Abstract
Objective: The aim of this study was to determine whether preoperative MRI has an impact on surgical planning in breast cancer patients. Tumor extent and molecular breast cancer subtypes were evaluated. Methods: This was a single-center study including 137 female patients with a first diagnosis of invasive breast cancer. Each patient had a standard clinical preoperative workup and an additional breast MRI. The interdisciplinary tumor board made written recommendations regarding the surgical therapy of each patient with and without the knowledge of the MRI findings. Results: The addition of MRI led to changes in surgical recommendations in 32 (23%) of the 137 patients. The highest rate of change in surgical therapy recommendations was observed in patients with multifocal tumors (53%). Molecular subtype had no influence on the changes in surgical therapy recommendations (p = 0.8). Conclusions: Patients with multifocal breast tumors were more likely to have a change in surgical therapy following MRI.
1. Introduction
Magnetic resonance imaging (MRI) of the female breast has become an essential tool in the preoperative evaluation of breast cancer. Unlike mammography and ultrasound, MRI provides high-resolution, three-dimensional images of the breast tissue, offering a more detailed assessment of tumor size, location, and spread [1]. This additional information can significantly impact surgical planning and overall treatment strategies, making breast MRI an important component of modern breast cancer management. MRI has increasingly been used for preoperative staging in patients with breast cancer [1]. Nevertheless, there is controversy among breast cancer care providers about the routine use of MRI to evaluate patients preoperatively. Some studies have reported that, compared with mammograms and ultrasound, breast MRI helps to measure tumor size more accurately and to detect multifocal and multicentric disease, which may lead to changes in treatment planning [2,3,4,5,6]. In addition to tumor size and multifocality, MRI can also provide important information about the tumor’s shape, margins, and enhancement patterns. These characteristics help distinguish between invasive and non-invasive tumors and can guide decisions regarding the need for additional biopsies before surgery. Another major benefit of preoperative breast MRI is its ability to detect cancer in the contralateral (opposite) breast. While most cases of breast cancer are unilateral, meaning that they affect only one breast, studies have shown that, in some cases, synchronous (simultaneous) cancer can be present in both breasts. Mammography alone may miss these contralateral tumors, particularly in women with dense breast tissue or those with lobular carcinoma, which can be difficult to visualize [7]. The ability of MRI to detect contralateral tumors is particularly important for patients with a high genetic risk of breast cancer, such as those with BRCA1 or BRCA2 mutations. For these patients, MRI is often used as part of a routine surveillance strategy, even in the absence of a known cancer diagnosis [7].
Breast cancer is a heterogeneous disease with many histological and molecular subtypes that have different prognoses and responses to therapy [8,9,10,11]. The main molecular subtypes of invasive breast cancer are related to the expression of hormone receptors and Her2 status [1]. The luminal A subtype is associated with a low proliferation index and accounts for 50–60% of all breast cancers [12]. The luminal B subtype is associated with a high expression of proliferation-related genes and accounts for 20% of all breast cancers [13,14]. The Her2 subtype accounts for 10% of all breast cancers and is characterized by the absence of hormone receptors and a high expression of Her2 [15,16]. The triple-negative subtype accounts for 7–16% of all breast cancers and is characterized by the absence of the expression of hormone receptors and Her2; it is associated with less differentiated invasive carcinomas and accounts for 70% of breast cancers in BRCA1-mutated females [1,17,18,19,20]. The different tumor types may also have different presentations on imaging studies [21,22,23,24,25,26]; however, to the best of our knowledge, only two studies have evaluated the use of preoperative MRI in patients with specific types of breast cancer [2,17].
The purpose of this study was to determine whether preoperative MRI has an impact on surgical planning in breast cancer patients. Furthermore, we investigated whether patients benefit differently depending on their histological type of cancer.
2. Methods
2.1. Study Design and Setting
This was a single-center retrospective study. It was conducted at a hospital in a medium-sized German city. All patients provided written informed consent for the MRI and other imaging and clinical treatments reported.
2.2. Patient Characteristics
Patients were eligible for inclusion if they were (1) female, (2) aged 18 or older, (3) had a first diagnosis of invasive breast cancer confirmed by percutaneous biopsy, (4) had undergone ultrasound and mammography, and (5) had undergone MRI between January 2017 and November 2019. Patients were excluded from the study if they met any one of the following criteria: (1) no invasive breast cancer, (2) metastatic disease at primary diagnosis, (3) MRI findings without histopathologic correlation, or (4) treatment planning and surgery at another hospital.
2.3. Routine Preoperative Imaging and Tumor Assessment
Each patient underwent a routine ultrasound, mammography, and computed tomography of the chest and abdomen for tumor staging. The presence of multifocal or multicentric tumor lesions, contralateral breast tumors, and immunohistochemical profiles were evaluated. Multifocal disease was defined as macroscopically recognizable, separate carcinoma foci in the same quadrant of the breast. Multicentric disease was defined as the occurrence of separate carcinoma foci in the ipsilateral breast within different quadrants [27,28].
2.4. Magnetic Resonance Imaging Protocol
MRI was performed with a 1.5-T system (Intera; Philips Medical Systems; Best, The Netherlands), using a double-breast 16-element surface coil. The imaging protocol, consisting of axial T2-weighted fast spin-echo sequences, was performed with and without fat-sat. Additionally, axial bilateral 2D-multisection gradient-echo dynamic series (repetition time msec/echo time msec: 250/4.6; flip angle: 90°) were performed with a full 512 × 512 acquisition matrix and a sensitivity encoding factor of two. Dynamic contrast-enhanced imaging was performed with identical anatomic parameters before and three times after a bolus injection of 0.1 mmol of gadobutrol per kilogram of body weight (Gadovist; Bayer; Leverkusen, Germany).
2.5. MRI Analysis
MRIs were evaluated by a radiologist who was experienced in breast imaging and blinded to all clinical evaluation data, except for the histologically confirmed diagnosis of breast cancer. The presence of monofocal, multifocal, multicentric, and/or contralateral tumor lesions was recorded.
2.6. Histopathology
The histology of breast lesions was classified using the WHO Classification of Breast Tumors [29]. Based on gene expression profiles, breast carcinomas were classified into four molecular subtypes: luminal A (positive for ER and/or PR, negative for Her2, and low Ki-67), luminal B (positive for ER and/or PR and high Ki-67 or positive for ER and/or PR and overexpressed Her2), Her2-positive (negative for ER and PR, with overexpressed Her2), and triple-negative (negative for ER, PR, and Her2 receptors) subtypes [27].
2.7. Tumor Board Recommendation
For each patient included in the study, the tumor board first made a clinical therapy recommendation based on all available information, except for the MRI findings. After case presentation and consideration of the MRI findings, the interdisciplinary tumor board made an additional recommendation. The board’s decisions with and without the additional breast MRI information are reported.
2.8. Statistical Analysis
All statistical calculations were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA). Descriptive statistics are used to summarize the data. Group comparisons of the relative frequencies of a variable were made using the Chi-squared test or Fischer’s exact test (when the preconditions for the Chi-squared test were not met). A two-sample t-test was used to compare the mean values of quantitative values between two groups. All tests were conducted as two-sided tests. The result of a statistical test was considered significant at p < 0.05.
3. Results
3.1. Patients
We collected data from 215 female patients who underwent breast MRI at our institution; however, 78 of them met the exclusion criteria. Thus, 137 patients who underwent preoperative MRI and had a first diagnosis of invasive breast cancer qualified for the analysis (Figure 1). All patients included in this study underwent percutaneous biopsy, followed by surgery of the breast. The mean (SD) age was 62.6 (14.0) years, and the age range was 30 to 88 years.
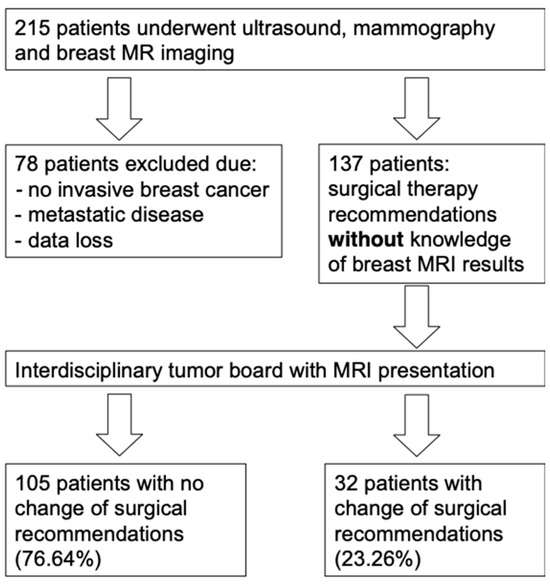
Figure 1.
Patient selection from the initial 215 patients.
3.2. Histological and Molecular Subtypes
Invasive breast cancer of no special type (NST) and special types of invasive breast carcinoma (i.e., invasive lobular carcinoma, metaplastic carcinoma, and mucinous carcinoma) were identified [19]. Based on gene expression profiles, breast carcinomas were classified into four molecular subtypes: luminal A (positive for ER and/or PgR, negative for Her2, and low Ki-67), luminal B (positive for ER and/or PgR and high Ki-67 or positive for ER and/or PgR and overexpressed Her2), Her2 (negative for ER and PgR, with overexpressed Her2), and triple-negative (negative for ER, PR, and Her2 receptors) subtypes.
The histological and molecular subtypes are summarized in Table 1.

Table 1.
Histological and molecular tumor subtypes and tumor manifestations.
3.3. Tumor Board Decision
After reviewing the additional information from the MRI, the surgical therapy recommendation was changed for 32 patients (23%) (95% confidence interval: 16.6% to 31.3%). The changes in surgical decisions were as follows: a wider excision in 15 patients in planned BCS therapy that included lumpectomy or quadrantectomy, additional excision in 9 patients (additional excision in another quadrant of the ipsilateral breast or tissue removal in the contralateral breast), and mastectomy in 8 patients (removal of the female mammary gland on one or both sides).
A change in the surgical therapy recommendation was especially frequent in the luminal B/Her2-negative subtypes in combination with multifocal tumors on MRI (in seven out of nine cases). In 128 patients with other combinations, a change in treatment was made in only 20% of cases. This difference was significant (p < 0.001).
Among the patients who received a surgical recommendation for mastectomy, the tumor board decision did not differ after the MRI findings were reported. In the BCS therapy group, 32 out of 113 patients (28%) had changes in their surgical therapy recommendations when the MRI findings were considered (Table 2).

Table 2.
Influence of MRI on surgical therapy recommendations.
In the two women with both NST and lobular carcinoma, the therapy recommendation was changed after considering the MRI findings. Although minor differences were apparent, the different molecular subtypes had no influence on whether the surgical therapy recommendation was changed (p = 0.7) (Table 3 and Table 4).

Table 3.
Change in surgical therapy recommendation following breast MRI, depending on molecular subtype.

Table 4.
Characteristics of patients with therapy modification.
4. Discussion
Our data suggest that preoperative MRI has an impact on surgical therapy recommendations in patients with invasive breast cancer. Molecular subtype alone had no influence on surgical therapy recommendations following MRI. We found that patients with multifocal (i.e., bifocal or more) breast tumors were more likely to have a change in surgical therapy following MRI.
MRI is particularly useful in cases where conventional imaging methods might not provide a complete picture of the tumor burden. By using strong magnetic fields and contrast agents, it enhances the visualization of cancerous lesions and helps determine the most appropriate surgical approach. While mammography remains the gold standard for breast cancer screening, preoperative MRI is increasingly recommended in specific clinical scenarios where more precise imaging is needed.
One of the primary advantages of preoperative breast MRI is its ability to more accurately define the extent of the tumor. Breast cancer can present as a single localized lesion; however, in many cases, additional cancerous areas may be present within the same breast. Conventional imaging techniques, such as mammography and ultrasound, may not always detect these additional lesions, particularly in women with dense breast tissue or those with lobular carcinoma, which tends to grow in a diffuse pattern. MRI has a much higher sensitivity than mammography and ultrasound in identifying multifocal and multicentric disease [2,5,9,29,30]. This is a critical factor in determining whether BCS surgery is feasible or whether mastectomy may be a better option [31]. Studies have shown that MRI findings lead to changes in the surgical plan in a significant percentage of patients, often preventing incomplete tumor removal and reducing the likelihood of local recurrence [2,5,9]. Compared to mammography and ultrasound, MRI of the breast has a higher sensitivity in the examination of tumor extension, especially for invasive lobular breast cancer and for the detection of multifocal, multicentric, and contralateral tumors [29,30].
Several other studies have shown that preoperative MRI can improve the detection of multifocal or multicentric breast cancer, a larger cancer, or a contralateral tumor, when added to conventional preoperative imaging at the time of the initial diagnosis, leading to changes in surgical therapy recommendations [29,32,33,34,35,36,37,38,39]. Nonetheless, MRI of the breast has not yet become a routine imaging modality in breast cancer patients who are to be treated with surgery.
Preoperative MRI has been shown to significantly impact surgical decision-making. In some cases, MRI findings may indicate that a tumor is larger or more extensive than initially thought, leading to a change in the surgical approach. This can prevent the need for additional surgeries by ensuring that all cancerous tissue is removed in the initial procedure. For patients undergoing BCS surgery, MRI helps determine whether clear surgical margins can be achieved. If MRI shows that the tumor is too large or has multiple satellite lesions, mastectomy may be recommended instead. Additionally, MRI can be useful in identifying cases where BCS surgery is still feasible despite an initially unfavorable mammographic appearance. In some cases, MRI may reveal that a tumor is well defined and can be safely removed with lumpectomy, sparing the patient from more aggressive surgery [40].
This is in line with our results showing that the consideration of MRI findings often led to changes in the treatment recommendations of breast cancer patients who previously only underwent mammography and ultrasound. Out of the 137 patients who underwent MRI in our study, 32 had a change in the surgical decision. In particular, a subgroup of patients with multifocal tumors of the breast seen on MRI was more likely to have changes in surgical therapy recommendation. Therefore, it could be argued that these patients are the most likely to benefit from MRI. Nonetheless, no randomized clinical trials have revealed an increase in survival or even a reduction in recurrence in breast cancer in patients who have undergone MRI for staging and treatment planning.
However, studies have shown contradictory results. For example, Young et al. reported that preoperative MRI confers no diagnostic advantage over breast ultrasound and mammography and that it may even lead to a worse outcome [15]. In particular, increased mastectomy rates without benefit may lead to unnecessary overtreatment. Delayed surgical reconstruction is subject to higher complication rates and a lack of recurrence reduction, despite more extensive surgery.
Furthermore, there is controversy about the numbers of additional surgeries carried out due to MRI findings and their impact in terms of morbidity and mortality in the medium and long term [1]. A German registry study with more than 142,000 cases reported that breast MRI was an independent factor predicting mastectomy (odds ratio: 1.42; 95% CI: 1.36–1.47) [28].
We also assessed whether tumor biology has an impact on presurgical planning. We found that a subgroup of patients with the luminal B/Her2-negative subtypes in combination with multifocal tumors also had a higher likelihood of changes in surgical therapy recommendations after MRI. This is partially consistent with a retrospective single-center analysis of 441 patients showing that MRI may helpful in patients with multifocal or multicentric disease in Her2-positive and luminal B tumor subtypes [2]. The authors suggested that women with these tumor subtypes may particularly benefit from MRI prior to treatment planning. Another study found significantly more multifocal and multicentric disease in the Her2-positive and luminal B subtypes in a data analysis of 299 patients [41]. Additionally, we investigated whether subgroups of patients according to histological type and subtype benefit differently from presurgical breast MRI. In our patients, we could not find a difference between the histological subtypes. This is in line with the findings of Bitencourt et al. who examined 160 patients and Jonna et al. who did not find significant differences in the frequency of the multicentric or multifocal spread of any molecular subtype [1,33]. However, these studies investigated the impact of molecular subtypes according to their appearance on MRI and not regarding surgical planning.
5. Limitations
Despite its many advantages, preoperative breast MRI is not without limitations.
One of the main concerns is its tendency to produce false-positive findings—areas that appear suspicious on MRI but are ultimately benign upon biopsy. This can lead to unnecessary additional testing, biopsies, and even overtreatment, which can increase patient anxiety and healthcare costs. MRI is also more expensive than other imaging modalities and requires specialized equipment and expertise. For these reasons, it is not routinely recommended for all breast cancer patients, but is instead reserved for specific cases where additional imaging is expected to impact treatment decisions.
Another consideration is the use of contrast agents, such as gadolinium, which are required for optimal tumor visualization. While generally safe, gadolinium-based contrast agents can rarely cause allergic reactions or kidney-related complications in susceptible patients.
This study has limitations: Data were obtained from a single center, and a medium sample size was used. No long-term follow-up data on recurrence and survival rates were obtained in this cohort. Additionally, patient selection was not randomized, which could have led to selection bias.
Confounders such as tumor size, histology, breast density, patient age, and the general ability to undergo MRI are factors likely to influence MRI use.
6. Conclusions
Preoperative MRI is an important tool in the evaluation and management of breast cancer, as it provides detailed imaging that surpasses the capabilities of mammography and ultrasound. The information obtained from preoperative MRI is invaluable in determining whether the most appropriate surgical approach is BCS surgery or mastectomy [31].
As imaging technology continues to advance, the role of MRI in preoperative breast cancer assessment will likely expand, further improving patient management and personalized treatment strategies. Our study found that preoperative breast MRI had an impact on surgical therapy recommendations in patients with invasive breast cancer, which is not new. However, molecular subtype alone had no influence on surgical therapy recommendations following MRI.
Patients with multifocal (i.e., bifocal or more) breast tumors were more likely to have a change in surgical therapy following MRI. Thus, this group of patients may benefit from the addition of breast MRI to the routine clinical workup before breast cancer surgery, rather than more general use of MRI.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by all authors. The first draft of the manuscript was written by K.M.-Z. and all authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The investigations were conducted in accordance with the principles outlined in the Declaration of Helsinki (1975, revised in 2013). A study-specific participant information leaflet and consent can be waived because of the retrospective analyses of routine clinical data according to the local IRB. Ethics Committee approval is given by the Ethics committee of the University of Heidelberg (https://www.medizinische-fakultaet-hd.uni-heidelberg.de/en/fakultaet/kommissionen/ethics-committee).
Informed Consent Statement
Patient consent was waived because this retrospective study used routinely collected clinical data, and local regulations do not require informed consent for such research.
Data Availability Statement
The datasets generated and/or analyzed during this study are not publicly available due to compromising individual privacy but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ALND | axillary lymph node dissection |
| BRCA1 | breast cancer 1 gene |
| ER | estrogen receptor |
| Her2 receptor | human epidermal growth factor receptor 2 |
| MRI | magnetic resonance imaging |
| NST | invasive breast cancer of no special type |
| PR | progesterone receptor |
| SLNE | sentinel lymph node excision |
| TB | tumor board |
References
- Bitencourt, A.G.; Pereira, N.P.; Franca, L.K.; Silva, C.B.; Paludo, J.; Paiva, H.L.; Graziano, L.; Guatelli, C.S.; Souza, J.A.; Marques, E.F. Role of MRI in the staging of breast cancer patients: Does histological type and molecular subtype matter? Br. J. Radiol. 2015, 88, 20150458. [Google Scholar] [CrossRef]
- Grimm, L.J.; Johnson, K.S.; Marcom, P.K.; Baker, J.A.; Soo, M.S. Can breast cancer molecular subtype help to select patients for preoperative MR imaging? Radiology 2015, 274, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Houssami, N.; Hayes, D.F. Review of preoperative magnetic resonance imaging (MRI) in breast cancer: Should MRI be performed on all women with newly diagnosed, early stage breast cancer? CA Cancer J. Clin. 2009, 59, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C.; Kuhn, W.; Braun, M.; Schild, H. Pre-operative staging of breast cancer with breast MRI: One step forward, two steps back? Breast 2007, 16 (Suppl. 2), S34–S44. [Google Scholar] [CrossRef] [PubMed]
- Sardanelli, F. Overview of the role of pre-operative breast MRI in the absence of evidence on patient outcomes. Breast 2010, 19, 3–6. [Google Scholar] [CrossRef]
- Schnall, M.D.; Blume, J.; Bluemke, D.A.; DeAngelis, G.A.; DeBruhl, N.; Harms, S.; Heywang-Köbrunner, S.H.; Hylton, N.; Kuhl, C.K.; Pisano, E.D.; et al. MRI detection of distinct incidental cancer in women with primary breast cancer studied in IBMC 6883. J. Surg. Oncol. 2005, 92, 32–38. [Google Scholar] [CrossRef]
- Brennan, M.E.; Houssami, N.; Lord, S.; Macaskill, P.; Irwig, L.; Dixon, J.M.; Warren, R.M.; Ciatto, S. Magnetic resonance imaging screening of the contralateral breast in women with newly diagnosed breast cancer: Systematic review and meta-analysis of incremental cancer detection and impact on surgical management. J. Clin. Oncol. 2009, 27, 5640–5649. [Google Scholar] [CrossRef]
- Houssami, N.; Turner, R.; Macaskill, P.; Turnbull, L.W.; McCready, D.R.; Tuttle, T.M.; Vapiwala, N.; Solin, L.J. An individual person data meta-analysis of preoperative magnetic resonance imaging and breast cancer recurrence. J. Clin. Oncol. 2014, 32, 392–401. [Google Scholar] [CrossRef]
- Houssami, N.; Turner, R.; Morrow, M. Preoperative magnetic resonance imaging in breast cancer: Meta-analysis of surgical outcomes. Ann. Surg. 2013, 257, 249–255. [Google Scholar] [CrossRef]
- Houssami, N.; Turner, R.M.; Morrow, M. Meta-analysis of pre-operative magnetic resonance imaging (MRI) and surgical treatment for breast cancer. Breast Cancer Res. Treat. 2017, 165, 273–283. [Google Scholar] [CrossRef]
- Jatoi, I.; Benson, J.R. The case against routine preoperative breast MRI. Future Oncol. 2013, 9, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.S.; Han, B.K.; Kim, R.B.; Ko, E.Y.; Shin, J.H.; Nam, S.Y.; Nam, M.; Nam, S.J.; Lee, J.E.; Kil, W.H.; et al. Analysis of the effect of breast magnetic resonance imaging on the outcome in women undergoing breast conservation surgery with radiation therapy. J. Surg. Oncol. 2013, 107, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Solin, L.J. Counterview: Pre-operative breast MRI (magnetic resonance imaging) is not recommended for all patients with newly diagnosed breast cancer. Breast 2010, 19, 7–9. [Google Scholar] [CrossRef]
- Solin, L.J.; Orel, S.G.; Hwang, W.T.; Harris, E.E.; Schnall, M.D. Relationship of breast magnetic resonance imaging to outcome after breast-conservation treatment with radiation for women with early-stage invasive breast carcinoma or ductal carcinoma in situ. J. Clin. Oncol. 2008, 26, 386–391. [Google Scholar] [CrossRef]
- Young, P.; Kim, B.; Malin, J.L. Preoperative breast MRI in early-stage breast cancer. Breast Cancer Res. Treat. 2012, 135, 907–912. [Google Scholar] [CrossRef]
- Voduc, K.D.; Cheang, M.C.; Tyldesley, S.; Gelmon, K.; Nielsen, T.O.; Kennecke, H. Breast cancer subtypes and the risk of local and regional relapse. J. Clin. Oncol. 2010, 28, 1684–1691. [Google Scholar] [CrossRef]
- Boisserie-Lacroix, M.; Hurtevent-Labrot, G.; Ferron, S.; Lippa, N.; Bonnefoi, H.; Mac Grogan, G. Correlation between imaging and molecular classification of breast cancers. Diagn. Interv. Imaging 2013, 94, 1069–1080. [Google Scholar] [CrossRef]
- Prat, A.; Ellis, M.J.; Perou, C.M. Practical implications of gene-expression-based assays for breast oncologists. Nat. Rev. Clin. Oncol. 2011, 9, 48–57. [Google Scholar] [CrossRef]
- Trop, I.; LeBlanc, S.M.; David, J.; Lalonde, L.; Tran-Thanh, D.; Labelle, M.; El Khoury, M.M. Molecular classification of infiltrating breast cancer: Toward personalized therapy. Radiographics 2014, 34, 1178–1195. [Google Scholar] [CrossRef]
- Whitman, G.J.; Albarracin, C.T.; Gonzalez-Angulo, A.M. Triple-negative breast cancer: What the radiologist needs to know. Semin. Roentgenol. 2011, 46, 26–39. [Google Scholar] [CrossRef]
- Chen, J.-H.; Agrawal, G.; Feig, B.; Baek, H.-M.; Carpenter, P.M.; Nalcioglu, O.; Su, M.-Y.; Mehta, R.S. Triple-negative breast cancer: MRI features in 29 patients. Ann. Oncol. 2007, 18, 2042–2043. [Google Scholar] [CrossRef]
- Chen, J.H.; Baek, H.M.; Nalcioglu, O.; Su, M.Y. Estrogen receptor and breast MR imaging features: A correlation study. J. Magn. Reson. Imaging 2008, 27, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Dogan, B.E.; Gonzalez-Angulo, A.M.; Gilcrease, M.; Dryden, M.J.; Yang, W.T. Multimodality imaging of triple receptor-negative tumors with mammography, ultrasound, and MRI. Am. J. Roentgenol. 2010, 194, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.M.; Han, B.-K.; Choi, D.H.; Rhee, S.J.; Cho, E.Y.; Huh, S.J.; Park, W.; Park, H.; Nam, S.J.; Lee, J.E.; et al. Association between BRCA Mutation Status, Pathological Findings, and Magnetic Resonance Imaging Features in Patients with Breast Cancer at Risk for the Mutation. J. Breast Cancer. 2013, 16, 308–314. [Google Scholar] [CrossRef]
- Teifke, A.; Behr, O.; Schmidt, M.; Victor, A.; Vomweg, T.W.; Thelen, M.; Lehr, H.-A. Dynamic MR imaging of breast lesions: Correlation with microvessel distribution pattern and histologic characteristics of prognosis. Radiology 2006, 239, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ikeda, D.M.; Narasimhan, B.; Longacre, T.A.; Bleicher, R.J.; Pal, S.; Jackman, R.J.; Jeffrey, S.S. Estrogen receptor-negative invasive breast cancer: Imaging features of tumors with and without human epidermal growth factor receptor type 2 overexpression. Radiology 2008, 246, 367–375. [Google Scholar] [CrossRef]
- Deutsche Gesellschaft für Gynäkologie, D.K. S3-Leitlinie für die Früherkennung, Diagnostik, Therapie und Nachsorge des Mammakarzinoms. Leitlinienprogramm Onkologie 2021. Available online: https://register.awmf.org/assets/guidelines/032-045OLl_S3_Mammakarzinom_2021-07.pdf (accessed on 30 March 2025).
- Wockel, A.; Festl, J.; Stuber, T.; Brust, K.; Stangl, S.; Heuschmann, P.U.; Albert, U.S.; Budach, W.; Follmann, M.; Janni, W.; et al. Interdisciplinary Screening, Diagnosis, Therapy and Follow-up of Breast Cancer. Guideline of the DGGG and the DKG (S3-Level, AWMF Registry Number 032/045OL, December 2017)—Part 1 with Recommendations for the Screening, Diagnosis and Therapy of Breast Cancer. Geburtshilfe Frauenheilkd. 2018, 78, 927–948. [Google Scholar] [CrossRef]
- Tsina, G.; Simon, P. Breast magnetic resonance imaging and its impact on the surgical treatment of breast cancer. Obstet. Gynecol. Int. 2014, 2014, 632074. [Google Scholar] [CrossRef]
- Berg, W.A.; Gutierrez, L.; NessAiver, M.S.; Carter, W.B.; Bhargavan, M.; Lewis, R.S.; Ioffe, O.B. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology 2004, 233, 830–849. [Google Scholar] [CrossRef]
- Sardanelli, F.; Trimboli, R.M.; Houssami, N.; Gilbert, F.J.; Helbich, T.H.; Benito, M.Á.; Balleyguier, C.; Bazzocchi, M.; Bult, P.; Calabrese, M.; et al. Magnetic resonance imaging before breast cancer surgery: Results of an observational multicenter international prospective analysis (MIPA). Eur. Radiol. 2022, 32, 1611–1623. [Google Scholar] [CrossRef]
- Bernard, J.R., Jr.; Vallow, L.A.; DePeri, E.R.; McNeil, R.B.; Feigel, D.G.; Amar, S.; Buskirk, S.J.; Perez, E.A. In newly diagnosed breast cancer, screening MRI of the contralateral breast detects mammographically occult cancer, even in elderly women: The mayo clinic in Florida experience. Breast J. 2010, 16, 118–126. [Google Scholar] [CrossRef]
- Gonzalez, V.; Sandelin, K.; Karlsson, A.; Aberg, W.; Lofgren, L.; Iliescu, G.; Eriksson, S.; Arver, B. Preoperative MRI of the breast (POMB) influences primary treatment in breast cancer: A prospective, randomized, multicenter study. World J. Surg. 2014, 38, 1685–1693. [Google Scholar] [CrossRef]
- Hlubocky, J.; Bhavnagri, S.; Swinford, A.; Mitri, C.; Rebner, M.; Pai, V. Does the use of pretreatment MRI change the management of patients with newly diagnosed breast cancer? Breast J. 2018, 24, 309–313. [Google Scholar] [CrossRef]
- Jonna, A.R.; Sam, K.Q.; Ebuoma, L.O.; Sedgwick, E.L.; Wang, T.; Benveniste, A.P. Detection of multicentric and contralateral breast cancers on MRI based on primary cancer biomarker status: Will this change surgical or medical management? Breast Cancer Res. Treat. 2017, 166, 623–629. [Google Scholar] [CrossRef]
- Lehman, C.D.; Gatsonis, C.; Kuhl, C.K.; Hendrick, R.E.; Pisano, E.D.; Hanna, L.; Peacock, S.; Smazal, S.F.; Maki, D.D.; Julian, T.B.; et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N. Engl. J. Med. 2007, 356, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Liberman, L.; Morris, E.A.; Kim, C.M.; Kaplan, J.B.; Abramson, A.F.; Menell, J.H.; Van Zee, K.J.; Dershaw, D.D. MR imaging findings in the contralateral breast of women with recently diagnosed breast cancer. Am. J. Roentgenol. 2003, 180, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Perono Biacchiardi, C.; Brizzi, D.; Genta, F.; Zanon, E.; Camanni, M.; Deltetto, F. Breast cancer preoperative staging: Does contrast-enhanced magnetic resonance mammography modify surgery? Int. J. Breast Cancer 2011, 2011, 757234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, W.P.; Chen, C.Y.; Lee, C.W.; Wu, H.K.; Chen, S.T.; Wu, Y.T.; Lin, Y.J.; Chen, D.R.; Kuo, S.J.; Lai, H.W. Impact of pre-operative breast magnetic resonance imaging on contralateral synchronous and metachronous breast cancer detection-A case control comparison study with 1468 primary operable breast cancer patients with mean follow-up of 102 months. PLoS ONE 2021, 16, e0260093. [Google Scholar] [CrossRef]
- Heil, J.; Rauch, G.; Szabo, A.Z.; Garcia-Etienne, C.A.; Golatta, M.; Domschke, C.; Badiian, M.; Kern, P.; Schuetz, F.; Wallwiener, M.; et al. Breast cancer mastectomy trends between 2006 and 2010: Association with magnetic resonance imaging, immediate breast reconstruction, and hospital volume. Ann. Surg. Oncol. 2013, 20, 3839–3846. [Google Scholar] [CrossRef]
- Ha, R.; Jin, B.; Mango, V.; Friedlander, L.; Miloshev, V.; Malak, S.; Wynn, R. Breast cancer molecular subtype as a predictor of the utility of preoperative MRI. Am. J. Roentgenol. 2015, 204, 1354–1360. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).