Abstract
COVID-19 antiviral medications approved or authorized for emergency use by the U.S. Food and Drug Administration are reported to have high efficacy in preventing severe illness, hospitalizations, and deaths. However, reports for some of these antivirals use relative risk reductions from clinical trials without absolute risk reductions. The present paper reappraises recently published clinical trial data for the COVID-19 antivirals paxlovid, remdesivir, and molnupiravir, and reports absolute risk reductions, relative risk reductions, as well as number needed to treat to reduce severe illness, hospitalizations, and deaths. Relative risk reductions are 88.88% for paxlovid (95% CI: 72.13–95.56%), 86.48% for remdesivir (95% CI: 41.41–96.88%), and 30.41% for molnupiravir (95% CI: 0.81–51.18%), while absolute risk reductions are much lower at 5.73% for paxlovid (95% CI: 3.79–7.68%), 4.58% for remdesivir (95% CI: 1.79–7.38%), and 2.96% for molnupiravir (95% CI: 0.09–5.83%). Low absolute risk reductions and the high number of patients needed to treat to reduce severe COVID-19 infections, hospitalizations, and deaths challenge the clinical efficacy of antivirals approved or authorized by the U.S Food and Drug Administration. These findings apply to other populations with similar control event rates. Accurate information should be disseminated to the public when selecting treatments for COVID-19.
1. Introduction
The U.S. National Institutes of Health (NIH) issued treatment guidelines for COVID-19 antiviral medications approved or authorized for emergency use by the U.S. Food and Drug Administration to treat mild-to-moderate symptoms of COVID-19: nirmatrelvir with ritonavir (paxlovid), remdesivir (veklury), and molnupiravir (lagevrio) [1]. COVID-19 antiviral drug mechanisms are designed to reduce replication of SARS-CoV-2 by inhibiting proteases and RNA polymerase, enzymes that break apart viral polyproteins into “essential subunits” and assemble proteins into viral genomes for replication and transcription, respectively [2]. Molnupiravir is a polymerase inhibitor that interferes with replication of genetic material in SARS-CoV-2 and in other RNA viruses, such as MERS-CoV, Ebola, and influenza [3]. Paxlovid uses the protease inhibitor nirmatrelvir, boosted with ritonavir, that blocks the proteolytic process that splits viral proteins into subunits [4]. Both drugs were repurposed for emergency use authorization against COVID-19 and are provided in pill form. Molnupiravir and paxlovid are intended for ambulatory adults with mild-to-moderate COVID-19 symptoms who have a high risk of severe disease. Gilead Sciences’ remdesivir is also a polymerase inhibitor. However, unlike molnupiravir, remdesivir is administered intravenously to hospitalized COVID-19 patients. As the present article goes to press, “Remdesivir is the only antiviral drug that is approved by the Food and Drug Administration (FDA) for the treatment of COVID-19” [5]. Long-term studies of safety and efficacy are lacking for paxlovid and molnupiravir, both of which received emergency use authorization during the COVID-19 pandemic. A systematic review and meta-analysis of antiviral clinical trials for nonsevere COVID-19 is available elsewhere [6].
Antivirals are prescribed to prevent mild-to-moderate SARS-CoV2 infections in adults from progressing to severe COVID-19 illness, hospitalizations, and deaths. Severity of COVID-19 illness in adults with SARS-CoV-2 infection (Table 1) is grouped into five general categories; definitions may overlap between categories [5].

Table 1.
COVID-19 illness severity levels.
Coinfections, including community-acquired bacterial pneumonia, influenza, and other respiratory viruses, may also present as infectious complications in patients with COVID-19. Hospital acquired complications may occur in COVID-19 patients, including pneumonia associated with ventilator and catheter use as well as bacterial associated diarrhea [5]. Other complications observed in patients include breakthrough infections following COVID-19 vaccination, SARS-CoV-2 reinfection following recovery from a previous infection, and continuation of symptoms and conditions following acute COVID-19 [5].
Importantly, recently published clinical trial results for efficacy of COVID-19 antivirals in reducing severe COVID-19 with paxlovid and reducing rehospitalizations and deaths with remdesivir are reported as relative risk reductions (RRRs) with no mention of absolute risk reductions (ARRs) (See Data Availability Statement at the end of the present Brief Report). Reported outcomes for paxlovid and remdesivir are inconsistent with FDA recommendations to report both RRRs and ARRs when communicating health risks and benefits to the public [7]:
“Risk communication is the term of art used for situations when people need good information to make sound choices. It is distinguished from public affairs (or public relations) communication by its commitment to accuracy and its avoidance of spin”.[7]
“Relative measures can exaggerate findings of modest clinical benefit and can often be uninterpretable, such as if control event rates are not reported” [8]. Guidelines from the CONSORT Group recommend reporting absolute measures in addition to relative measures in randomized trials [9]. Yet, of 344 articles reviewed on research in health inequalities, relative measures were the only measures reported in 88% of the article abstracts; absolute measures were reported in 9% of the abstracts and both relative and absolute measures were reported in just 2% of the abstracts [10]. The present brief report reappraises recent clinical trial results of COVID-19 antiviral medications authorized for emergency use or approved by the FDA, and reports absolute risk reductions, number needed to treat (NNT), and relative risk reductions along with 95% confidence intervals.
2. Results
2.1. Nirmatrelvir with Ritonavir (Paxlovid)
The following analysis (Table 2) is based on peer-reviewed published data from the Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients (EPIC-HR), a phase 2–3 clinical trial for efficacy of Pfizer’s paxlovid in preventing severe illness in COVID-19 patients [11].

Table 2.
Nirmatrelvir with ritonavir (paxlovid) EPIC-HR trial data to prevent severe COVID-19 in outpatients.
2.2. Remdesivir (Veklury)
The following analysis (Table 3) is based on peer-reviewed published data from the PINETREE clinical trial for efficacy of Gilead Sciences’ remdesivir to prevent hospitalization or death in outpatients with COVID-19 [12].

Table 3.
Remdesivir (veklury) PINETREE trial data to prevent severe hospitalization or death in COVID-19 outpatients.
2.3. Molnupiravir (Lagevrio)
The following analysis (Table 4) is based on peer-reviewed published data from the MOVe-OUT phase 3 clinical trial for efficacy of Merck, Sharp and Dohme’s molnupiravir to prevent severe COVID-19 in outpatients [13].

Table 4.
Molnupiravir (lagevrio) MOVe-OUT trial data to prevent severe COVID-19 in outpatients.
3. Discussion
Reappraising absolute risk reductions of COVID-19 antiviral medications reveals the unreported low efficacy of treatments with paxlovid and remdesivir. Compared to the relative risk reductions of these drugs, misrepresented to the public with reports of treatment efficacies in the upper 80% range, absolute risk reductions and their 95% confidence intervals are more than 15- to 18-fold lower, in the single-digit range: 5.73% for paxlovid (95% CI: 3.79–7.68%), 4.58% for remdesivir (95% CI: 1.79–7.38%), and 2.96% for molnupiravir (95% CI: 0.09–5.83%); these values are compared to relative risk reductions of 88.88% for paxlovid (95% CI: 72.13–95.56%), 86.48% for remdesivir (95% CI: 41.41–96.88%), and 30.41% for molnupiravir (95% CI: 0.81–51.18%). Additionally, based on the number needed to treat, only one out of every 18–22 patients treated with paxlovid or remdesivir could expect to see a single-digit reduction in severe COVID-19. Thus, the present reappraisal infers that patients, clinicians, and the public have been misled about the true efficacies of these antivirals to prevent severe COVID-19 due to misinformation published in medical journals and disseminated by pharmaceutical companies and public health agencies. Furthermore, phase 3 trials for paxlovid and molnupiravir reappraised in the present brief report did not measure hospitalizations and deaths as primary endpoints; these are implied outcomes associated with severe COVID-19. Accordingly, low absolute risk reductions in severe COVID-19 for paxlovid and molnupiravir do not support implied reductions in related hospitalizations and deaths associated with these antivirals.
Although Merck, Sharpe and Dohme did not report the RRR of the MOVe-OUT clinical trial for molnupiravir, the present analysis shows that the antiviral’s RRR is approximately 30%, much lower than the upper 80% RRRs of competitive COVID-19 antivirals from Pfizer and Gilead Sciences. On the other hand, molnupiravir’s reported ARR, within the single-digit range at approximately 3%, is competitive with the unreported single digit ARRs of paxlovid and remdesivir, although molnupiravir’s NNT is much higher at 34 treated patients needed for one reduction in severe COVID-19. Recently, the PANORAMIC study found no reduced risk of hospitalization or deaths in vaccinated patients receiving molnupiravir plus usual care compared to usual care alone [14]. The authors of the PANORAMIC study cited the RRR of the MOVe-OUT study, in which “molnupiravir was associated with a relative reduction of roughly 30% in the primary outcome.”
Low absolute risk reductions reported in the present brief report for COVID-19 antivirals approved and authorized for emergency use by the FDA seem plausible when considering the many underlying risk factors in people ≥65 years, who are at greatest risk for severe illness, hospitalization, and death.
“Data on comorbid health conditions among patients with COVID-19 indicate that patients with cardiovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, diabetes with complications, neurocognitive disorders, and obesity are at increased risk of severe COVID-19. The risk appears to be higher in patients with multiple comorbid conditions”.[5]
Is it plausible that a single antiviral drug can sufficiently reduce the combined deleterious health effects of so many comorbidities? Other conditions potentially increasing risk for severe COVID-19 include “cancer, cystic fibrosis, immunocompromising conditions, liver disease (especially in patients with cirrhosis), pregnancy, and sickle cell disease,” in addition to potentially higher risks in patients using immunosuppressive drugs [5]. Furthermore, SARS-CoV-2 reinfection following recovery after paxlovid use, a “rebound” effect [15], is potentially explained by evidence in the present reappraisal showing that antivirals have little impact on reducing SARS-CoV-2 infections.
The decision to prescribe COVID-19 antivirals is usually guided by a patient-based benefit-risk assessment [16] that should include assessments of non-misleading drug efficacy reports from clinical trials, financial costs of the drug, reported adverse effects [17,18,19,20], and drug interactions with other drugs [19,20,21,22]. For example, “ritonavir-boosted nirmatrelvir [paxlovid] has significant drug-drug interactions, primarily due to the ritonavir component of the combination,” and paxlovid should not be used with specific anticonvulsants, anti-infectives, immunosuppressants, and medications to treat cardiovascular conditions, pulmonary hypertension, or neuropsychiatric disorders [5]. Regarding examples of adverse effects, “reproductive toxicity has been reported in animal studies of molnupiravir, and molnupiravir may be mutagenic during pregnancy,” in addition to causing nausea, diarrhea, and dizziness [5]. As well, hypertension, myalgia, dysgeusia, and diarrhea are adverse reactions caused by paxlovid; hypersensitivity reactions and gastrointestinal symptoms are among adverse reactions caused by remdesivir, along with concerns for use by patients with renal insufficiency [5].
Hypothetically, benefits and financial costs of an antiviral having moderate adverse effects and several drug interactions may appear acceptable to a patient if drug efficacy for protection against severe COVID-19 is presented as a high relative risk reduction, but patients’ preferences and perceptions of benefits, costs, and intention to use the antiviral may change if a low absolute risk reduction is presented. A 2010 editorial in BMJ noted public and clinician overestimations of benefits from mammography screening based on misleading reports of lower breast cancer mortality [23]. The author’s comment that “the problem of misleading reporting has not gone away” applies equally as much in 2023. The author further stated, “relative risks do not inform about the baseline risk—for example, whether twofold means from one to two or from 50 to 100 in 7000—and without this information, people overestimate benefits or harms.” In other words, the absolute risk reduction changes as the baseline risk changes, even if the relative risk reduction remains the same. Up-to-date research is needed to investigate overestimation of benefits from COVID-19 antivirals due to misleading risks communicated in patient-based benefit-risk assessments.
Nevertheless, some clinicians continue to stress the importance of presenting relative risk reductions in order to promote higher use of a drug [24], but clinicians should not allow their opinions and presumptions to override clinical facts as represented by absolute risk reductions. Additionally, if a treatment’s absolute risk reduction of 1% can potentially benefit 10,000 patients when prescribed to one million people, why completely omit mentioning the absolute risk reduction to patients and clinicians, other than to frame benefits in a misleading manner using only relative risk measures? The best course of action is to communicate to the public both relative and absolute risk reductions of COVID-19 antivirals [7] with clear explanations of differences in clinical trial risk measures, in accordance with ethical principles of informed consent [25]. Furthermore, ARRs are dependent on the baseline risk, and ARRs with reciprocal NNTs (1/ARR) should not be extrapolated to other populations unless the baseline risk is similar.
Biostatistician Jerome Cornfield described how relative measures appraise “possible noncausal” effects, or associations, while absolute measures appraise effects “known to be causal” [26]. Causative and associative pathways in clinical epidemiology are illustrated using directed acyclic graphs [27]. A directed acyclic graph (Figure 1) illustrates the noncausal association between high relative risk reductions of COVID-19 antivirals and reduced COVID-19 severity, hospitalizations, and deaths (dashed arrow pathway), which is causatively mediated by low absolute risk reductions of COVID-19 antivirals (solid arrow pathways).
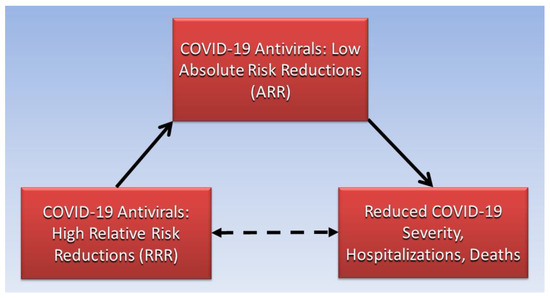
Figure 1.
High RRRs of COVID-19 antivirals are mediated by low ARRs for severity, hospitalizations, and deaths.
4. Methods
Risk in a randomized controlled clinical trial is measured as the proportion or percentage of events that occur in the treatment or experimental group, the experimental event rate (EER), and in the placebo or control group, the control event rate (CER) [28]. That is, the EER and CER are calculated as the number of people in each group in which an endpoint event occurs, such as a disease symptom, divided by the total number of people in the respective group.
Unlike randomized controlled clinical trials that measure causative effects, observational studies, such as cohort studies, that investigate disease incidence cannot control confounding factors and measure only associations of disease risk with exposures [28]. The EER in an uncontrolled study is divided by the CER, which is also known as the baseline risk, to calculate the relative risk (RR). The RR is the ratio of the risk associated with exposure to some factor in one group relative to the risk associated without exposure to that factor in the other group. If there is no risk difference between the EER and CER, the relative risk has the null value of 1. The relative risk reduction is calculated by subtracting the RR from the null value of 1. For example, a relative risk of 0.4 has a RRR of 0.6. RRRs are used in meta-analyses to compare results of uncontrolled cohort studies.
By contrast, relative risks that measure associations can be misleading and do not provide meaningful information about causative effects in randomized controlled trials, where absolute risk measures are required [28]. The absolute risk reduction is the simple mathematical difference when the EER is subtracted from the CER. Randomization of participants assigned to the vaccine and placebo groups in a clinical trial controls confounding factors that may affect outcomes, such as different rates of viral transmission/infection. The reciprocal of the ARR, 1/ARR, is the number of patients needed to treat to reduce one event (NNT), usually rounded up to the nearest whole number. Dividing the ARR by the baseline risk, the CER, is an alternate way to calculate the RRR. RRRs and ARRs, with their respective 95% confidence intervals, and NNTs are calculated for COVID-19 antiviral medications in the present brief report. When performing calculations, decimal numbers are converted to numbers with percentage signs (%) by multiplying by 100 and moving the decimal point two places to the right. Confidence intervals for ARRs and RRRs are calculated according to formulas described elsewhere [29].
5. Conclusions
Antivirals are prescribed to prevent mild-to-moderate SARS-CoV2 infections in adults from progressing to severe COVID-19 illness, hospitalizations, and deaths. COVID-19 antiviral mechanisms reduce SARS-CoV-2 replication by inhibiting proteases and RNA polymerase. The problem of misleading reporting is evident in recent clinical trials for COVID-19 antiviral medications approved or authorized for emergency use by the FDA. Relative risk reductions in the present reappraisal of clinical trials for COVID-19 antiviral medications are 88.88% for paxlovid (95% CI: 72.13–95.56%), 86.48% for remdesivir (95% CI: 41.41–96.88%), and 30.41% for molnupiravir (95% CI: 0.81–51.18%), while absolute risk reductions are much lower at 5.73% for paxlovid (95% CI: 3.79–7.68%), 4.58% for remdesivir (95% CI: 1.79–7.38%), and 2.96% for molnupiravir (95% CI: 0.09–5.83%). The number of patients needed to treat to reduce severe COVID-19 are 18, 22, and 34 for paxlovid, remdesivir, and molnupiravir, respectively. Low ARRs and high NNTs call into question the clinical efficacy of these antivirals, and more accurate information should be communicated to the public to allow sound choices of treatments for COVID-19 while avoiding spin from public health agencies, medical journals, and pharmacotherapy manufacturers.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19 [11]. Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients [12]. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients [13]. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial [14].
Conflicts of Interest
The author declares no conflict of interest.
References
- cdc.gov. COVID-19 Treatments and Medications. Available online: https://www.cdc.gov/coronavirus/2019-ncov/your-health/treatments-for-severe-illness.html (accessed on 23 December 2022).
- Jin, Z.; Wang, H.; Duan, Y.; Yang, H. The main protease and RNA-dependent RNA polymerase are two prime targets for SARS-CoV-2. Biochem. Biophys. Res. Commun. 2021, 538, 63–71. [Google Scholar] [CrossRef] [PubMed]
- IDSA. Molnupiravir. Available online: https://www.idsociety.org/covid-19-real-time-learning-network/therapeutics-and-interventions/molnupiravir/ (accessed on 18 February 2023).
- IDSA. IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Available online: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/#Recommendation26:Nirmatrelvir/ritonavir (accessed on 19 February 2023).
- nih.gov. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 19 February 2023).
- Pitre, T.; Van Alstine, R.; Chick, G.; Leung, G.; Mikhail, D.; Cusano, E.; Khalid, F.; Zeraatkar, D. Antiviral drug treatment for nonsevere COVID-19: A systematic review and network meta-analysis. Can. Med. Assoc. J. 2022, 194, E969–E980. [Google Scholar] [CrossRef] [PubMed]
- Fischhoff, B.; Brewer, N.; Downs, J. Communicating Risks and Benefits: An Evidence-Based User’s Guide; Food and Drug Administration (FDA), US Department of Health and Human Services: Silver Spring, MA, USA, 2011.
- Heneghan, C.; Goldacre, B.; Mahtani, K.R. Why clinical trial outcomes fail to translate into benefits for patients. Trials 2017, 18, 122. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef]
- King, N.B.; Harper, S.; Young, M.E. Use of relative and absolute effect measures in reporting health inequalities: Structured review. BMJ 2012, 345, e5774. [Google Scholar] [CrossRef]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef]
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, B.U.; Brown, M.; et al. Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients. N. Engl. J. Med. 2021, 386, 305–315. [Google Scholar] [CrossRef]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2021, 386, 509–520. [Google Scholar] [CrossRef]
- Butler, C.C.; Hobbs, F.D.R.; Gbinigie, O.A.; Rahman, N.M.; Hayward, G.; Richards, D.B.; Dorward, J.; Lowe, D.M.; Standing, J.F.; Breuer, J.; et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): An open-label, platform-adaptive randomised controlled trial. Lancet 2022, 401, 281–293. [Google Scholar] [CrossRef]
- Rubin, R. From Positive to Negative to Positive Again-The Mystery of Why COVID-19 Rebounds in Some Patients Who Take Paxlovid. JAMA 2022, 327, 2380–2382. [Google Scholar] [CrossRef]
- El Masri, H.; McGuire, T.M.; van Driel, M.L.; Benham, H.; Hollingworth, S.A. Dynamics of Patient-Based Benefit-Risk Assessment of Medicines in Chronic Diseases: A Systematic Review. Patient Prefer. Adherence 2022, 16, 2609–2637. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Zadeh, N.; Mashinchi Asl, N.S.; Forouharnejad, K.; Ghadimi, K.; Parsa, S.; Mohammadi, S.; Omidi, A. Mechanism and adverse effects of COVID-19 drugs: A basic review. Int. J. Physiol. Pathophysiol. Pharmacol. 2021, 13, 102–109. [Google Scholar] [PubMed]
- Liu, D.; Zeng, X.; Ding, Z.; Lv, F.; Mehta, J.L.; Wang, X. Adverse Cardiovascular Effects of Anti-COVID-19 Drugs. Front. Pharmacol. 2021, 12, 699949. [Google Scholar] [CrossRef] [PubMed]
- Saravolatz, L.D.; Depcinski, S.; Sharma, M. Molnupiravir and Nirmatrelvir-Ritonavir: Oral Coronavirus Disease 2019 Antiviral Drugs. Clin. Infect. Dis. 2022, 76, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Pfizer. Fact Sheet for Healthcare Providers: Emergency Use Authorization for Paxlovid. Available online: https://www.fda.gov/media/155050/download (accessed on 18 February 2023).
- Larsen, C.S. Assessing the proportion of the Danish population at risk of clinically significant drug-drug interactions with new oral antivirals for early treatment of COVID-19. Int. J. Infect. Dis. 2022, 122, 599–601. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.; Nohria, A.; Neilan, T.G.; Asnani, A.; Saji, A.M.; Shah, J.; Lech, T.; Grossman, J.; Abraham, G.M.; McQuillen, D.P.; et al. Cardiovascular Drug Interactions With Nirmatrelvir/Ritonavir in Patients With COVID-19: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2022, 80, 1912–1924. [Google Scholar] [CrossRef]
- Gigerenzer, G.; Wegwarth, O.; Feufel, M. Misleading communication of risk. BMJ 2010, 341, c4830. [Google Scholar] [CrossRef]
- Chapelle, N.; Martel, M.; Barkun, A.N.; Bardou, M. Relative risk rather than absolute risk reduction should be preferred to sensitise the public to preventive actions. Gut 2022, 71, 1045–1046. [Google Scholar] [CrossRef]
- Holm, S.; Lewis, J.; Dal-Ré, R. Equipoise, standard of care and consent: Responding to the authorisation of new COVID-19 treatments in randomised controlled trials. J. Med. Ethics 2022, 1–6. [Google Scholar] [CrossRef]
- Cornfield, J.; Haenszel, W.; Hammond, E.C.; Lilienfeld, A.M.; Shimkin, M.B.; Wynder, E.L. Smoking and lung cancer: Recent evidence and a discussion of some questions. J. Natl. Cancer Inst. 1959, 22, 173–203. [Google Scholar] [CrossRef]
- Digitale, J.C.; Martin, J.N.; Glymour, M.M. Tutorial on directed acyclic graphs. J. Clin. Epidemiol. 2022, 142, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.B. Relative risk reduction: Misinformative measure in clinical trials and COVID-19 vaccine efficacy. Dialogues Health 2022, 1, 100074. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.B. Outcome Reporting Bias in COVID-19 mRNA Vaccine Clinical Trials. Medicina 2021, 57, 199. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).