CNS Axon Regeneration in the Long Primary Afferent System in E15/E16 Hypoxic-Conditioned Fetal Rats: A Thrust-Driven Concept
Abstract
1. Introduction
2. Materials and Methods
2.1. A Summary
| 1 | 2 | 3 | 1 | 2 | 3 |
|---|---|---|---|---|---|
| Case ID | litter-ratio | Figure | Case ID | litter-ratio | Figure |
| (Tx: n = p0) | (Tx: n = p0) | ||||
| C39.E18.E20 | N/A | T47.E16.p4 | (1:2 = 2) | ||
| C44.Sx.p4 | N/A | T60.E16+12h.p135 | (1:10 = 10) | ||
| D14.D5.E21 | N/A | T62.E16.5.p17 | (2:11 = 11) | ||
| D15.E17.E20 | N/A | T65.E16.5.p48 | (1:10 = 10) | ||
| D20.D17.E18 | N/A | T68.E16.5.p370 | (1:11 = 11) | ||
| N45.E17.p8 | (1:4 = 4) | T77.E17.p35 | (1:12 = 12) | ||
| N46.E18.p10 | (2:15 = 15) | T78.E16+2h.p40 | (1:8) = 7 | Figure 3 | |
| P1.E18.p48 | (2:15 = 14 + 1 †) | V1.E16+6h.p600 | (1:10 = 9) | Figure 5 | |
| P17.E17.p90 | (2:10 = 10) | V2.E16-1h.p240 | (2:14 = 13) | ||
| P20.E18.p600 | (2:9 = 8) | V3.E16-1h.p195 | (1:13 = 12 + 1 †) | ||
| R9.s6.E17.p44 | (4:11 = 11) | V31.E16-3h.p145 | (1:13 = 13) | ||
| T8.E17.p180 | (2:15 = 15) | V38.E16-3h.p210 | (1:15 = 15) | ||
| T9.2.E18.p225 | (3:15 = 12) | V51.E16-4h.p2 | (1:9 = 9) | ||
| T12.E17.p7 | (2:15 = 15) | V63.E16-2h.p1.5 | (1:11 = 11) | ||
| T13.E17.p7 | (2:14 = 12) | V67.E16+7h.p9 | (1:7 = 7) | ||
| T15.E17.p305 | (3:16 = 14) | V72.E16+8h.p1.5 | (1:14 = 14) | ||
| T16.E17.p40 | (2:15 = 13) | W2.E16-7h.p9 | (2:10 = 10) | Figure 6A,D | |
| T20.E17.p700 | (1:9 = 7) | W2.E16-6.5h.p42 | (2:10 = 10) | Figure 6E,H | |
| T23.E16.p360 | (1:6 = 6) | W6.E16.p32 | (2:14 = 14) | ||
| T34.E17.p100 | (1:8 = 8) | W14.E16-8h.p14 | (2:15 = 15) | Figure 8 | |
| T42.E16.p240 | (1:9 = 9) | Figure 4 | W17.E16.p64 | (1:14 = 14) | |
| T45.E16.p7 | (1:4 = 4) | W20.E16-9h.p6 | (2:11 = 9) | Figure 7 | |
| T46.E16.p3 | (1:13 = 12 + 1 †) |
2.2. Preamble to the Long Primary Afferent System’s Development
 . The colored bars represent the current dorsal columns, harboring the dominant upstream-stage axons. The green line in the right panel delineates the critical period, i.e., the upstream time window at spring tide. (D,F) The two-line graphics reflect how the former stage of axons develops after the myelotomy. The lines serve as a pars pro toto for axons from the left DRG L4–L6 neurons. The high-tide axons target the gracile nuclei (g.nu), and the low-tide axons target Clarke’s nucleus (C.nu). t = time. d = axon length.
. The colored bars represent the current dorsal columns, harboring the dominant upstream-stage axons. The green line in the right panel delineates the critical period, i.e., the upstream time window at spring tide. (D,F) The two-line graphics reflect how the former stage of axons develops after the myelotomy. The lines serve as a pars pro toto for axons from the left DRG L4–L6 neurons. The high-tide axons target the gracile nuclei (g.nu), and the low-tide axons target Clarke’s nucleus (C.nu). t = time. d = axon length.
 . The colored bars represent the current dorsal columns, harboring the dominant upstream-stage axons. The green line in the right panel delineates the critical period, i.e., the upstream time window at spring tide. (D,F) The two-line graphics reflect how the former stage of axons develops after the myelotomy. The lines serve as a pars pro toto for axons from the left DRG L4–L6 neurons. The high-tide axons target the gracile nuclei (g.nu), and the low-tide axons target Clarke’s nucleus (C.nu). t = time. d = axon length.
. The colored bars represent the current dorsal columns, harboring the dominant upstream-stage axons. The green line in the right panel delineates the critical period, i.e., the upstream time window at spring tide. (D,F) The two-line graphics reflect how the former stage of axons develops after the myelotomy. The lines serve as a pars pro toto for axons from the left DRG L4–L6 neurons. The high-tide axons target the gracile nuclei (g.nu), and the low-tide axons target Clarke’s nucleus (C.nu). t = time. d = axon length.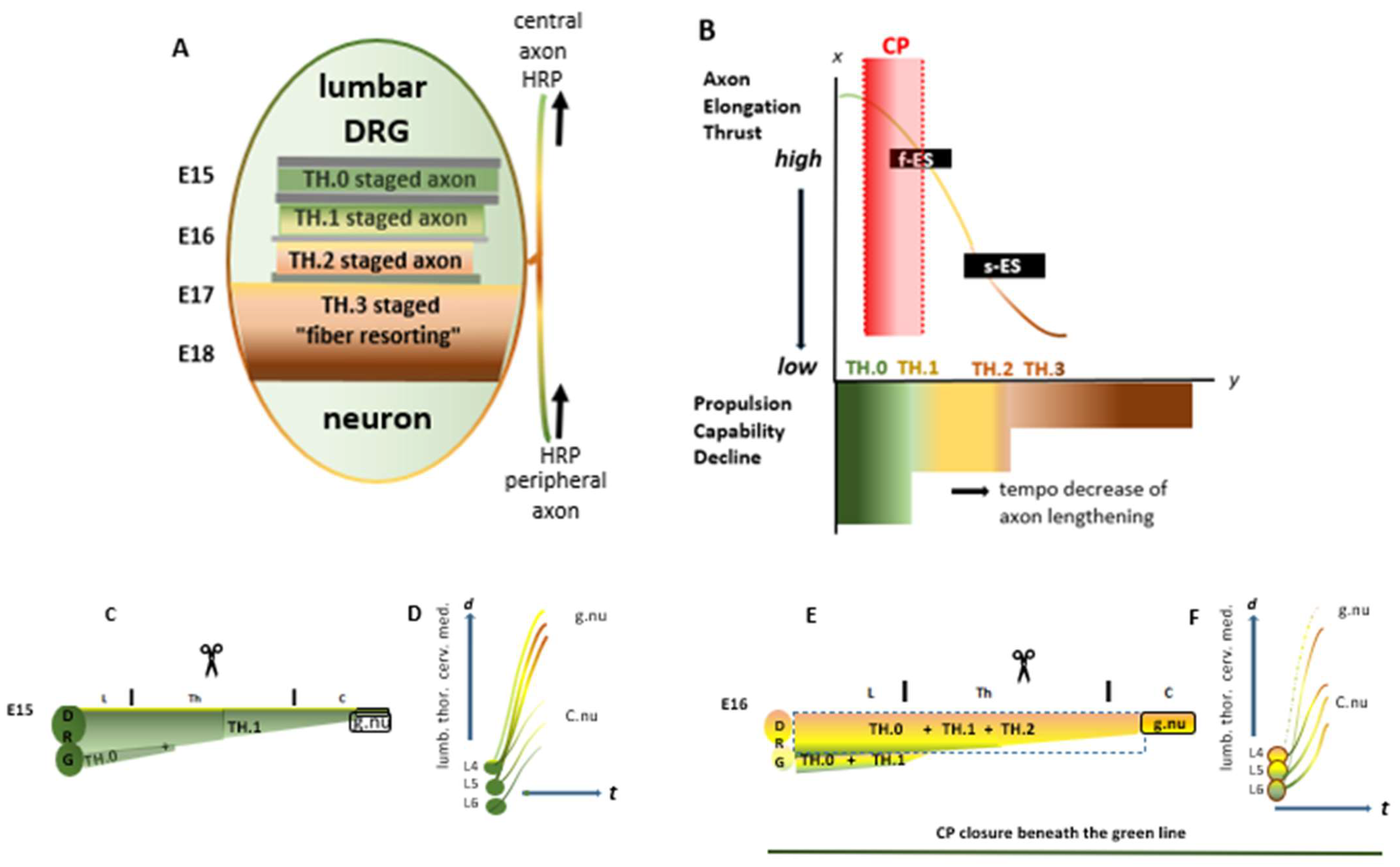
2.3. The Impact of Hypoxia During the Critical Period
 and
and  , respectively, the axon lengths may parallel the energy generated for elongation. The reprogrammed i-FS’s phenotypes are yellowish-tinted. Within the upstream CP (red-shaded area/line), the i-FSs regenerate up to the medulla, as depicted in the following figure
, respectively, the axon lengths may parallel the energy generated for elongation. The reprogrammed i-FS’s phenotypes are yellowish-tinted. Within the upstream CP (red-shaded area/line), the i-FSs regenerate up to the medulla, as depicted in the following figure  . The high-tide a-FS of joined TH.1 (*) i-FSs abuts the lesion site caudally. Beyond the a-FS (the red line changes color), severed axons gradually increase their distance to the rostral lesion site due to failing thrust. The numbers (3)–(8) represent seven cases, including twins, with Txs positioned at their estimated M0 spots on the assembly line. (*) and (**): single and tandem asterisks displayed in some figures indicate TH.1 and TH.2 i-FSs.
. The high-tide a-FS of joined TH.1 (*) i-FSs abuts the lesion site caudally. Beyond the a-FS (the red line changes color), severed axons gradually increase their distance to the rostral lesion site due to failing thrust. The numbers (3)–(8) represent seven cases, including twins, with Txs positioned at their estimated M0 spots on the assembly line. (*) and (**): single and tandem asterisks displayed in some figures indicate TH.1 and TH.2 i-FSs.
 and
and  , respectively, the axon lengths may parallel the energy generated for elongation. The reprogrammed i-FS’s phenotypes are yellowish-tinted. Within the upstream CP (red-shaded area/line), the i-FSs regenerate up to the medulla, as depicted in the following figure
, respectively, the axon lengths may parallel the energy generated for elongation. The reprogrammed i-FS’s phenotypes are yellowish-tinted. Within the upstream CP (red-shaded area/line), the i-FSs regenerate up to the medulla, as depicted in the following figure  . The high-tide a-FS of joined TH.1 (*) i-FSs abuts the lesion site caudally. Beyond the a-FS (the red line changes color), severed axons gradually increase their distance to the rostral lesion site due to failing thrust. The numbers (3)–(8) represent seven cases, including twins, with Txs positioned at their estimated M0 spots on the assembly line. (*) and (**): single and tandem asterisks displayed in some figures indicate TH.1 and TH.2 i-FSs.
. The high-tide a-FS of joined TH.1 (*) i-FSs abuts the lesion site caudally. Beyond the a-FS (the red line changes color), severed axons gradually increase their distance to the rostral lesion site due to failing thrust. The numbers (3)–(8) represent seven cases, including twins, with Txs positioned at their estimated M0 spots on the assembly line. (*) and (**): single and tandem asterisks displayed in some figures indicate TH.1 and TH.2 i-FSs.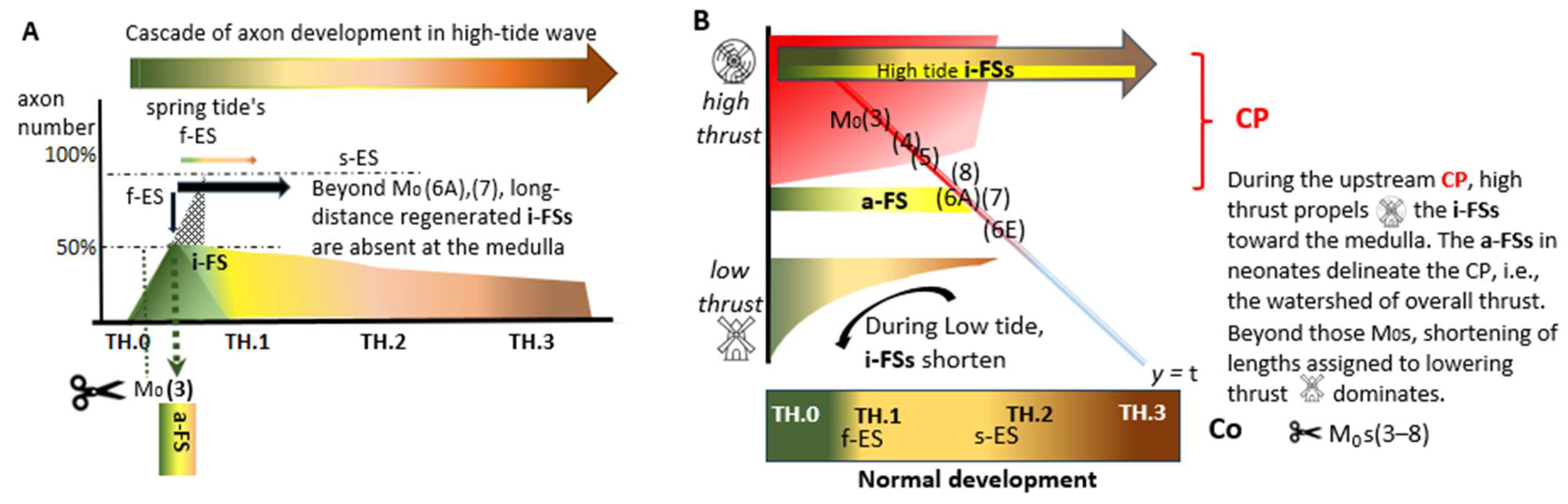
2.4. The Watershed: Axons Share the Critical Period Restricting Intrinsic Regeneration
3. Results
3.1. Regeneration Comes to a Halt Before Neap Tide
3.2. Six Bell Ringer Cases Exhibiting All Dynamic Features
| * | asterisk | a single asterisk indicates a TH.1 i-FS | ||||||
| ** | asterisks | a tandem asterisk indicates a TH.2 i-FS | ||||||
| *** | asterisks | three asterisks indicate downstream TH.3 colls | ||||||
| g.nu | gracile nucleus | |||||||
| 4th V | fourth ventricle | |||||||
| L|Th|C | Lumbar|Thoracic|Cervical segment of the spinal cord | |||||||
| c <== ==> r | caudal <== ==> rostral direction of the spinal cord | |||||||
| d <== ==> v | dorsal <== ==> ventral in a sagittal section of the spinal cord | |||||||
| L/R | left side/right side | of the spinal cord in a horizontal section | ||||||
| N | fix pinhole | an artifact from tissue processing; a number identifies the spinal cord’s level derived from the gelatine block’s count | ||||||
 | spinal level of Tx | |||||||
 | depicted level in the Figure | |||||||
 denotes the spinal levels at C and D (horizontal sections). (B) The lines (in yellow and ochre) represent the TH.1 (*) and TH.2 (**) i-FSs from within the CP’s first half and TH.3 (***) colls (in brown) originating from parent axons at the medulla from within the CP’s second half. (C)
denotes the spinal levels at C and D (horizontal sections). (B) The lines (in yellow and ochre) represent the TH.1 (*) and TH.2 (**) i-FSs from within the CP’s first half and TH.3 (***) colls (in brown) originating from parent axons at the medulla from within the CP’s second half. (C)  The p40 young adult exhibited a medulla with multi-stage axons in both gracile nuclei. The spotty TH.3 (***) colls in the left gracile nucleus indicated accomplished development due to presumably sustained high thrust levels. Phenotypically altered TH.1 (*) and TH.2 (**) i-FSs outnumbered others in the right DC. The increased labeling on the right side confirmed that many axons had regenerated across the damaged midline septum. (D)
The p40 young adult exhibited a medulla with multi-stage axons in both gracile nuclei. The spotty TH.3 (***) colls in the left gracile nucleus indicated accomplished development due to presumably sustained high thrust levels. Phenotypically altered TH.1 (*) and TH.2 (**) i-FSs outnumbered others in the right DC. The increased labeling on the right side confirmed that many axons had regenerated across the damaged midline septum. (D)  Caudally from the medulla, occasional TH.1 (*) and TH.2 (**) i-FSs were present in the DCs. t = time. N: iron fix pinhole. Bars: 100 µm.
Caudally from the medulla, occasional TH.1 (*) and TH.2 (**) i-FSs were present in the DCs. t = time. N: iron fix pinhole. Bars: 100 µm.
 denotes the spinal levels at C and D (horizontal sections). (B) The lines (in yellow and ochre) represent the TH.1 (*) and TH.2 (**) i-FSs from within the CP’s first half and TH.3 (***) colls (in brown) originating from parent axons at the medulla from within the CP’s second half. (C)
denotes the spinal levels at C and D (horizontal sections). (B) The lines (in yellow and ochre) represent the TH.1 (*) and TH.2 (**) i-FSs from within the CP’s first half and TH.3 (***) colls (in brown) originating from parent axons at the medulla from within the CP’s second half. (C)  The p40 young adult exhibited a medulla with multi-stage axons in both gracile nuclei. The spotty TH.3 (***) colls in the left gracile nucleus indicated accomplished development due to presumably sustained high thrust levels. Phenotypically altered TH.1 (*) and TH.2 (**) i-FSs outnumbered others in the right DC. The increased labeling on the right side confirmed that many axons had regenerated across the damaged midline septum. (D)
The p40 young adult exhibited a medulla with multi-stage axons in both gracile nuclei. The spotty TH.3 (***) colls in the left gracile nucleus indicated accomplished development due to presumably sustained high thrust levels. Phenotypically altered TH.1 (*) and TH.2 (**) i-FSs outnumbered others in the right DC. The increased labeling on the right side confirmed that many axons had regenerated across the damaged midline septum. (D)  Caudally from the medulla, occasional TH.1 (*) and TH.2 (**) i-FSs were present in the DCs. t = time. N: iron fix pinhole. Bars: 100 µm.
Caudally from the medulla, occasional TH.1 (*) and TH.2 (**) i-FSs were present in the DCs. t = time. N: iron fix pinhole. Bars: 100 µm.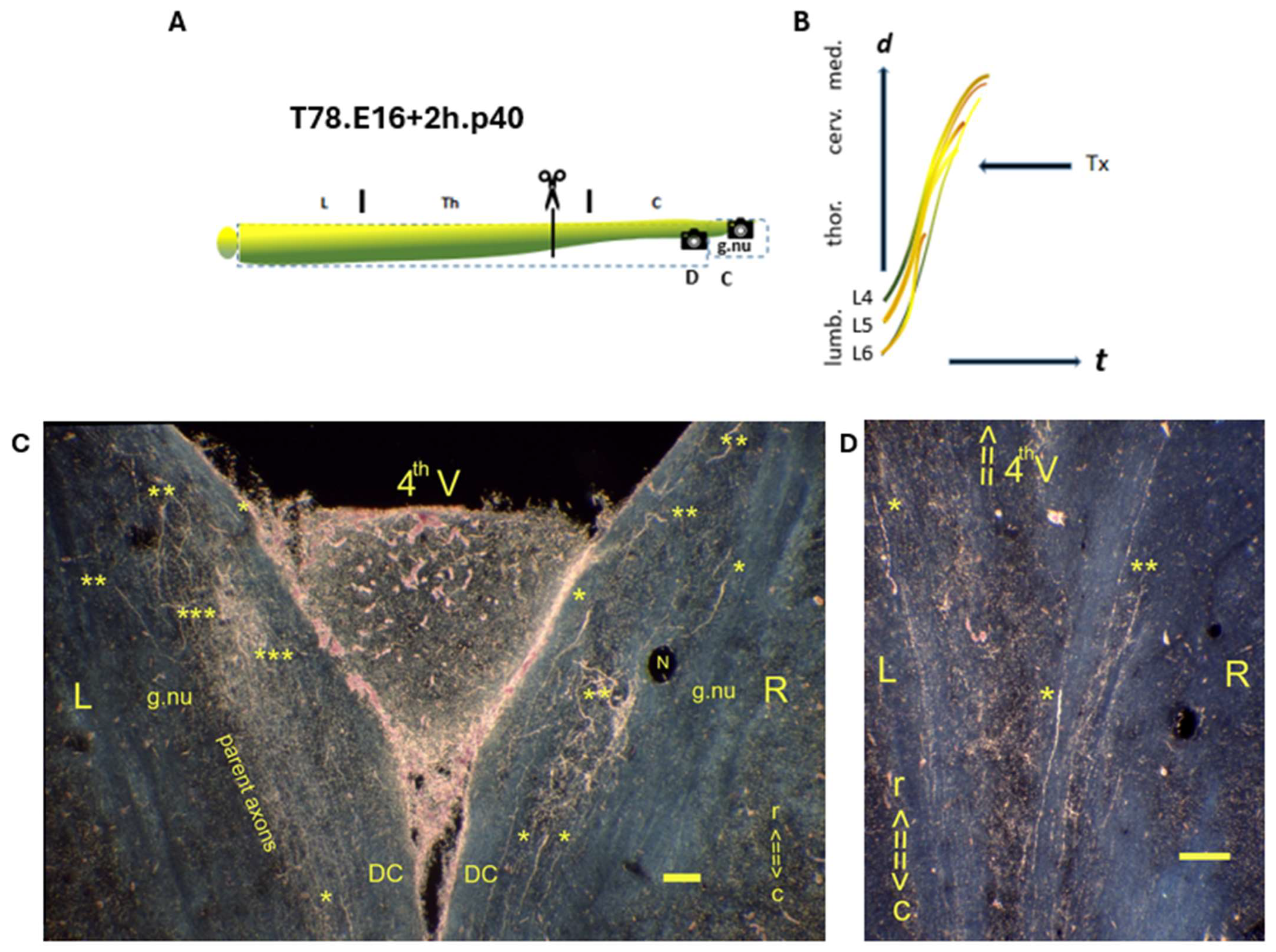
 denotes the spinal levels at (C,D). (B) The axons in the medulla demonstrated that the CP had not been closed. (C)
denotes the spinal levels at (C,D). (B) The axons in the medulla demonstrated that the CP had not been closed. (C)  Horizontal section of gracile nuclei. The p600 male showed TH.1 (*) and TH.2 (**) i-FSs in the medulla. TH.3 (***) colls cannot be ruled out. (D)
Horizontal section of gracile nuclei. The p600 male showed TH.1 (*) and TH.2 (**) i-FSs in the medulla. TH.3 (***) colls cannot be ruled out. (D)  Sagittal section of the mid-thoracic spinal cord. The multilevel TH.1 (*) i-FSs elongated to caudal levels distant from the lesion site. Fading thrust was thought to determine their shortened lengths. t = time. N: iron fix pinhole. Bars: 100 µm.
Sagittal section of the mid-thoracic spinal cord. The multilevel TH.1 (*) i-FSs elongated to caudal levels distant from the lesion site. Fading thrust was thought to determine their shortened lengths. t = time. N: iron fix pinhole. Bars: 100 µm.
 denotes the spinal levels at (C,D). (B) The axons in the medulla demonstrated that the CP had not been closed. (C)
denotes the spinal levels at (C,D). (B) The axons in the medulla demonstrated that the CP had not been closed. (C)  Horizontal section of gracile nuclei. The p600 male showed TH.1 (*) and TH.2 (**) i-FSs in the medulla. TH.3 (***) colls cannot be ruled out. (D)
Horizontal section of gracile nuclei. The p600 male showed TH.1 (*) and TH.2 (**) i-FSs in the medulla. TH.3 (***) colls cannot be ruled out. (D)  Sagittal section of the mid-thoracic spinal cord. The multilevel TH.1 (*) i-FSs elongated to caudal levels distant from the lesion site. Fading thrust was thought to determine their shortened lengths. t = time. N: iron fix pinhole. Bars: 100 µm.
Sagittal section of the mid-thoracic spinal cord. The multilevel TH.1 (*) i-FSs elongated to caudal levels distant from the lesion site. Fading thrust was thought to determine their shortened lengths. t = time. N: iron fix pinhole. Bars: 100 µm.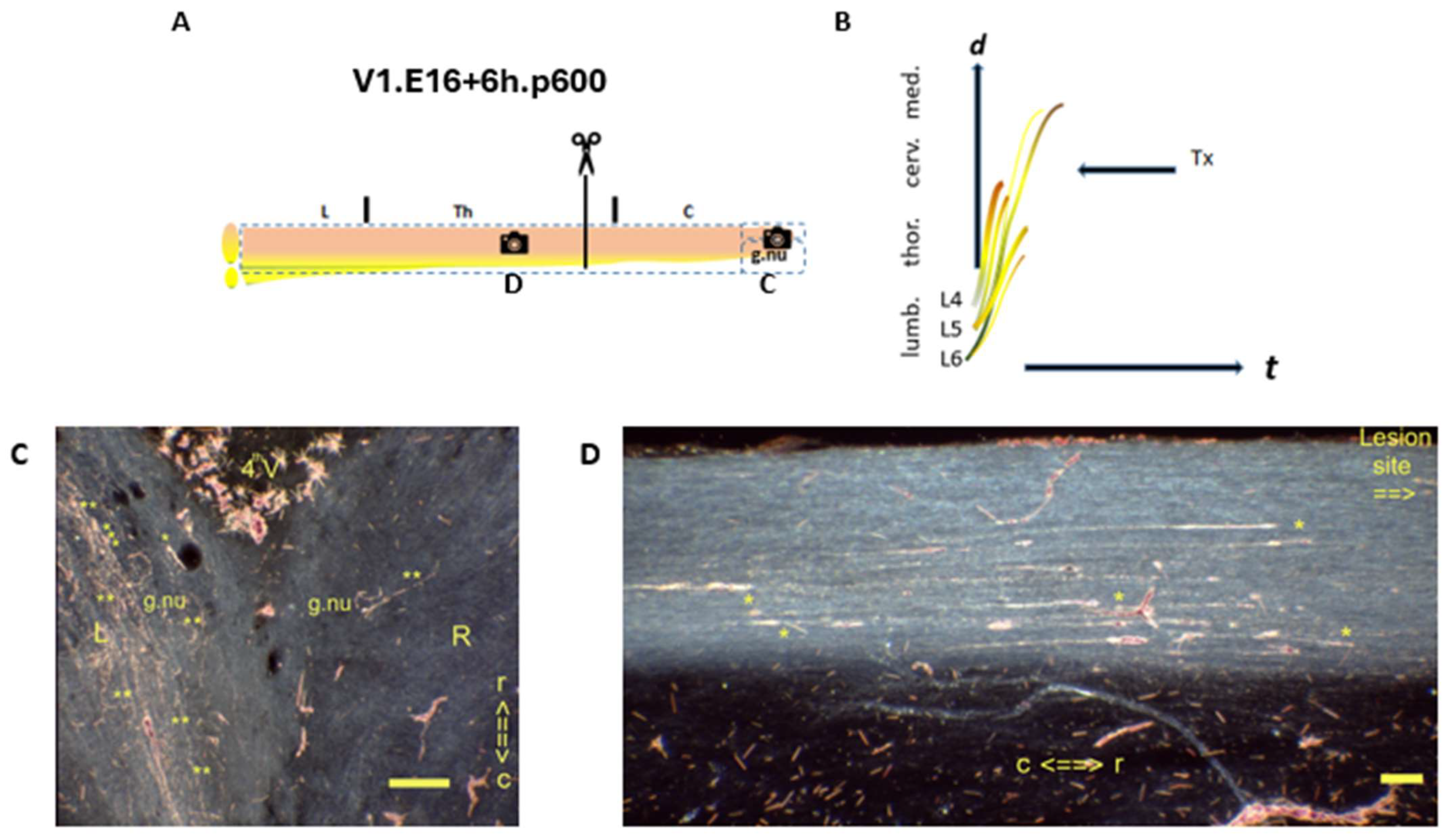
 denotes the spinal levels at (C,D). (B) Various i-FSs had crossed the lesion site. (C)
denotes the spinal levels at (C,D). (B) Various i-FSs had crossed the lesion site. (C)  Horizontal section of gracile nuclei. The p240 female exhibited the left gracile nucleus labeled possibly with a few TH.2 (**) i-FS. The presence of TH.3 colls would have required parent axons. That is questionable considering the estimated M0 downward on the cascade compared with the previous case. (D)
Horizontal section of gracile nuclei. The p240 female exhibited the left gracile nucleus labeled possibly with a few TH.2 (**) i-FS. The presence of TH.3 colls would have required parent axons. That is questionable considering the estimated M0 downward on the cascade compared with the previous case. (D)  Sagittal section at the lesion site. A fibrous attachment marked the lesion site covering the CG. A few age-morphed TH.1 (*) i-FSs (*) abutted the lesion site caudally. The a-FS in the neonate had been morphed with age; these few dispersed i-FSs had remained, exemplifying the morphed feature into the adult state over time. These axons re-elongated close to the level of the Tx. Bypassing the lesion site ventrally, another few fibers were present. These TH.1 or TH.2 i-FSs might have labeled the left gracile nucleus. t = time. N: iron fix pinhole. Bars: 100 µm.
Sagittal section at the lesion site. A fibrous attachment marked the lesion site covering the CG. A few age-morphed TH.1 (*) i-FSs (*) abutted the lesion site caudally. The a-FS in the neonate had been morphed with age; these few dispersed i-FSs had remained, exemplifying the morphed feature into the adult state over time. These axons re-elongated close to the level of the Tx. Bypassing the lesion site ventrally, another few fibers were present. These TH.1 or TH.2 i-FSs might have labeled the left gracile nucleus. t = time. N: iron fix pinhole. Bars: 100 µm.
 denotes the spinal levels at (C,D). (B) Various i-FSs had crossed the lesion site. (C)
denotes the spinal levels at (C,D). (B) Various i-FSs had crossed the lesion site. (C)  Horizontal section of gracile nuclei. The p240 female exhibited the left gracile nucleus labeled possibly with a few TH.2 (**) i-FS. The presence of TH.3 colls would have required parent axons. That is questionable considering the estimated M0 downward on the cascade compared with the previous case. (D)
Horizontal section of gracile nuclei. The p240 female exhibited the left gracile nucleus labeled possibly with a few TH.2 (**) i-FS. The presence of TH.3 colls would have required parent axons. That is questionable considering the estimated M0 downward on the cascade compared with the previous case. (D)  Sagittal section at the lesion site. A fibrous attachment marked the lesion site covering the CG. A few age-morphed TH.1 (*) i-FSs (*) abutted the lesion site caudally. The a-FS in the neonate had been morphed with age; these few dispersed i-FSs had remained, exemplifying the morphed feature into the adult state over time. These axons re-elongated close to the level of the Tx. Bypassing the lesion site ventrally, another few fibers were present. These TH.1 or TH.2 i-FSs might have labeled the left gracile nucleus. t = time. N: iron fix pinhole. Bars: 100 µm.
Sagittal section at the lesion site. A fibrous attachment marked the lesion site covering the CG. A few age-morphed TH.1 (*) i-FSs (*) abutted the lesion site caudally. The a-FS in the neonate had been morphed with age; these few dispersed i-FSs had remained, exemplifying the morphed feature into the adult state over time. These axons re-elongated close to the level of the Tx. Bypassing the lesion site ventrally, another few fibers were present. These TH.1 or TH.2 i-FSs might have labeled the left gracile nucleus. t = time. N: iron fix pinhole. Bars: 100 µm.
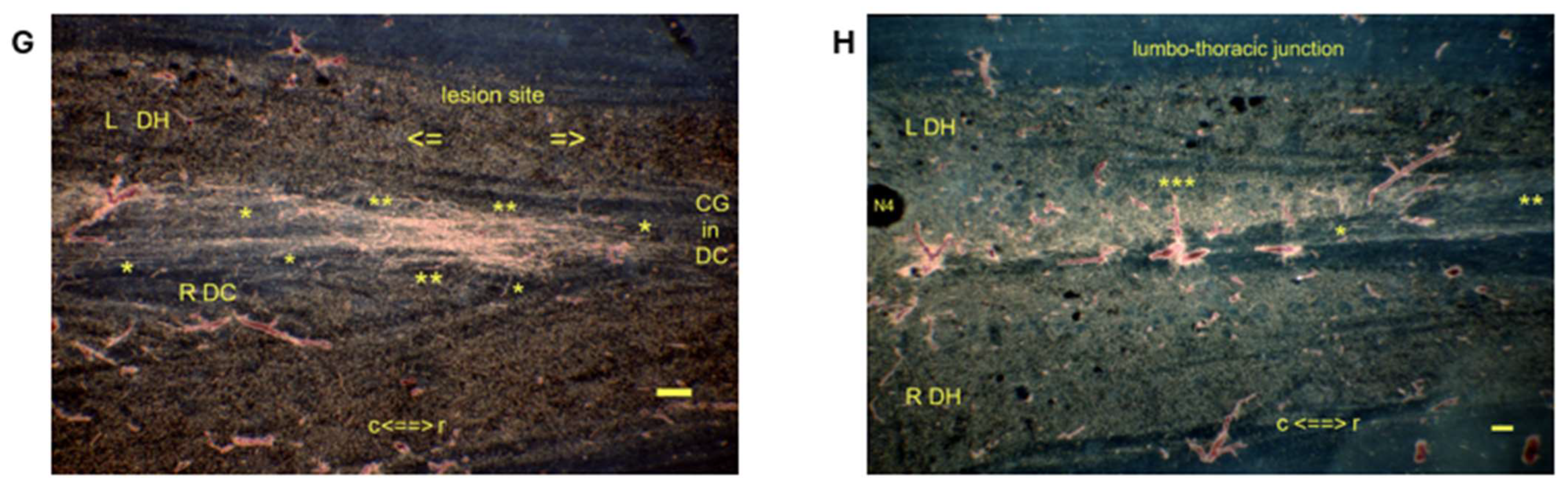
 denotes the spinal levels at C (sagittal section) and D (transverse section). (B) A few TH.2 (**) i-FSs might have regenerated into the medulla. (C) The lesion site was marked with a dorsal hump. The hallmark a-FS abutted the caudal lesion site. Various TH.1 (*) and TH.2 (**) i-FS were visible in the neuropil. (D) The labeled left gracile nucleus might exhibit an empty core fringed with labeled TH.2 (**) i-FSs. (E–H) In the W2.E16-6.5.p42 female, the configuration turned monomorphic, with decreased dynamic features. (E) The Tx level was located at an upper thoracic level.
denotes the spinal levels at C (sagittal section) and D (transverse section). (B) A few TH.2 (**) i-FSs might have regenerated into the medulla. (C) The lesion site was marked with a dorsal hump. The hallmark a-FS abutted the caudal lesion site. Various TH.1 (*) and TH.2 (**) i-FS were visible in the neuropil. (D) The labeled left gracile nucleus might exhibit an empty core fringed with labeled TH.2 (**) i-FSs. (E–H) In the W2.E16-6.5.p42 female, the configuration turned monomorphic, with decreased dynamic features. (E) The Tx level was located at an upper thoracic level.  denotes the spinal levels at (G,H) (horizontal sections). (F) The probably low-tide severed TH.1 (*) and all the TH.2 (**) i-FSs terminated caudally into the lesion site. The severed high-tide axons could also have contributed to the enhancement, leaving the gracile nuclei without label. (G) The Tx rendered the CG in disarray. The left-sided TH.2 (**) i-FSs crossed the midline, exhibiting downstream dynamics in contrast to those that had lost dynamics and remained on the left side. (H) The impaired labeling of Clarke’s nucleus matches the current dissociation. The presence of TH.3 (***) colls of low-tide parent axons targeting Clarke’s nucleus is also considered possible. t = time. Bars: 100 µm.
denotes the spinal levels at (G,H) (horizontal sections). (F) The probably low-tide severed TH.1 (*) and all the TH.2 (**) i-FSs terminated caudally into the lesion site. The severed high-tide axons could also have contributed to the enhancement, leaving the gracile nuclei without label. (G) The Tx rendered the CG in disarray. The left-sided TH.2 (**) i-FSs crossed the midline, exhibiting downstream dynamics in contrast to those that had lost dynamics and remained on the left side. (H) The impaired labeling of Clarke’s nucleus matches the current dissociation. The presence of TH.3 (***) colls of low-tide parent axons targeting Clarke’s nucleus is also considered possible. t = time. Bars: 100 µm.
 denotes the spinal levels at C (sagittal section) and D (transverse section). (B) A few TH.2 (**) i-FSs might have regenerated into the medulla. (C) The lesion site was marked with a dorsal hump. The hallmark a-FS abutted the caudal lesion site. Various TH.1 (*) and TH.2 (**) i-FS were visible in the neuropil. (D) The labeled left gracile nucleus might exhibit an empty core fringed with labeled TH.2 (**) i-FSs. (E–H) In the W2.E16-6.5.p42 female, the configuration turned monomorphic, with decreased dynamic features. (E) The Tx level was located at an upper thoracic level.
denotes the spinal levels at C (sagittal section) and D (transverse section). (B) A few TH.2 (**) i-FSs might have regenerated into the medulla. (C) The lesion site was marked with a dorsal hump. The hallmark a-FS abutted the caudal lesion site. Various TH.1 (*) and TH.2 (**) i-FS were visible in the neuropil. (D) The labeled left gracile nucleus might exhibit an empty core fringed with labeled TH.2 (**) i-FSs. (E–H) In the W2.E16-6.5.p42 female, the configuration turned monomorphic, with decreased dynamic features. (E) The Tx level was located at an upper thoracic level.  denotes the spinal levels at (G,H) (horizontal sections). (F) The probably low-tide severed TH.1 (*) and all the TH.2 (**) i-FSs terminated caudally into the lesion site. The severed high-tide axons could also have contributed to the enhancement, leaving the gracile nuclei without label. (G) The Tx rendered the CG in disarray. The left-sided TH.2 (**) i-FSs crossed the midline, exhibiting downstream dynamics in contrast to those that had lost dynamics and remained on the left side. (H) The impaired labeling of Clarke’s nucleus matches the current dissociation. The presence of TH.3 (***) colls of low-tide parent axons targeting Clarke’s nucleus is also considered possible. t = time. Bars: 100 µm.
denotes the spinal levels at (G,H) (horizontal sections). (F) The probably low-tide severed TH.1 (*) and all the TH.2 (**) i-FSs terminated caudally into the lesion site. The severed high-tide axons could also have contributed to the enhancement, leaving the gracile nuclei without label. (G) The Tx rendered the CG in disarray. The left-sided TH.2 (**) i-FSs crossed the midline, exhibiting downstream dynamics in contrast to those that had lost dynamics and remained on the left side. (H) The impaired labeling of Clarke’s nucleus matches the current dissociation. The presence of TH.3 (***) colls of low-tide parent axons targeting Clarke’s nucleus is also considered possible. t = time. Bars: 100 µm.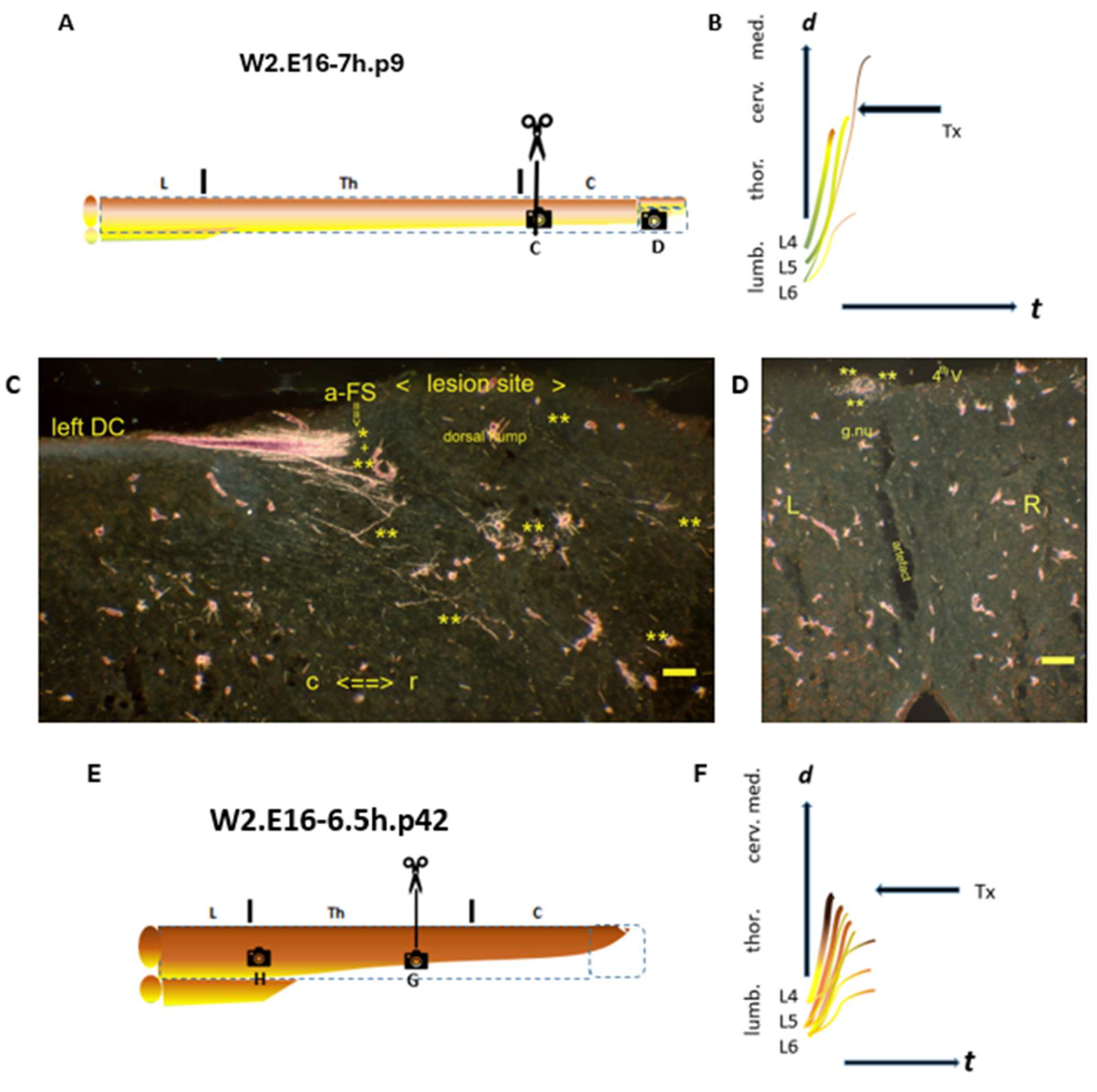
 denotes the spinal levels at (C,D) (horizontal sections). (B) The closure of the CP is imminent. (C)
denotes the spinal levels at (C,D) (horizontal sections). (B) The closure of the CP is imminent. (C)  In the left rostral DC, the final TH.2 (**) i-FS did not reach the medulla. (D)
In the left rostral DC, the final TH.2 (**) i-FS did not reach the medulla. (D)  The lesion site’s level was first and foremost identifiable by the midline cyst. The a-FS showed a typical feature of bundled TH.1 (*) i-FSs in a neonate. +: The a-FS may also show a plausible mix of TH.1 and TH.2 i-FSs. Several i-FSs might have regenerated beyond the lesion site without reaching the medulla. The TH.3 (***) colls exhibited an unusual growth pattern of variable lengths throughout the CG. Their dystopic levels in the upper thoracic spinal cord might indicate they originated from low-tide parent axons, aligning with dissociation. t = time. Bars: 100 µm.
The lesion site’s level was first and foremost identifiable by the midline cyst. The a-FS showed a typical feature of bundled TH.1 (*) i-FSs in a neonate. +: The a-FS may also show a plausible mix of TH.1 and TH.2 i-FSs. Several i-FSs might have regenerated beyond the lesion site without reaching the medulla. The TH.3 (***) colls exhibited an unusual growth pattern of variable lengths throughout the CG. Their dystopic levels in the upper thoracic spinal cord might indicate they originated from low-tide parent axons, aligning with dissociation. t = time. Bars: 100 µm.
 denotes the spinal levels at (C,D) (horizontal sections). (B) The closure of the CP is imminent. (C)
denotes the spinal levels at (C,D) (horizontal sections). (B) The closure of the CP is imminent. (C)  In the left rostral DC, the final TH.2 (**) i-FS did not reach the medulla. (D)
In the left rostral DC, the final TH.2 (**) i-FS did not reach the medulla. (D)  The lesion site’s level was first and foremost identifiable by the midline cyst. The a-FS showed a typical feature of bundled TH.1 (*) i-FSs in a neonate. +: The a-FS may also show a plausible mix of TH.1 and TH.2 i-FSs. Several i-FSs might have regenerated beyond the lesion site without reaching the medulla. The TH.3 (***) colls exhibited an unusual growth pattern of variable lengths throughout the CG. Their dystopic levels in the upper thoracic spinal cord might indicate they originated from low-tide parent axons, aligning with dissociation. t = time. Bars: 100 µm.
The lesion site’s level was first and foremost identifiable by the midline cyst. The a-FS showed a typical feature of bundled TH.1 (*) i-FSs in a neonate. +: The a-FS may also show a plausible mix of TH.1 and TH.2 i-FSs. Several i-FSs might have regenerated beyond the lesion site without reaching the medulla. The TH.3 (***) colls exhibited an unusual growth pattern of variable lengths throughout the CG. Their dystopic levels in the upper thoracic spinal cord might indicate they originated from low-tide parent axons, aligning with dissociation. t = time. Bars: 100 µm.
 denotes the spinal levels at (C,D) (horizontal sections). (B) The yellow WM-facing TH.1 (*) i-FSs regenerated into the medulla. (C)
denotes the spinal levels at (C,D) (horizontal sections). (B) The yellow WM-facing TH.1 (*) i-FSs regenerated into the medulla. (C)  The left DC (open arrow) covering the gracile nucleus harbored likely the axons connected to the a-FS, which regenerated to a remarkable rostral medullary level. The axons had bypassed the left gracile nucleus. The elongating axons from L5 and/or L6 DRGs might have created this unusual a-FS when severed just before and/or just after the transition assigned to the f-ES phenomenon at the medulla. (D)
The left DC (open arrow) covering the gracile nucleus harbored likely the axons connected to the a-FS, which regenerated to a remarkable rostral medullary level. The axons had bypassed the left gracile nucleus. The elongating axons from L5 and/or L6 DRGs might have created this unusual a-FS when severed just before and/or just after the transition assigned to the f-ES phenomenon at the medulla. (D)  The lesion site exhibited the traumatic origin of a diastematomyelia at the thoracic level. The left DC contained TH.1 (*) i-FSs regenerated into the medulla. t = time. Bar: 100 µm.
The lesion site exhibited the traumatic origin of a diastematomyelia at the thoracic level. The left DC contained TH.1 (*) i-FSs regenerated into the medulla. t = time. Bar: 100 µm.
 denotes the spinal levels at (C,D) (horizontal sections). (B) The yellow WM-facing TH.1 (*) i-FSs regenerated into the medulla. (C)
denotes the spinal levels at (C,D) (horizontal sections). (B) The yellow WM-facing TH.1 (*) i-FSs regenerated into the medulla. (C)  The left DC (open arrow) covering the gracile nucleus harbored likely the axons connected to the a-FS, which regenerated to a remarkable rostral medullary level. The axons had bypassed the left gracile nucleus. The elongating axons from L5 and/or L6 DRGs might have created this unusual a-FS when severed just before and/or just after the transition assigned to the f-ES phenomenon at the medulla. (D)
The left DC (open arrow) covering the gracile nucleus harbored likely the axons connected to the a-FS, which regenerated to a remarkable rostral medullary level. The axons had bypassed the left gracile nucleus. The elongating axons from L5 and/or L6 DRGs might have created this unusual a-FS when severed just before and/or just after the transition assigned to the f-ES phenomenon at the medulla. (D)  The lesion site exhibited the traumatic origin of a diastematomyelia at the thoracic level. The left DC contained TH.1 (*) i-FSs regenerated into the medulla. t = time. Bar: 100 µm.
The lesion site exhibited the traumatic origin of a diastematomyelia at the thoracic level. The left DC contained TH.1 (*) i-FSs regenerated into the medulla. t = time. Bar: 100 µm.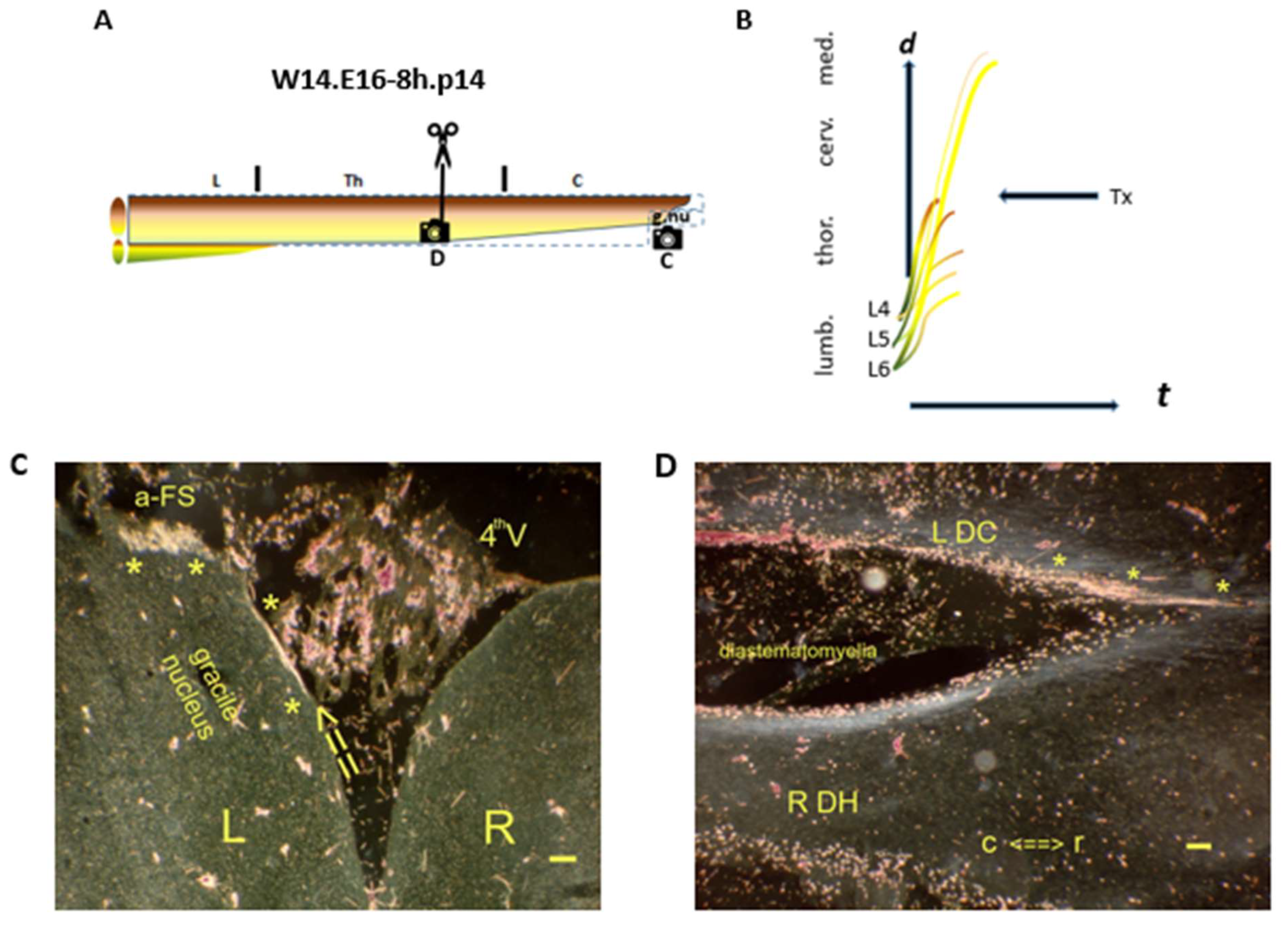
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Glossary
| a-FS | abrupt front stop | A bundle of WM-facing i-FSs manifests in neonates. The a-FS abuts the rostral lesion site, and the phenotype demonstrates the blocked development [12] |
| CP | critical period | Upstream time slot(s) delineating elongation in each (and all) pioneering long primary afferent axon(s), as well as axon regeneration |
| CG | central gray | neuropil |
| colls | TH.3-staged collaterals = collateral sprouting after the TH.3 transit. They confirm accomplished development, e.g., in gracile nuclei | |
| DRG | dorsal root ganglion | involving the lumbar segments L4, L5, and L6 (HRP-tracing at the left side, only) |
| DC | dorsal column | |
| E16-8h | 15th day of gestation | M0 is scheduled 15 days + 16 h after mating, restricted to 1 h |
| E16 | day of conception without surveillance of mating time | |
| f-ES | fast elongation stop | a hypothetical phenomenon of pioneering TH.0-staged axons arriving at the medulla and swiftly slowing down |
| fringe | The CG adjacent to the DC white matter | |
| high tide | In the rostral DC, pioneering axons target the gracile nuclei in high-tide waves at spring tide | |
| i-FS | intrinsic front stop | The hypothetical TH.1- and TH.2-stage axon mimicries generate phenotypes, which demonstrate blocked development. At high tide, the i-FSs regenerate toward the medulla. At low tide, the TH.1 fiber tips remain caudally distanced from the lesion site, while the TH.2 i-FSs terminate caudally in the lesion site. |
| HRP | Horseradish peroxidase | |
| low tide | In the lower thoracic DC, pioneering axons target Clarke’s nucleus in low-tide waves at neap tide Horseradish peroxidase | |
| M0 | The moment of Tx is referenced to the hour (or day) of conception and serves as the case ID in combination with survival time (Table 1). M0 has a temporospatial link with a location on the assembly line determined by the axon features, demonstrating variability. This underlines that the development of fetuses is prone to differ | |
| neap tide | See low tide | |
| s-ES | slow elongation switch | The hypothetical phenomenon of TH.1 axons transitioning into the consecutive TH.2 stage |
| spring tide | See high tide | |
| tc | Terminal club: fiber termination with a configuration comparable to i-FS | |
| THs | transition hubs | TH.0, TH.1, TH.2, and TH.3 are hypothetical transition hubs situated on the developmental cascade. TH.0 axons were reprogrammed into the TH.1 i-FS mimicry, and TH.1 into the TH.2 i-FS substitute |
| TH.0 | staged axon | Pioneering primary afferent axon in a high-tide, as well as a low-tide wave |
| Tx | dorsal myelotomy | The microsurgical procedure |
References
- Bourguignon, L.; Tong, B.; Geisler, F.; Schubert, M.; Röhrich, F.; Sauer, M.; Weidner, N.; Rupp, R.; Kalke, Y.-B.B.; Abel, R.; et al. International surveillance study in acute spinal cord injury confirms viability of multinational clinical trials. BMC Med. 2022, 20, 225. [Google Scholar] [CrossRef] [PubMed]
- Dietz, V.A.; Roberts, N.; Knox, K.; Moore, S.; Pitonak, M.; Barr, C.; Centeno, J.; Leininger, S.; New, K.C.; Nowell, P.; et al. Fighting for recovery on multiple fronts: The past, present, and future of clinical trials for spinal cord injury. Front. Cell. Neurosci. 2022, 16, 977679. [Google Scholar] [CrossRef] [PubMed]
- Jagrit, V.; Koffler, J.; Dulin, J.N. Combinatorial strategies for cell transplantation in traumatic spinal cord injury. Front. Neurosci. 2024, 18, 1349446. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, P.; Zhang, F.; O’Neill, P. Changes in spinal cord regenerative ability through phylogenesis and development: Lessons to be learned. Dev. Dyn. 2003, 226, 245–256. [Google Scholar] [CrossRef]
- Rodemer, W.; Hu, J.; Selzer, M.E.; Shifman, M.I. Heterogeneity in the regenerative abilities of central nervous system axons within species: Why do some neurons regenerate better than others? Neur Reg. Res. 2020, 15, 996–1005. [Google Scholar]
- Norris, V. Successive paradigm shifts in the bacterial cell cycle and related subjects. Life 2019, 9, 27. [Google Scholar] [CrossRef]
- Illis, L.S. Central nervous system regeneration does not occur. Spinal Cord 2012, 50, 259–263. [Google Scholar] [CrossRef]
- Mothe, A.J.; Tator, C.H. Review of transplantation of neural stem/progenitor cells for spinal cord injury. Int. J. Devl. Neurosci. 2013, 31, 701–713. [Google Scholar] [CrossRef]
- Olivero, R.S.; Bello, S.; Biering-Sørensen, F. Mesenchymal stem cells improve locomotor recovery in traumatic spinal cord injury: Systematic review with meta-analyses of rat models. Neurobiol. Dis. 2014, 62, 338–353. [Google Scholar] [CrossRef]
- Xu, X.-M. Breaking news in spinal cord injury research. Neur Reg. Res. 2012, 7, 1685–1687. [Google Scholar]
- Ahuja, C.S.; Mothe, A.; Khazaei, M.; Badhiwala, J.H.; Gilbert, E.A.; Kooy, D.; Morshead, C.M.; Tator, C.; Fehlings, M.G. The leading edge: Emerging neuroprotective cell-based therapies for spinal cord injury. Stem Cells Transl. Med. 2020, 9, 1509–1530. [Google Scholar] [CrossRef]
- De Beer, F.C.; Steinbusch, H.W. On the blueprint of the long primary afferent axons and the dichotomous axon trajectory of Clarke’s nucleus. A morphological tracing study on the effect of hypoxia during development. Anatomia 2023, 2, 414–449. [Google Scholar] [CrossRef]
- Schwab, M.E.; Bartholdi, D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol. Rev. 1996, 76, 319–370. [Google Scholar] [CrossRef] [PubMed]
- Quraishe, S.; Forbes, L.H.; Andrews, M.R. The extracellular environment of the CNS: Influence on plasticity, sprouting, and axonal regeneration after spinal cord injury. Neural Plast. 2018, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S. Induced pluripotent stem cells: Past, Present, and Future. Cell Stem Cell 2012, 10, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Gearhart, J.; Oster-Granite, M.L.; Guth, L. Histological changes after transection of the spinal cord of fetal and neonatal mice. Exp. Neurol. 1979, 66, 1–15. [Google Scholar] [CrossRef]
- Buyan Dent, L.J.; McCasland, J.S.; Stelzner, D.J. Attempts to facilitate dorsal column axonal regeneration in a neonatal spinal environment. J. Comp. Neurol. 1996, 372, 435–456. [Google Scholar] [CrossRef]
- Wree, A.; Fiekas, D.; De Beer, F.C.; Beck, T. Lokale zerebrale Glukose-Utilisation des Riickenmarkes und der Hinterstrangkerne der Ratte nach pränataler dorsaler Myelotomie in Höhe Th12: Eine [14C]2-Desoxyglukose-Studie. Eur. J. Neurosci. 1998, 10, 62. [Google Scholar]
- Steward, O.; Zheng, B.; Tessier-Lavigne, M. False resurrections: Distinguishing regenerated from spared axons in the injured central nervous system. J. Comp. Neurol. 2003, 459, 1–8. [Google Scholar] [CrossRef]
- Mesulam, M.M.; Brushart, T.M. Transganglionic and anterograde transport of horseradish peroxidase across dorsal root ganglia: A tetramethylbenzidine method for tracing central sensory connections of muscles and peripheral nerves. Neuroscience 1979, 4, 1107–1117. [Google Scholar] [CrossRef]
- De Beer, F.C. Dorsal myelotomy in E15-E16 fetal rat: A promising paradigm in regeneration research with serendipitous transcriptomic effects on development of the long primary afferent system. J. Neurosci. Methods 2022, 366, 109402. [Google Scholar] [CrossRef]
- Cox-Limpens, K.; Strackx, E.; Van den Hove, D.L.A.; Van Ekkendonk, J.R.A.; De Jong, M.; Zimmermann, L.J.I.; Steinbusch, H.W.M.; Vles, J.S.H.; Gavilanes, A.W.D. Fetal asphyctic preconditioning protects against perinatal asphyxia-induced apoptosis and astrogliosis in neonatal brain. CNS Neurol. Disord. Drug Targets 2015, 14, 33–40. [Google Scholar] [CrossRef]
- Rivkees, S.A.; Wendler, C.C. Long-term consequences of disrupting adenosine signaling during embryonic development. Mol. Aspects Med. 2017, 55, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Bustelo, M.; Barkhuizen, M.; Van den Hove, D.L.A.; Steinbusch, H.W.M.; Bruno, M.A.; Loidl, C.F.; Gavilanes, A.W.D. Clinical implications of epigenetic dysregulation in perinatal hypoxic-ischemic brain damage. Front. Neurol. 2020, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Dissecting spinal cord regeneration. Nature 2018, 557, 344. [Google Scholar] [CrossRef] [PubMed]
- Stepien, B.K.; Wielockx, B. From vessels to neurons—The role of hypoxia pathway proteins in embryonic neurogenesis. Cells 2024, 13, 621–651. [Google Scholar] [CrossRef]
- Bjelke, B.; Andersson, K.; Ögren, S.O.; Bolme, P. Asphyctic lesion: Proliferation of tyrosine hydroxylase-immunoreactive nerve cell bodies in the rat substantia nigra and functional changes in dopamine neurotransmission. Brain Res. 1991, 543, 1–9. [Google Scholar] [CrossRef]
- Pires Monteiro, S.; Voogd, E.; Muzzi, L.; De Vecchis, G.; Mossink, B.; Levers, M.; Hassink, G.; Van Putten, M.; Le Feber, J.; Hofmeijer, J.; et al. Neuroprotective effect of hypoxic preconditioning and neuronal activation in an in vitro human model of the ischemic penumbra. J. Neural Eng. 2021, 18, 036016. [Google Scholar] [CrossRef]
- Stefanova, E.E.; Scott, A.L. Purinergic signaling systems across comparative models of spinal cord injury. Neural Regen. Res. 2022, 17, 2391–2398. [Google Scholar] [CrossRef]
- Lanfranchi, M.; Yandiev, S.; Meyer-Dilhet, G.; Ellouze, S.; Kerkhofs, M.; Dos Reis, R.; Garcia, A.; Blondet, C.; Amar, A.; Kneppers, A.; et al. The AMPK-related kinase NUAK1 controls cortical axon branching by locally modulating mitochondrial metabolic functions. Nat. Commun. 2024, 15, 2487–2504. [Google Scholar] [CrossRef]
- Silagi, E.S.; Nduka, E.; Pazyra-Murphy, M.F.; Paiz, J.Z.; Bhuiyan, S.; Segal, R.A. Profiling local translatomes and RNA binding proteins of somatosensory neurons reveals specializations of individual axons. bioRxiv 2025. [CrossRef]
- Lu, P.; Woodruff, G.; Wang, Y.; Graham, L.; Hunt, M.; Wu, D.; Boehle, E.; Ahmad, R.; Poplawski, G.; Brock, J.; et al. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron 2014, 83, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Tuszynski, M.H.; Steward, O. Concepts and methods for the study of axonal regeneration in the CNS. Neuron 2012, 74, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Tedeschi, A.; Park, K.K.; He, Z. Neuronal intrinsic mechanisms of axon regeneration. Ann. Rev. Neurosci. 2011, 34, 131–152. [Google Scholar] [CrossRef] [PubMed]
- Lindhout, F.W.; Szafranska, H.M.; Imaz-Rosshandler, I.; Guglielmi, L.; Morefian, M.; Voitiuk, K.; Zernicka-Glover, N.K.; Boulanger, J.; Schulze, U.; Lloyd-Davies Sanchez, D.J.; et al. Calcium dynamics tune developmental tempo to generate evolutionary divergent tract lengths. bioRxiv 2024. [CrossRef]
- Jacobson, K.A.; Pradhan, B.; Wen, Z.; Pramanik, A. New paradigms in purinergic receptor ligand discovery. Neuropharmacology 2023, 230, 10950. [Google Scholar] [CrossRef]
- Vissers, C.; Sinha, A.; Ming, G.-L.; Song, H. The epitranscriptome in stem cell biology and neural development. Neurobiol. Dis. 2020, 146, 105139. [Google Scholar] [CrossRef]
- Coquand, L.; Avalos, C.B.; Macé, A.-S.; Farcy, S.; Di Cicco, A.; Lampic, M.; Wimmer, R.; Bessières, B.; Attie-Bitach, T.; Fraisier, V.; et al. A cell fate decision map reveals abundant direct neurogenesis bypassing intermediate progenitors in the human developing neocortex. Nat. Cell Biol. 2024, 26, 698–709. [Google Scholar] [CrossRef]
- Hartman, A.; Satija, R. Comparative analysis of multiplexed in situ gene expression profiling technologies. bioRxiv 2024. [Google Scholar] [CrossRef]
- He, Z.; Jin, Y. Intrinsic control of axon regeneration. Neuron 2016, 90, 437–451. [Google Scholar] [CrossRef]
- Tedeschi, A.; Dupraz, S.; Curcio, M.; Laskowski, C.J.; Schaffran, B.; Flynn, K.C.; Santos, T.E.; Stern, S.; Hilton, B.J.; Larson, M.J.E.; et al. ADF/Cofilin-mediated actin turnover promotes axon regeneration in the adult CNS. Neuron 2019, 103, 1073–1085. [Google Scholar] [CrossRef]
- Martins-Costa, C.; Wiegers, A.; Pham, V.A.; Sidhaye, J.; Doleschall, B.; Novatchkova, M.; Lendl, T.; Piber, M.; Peer, A.; Möseneder, P.; et al. ARID1B controls transcriptional programs of axon projection in an organoid model of the human corpus callosum. Cell Stem Cell 2024, 31, 866–885.e14. [Google Scholar] [CrossRef]
- Leite, S.C.; Pinto-Costa, R.; Sousa, M.M. Actin dynamics in the growth cone: A key player in axon regeneration. Curr. Opin. Neurobiol. 2021, 69, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Marichal, N.; Fabbiani, G.; Trujillo-Cenóz, O.; Russo, R.E. Purinergic signaling in a latent stem cell niche of the rat spinal cord. Purinergic Signal 2016, 12, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic nerves. Pharmacol. Rev. 1972, 24, 509–557. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 2007, 87, 659–797. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Arias, J.C.; Van der Slagt, E.; Vecchiarelli, H.A.; Candlish, R.C.; York, N.; Young, P.A.; Shevtsova, O.; Juma, A.; Tremblay, M.-E.; Swayne, L.A. Purinergic signaling in nervous system health and disease: Focus on pannexin 1. Pharmacol. Ther. 2021, 225, 107840. [Google Scholar] [CrossRef]
- Illes, P.; Di Virgilio, F.; Tang, Y. Purinergic Signaling: 50 years. Neuropharmacology 2024, 245, 109826. [Google Scholar] [CrossRef]
- Fawcett, J.W. Why has it been so difficult to make CNS axons regenerate? Neurochem. Res. 2020, 45, 144–158. [Google Scholar] [CrossRef]
- Colonna, M.; Konopka, G.; Liddelow, S.A.; Nowakowski, T.; Awatramani, R.; Bateup, H.S.; Cadwell, C.R.; Caglayan, E.; Chen, J.L.; Gillis, J.; et al. Implementation and validation of single-cell genomics experiments in neuroscience. Nat. Neurosci. 2024, 27, 2310–2325. [Google Scholar] [CrossRef]
- Blesch, A.; Lu, P.; Tsukada, S.; Alto, L.T.; Roet, K.; Coppola, G.; Geschwind, D.; Tuszynski, M.H. Conditioning lesions before or after spinal cord injury recruit broad genetic mechanisms that sustain axonal regeneration: Superiority to clamp-mediated effects. Exp. Neurol. 2012, 235, 162–173. [Google Scholar] [CrossRef]
- Griffin, J.M.; Bradke, F. Therapeutic repair for spinal cord injury: Combinatory approaches to address a multifaceted problem. EMBO Mol. Med. 2020, 12, e11505. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Beer, F.C.; Steinbusch, H.W.M. CNS Axon Regeneration in the Long Primary Afferent System in E15/E16 Hypoxic-Conditioned Fetal Rats: A Thrust-Driven Concept. Anatomia 2025, 4, 12. https://doi.org/10.3390/anatomia4030012
de Beer FC, Steinbusch HWM. CNS Axon Regeneration in the Long Primary Afferent System in E15/E16 Hypoxic-Conditioned Fetal Rats: A Thrust-Driven Concept. Anatomia. 2025; 4(3):12. https://doi.org/10.3390/anatomia4030012
Chicago/Turabian Stylede Beer, Frits C., and Harry W. M. Steinbusch. 2025. "CNS Axon Regeneration in the Long Primary Afferent System in E15/E16 Hypoxic-Conditioned Fetal Rats: A Thrust-Driven Concept" Anatomia 4, no. 3: 12. https://doi.org/10.3390/anatomia4030012
APA Stylede Beer, F. C., & Steinbusch, H. W. M. (2025). CNS Axon Regeneration in the Long Primary Afferent System in E15/E16 Hypoxic-Conditioned Fetal Rats: A Thrust-Driven Concept. Anatomia, 4(3), 12. https://doi.org/10.3390/anatomia4030012








