Abstract
Background: The brachial plexus is a network of nerves responsible for the motor and sensory innervation of the upper limb. Variations in the formation and course of the brachial plexus are well documented, though combinations of multiple unilateral abnormalities are rare. The complex pathology of this structure nerve may result in clinical consequences. We present a unique set of brachial plexus abnormalities involving the C4–C6 nerve roots, superior and middle trunks, additional communicating branches, and delayed median nerve union. Case Presentation: During the routine dissection of a 70-year-old female cadaver, several unique variations in the brachial plexus anatomy were identified. The C4 root contributed to C5 before the superior trunk formed, resulting in a superior trunk composed of C4–C6. The C5 root was located anterior to the anterior scalene muscle, whereas C6 maintained its usual posterior position. Additionally, an anterior communicating branch from the middle trunk to the posterior cord was observed. A communicating branch between the lateral and medial cords split into two terminal branches: one merged with the ulnar nerve, and the other joined the medial contribution of the median nerve. The median nerve contributions from the lateral and medial cords merged approximately two inches above the elbow. Conclusions: This rare combination of brachial plexus anomalies has not been previously described in the literature and is of significant clinical relevance. The additional anterior communicating branch from the middle trunk may suggest potential flexor muscle innervation by the posterior cord, which typically innervates extensor muscles. Additionally, the delayed convergence of the median nerve may provide a protective mechanism in cases of midshaft humeral fracture. Awareness of these peripheral nerve abnormalities is important for diagnostic imaging, surgery, or peripheral nerve blocks. Knowledge of such variations is critical for clinicians managing upper limb pathologies.
1. Introduction
The brachial plexus is a peripheral nervous system (PNS) structure that provides motor and cutaneous innervation to the shoulders, arms, and upper thorax. It is located between the neck’s anterior and middle scalene muscles and is derived from five nerve roots that arise from the anterior rami of the C5-T1 myelomeres. The roots unite to form three trunks: superior, middle, and inferior [1]. Roots C5 and C6 combine to form the superior trunk, root C7 forms the middle trunk, and roots C8 and T1 form the inferior trunk. Each trunk divides into two divisions: the anterior, and the posterior. The posterior divisions of the three trunks form the posterior cord. The anterior divisions of the superior and middle trunks form the lateral cord, and the anterior division of the inferior trunk alone forms the medial cord [2]. The cords give rise to the five terminal branches of the brachial plexus which supply the muscles and skin of the shoulder, arm, forearm, and hands [1,2]. The terminal branches comprise the musculocutaneous, radial, axillary, median, and ulnar nerves. Other nerves and communicating branches are also present along the brachial plexus and arise from different levels of the roots, trunks, and cords of the plexus.
Numerous variations in the brachial plexus have been described in the literature. One of the variations seen at the level of the roots is known as a prefixed brachial plexus. In this irregularity, the C4 nerve root provides a great contribution to the formation of the plexus, while the T1 root plays a minimal role [3,4]. A less common variant is the postfixed brachial plexus, where the C4 root is not involved, and the T1 and T2 roots are the ones that provide significant branches. This alternative communication of the T2 root to the brachial plexus is known as the nerve of Kuntz [5,6]. There are a wide number of anomalies found in the trunks of the brachial plexus, and some of them are a result of root abnormalities. An example of this is seen in the prefixed variant, where the roots of C4 and C5 are the ones that fuse to create the superior trunk, and the C6 root continues to individually produce the anterior and posterior divisions that later join the lateral and posterior cords [6]. Multiple cases have also been reported where the C5 and C6 roots never join to form the upper trunk, and each nerve root independently gives rise to an anterior and a posterior division [5]. There are instances in which the inferior trunk does not form, and the C8 root splits into multiple branches that contribute to the posterior and medial cords.
Division variants are not fairly common, but there can be some discrepancies in the cords. There can be a contribution to the lateral cord from the lower trunk or the C8 nerve root, both of which normally only take part in the formation of the medial cord. A phenomenon known as an absence of the lateral cord can also be seen when the aforementioned does not receive branches from the middle trunk. This will cause the musculocutaneous nerve and the lateral portion of the median nerve to arise from the anterior division of the upper trunk only [4,5]. Not many functional variations relating to the terminal nerves have been reported in the literature, but one publication reported 29 cases in which the median nerves were formed by three branches, one from the medial cord and two from the lateral cord [6].
It is extremely important to be aware of these anomalies because they can be integral when diagnosing and treating plexopathies and nerve entrapments. By knowing these variations, physicians can offer patients more suited surgical and medical management for their pathologies. In this case report, we present a series of variations in the brachial plexus including, a superior trunk composed of C4–C6 nerve roots, a C5 root located anteriorly to the anterior scalene muscle, an anterior communicating branch from the middle trunk to the posterior cord, a communicating branch between the lateral and medial cords, and delayed joining of the lateral and medial cord contributions to the median nerve.
2. Materials and Methods
2.1. Disclaimer Regarding Use of Human Donor Patients
The cadaver referenced in this study was obtained through a university anatomical donation program. Before her demise, the patient provided written informed consent for the utilization of her remains for the purposes of medical education and research. After confirming the patient’s inclusion as a student cadaver, the body was drained of fluids and embalmed with a mixture of formaldehyde (35%), methanol (15%), phenol (5%), glycerin (10%), and water. The cadaver was then kept at 4 °C, occasionally being moved to 0 °C storage to maintain preservation for the duration of the study. The dissection of the donor body, specimen preparation, and variation discovery were performed following ethical guidelines and established university protocols of good practice. All students involved in this project offer their sincere thanks to anyone who has participated in an anatomical donation program. Anatomical donations help ensure that future physicians receive a world-class education.
2.2. Anatomical Dissection
The anomaly was discovered during a routine dissection of a 70-year-old Caucasian female human body donor in a medical student anatomy laboratory. The neck, shoulder, and upper limb regions were intact prior to the dissection with no apparent deformities, surgical intervention, or significant trauma that could affect the brachial plexus. The cadaver was placed in a supine position to provide optimal access and an incision was made. After removing the superficial fascia, we reflected the pectoralis muscles. The long head of the biceps brachii muscles was parted, revealing the brachial artery. This artery was traced back to the axillary artery, and plexus trunks, divisions, and terminal nerves were carefully teased from the surrounding connective tissue. Following the discovery of the anatomical variation, the right side was dissected and investigated to determine if the variation was bilateral. Using a Nikon D7500 camera (Nikon Corp., Tokyo, Japan), photographs were taken to document the anatomical variations. Figures were prepared using the CorelDrew software (version 24.2).
3. Case Report
During routine dissection, an abnormal brachial plexus structure was observed. The donor’s cause of death was congestive heart failure with no indication of neurological or musculoskeletal diseases. Therefore, this anomaly may have gone unnoticed without the gross anatomical dissection. To begin the dissection, the skin, subcutaneous fat, and superficial fascia of both right and left upper limbs were dissected out to review symmetry. Muscles surrounding the region including the pectoralis major and minor and biceps were reflected back to see the entirety of the plexus. The nerves within the plexus were then observed and examined for proper origin, orientation, branching, and path of innervation. Only on the cadaver’s left side were the following abnormalities observed.
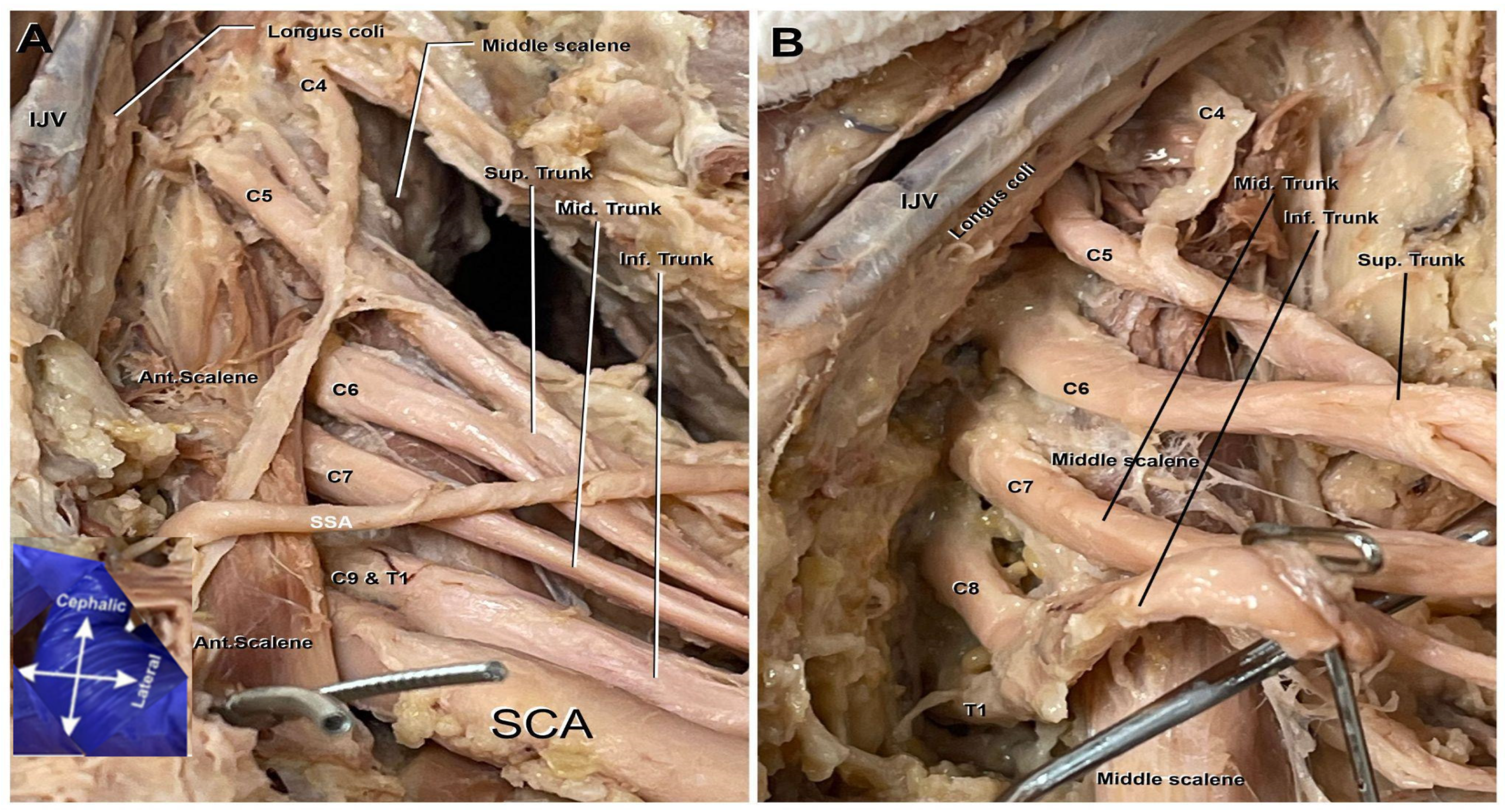
The superior trunk of the brachial plexus typically comprises the roots of C5–C6. However, on the cadaver’s left plexus, C4 was an additional contributor to the superior trunk as seen in Figure 1A,B. Also noted in Figure 1A,B, it can be observed that the C5 nerve root lies anterior to the anterior scalene muscle. Typically, the entirety of the nerve roots of this plexus lay posterior to the anterior scalene muscle; C6-T1 uphold their anatomical positioning and remain posterior to this scalene muscle.
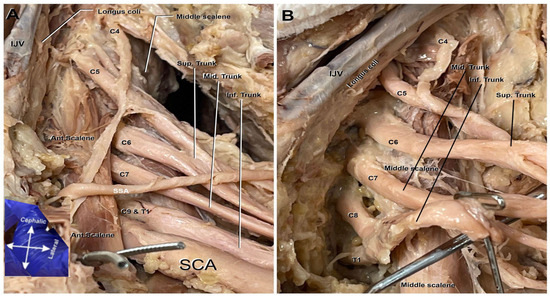
Figure 1.
Roots and trunks of the left brachial plexus. C4 root merging with C5 contributes to the formation of the plexus. (A). Anterior view of deep dissection including the anterior scalene muscle (Ant. Scalene), subscapular artery (SSA), subclavian artery (SCA), internal jugular vein (IJV), and the C4 nerve root merging with C5 and contributing to the formation of the plexus. (B). A zoomed-in view of the brachial plexus roots and trunks following the removal of the surrounding vasculature and anterior scalene muscle. The image also includes the internal jugular vein (IJV) and the Longus coli.
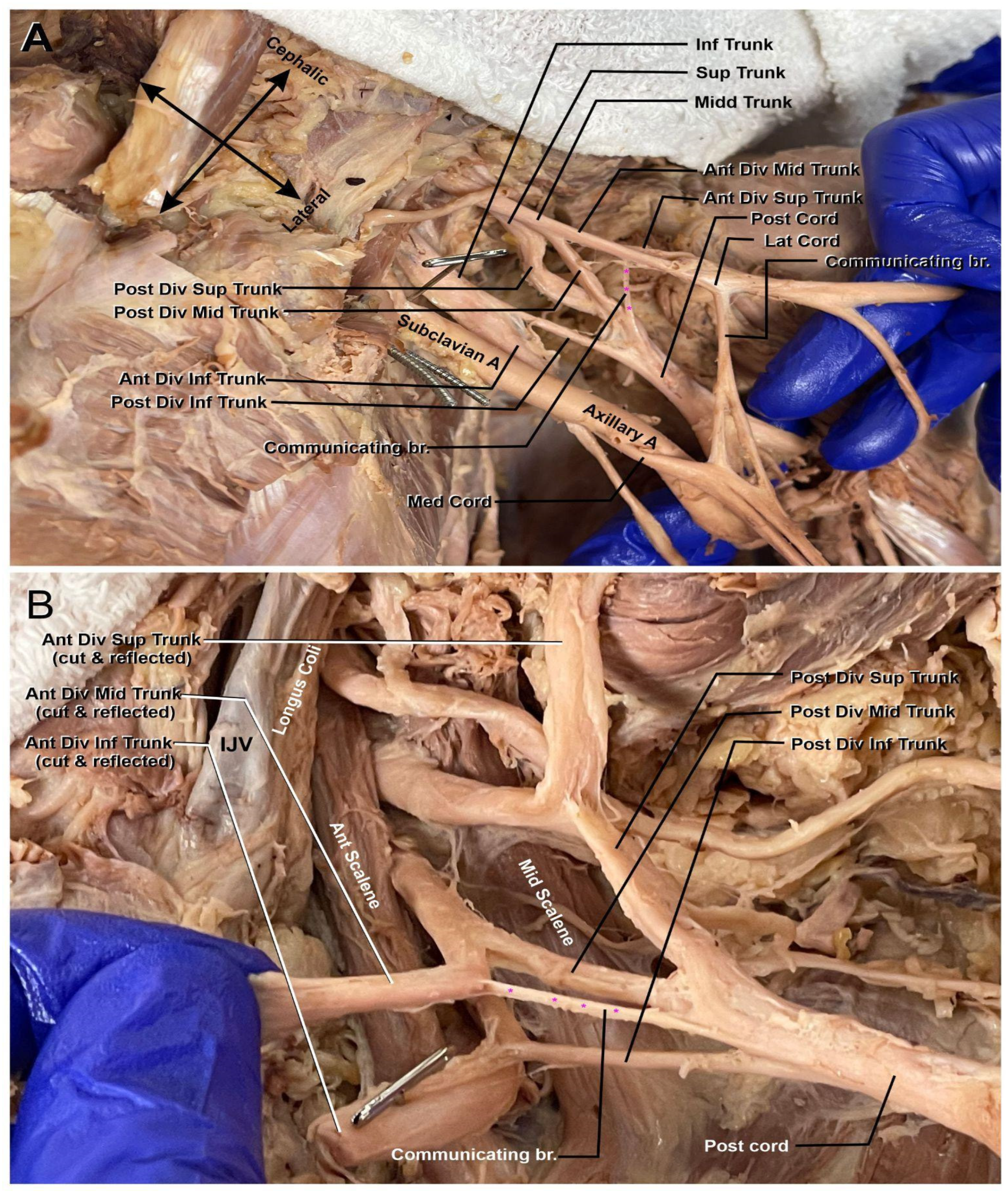
Moving distally along the plexus, the next abnormality can be identified (marked in Figure 2A,B). There is an anterior communicating branch from the middle trunk that is contributing to the makeup of the posterior cord. The branch comes off the anterior division of the middle trunk proximally to the beginning of the cords within the plexus. The addition of this middle trunk branch into the posterior cord could possibly indicate that the latter contributed to some flexor muscle innervation while still maintaining its extensor muscle innervation via the axillary and radial nerves.
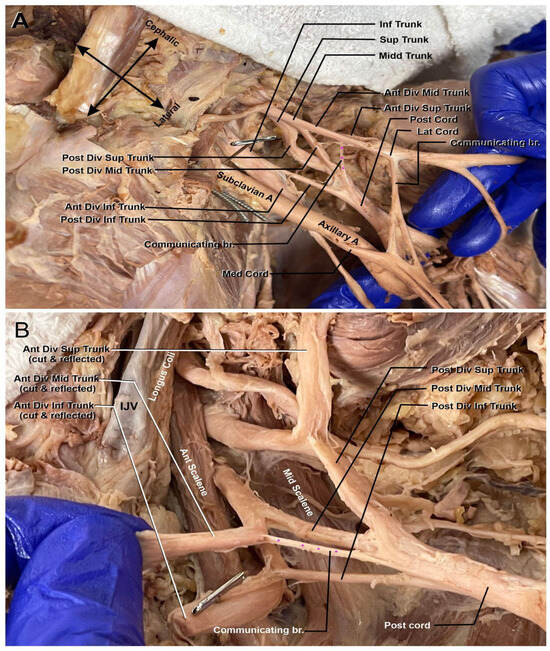
Figure 2.
Left brachial plexus divisions. (A) Anterior view of the communicating branch of the anterior division of the superior trunk to the posterior cord (highlighted with purple asterisk). The subclavian artery (Subclavian A) is seen coursing through the plexus to become the axillary artery (Axillary A.). (B) A view of the communicating branch of the anterior division of the middle trunk to the posterior cord (highlighted with purple asterisk). Internal jugular vein (IJV), anterior scalene muscle (Ant. Scalene), and middle scalene muscle (Mid. Scalene) can be appreciated.
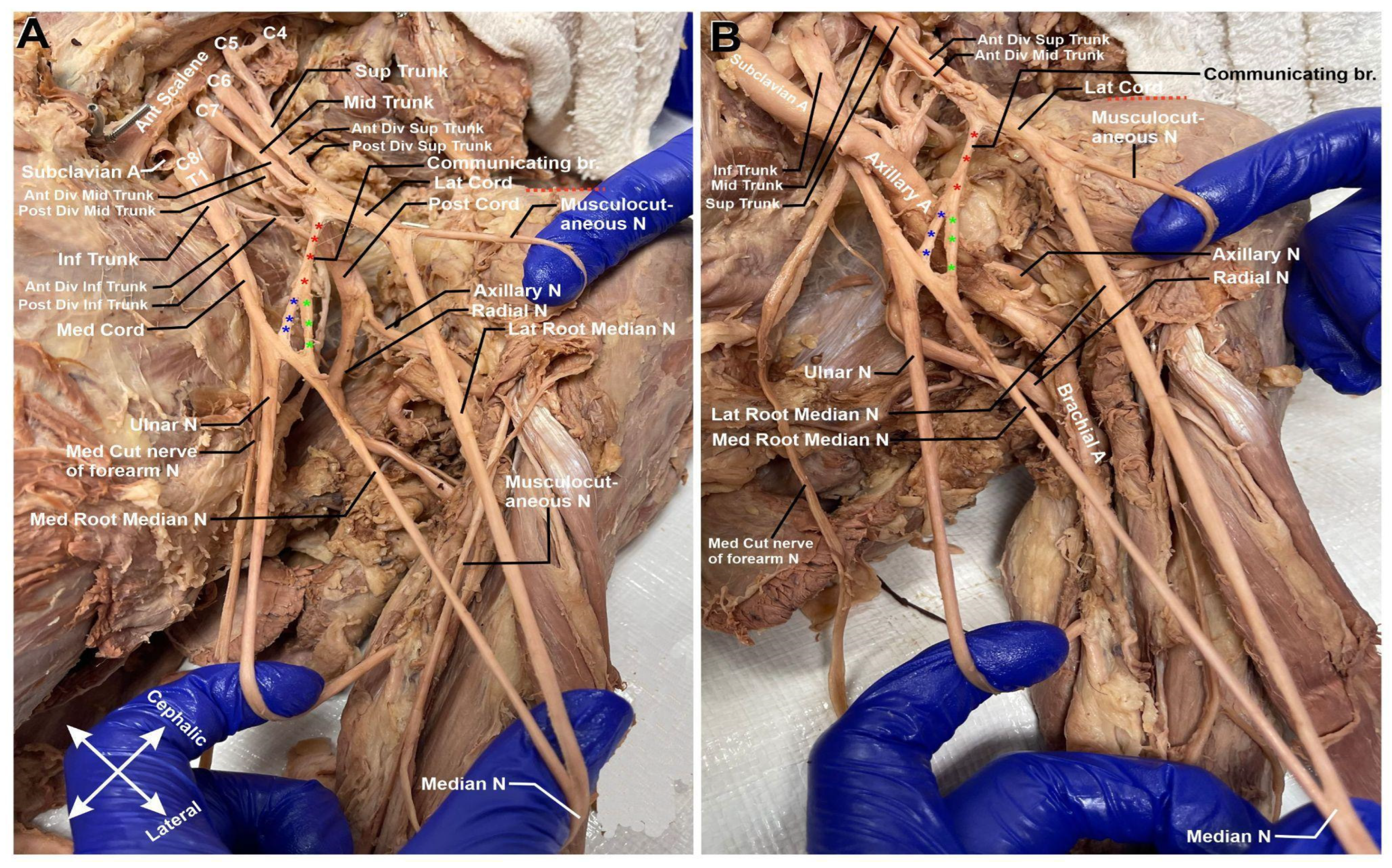
Descending down the arm, there is a notable communication branch between the lateral and medial cords (Figure 3A,B). This communicating branch (highlighted in red asterisk) bifurcates into two separate nerves midway through its path. One of these nerves joins the medial cord prior to its split into the terminal branches of the median and ulnar nerve (highlighted in blue asterisk). The second branch (highlighted in green asterisk) binds solely to the medial cord’s contribution to the median nerve.
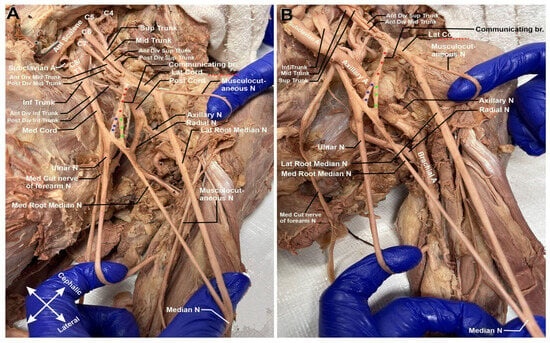
Figure 3.
Left brachial plexus cords. (A) Following the medial root of the median nerve (Med. Root median N) and the lateral root of the median nerve (Lat. Root Median N.), there is a delayed median nerve intersection. (B) Communicating branches of medial contribution of median nerve. Communicating branch is highlighted with red asterisks. The branch binding to the median cord prior to the split into the median and ulnar nerves is highlighted with blue asterisks. The branch binding to the medial contribution to the median cord is highlighted with green asterisks.
In Figure 3A,B, it is also noteworthy to add that there is delayed median nerve joining. Typically, the median nerve receives its input from the medial and lateral cords at a point closer to the shoulder joint; in this cadaver, the fusion takes place further down the arm at about 5 cm from the elbow joint (refer to Figure 4). Clinically, this could be significant if there were to be a midshaft humeral fracture. It could be possible that only one cord contributor to the median nerve be severed, and the patient would retain median nerve innervation further down the arm.
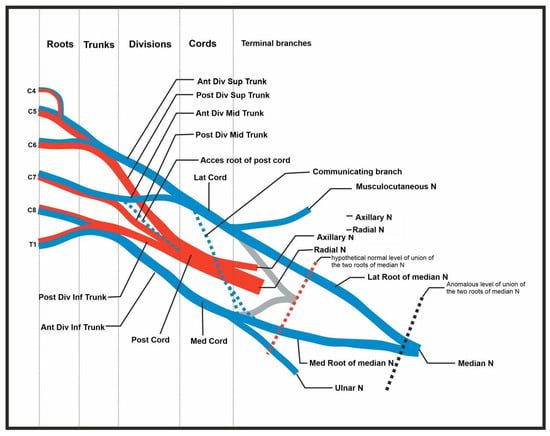
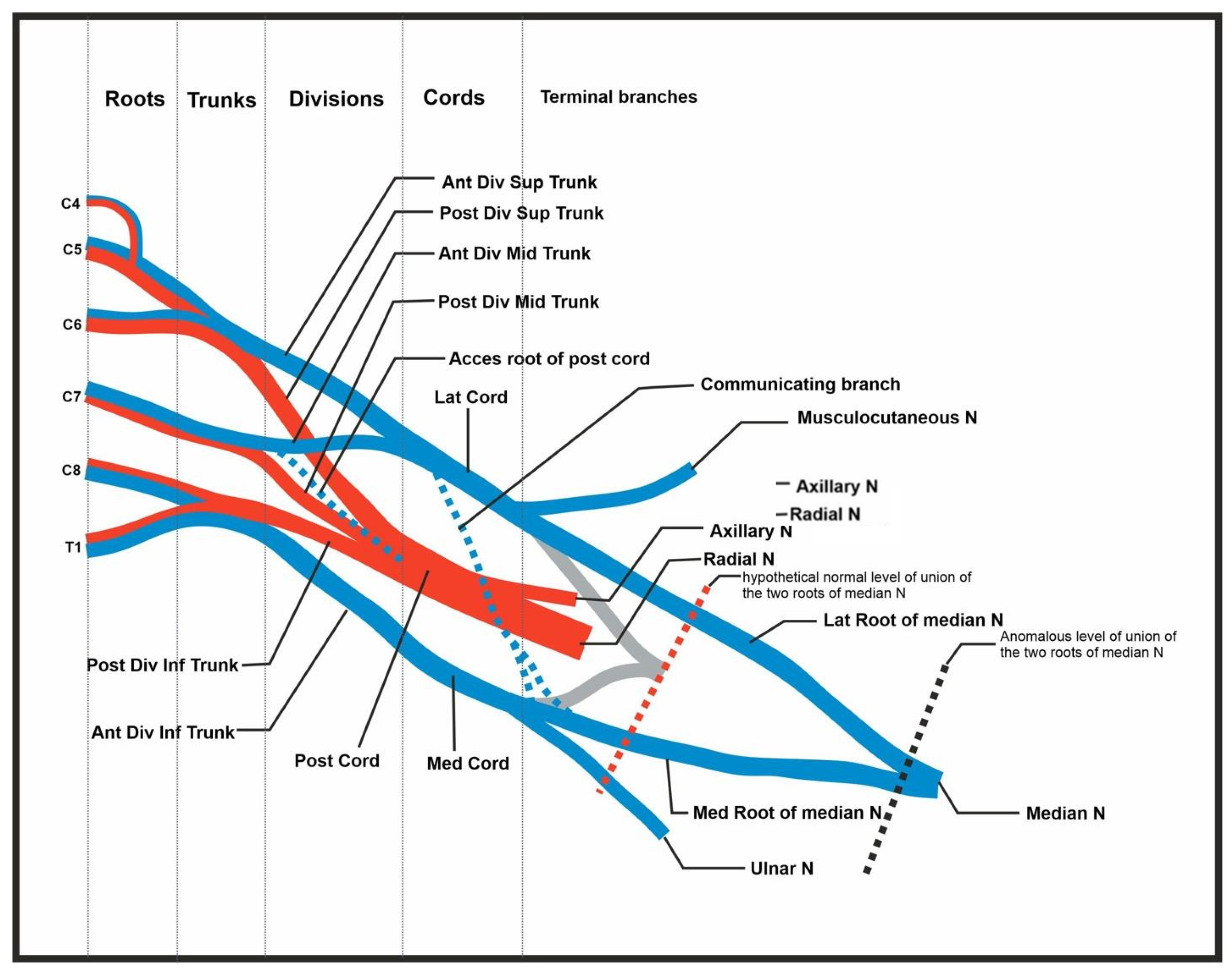
Figure 4.
Summary of abnormal findings. Figure includes the C4 root contribution, the accessory root of the posterior cord, the communicating branch (and splitting of branch) of the lateral contribution of the median nerve, and the delayed union of the median nerve.
4. Discussion
The brachial plexus plays a critical role in the motor and sensory innervation of the upper limb. As a highly complex network of nerves, anomalies can occur, and although often asymptomatic, these variations may cause significant challenges in medical and surgical procedures. Asymptomatic anomalies can lead to false positives on imaging or cause surgeons to lose orientation during operations. In contrast, symptomatic abnormalities are easier to identify through imaging or patient-reported symptoms such as pain, numbness, or paresthesia radiating into the arm. Advanced imaging techniques, such as AI-assisted diagnostics, have been explored to improve the identification of complex nerve variations, though their clinical application remains in the early stages [7,8].
In this cadaver, we hypothesized that the delayed joining of the median nerve might have affected the patient’s hand function during life, possibly causing numbness or weakness in the radial digits. However, upon reviewing the cadaver’s medical history, it was confirmed that the patient never reported any such symptoms. This aligns with previous studies demonstrating that many nerve anomalies remain clinically silent, only becoming significant when additional compression or trauma occurs [7]. Additionally, these symptoms, just like the anatomical anomalies, would be unilateral, which is much more common in brachial plexus abnormalities [1,6].
4.1. C4 Contribution to C5 and Formation of the Superior Trunk
The contribution of C4 to C5, resulting in the formation of the superior trunk, is an example of a prefixed brachial plexus, a variation well documented in the literature [9,10]. In this case, the superior trunk was composed of C4, C5, and C6 nerve roots. A notable feature of this case is the additional finding of C5 positioned anterior to the anterior scalene muscle, which adds further complexity to this variation.
Prefixed brachial plexuses are clinically significant, particularly when performing procedures like interscalene blocks, where an expanded area of nerve roots requires anesthetic coverage. This variation can also affect motor and sensory innervation to the upper limb, leading to unexpected outcomes during surgeries or trauma management involving the neck or shoulder [11,12]. Studies on surgical robotics have suggested that robotic-assisted dissection may help navigate such complex anatomical variations with increased precision, reducing the risk of nerve injury [8].
4.2. C5 Positioned Anterior to the Anterior Scalene Muscle
In this case, the C5 nerve root was found anterior to the anterior scalene muscle, an unusual deviation from the typical anatomy where the nerve roots pass posterior to this muscle. This positional variation has been noted in the literature in conjunction with C4 contributions [13]. Interestingly, while C5 was anterior, C6 remained posterior, following its typical path behind the anterior scalene muscle.
This positional anomaly may increase the risk of compression injuries, particularly with external pressure such as from heavy backpacks or trauma to the neck. Additionally, this abnormal position could complicate nerve blocks, as the altered nerve path could necessitate increased anesthetic or alternative techniques [14]. Because C5 lies in a more superficial position, it may also be more susceptible to injury in cases of neck or shoulder trauma [12].
4.3. Anterior Contribution from the Middle Trunk to the Posterior Cord
Another notable anomaly observed in this cadaver was the presence of a communicating branch from the middle trunk to the posterior cord. Typically, the posterior cord is responsible for innervating extensor muscles via the radial and axillary nerves. However, this anterior contribution from the middle trunk suggests a potential for some flexor muscle innervation to originate from the posterior cord, which is atypical [12].
This anomaly introduces the possibility of altered motor innervation patterns, potentially affecting both flexor and extensor muscle groups. This unusual connection could also have important implications during trauma or surgical intervention, as the standard nerve pathways may be affected, leading to unexpected motor deficits. Additionally, this variation may complicate nerve blocks, making it difficult to predict the spread of local anesthetics [9].
4.4. Communicating Branch Between Lateral and Medial Cords
We also identified a communicating branch between the lateral and medial cords, which split into two terminal branches on the medial cord. The first branch connected just before the medial cord divided into the medial contribution of the median nerve and ulnar nerve, while the second branch linked directly with the medial contribution of the median nerve.
Although communication between the lateral and medial cords has been reported in other cases, such variations are rare [15]. This type of communication can significantly impact the innervation patterns of both the median and ulnar nerves, leading to altered motor and sensory function. Variations like this can also modify the typical “M” structure of the brachial plexus, a feature seen in approximately 40% of individuals [6]. In this case, the dual contribution to the median nerve could lead to unique innervation patterns in the forearm and hand, relevant during injury or surgery.
4.5. Delayed Median Nerve Joining
A rare finding in this case was the delayed joining of the median nerve, which did not fully form until approximately 2 inches above the elbow, rather than at the shoulder as is typical. This delayed joining could have significant clinical implications in cases of midshaft humeral fractures. The anomaly suggests that partial innervation of the median nerve could remain intact even if one of the contributing branches is severed [16,17].
This anatomical variation could lead to atypical presentations of motor or sensory deficits in the arm following trauma. In cases of nerve-sparing surgery, this variation could provide an advantage, as it might preserve some median nerve function despite injury to one branch due to trauma to the arm or shoulder [18,19].
5. Conclusions
The brachial plexus anomalies presented in this case highlight the complexity and variability of this nerve network. Understanding these anomalies is crucial in clinical practice, as they can pose challenges during imaging, nerve blocks, or surgical interventions. Accounting for such variations can help prevent complications, reduce hospitalization time, and improve patient outcomes. By documenting rare cases like these, we advance our knowledge of the brachial plexus and improve the quality of care for patients with similar anatomical variations.
This case report is limited in scope due to the inclusion of only one cadaver in this study. Additionally, while we did receive information on any pertinent medical history for the cadaver patient, this was still a post-mortem case report, so we do not know for certain how the abnormality could have affected patient functionality. Further studies focused on these anomalies in live patients would be needed to understand more about the plexopathy.
Author Contributions
Conceptualization, A.L. and N.B.D.II; methodology, A.L.; software, A.M.; validation, A.L., A.M. and N.B.D.II; formal analysis, A.L.; investigation, A.L., A.T.M. and N.B.D.II; resources, A.L. and A.M.; data curation, A.M.; writing—original draft preparation, A.L., N.B.D.II, A.T.M., C.T. and E.O.; writing—review and editing, A.L.; visualization, A.L.; supervision, A.M. and A.L.; project administration, A.M.; funding acquisition, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
All donors contributing to the anatomy laboratory at the University of Toledo College of Medicine and Life Sciences are required to complete documentation prior to donating their remains for educational purposes.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors of this case report are thankful to the medical educators at the University of Toledo College of Medicine and Life Sciences, especially those assisting in the anatomy laboratory. Their guidance towards medical students is paramount to the comprehensive medical curriculum. The opportunity given to students to learn from these educators through cadaveric dissection in an anatomy laboratory is difficult to obtain anywhere else.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Orebaugh, S.L.; Williams, B.A. Brachial plexus anatomy: Normal and variant. Sci. World J. 2009, 9, 300–312. [Google Scholar] [CrossRef]
- Leinberry, C.F.; Wehbé, M.A. Brachial plexus anatomy. Hand Clin. 2004, 20, 1–5. [Google Scholar] [CrossRef]
- Leijnse, J.N.; de Bakker, B.S.; D’Herde, K. The brachial plexus—Explaining its morphology and variability by a generic developmental model. J. Anat. 2020, 236, 862–882. [Google Scholar] [CrossRef]
- Gilcrease-Garcia, B.M.; Deshmukh, S.D.; Parsons, M.S. Anatomy, Imaging, and Pathologic Conditions of the Brachial Plexus. Radiographics 2020, 40, 1686–1714. [Google Scholar] [CrossRef] [PubMed]
- Kirik, A.; Mut, S.E.; Daneyemez, M.K.; Seçer, H.İ. Anatomical variations of brachial plexus in fetal cadavers. Turk. Neurosurg. 2018, 28, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Bergman, R.A.; Tubbs, R.S.; Shoja, M.M.; Loukas, M. (Eds.) Bergman’s Comprehensive Encyclopedia of Human Anatomic Variation; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- De Simone, M.; Choucha, A.; Ciaglia, E.; Conti, V.; Pecoraro, G.; Santurro, A.; Puca, A.A.; Cascella, M.; Iaconetta, G. Discogenic Low Back Pain: Anatomic and Pathophysiologic Characterization, Clinical Evaluation, Biomarkers, AI, and Treatment Options. J. Clin. Med. 2024, 13, 5915. [Google Scholar] [CrossRef] [PubMed]
- Choucha, A.; Travaglini, F.; De Simone, M.; Evin, M.; Farah, K.; Fuentes, S. The Da Vinci Robot, a promising yet underused minimally invasive tool for spine surgery: A scoping review of its current role and limits. Neuro-Chir. 2024, 71, 101624. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.H.; Mohammed, A.M.A.; Grebballa, A.; Rizig, S. Absence of upper trunk of the brachial plexus. Int. J. Appl. Basic Med. Res. 2011, 1, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Polcaro, L.; Charlick, M.; Daly, D.T. Anatomy, Head and Neck: Brachial Plexus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024; Available online: http://www.ncbi.nlm.nih.gov/books/NBK531473/ (accessed on 23 November 2024).
- Patel, N.T.; Smith, H.F. Clinically Relevant Anatomical Variations in the Brachial Plexus. Diagnostics 2023, 13, 830. [Google Scholar] [CrossRef] [PubMed]
- Keet, K.; Louw, G. Variation of the brachial plexus roots in the interscalene groove: Relevance in interscalene blocks. Anatomy 2019, 13, 40–48. [Google Scholar] [CrossRef]
- Aheer, G.K.; Villella, J. Scalenus muscle and the C5 root of the brachial plexus: Bilateral anatomical variation and its clinical significance. J. Can. Chiropr. Assoc. 2021, 65, 229–233. [Google Scholar] [PubMed]
- Mendonsa, M.; Busch, M.; Faheem, H.; Nawras, Y.; Lassiter, E.; Maklad, A. A Prefixed Four-Trunk Brachial Plexus with Anomalous Anatomy of All Subsequent Divisions, Cords, and Terminal Branches: A Cadaveric Case Report. Am. J. Case Rep. 2024, 25, e943866. [Google Scholar] [CrossRef] [PubMed]
- Natsis, K.; Giannakopoulou, A.; Piagkou, M.; Lazaridis, N.; Tegos, T.; Colonna, M.R. Connections between radial and ulnar nerve at high humeral level in cadavers: Incidence, topography, and literature review. Surg. Radiol. Anat. 2018, 40, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Harjeet, K.; Sahni, D.; Aggarwal, A. Bilateral multiple complex variations in the formation and branching pattern of brachial plexus. Surg. Radiol. Anat. 2009, 31, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, J.; Singh, V.; Ohtsuka, A.; Hwang, Y.; Kim, H.; Moryś, J.; Ravi, K.S.; Ribatti, D.; Trainor, P.A.; Sañudo, J.R.; et al. Acknowledging the use of human cadaveric tissues in research papers: Recommendations from anatomical journal editors. Clin. Anat. 2021, 34, 2–4. [Google Scholar] [CrossRef] [PubMed]
- DiGiacomo, D.; Cruz, P.P.; Lassiter, E.M.; Sood, A.; Lackey-Cornelison, W.; Maklad, A. Independent C5 And C6 Superior Trunks of the Brachial Plexus with an Anomalous Origin of the Pectoral Nerves. Arch. Neurol. Neurosci. 2024, 16, ANN.MS.ID.000891. [Google Scholar]
- Mchonde, G.; Fabian, F.M.; Nondoli, H.G. Bilateral multiple variations in the formation of the brachial plexus and its terminal nerves: A case report. Int. J. Anat. Res. 2013, 2, 78–82. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).