An Improved Approach to Protoplast Regeneration and Transfection in Banana (Musa acuminata AAA cv. Williams)
Abstract
1. Introduction
2. Materials and Methods
2.1. Buffers and Media
2.2. Protoplast Isolation and Purification
2.3. Preparation of Conditioned Media
2.4. Protoplast Culture and Plant Regeneration
2.5. Protoplast Transfection
2.6. Post Transfection and Culture Maintenance
2.7. Statistical Analysis
3. Results
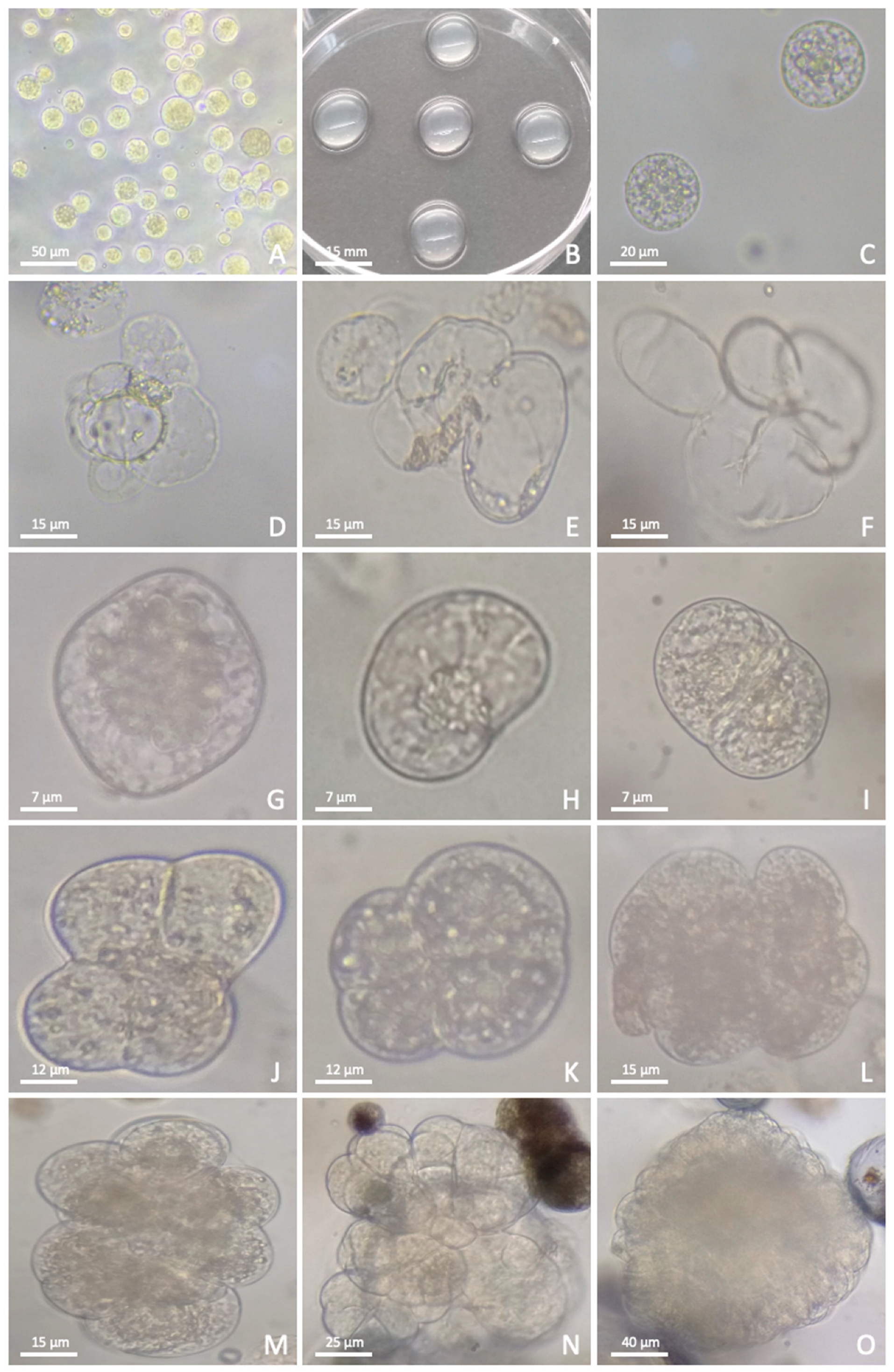
3.1. Protoplast Isolation
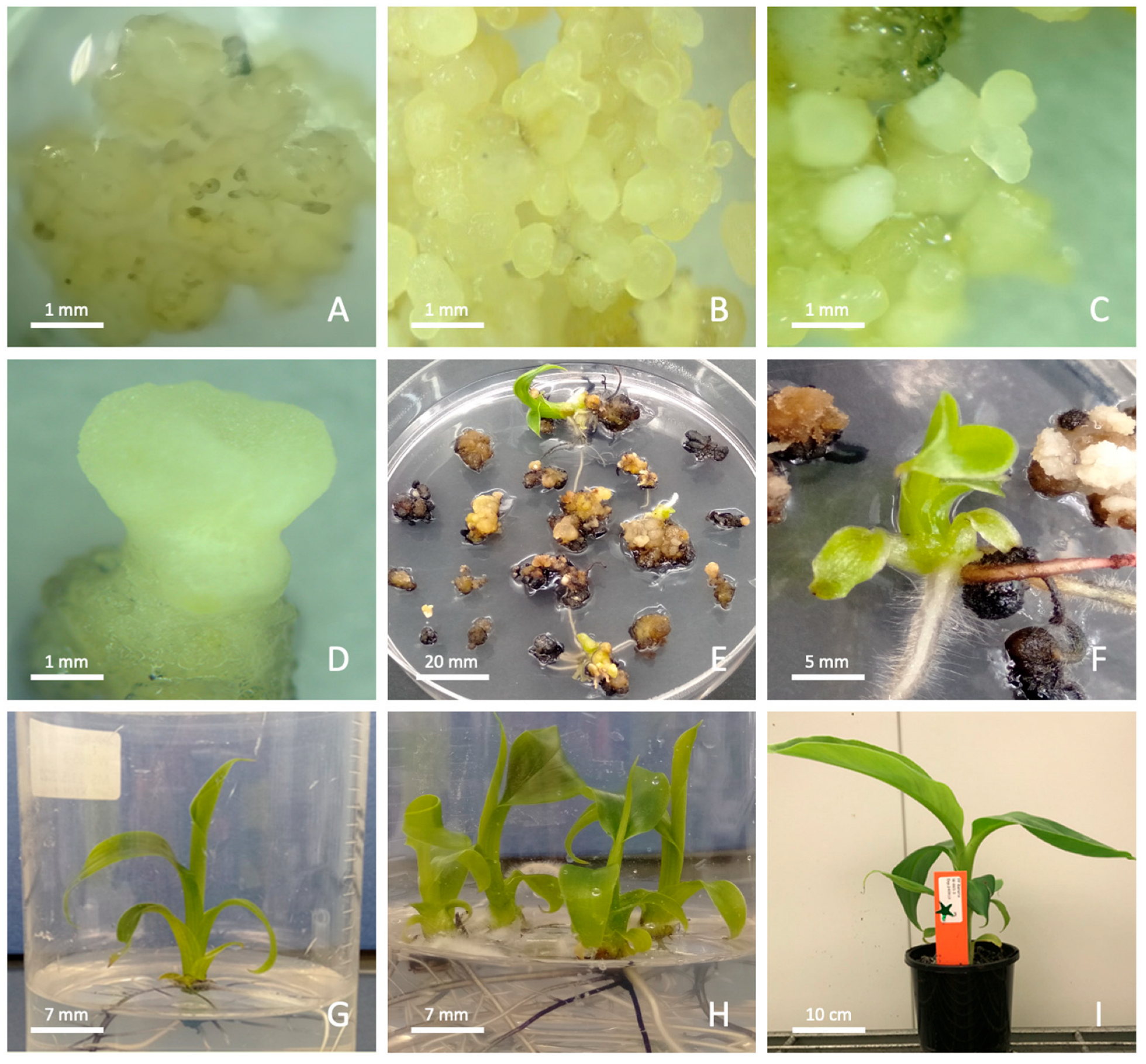
3.2. Protoplast Culture and Plant Regeneration
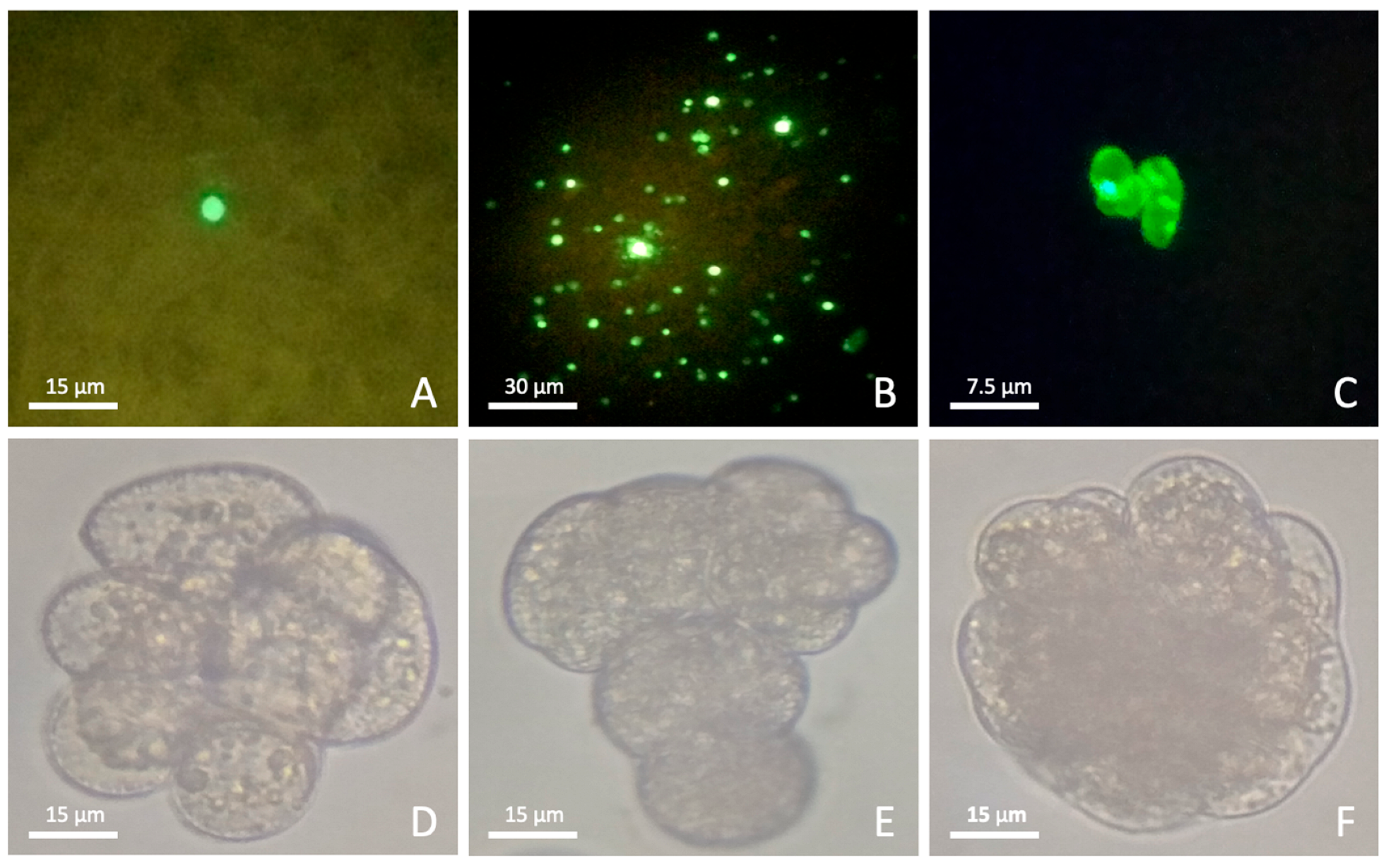
3.3. Transfection
3.4. Culture of Transfected Protoplasts
4. Discussion
4.1. Protoplast Isolation
4.2. Protoplast Regeneration
4.3. Protoplast Transfection
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Voora, V.; Larrea, C.; Bermudez, S. Global Market Report: Banana Prices and Sustainability. 2023. Available online: https://www.iisd.org/system/files/2023-03/2023-global-market-report-banana.pdf (accessed on 6 February 2024).
- Varma, V.; Bebber, D.P. Climate Change Impacts on Banana Yields around the World. Nat. Clim. Change 2019, 9, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Drenth, A.; Kema, G. The Vulnerability of Bananas to Globally Emerging Disease Threats. Phytopathology 2021, 111, 2146–2161. [Google Scholar] [CrossRef]
- Khanna, H.K.; Deo, P.C. Novel Gene Transfer Technologies. In Banana: Genomics and Transgenic Approaches for Genetic Improvement; Mohandas, S., Ravishankar, K.V., Eds.; Springer Singapore: Singapore, 2016; pp. 127–140. [Google Scholar] [CrossRef]
- Tripathi, L.; Tripathi, J.N.; Kiggundu, A.; Korie, S.; Shotkoski, F.; Tushemereirwe, W.K. Field Trial of Xanthomonas Wilt Disease-Resistant Bananas in East Africa. Nat. Biotechnol. 2014, 32, 868–870. [Google Scholar] [CrossRef]
- Tripathi, L.; Ntui, V.O.; Tripathi, J.N. Control of Bacterial Diseases of Banana Using CRISPR/Cas-Based Gene Editing. Int. J. Mol. Sci. 2022, 23, 3619. [Google Scholar] [CrossRef]
- Dale, J.; James, A.; Paul, J.-Y.; Khanna, H.; Smith, M.; Peraza-Echeverria, S.; Garcia-Bastidas, F.; Kema, G.; Waterhouse, P.; Mengersen, K.; et al. Transgenic Cavendish Bananas with Resistance to Fusarium Wilt Tropical Race 4. Nat. Commun. 2017, 8, 1496. [Google Scholar] [CrossRef]
- Paul, J.; Khanna, H.; Kleidon, J.; Hoang, P.; Geijskes, J.; Daniells, J.; Zaplin, E.; Rosenberg, Y.; James, A.; Mlalazi, B.; et al. Golden Bananas in the Field: Elevated Fruit Pro-vitamin A from the Expression of a Single Banana Transgene. Plant Biotechnol. J. 2017, 15, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Alok, A.; Shivani; Kaur, N.; Pandey, P.; Awasthi, P.; Tiwari, S. CRISPR/Cas9-Mediated Efficient Editing in Phytoene Desaturase (PDS) Demonstrates Precise Manipulation in Banana cv. Rasthali Genome. Funct. Integr. Genom. 2018, 18, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Alok, A.; Shivani; Kumar, P.; Kaur, N.; Awasthi, P.; Chaturvedi, S.; Pandey, P.; Pandey, A.; Pandey, A.K.; et al. CRISPR/Cas9 Directed Editing of Lycopene Epsilon-Cyclase Modulates Metabolic Flux for β-Carotene Biosynthesis in Banana Fruit. Metab. Eng. 2020, 59, 76–86. [Google Scholar] [CrossRef]
- Tripathi, L.; Ntui, V.O.; Tripathi, J.N. CRISPR/Cas9-Based Genome Editing of Banana for Disease Resistance. Curr. Opin. Plant Biol. 2020, 56, 118–126. [Google Scholar] [CrossRef]
- Tripathi, J.N.; Ntui, V.O.; Shah, T.; Tripathi, L. CRISPR/Cas9-mediated Editing of DMR6 Orthologue in Banana (Musa spp.) Confers Enhanced Resistance to Bacterial Disease. Plant Biotechnol. J. 2021, 19, 1291–1293. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Wu, S.; Dou, T.; Zhu, H.; Hu, C.; Huo, H.; He, W.; Deng, G.; Sheng, O.; Bi, F.; et al. Using CRISPR/Cas9 Genome Editing System to Create MaGA20ox2 Gene-modified Semi-dwarf Banana. Plant Biotechnol. J. 2020, 18, 17–19. [Google Scholar] [CrossRef]
- Hu, C.; Sheng, O.; Deng, G.; He, W.; Dong, T.; Yang, Q.; Dou, T.; Li, C.; Gao, H.; Liu, S.; et al. CRISPR/Cas9-mediated Genome Editing of MaACO1 (Aminocyclopropane-1-carboxylate Oxidase 1) Promotes the Shelf Life of Banana Fruit. Plant Biotechnol. J. 2021, 19, 654–656. [Google Scholar] [CrossRef]
- Ntui, V.O.; Tripathi, J.N.; Shah, T.; Tripathi, L. Targeted Knockout of Early Nodulin-like 3 (MusaENODL3) Gene in Banana Reveals Its Function in Resistance to Xanthomonas Wilt Disease. Plant Biotechnol. J. 2024, 22, 1101–1112. [Google Scholar] [CrossRef]
- Awasthi, P.; Khan, S.; Lakhani, H.; Chaturvedi, S.; Shivani; Kaur, N.; Singh, J.; Kesarwani, A.K.; Tiwari, S. Transgene-Free Genome Editing Supports CCD4 Role as a Negative Regulator of β-Carotene in Banana. J. Exp. Bot. 2022, 73, 3401–3416. [Google Scholar] [CrossRef]
- Yue, J.-J.; Yuan, J.-L.; Wu, F.-H.; Yuan, Y.-H.; Cheng, Q.-W.; Hsu, C.-T.; Lin, C.-S. Protoplasts: From Isolation to CRISPR/Cas Genome Editing Application. Front. Genome Ed. 2021, 3, 717017. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, K.; Li, T.; Zhang, Y.; Wang, Y.; Zhao, Q.; Liu, J.; Zhang, H.; Liu, C.; Ran, Y.; et al. Efficient DNA-Free Genome Editing of Bread Wheat Using CRISPR/Cas9 Ribonucleoprotein Complexes. Nat. Commun. 2017, 8, 14261. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhu, H.; Liu, J.; Yang, Q.; Shao, X.; Bi, F.; Hu, C.; Huo, H.; Chen, K.; Yi, G. Establishment of a PEG-Mediated Protoplast Transformation System Based on DNA and CRISPR/Cas9 Ribonucleoprotein Complexes for Banana. BMC Plant Biol. 2020, 20, 425. [Google Scholar] [CrossRef] [PubMed]
- Demirer, G.S.; Zhang, H.; Goh, N.S.; González-Grandío, E.; Landry, M.P. Carbon Nanotube–Mediated DNA Delivery without Transgene Integration in Intact Plants. Nat. Protoc. 2019, 14, 2954–2971. [Google Scholar] [CrossRef] [PubMed]
- Demirer, G.S.; Zhang, H.; Matos, J.L.; Goh, N.S.; Cunningham, F.J.; Sung, Y.; Chang, R.; Aditham, A.J.; Chio, L.; Cho, M.-J.; et al. High Aspect Ratio Nanomaterials Enable Delivery of Functional Genetic Material without DNA Integration in Mature Plants. Nat. Nanotechnol. 2019, 14, 456–464. [Google Scholar] [CrossRef]
- Panis, B.; Van Wauwe, A.; Swennen, R. Plant Regeneration through Direct Somatic Embryogenesis from Protoplasts of Banana (Musa spp.). Plant Cell Rep. 1993, 12, 403–407. [Google Scholar] [CrossRef]
- Assani, A.; Haicour, R.; Wenzel, G.; Côte, F.; Bakry, F.; Foroughi-Wehr, B.; Ducreux, G.; Aguillar, M.-E.; Grapin, A. Plant Regeneration from Protoplasts of Dessert Banana cv. Grande Naine (Musa spp., Cavendish Sub-Group AAA) via Somatic Embryogenesis. Plant Cell Rep. 2001, 20, 482–488. [Google Scholar] [CrossRef]
- Assani, A.; Haïcour, R.; Wenzel, G.; Foroughi-Wehr, B.; Bakry, F.; Côte, F.-X.; Ducreux, G.; Ambroise, A.; Grapin, A. Influence of Donor Material and Genotype on Protoplast Regeneration in Banana and Plantain Cultivars (Musa spp.). Plant Sci. 2002, 162, 355–362. [Google Scholar] [CrossRef]
- Haïcour, R.; Assani, A.; BuiTrang, V.; Guedira, A. Protoplast Isolation and Culture for Banana Regeneration via Somatic Embryogenesis. Fruits 2009, 64, 261–269. [Google Scholar] [CrossRef]
- Dai, X.-M.; Xiao, W.; Huang, X.; Zhao, J.-T.; Chen, Y.-F.; Huang, X.-L. Plant Regeneration from Embryogenic Cell Suspensions and Protoplasts of Dessert Banana cv. ‘Da Jiao’ (Musa paradisiaca ABB Linn.) via Somatic Embryogenesis. Vitr. Cell. Dev. Biol. Plant 2010, 46, 403–410. [Google Scholar] [CrossRef]
- Panis, B.; Sagi, L.; Swennen, R. Regeneration of Plants from Protoplasts of Musa Species (Banana). In Plant Protoplasts and Genetic Engineering V; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1994; Volume 29, pp. 102–114. [Google Scholar] [CrossRef]
- Dowling, P.; Clynes, M. Conditioned Media from Cell Lines: A Complementary Model to Clinical Specimens for the Discovery of Disease-specific Biomarkers. Proteomics 2011, 11, 794–804. [Google Scholar] [CrossRef]
- Couillerot, J.-P.; Windels, D.; Vazquez, F.; Michalski, J.-C.; Hilbert, J.-L.; Blervacq, A.-S. Pretreatments, Conditioned Medium and Co-Culture Increase the Incidence of Somatic Embryogenesis of Different Cichorium Species. Plant Signal. Behav. 2012, 7, 121–131. [Google Scholar] [CrossRef]
- Deryckere, D.; Eeckhaut, T.; Van Huylenbroeck, J.; Van Bockstaele, E. Low Melting Point Agarose Beads as a Standard Method for Plantlet Regeneration from Protoplasts within the Cichorium Genus. Plant Cell Rep. 2012, 31, 2261–2269. [Google Scholar] [CrossRef]
- Kiełkowska, A.; Adamus, A. An Alginate-Layer Technique for Culture of Brassica oleracea L. Protoplasts. Vitr. Cell. Dev. Biol.-Plant 2012, 48, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Maćkowska, K.; Jarosz, A.; Grzebelus, E. Plant Regeneration from Leaf-Derived Protoplasts within the Daucus Genus: Effect of Different Conditions in Alginate Embedding and Phytosulfokine Application. Plant Cell Tiss. Organ. Cult. 2014, 117, 241–252. [Google Scholar] [CrossRef]
- Sagi, L.; Remy, S.; Panis, B.; Swennen, R.; Volckaert, G. Transient Gene Expression in Electroporated Banana (Musa spp., cv. Bluggoe, ABB Group) Protoplasts Isolated from Regenerable Embryogenetic Cell Suspensions. Plant Cell Rep. 1994, 13, 262–266. [Google Scholar] [CrossRef]
- Zhao, C.; Li, S.; Du, C.; Gao, H.; Yang, D.; Fu, G.; Cui, H. Establishment of a Protoplasts-Based Transient Expression System in Banana (Musa spp.). Agronomy 2022, 12, 2648. [Google Scholar] [CrossRef]
- Morel, G.; Wetmore, R.H. Fern Callus Tissue Culture. Am. J. Bot. 1951, 38, 141–143. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Schenk, R.U.; Hildebrandt, A.C. Medium and Techniques for Induction and Growth of Monocotyledonous and Dicotyledonous Plant Cell Cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Chu, C.C.; Wang, C.C.; Sun, C.S.; Hsu, C.; Yin, K.C.; Chu, C.Y.; Bi, F.Y. Establishment of an Efficient Medium for Anther Culture of Rice Through Comparative Experiments on the Nitrogen Sources. Sci. Sin. 1975, 18, 659–669. [Google Scholar]
- Kao, K.N.; Michayluk, M.R. Nutritional Requirements for Growth of Vicia Hajastana Cells and Protoplasts at a Very Low Population Density in Liquid Media. Planta 1975, 126, 105–110. [Google Scholar] [CrossRef]
- Becker, D.K.; Dale, J.L. Transformation of Banana Using Microprojectile Bombardment. In Transgenic Crops of the World; Curtis, I.S., Ed.; Springer: Dordrecht, The Netherlands, 2004; pp. 131–143. [Google Scholar] [CrossRef]
- Yoo, S.-D.; Cho, Y.-H.; Sheen, J. Arabidopsis Mesophyll Protoplasts: A Versatile Cell System for Transient Gene Expression Analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef]
- Tricoli, D.M. Interim Progress Report for CDFA Agreement Number # 18-0397 Grape Protoplast Isolation and Regeneration of Plants for Use in Gene Editing Technology Project. Available online: https://www.semanticscholar.org/paper/6ee0998387de581fea1820aab88aae247051d7fb (accessed on 8 February 2024).
- Verchot-Lubicz, J.; Goldstein, R.E. Cytoplasmic Streaming Enables the Distribution of Molecules and Vesicles in Large Plant Cells. Protoplasma 2010, 240, 99–107. [Google Scholar] [CrossRef]
- Kidanemariam, D.B.; Sukal, A.C.; Crew, K.; Jackson, G.V.H.; Abraham, A.D.; Dale, J.L.; Harding, R.M.; James, A.P. Characterization of an Australian Isolate of Taro Bacilliform Virus and Development of an Infectious Clone. Arch. Virol. 2018, 163, 1677–1681. [Google Scholar] [CrossRef] [PubMed]
- Chamani, E.; Tahami, S.K.; Zare, N.; Asghari-Zakaria, R.; Mohebodini, M.; Joyce, D. Effect of Different Cellulase and Pectinase Enzyme Treatments on Protoplast Isolation and Viability in Lilium ledebeourii Bioss. Not. Bot. Hort. Agrobot. Cluj. 2012, 40, 123. [Google Scholar] [CrossRef]
- Wang, P.; Pu, Y.; Abid, M.A.; Kang, L.; Ye, Y.; Zhang, M.; Liang, C.; Wei, Y.; Zhang, R.; Meng, Z. A Rapid and Efficient Method for Isolation and Transformation of Cotton Callus Protoplast. Int. J. Mol. Sci. 2022, 23, 8368. [Google Scholar] [CrossRef]
- Ishii, S. Factors Influencing Protoplast Viability of Suspension-Cultured Rice Cells during Isolation Process. Plant Physiol. 1988, 88, 26–29. [Google Scholar] [CrossRef]
- Jones, A.M.P.; Shukla, M.R.; Biswas, G.C.G.; Saxena, P.K. Protoplast-to-Plant Regeneration of American Elm (Ulmus Americana). Protoplasma 2015, 252, 925–931. [Google Scholar] [CrossRef]
- Taylor, R.J.; Ruby, C.L.; Secor, G.A. Effects of Enzyme Dialysis, Cellulase Concentration, Bovine Serum Albumin and Origin of Source Leaflet Tissue on Yield and Plating Efficiency of Potato Mesophyll Protoplasts. Biochem. Und Physiol. Der Pflanz. 1989, 185, 227–232. [Google Scholar] [CrossRef]
- Ribnicky, D.M.; Ilic, N.; Cohen, J.D.; Cooke, T.J. The Effects of Exogenous Auxins on Endogenous Indole-3-Acetic Acid Metabolism (The Implications for Carrot Somatic Embryogenesis). Plant Physiol. 1996, 112, 549–558. [Google Scholar] [CrossRef]
- Cao, X.; Yang, H.; Shang, C.; Ma, S.; Liu, L.; Cheng, J. The Roles of Auxin Biosynthesis YUCCA Gene Family in Plants. Int. J. Mol. Sci. 2019, 20, 6343. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Kawamura, A.; Suzuki, T.; Segami, S.; Maeshima, M.; Polyn, S.; De Veylder, L.; Sugimoto, K. Transcriptional Activation of Auxin Biosynthesis Drives Developmental Reprogramming of Differentiated Cells. Plant Cell 2022, 34, 4348–4365. [Google Scholar] [CrossRef]
- Papadakis, A.K.; Siminis, C.I.; Roubelakis-Angelakis, K.A. Reduced Activity of Antioxidant Machinery Is Correlated with Suppression of Totipotency in Plant Protoplasts. Plant Physiol. 2001, 126, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Blake, T.J. Comparison of Polyethylene Glycol 3350 Induced Osmotic Stress and Soil Drying for Drought Simulation in Three Woody Species. Trees 1997, 11, 342. [Google Scholar] [CrossRef]
- Li, X.; Sandgrind, S.; Moss, O.; Guan, R.; Ivarson, E.; Wang, E.S.; Kanagarajan, S.; Zhu, L.-H. Efficient Protoplast Regeneration Protocol and CRISPR/Cas9-Mediated Editing of Glucosinolate Transporter (GTR) Genes in Rapeseed (Brassica napus L.). Front. Plant Sci. 2021, 12, 680859. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Nakata, P.A. Development of a Rapid and Efficient Protoplast Isolation and Transfection Method for Chickpea (Cicer arietinum). MethodsX 2020, 7, 101025. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Song, D.; Gao, L.; Ajayo, B.S.; Wang, Y.; Huang, H.; Zhang, J.; Liu, H.; Liu, Y.; Yu, G.; et al. Optimization of Isolation and Transfection Conditions of Maize Endosperm Protoplasts. Plant Methods 2020, 16, 96. [Google Scholar] [CrossRef] [PubMed]

| Buffers and Media | Components * |
|---|---|
| Banana Cell Culture Media (BL) | MS macro and micronutrients (1×), MS-Fe-EDTA (1×), sucrose (20 g/L), Bluggoe vitamins (1×), ascorbic acid (50 mg/L), 2,4-D (1.1 mg/L), zeatin (0.25 mg/L), pH 5.7 |
| Protoplast Isolation Buffer (PIB) | CaCl2.2H2O (7 mM), NaH2PO4.2H2O (0.7 mM), mannitol (0.55 M), MES (3 mM), pH 5.7 |
| Protoplast Wash Buffer (PWB) | KCl (0.4 M), CaCl2.2H2O (0.034 M), mannitol (0.55 M), MES (3.5 mM), pH 5.7 |
| Antioxidant Mix (AOM) | Ascorbic acid (50 mg/L), citric acid (5 mg/L), L-cysteine (50 mg/L) |
| Protoplast Culture Media-8G (PCM-8G) | N6 salts (1×), KM vitamins (1×), KM sugars (1×), KM organic acids (1×), KH2PO4 (0.25 g/L), glucose (80 g/L), MES (0.5 mM), pH 5.7 |
| Protoplast Culture Media-2G (PCM-2G) | N6 salts (1×), KM vitamins (1×), KM sugars (1×), KM organic acids (1×), KH2PO4 (0.25 g/L), glucose (20 g/L), sucrose (1 g/L), MES (0.5 mM), pH 5.7 |
| Protoplast Culture Medium for Embryogenesis (PCM-E) | SH macro and micro salts (½×), MS micronutrients (½×), KM vitamins (½×), sucrose (20 g/L), glucose (2 g/L), MES (0.5 mM), pH 5.7 |
| Regeneration Medium-I (RM-I) | MS macro and micronutrients (1×), MS-Fe-EDTA (1×), MW vitamins (1×), sucrose (30 g/L), IAA (0.1 mg/L), BAP (0.5 mg/L), MI (100 mg/L), pH 5.7 |
| Regeneration Medium-II (RM-II) | MS macro and micronutrients (1×), MS-Fe-EDTA (1×), MW vitamins (1×), sucrose (30 g/L), BAP (0.05 mg/L), MI (100 mg/L), pH 5.7 |
| MS | MS macro and micronutrients (1×), MS-Fe-EDTA (1×), MS vitamins (1×), sucrose (30 g/L), pH 5.7 |
| MaMg | MgCl2.6H2O (15 mM), mannitol (0.5 M), MES (4 mM), pH 5.7 |
| PEG-MaMg | MgCl2.6H2O (15 mM), mannitol (0.55 M), MES (5.12 mM), pH 5.7, PEG-3350 (20%) |
| Stop Buffer | Mannitol (0.5 M), KCl (5 mM), MES (4 mM), pH 5.7 |
| Enzyme Combinations | Enzymes (%) | |||
|---|---|---|---|---|
| Cellulase | Macerozyme | Driselase | Pectinase | |
| E1 | 1 | 0.5 | 0 | 0 |
| E2 | 1 | 1 | 0 | 0 |
| E3 | 1 | 1 | 0.5 | 0 |
| E4 | 1 | 1 | 0 | 0.5 |
| E5 | 1 | 1 | 0.5 | 0.5 |
| Tested Variable | Comparative Results * | |
|---|---|---|
| Protoplast and DNA incubation (min) | 1 | +++ |
| 5 | - | |
| PEG size (ave. molecular weight) | 3350 | +++ |
| 4000 | - | |
| PEG-3350 concentration (%) | 10 | +++ |
| 20 | + | |
| PEG-3350 incubation time (min) | 5 | +++ |
| 10 | - | |
| PEG-3350 transfection buffer | MaMg a | +++ |
| CaCl2 b | - | |
| PEG-3350 transfection buffer pH | 5.6 | +++ |
| 7 | - | |
| PEG-3350 dilution regime | 20× | - |
| 40× | +++ | |
| Transfection culture vessel | Falcon tubes | - |
| Microfuge tubes | +++ | |
| ECS subculture regime (days) | 7 | +++ |
| 10 | + | |
| Protoplast recovery | Ice (30 min) | - |
| RT (60 min) | +++ | |
| Temperature shock (5 min) | Ice | +++ |
| 45 °C | + | |
| Protoplast culture post-transfection | Liquid medium | +++ |
| Solid medium | +++ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deo, P.C.; Paul, J.-Y.; James, A.; Harding, R.; Dale, J. An Improved Approach to Protoplast Regeneration and Transfection in Banana (Musa acuminata AAA cv. Williams). Appl. Biosci. 2025, 4, 42. https://doi.org/10.3390/applbiosci4030042
Deo PC, Paul J-Y, James A, Harding R, Dale J. An Improved Approach to Protoplast Regeneration and Transfection in Banana (Musa acuminata AAA cv. Williams). Applied Biosciences. 2025; 4(3):42. https://doi.org/10.3390/applbiosci4030042
Chicago/Turabian StyleDeo, Pradeep Chand, Jean-Yves Paul, Anthony James, Rob Harding, and James Dale. 2025. "An Improved Approach to Protoplast Regeneration and Transfection in Banana (Musa acuminata AAA cv. Williams)" Applied Biosciences 4, no. 3: 42. https://doi.org/10.3390/applbiosci4030042
APA StyleDeo, P. C., Paul, J.-Y., James, A., Harding, R., & Dale, J. (2025). An Improved Approach to Protoplast Regeneration and Transfection in Banana (Musa acuminata AAA cv. Williams). Applied Biosciences, 4(3), 42. https://doi.org/10.3390/applbiosci4030042






