: https://www.samhsa.gov/data/ (accessed on 5 March 2023).
- Miller, T.R.; Swedler, D.I.; Lawrence, B.A.; Ali, B.; Rockett, I.R.; Carlson, N.N.; Leonardo, J. Incidence and lethality of suicidal overdoses by drug class. JAMA Netw. Open 2020, 3, e200607. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Tian, C.; Wu, L.; Liu, M.; Lu, H. Prescribed opioid use is associated with increased all-purpose emergency department visits and hospitalizations in community-dwelling older adults in the United States. Front. Psychiatry 2022, 13, 1092199. [Google Scholar] [CrossRef] [PubMed]
- ElDesoky, E.S. Pharmacokinetic-pharmacodynamic crisis in the elderly. Am. J. Ther. 2007, 14, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Klotz, U. Age-related changes in pharmacokinetics. Curr. Drug Metab. 2011, 12, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Lau Hing Yim, C.; Wong, E.W.W.; Jellie, L.J.; Lim, A.K.H. Illicit drug use and acute kidney injury in patients admitted to hospital with rhabdomyolysis. Intern. Med. J. 2019, 49, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Thomas, I.C.; Nishimura, M.; Ma, J.; Dickson, S.D.; Alshawabkeh, L.; Adler, E.; Greenberg, B. Clinical characteristics and outcomes of patients with heart failure and methamphetamine abuse. J. Card. Fail. 2020, 26, 202–209. [Google Scholar] [CrossRef]
- Soder, H.E.; Berumen, A.M.; Gomez, K.E.; Green, C.E.; Suchting, R.; Wardle, M.C.; Lane, S.D. Elevated neutrophil to lymphocyte ratio in older adults with cocaine use disorder as a marker of chronic inflammation. Clin. Psychopharmacol. Neurosci. 2020, 18, 32–40. [Google Scholar] [CrossRef]
- Frazer, K.M.; Richards, Q.; Keith, D.R. The long-term effects of cocaine use on cognitive functioning: A systematic critical review. Behav. Brain Res. 2018, 348, 241–262. [Google Scholar] [CrossRef] [PubMed]
- Zolopa, C.; Høj, S.B.; Minoyan, N.; Bruneau, J.; Makarenko, I.; Larney, S. Ageing and older people who use illicit opioids; cocaine or methamphetamine: A scoping review and literature map. Addiction 2022, 117, 2168–2188. [Google Scholar] [CrossRef]
- Moran, L.V.; Masters, G.A.; Pingali, S.; Cohen, B.M.; Liebson, E.; Rajarethinam, R.P.; Ongur, D. Prescription stimulant use is associated with earlier onset of psychosis. J. Psychiatr. Res. 2015, 71, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Buoli, M.; Serati, M.; Cahn, W. Alternative pharmacological strategies for adult ADHD treatment: A systematic review. Expert Rev. Neurother. 2016, 16, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Death Rate Maps & Graphs; National Center for Injury Prevention and Control: Atlanta, GA, USA, 2022. Available online: https://www.cdc.gov/drugoverdose/deaths/index.html (accessed on 15 April 2023).
| (1) Unintentional Exposure 4325 (58.4%) | (2) Suspected Suicide 2100 (28.4%) | (3) Intentional Misuse 977 (13.2%) | P: among 3 Groups | P: between (2) and (3) | |
|---|---|---|---|---|---|
| Year (%) | 0.030 | 0.067 | |||
| 2015 | 13.2 | 12.9 | 14.6 | ||
| 2016 | 13.3 | 13.3 | 13.9 | ||
| 2017 | 13.7 | 14.2 | 15.1 | ||
| 2018 | 13.9 | 14.9 | 11.5 | ||
| 2019 | 14.0 | 14.3 | 16.1 | ||
| 2020 | 14.6 | 15.0 | 15.8 | ||
| 2021 | 17.3 | 15.3 | 13.0 | ||
| US census region (%) | <0.001 | 0.416 | |||
| Northeast | 12.2 | 14.1 | 15.8 | ||
| Midwest | 23.5 | 26.5 | 25.4 | ||
| South | 41.9 | 40.7 | 38.5 | ||
| West | 22.0 | 18.5 | 20.4 | ||
| Puerto Rico/unknown | 0.4 | 0.1 | 0 | ||
| Age group (in years; %) | <0.001 | 0.017 | |||
| 50–59 | 55.9 | 73.4 | 75.1 | ||
| 60–69 | 29.5 | 21.6 | 22.1 | ||
| 70+ | 14.5 | 5.0 | 2.8 | ||
| Sex (%) | <0.001 | <0.001 | |||
| Female | 74.4 | 64.6 | 45.7 | ||
| Male | 25.6 | 35.4 | 54.3 | ||
| Route of administration (%) | |||||
| Ingestion | 77.5 | 85.0 | 81.6 | <0.001 | 0.020 |
| Inhalation/nasal | 12.5 | 15.5 | 13.2 | 0.004 | 0.101 |
| Injection | 2.3 | 3.2 | 3.5 | 0.031 | 0.746 |
| Management/care site (%) | <0.001 | <0.001 | |||
| On-site (non-HCF) | 71.2 | 0 | 5.4 | ||
| Healthcare facility treated/ evaluated and released | 15.2 | 16.4 | 26.6 | ||
| Admitted to a psychiatric facility | 0.4 | 21.4 | 7.5 | ||
| Admitted to a noncritical care unit | 3.0 | 19.0 | 18.6 | ||
| Admitted to a critical care unit | 1.8 | 38.7 | 31.6 | ||
| No-show/lost to follow-up/left against medical advice/unknown | 8.4 | 4.5 | 10.2 | ||
| No. of substances involved, M (SD) | 2.09 (2.14) | 3.51 (2.45) | 2.38 (1.82) | <0.001 | <0.001 |
| Other substances used (%) | |||||
| Benzodiazepine | 3.5 | 34.9 | 15.4 | <0.001 | <0.001 |
| Antidepressants | 8.9 | 19.4 | 7.4 | <0.001 | <0.001 |
| Prescription opioids | 2.7 | 18.2 | 16.5 | <0.001 | 0.264 |
| Drugs for cardiovascular disease | 13.1 | 13.7 | 4.8 | <0.001 | <0.001 |
| Antipsychotics | 5.8 | 19.6 | 8.2 | <0.001 | <0.001 |
| Antihistamine | 3.0 | 6.5 | 3.8 | <0.001 | 0.002 |
| Muscle relaxant | 1.4 | 7.8 | 4.6 | <0.001 | 0.002 |
| Alcohol | 0.5 | 18.1 | 10.6 | <0.001 | <0.001 |
| Marijuana | 0.1 | 2.5 | 4.4 | <0.001 | 0.007 |
| Methadone | 0.3 | 1.2 | 1.7 | <0.001 | 0.243 |
| Illicit drug 1 | 0.2 | 3.0 | 12.0 | <0.001 | <0.001 |
| Medical outcomes (%) | |||||
| Major effect/death | 0.7 | 18.8 | 17.0 | <0.001 | 0.229 |
| All other | 99.3 | 81.2 | 83.0 |
| Suspected Suicide vs. Intentional Misuse/Abuse | Major Effect/Death vs. All Other Outcomes | |
|---|---|---|
| IRR (95% CI) | IRR (95% CI) | |
| Suspected suicide: vs. Intentional misuse/abuse | 1.06 (0.87–1.28) | |
| Amphetamine vs. Methylphenidate | 0.97 (0.91–1.04) | 1.24 (0.99–1.56) |
| Benzodiazepine | 1.09 (1.03–1.16) ** | 1.17 (0.98–1.41) |
| Antidepressants | 1.07 (0.99–1.16) | 1.43 (1.16–1.76) ** |
| Prescription opioids | 1.00 (0.93–1.08) | 1.48 (1.21–1.82) *** |
| Drugs for cardiovascular disease | 1.07 (0.90–1.13) | 1.51 (1.20–1.90) ** |
| Antipsychotics | 1.06 (0.98–1.14) | 1.26 (1.02–1.55) * |
| Antihistamine | 1.01 (0.90–1.13) | 0.87 (0.61–1.24) |
| Muscle relaxant | 1.01 (0.91–1.13) | 1.13 (0.84–1.52) |
| Alcohol | 1.06 (0.99–1.14) | 0.87 (0.68–1.11) |
| Marijuana | 0.97 (0.82–1.15) | 1.14 (0.75–1.73) |
| Methadone | 0.97 (0.76–1.25) | 1.19 (0.68–2.08) |
| Any illicit drug | 0.83 (0.73–0.95) ** | 2.40 (1.82–3.15) *** |
| Year: vs. 2015 | ||
| 2016 | 1.03 (0.92–1.14) | 1.00 (0.71–1.42) |
| 2017 | 1.02 (0.91–1.13) | 1.08 (0.78–1.50) |
| 2018 | 1.05 (0.95–1.17) | 1.19 (0.85–1.65) |
| 2019 | 1.01 (0.91–1.12) | 1.44 (1.05–1.97) * |
| 2020 | 1.03 (0.93–1.14) | 1.27 (0.93–1.75) |
| 2021 | 1.05 (0.95–1.16) | 1.13 (0.82–1.57) |
| US census region 1: vs. South | ||
| Northeast | 0.98 (0.90–1.06) | 1.43 (1.11–1.84) ** |
| Midwest | 1.00 (0.93–1.07) | 1.50 (1.22–1.84) *** |
| West | 1.00 (0.93–1.08) | 1.13 (0.88–1.44) |
| Age: vs. 50–59 years | ||
| 60–69 years | 1.00 (0.93–1.07) | 1.10 (0.90–1.34) |
| 70+ years | 1.05 (0.92–1.20) | 1.25 (0.85–1.83) |
| Female vs. Male | 1.07 (1.01–1.13) * | 1.10 (0.92–1.31) |
| N | 3072 | 3072 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, N.G.; Choi, B.Y.; Baker, S.D. Suspected Suicide Attempt and Intentional Misuse Cases Aged 50+ Involving Amphetamine or Methylphenidate and Medical Outcomes: Associations with Co-Used Other Substances. Pharmacoepidemiology 2023, 2, 236-248. https://doi.org/10.3390/pharma2030020
Choi NG, Choi BY, Baker SD. Suspected Suicide Attempt and Intentional Misuse Cases Aged 50+ Involving Amphetamine or Methylphenidate and Medical Outcomes: Associations with Co-Used Other Substances. Pharmacoepidemiology. 2023; 2(3):236-248. https://doi.org/10.3390/pharma2030020
Chicago/Turabian StyleChoi, Namkee G., Bryan Y. Choi, and S. David Baker. 2023. "Suspected Suicide Attempt and Intentional Misuse Cases Aged 50+ Involving Amphetamine or Methylphenidate and Medical Outcomes: Associations with Co-Used Other Substances" Pharmacoepidemiology 2, no. 3: 236-248. https://doi.org/10.3390/pharma2030020
APA StyleChoi, N. G., Choi, B. Y., & Baker, S. D. (2023). Suspected Suicide Attempt and Intentional Misuse Cases Aged 50+ Involving Amphetamine or Methylphenidate and Medical Outcomes: Associations with Co-Used Other Substances. Pharmacoepidemiology, 2(3), 236-248. https://doi.org/10.3390/pharma2030020






