Evolution of the Genetic Diversity and Spatial Distribution of Self-Establishing Black Locust (Robinia Pseudoacacia L.) Stands
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. DNA Extraction
2.3. SSR Analysis
2.4. Statistical Analysis
3. Results
3.1. Locus Diversity
3.2. Genetic Diversity in Plots
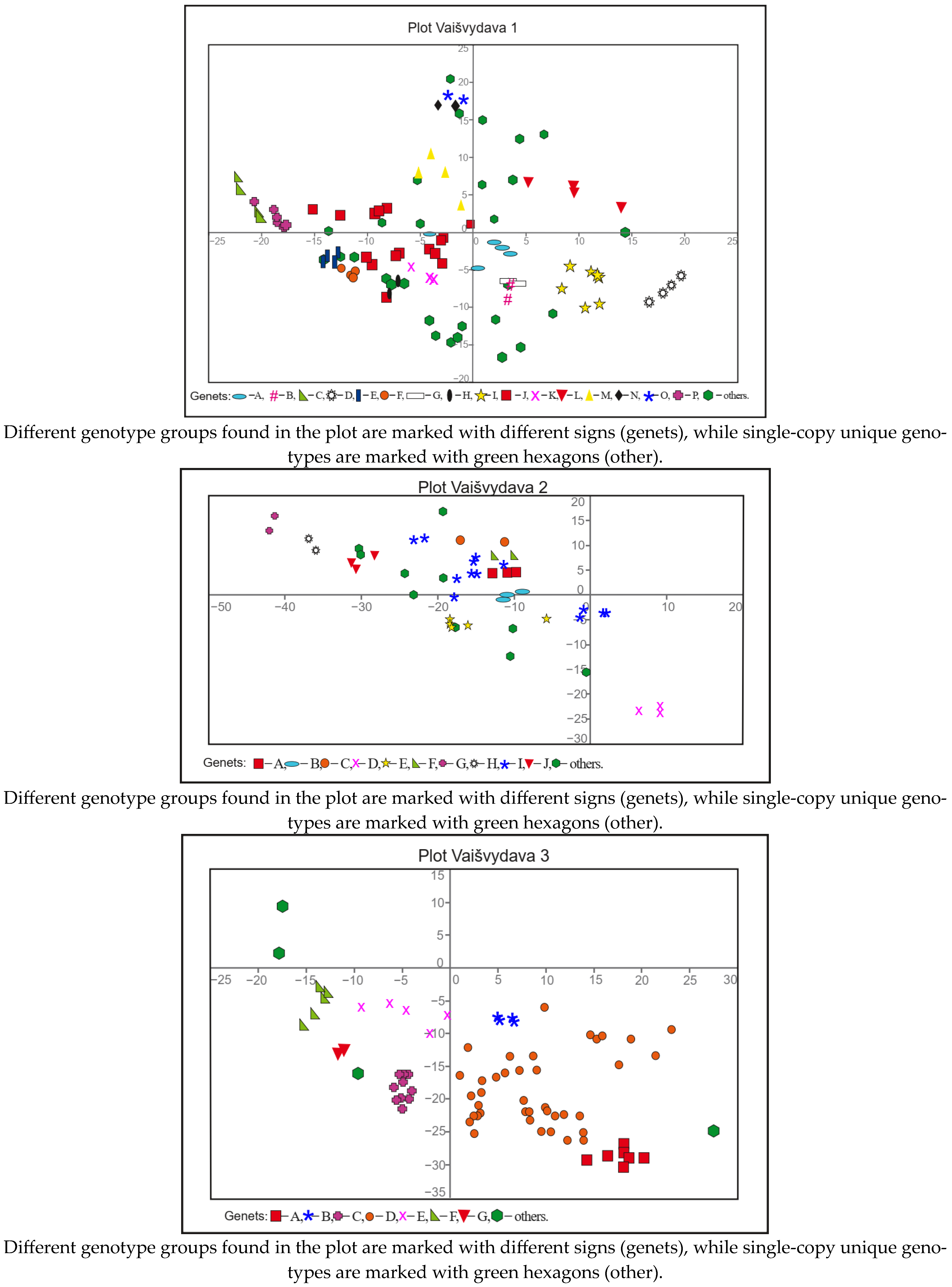
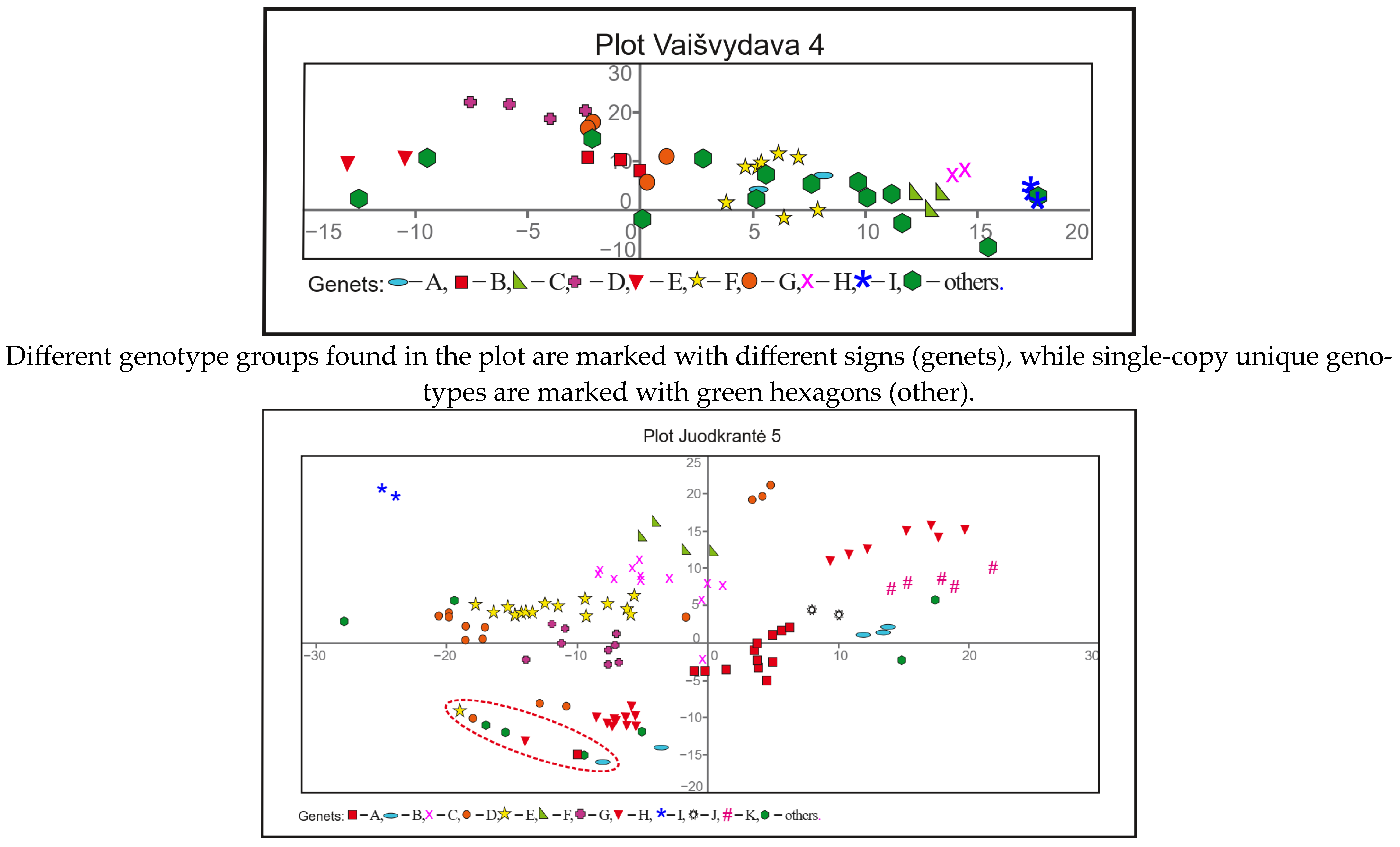
3.3. Genotypic Diversity and Spatial Structure
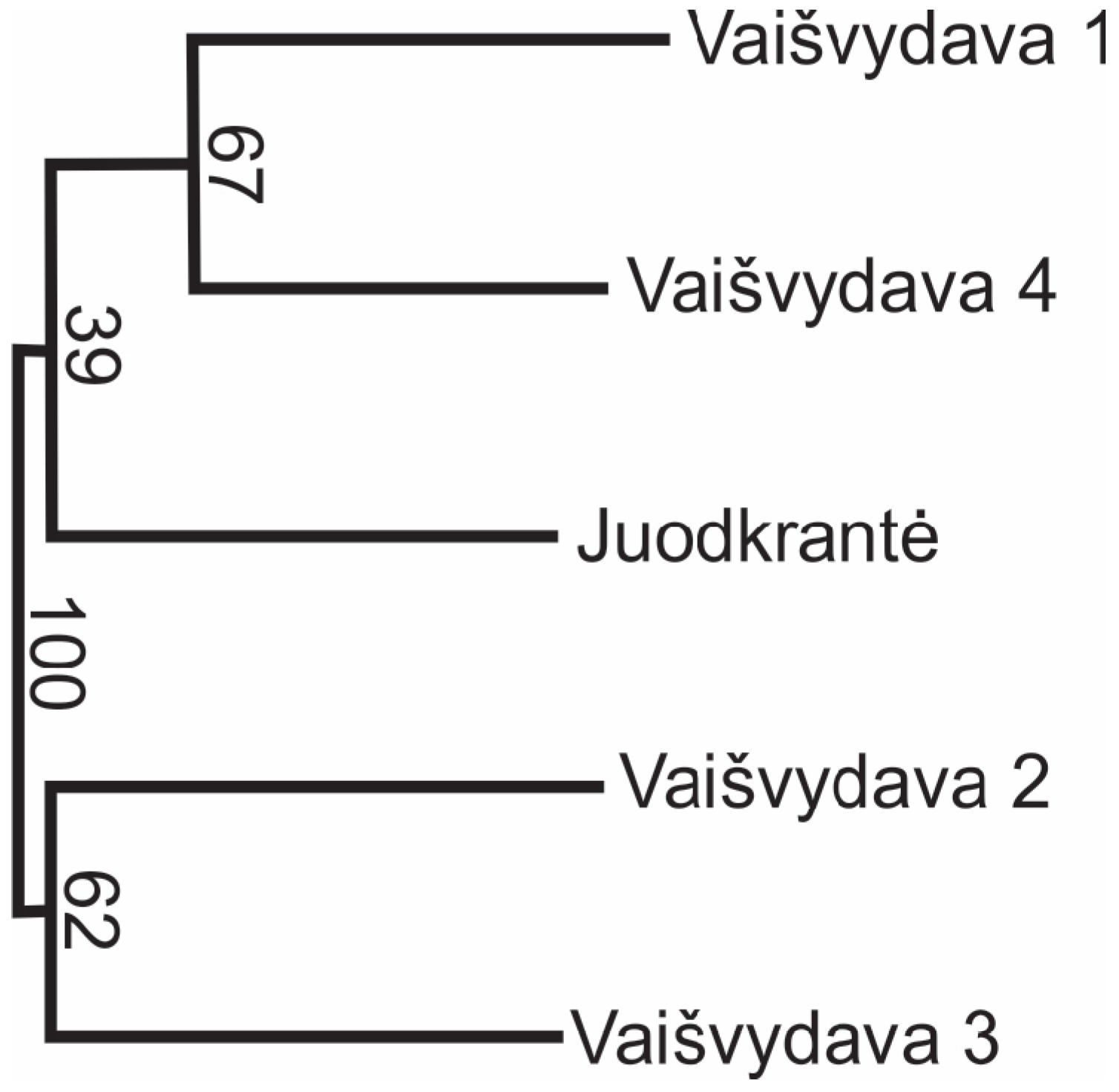
3.4. Genetic Differentiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boring, L.R.; Swank, W.T. The role of black locust (Robinia pseudoacacia) in forest succession. J. Ecol. 1984, 72, 749–766. [Google Scholar] [CrossRef]
- Martin, G.D. Addressing geographical bias: A review of Robinia pseudoacacia (black locust) in the Southern Hemisphere. S. Afr. J. Bot. 2019, 125, 481–492. [Google Scholar] [CrossRef]
- Richardson, D.M.; Rejmánek, M. Trees and shrubs as invasive alien species—A global review. Divers. Distrib. 2011, 17, 788–809. [Google Scholar] [CrossRef]
- Rumlerová, Z.; Vilà, M.; Pergl, J.; Nentwig, W.; Pyšek, P. Scoring environmental and socioeconomic impacts of alien plants invasive in Europe. Biol. Invasion 2016, 18, 3697–3711. [Google Scholar] [CrossRef]
- Roy, D.; Alderman, D.; Anastasiu, P.; Arianoutsou, M.; Augustin, S.; Bacher, S.; Basnou, C.; Beisel, J.; Bertolino, S.; Bonesi, L.; et al. DAISIE—Inventory of Alien Invasive Species in Europe. Version 1.6. Research Institute for Nature and Forest (INBO). 2019. Available online: https://doi.org/10.15468/ybwd3x (accessed on 24 February 2025). [CrossRef]
- Rédei, K.; Csiha, I.; Keserű, Z.; Rásó, J.; Kamandiné, V.Á.; Antal, B. Growth and yield of black locust (Robinia pseudoacacia L.) stands in Nyírség Growing Region (North-East Hungary). South-East Eur. For. SEEFOR 2014, 5, 13–22. [Google Scholar] [CrossRef]
- Wilkaniec, A.; Borowiak-Sobkowiak, B.; Irzykowska, L.; Breś, W.; Świerk, D.; Pardela, Ł.; Wielgus, K. Biotic and abiotic factors causing the collapse of Robinia pseudoacacia L. veteran trees in urban environments. PLoS ONE 2021, 16, e0245398. [Google Scholar] [CrossRef]
- Vitkova, M.; Mullerova, M.; Sadlo, J.; Pergl, J.; Pyšek, P. Black locust (Robinia pseudoacacia) beloved and despised: A story of an invasive tree in Central Europe. For. Ecol. Manag. 2017, 384, 287–302. [Google Scholar] [CrossRef]
- Ciuvăt, A.L.; Abrudan, I.V.; Ciuvăt, C.G.; Marcu, C.; Lorent, A.; Dincăa, L.; Szilard, B. Black locust (Robinia pseudoacacia L.) in Romanian Forestry. Diversity 2022, 14, 780. [Google Scholar] [CrossRef]
- Ivinskis, P.; Rimšaitė, J. Records of Phyllonorycter robiniella (Clemens, 1859) and Parectopa robiniella Clemens, 1863 (Lepidoptera, Gracillariidae) in Lithuania. Acta Zool. Litu. 2008, 18, 130–133. [Google Scholar] [CrossRef]
- Cierjacks, A.; Kowarik, I.; Joshi, J.; Hempel, S.; Ristow, M.; von der Lippe, M.; Weber, E. Biological flora of the British Isles: Robinia pseudoacacia. J. Ecol. 2013, 101, 1623–1640. [Google Scholar] [CrossRef]
- Środek, D.; Rahmonov, O. The Properties of Black Locust Robinia pseudoacacia L. to Selectively Accumulate Chemical Elements from Soils of Ecologically Transformed Areas. Forests 2022, 13, 7. [Google Scholar] [CrossRef]
- Redei, K.; Bujtas, Z.O.; Veperdi, I. Black locust (Robinia pseudoacacia L.) improvement in Hungary: A review. Acta Silv. Lign. Hung. 2008, 4, 127–132. [Google Scholar] [CrossRef]
- Saulino, L.; Rita, A.; Stinca, A.; Liuzzi, G.; Silvestro, R.; Rossi, S.; Saracino, A. Wildfire promotes the invasion of Robinia pseudoacacia in the unmanaged Mediterranean Castanea savita coppice forests. Fire For. 2023, 6, 1177551. [Google Scholar] [CrossRef]
- De Marco, A.; Napoletano, P.; Panico, S.C.; Memoli, V.; Santorufo, L.; Rugiero, A.G. Combined effect of black locust invasion and fire on soils of Mediterranean shrublands and pine forests. Catena 2023, 220, 106656. [Google Scholar] [CrossRef]
- Jung, S.C.; Matsushita, N.; Wu, B.Y.; Kondo, N.; Shiraishi, A.; Hogetsu, T. Reproduction of a Robinia pseudoacacia population in a coastal Pinus thunbergii windbreak along the Kujukurihama Coast, Japan. J. For. Res. 2009, 14, 101–110. [Google Scholar] [CrossRef]
- Kurokochi, H.; Toyama, K.; Hogetsu, T. Regeneration of Robinia pseudoacacia riparian forests after clear-cutting along the Chikumagawa River in Japan. Plant Ecol. 2010, 210, 31–41. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kato, M. Invasive characteristics of Robinia pseudoacacia and its impacts on species diversity. Diversity 2024, 16, 773. [Google Scholar] [CrossRef]
- Nadal-Sala, D.; Hartig, F.; Gracia, C.A.; Sabaté, S. Global warming likely to enhance black locust (Robinia pseudoacacia L.) growth in a Mediterranean riparian forest. For. Ecol. Manag. 2019, 449, 117448. [Google Scholar] [CrossRef]
- Patalauskaitė, D. Possibility of survival of Robinia pseudaccacia in decidous forest. Acta Biol. Univ. Daugavp. 2007, 7, 129–132. Available online: https://du.lv/wp-content/uploads/2022/02/Zurnals_7_2.pdf (accessed on 14 March 2025).
- Dyderski, M.K.; Jagodziński, A.M. Seedling survival of Prunus serotina Ehrh., Quercus rubra L. and Robinia pseudoacacia L. in temperate forests of Western Poland. For. Ecol. Manag. 2019, 450, 117498. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Paź, S.; Frelich, L.E.; Jagodziński, A.M. How much does climate change threaten European forest tree species distributions? Glob. Change Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Vítková, M.; Kolbek, J. Vegetation classification and synecology of Bohemian Robinia pseudacacia stands in a Central European context. Phytocoenologia 2010, 40, 205–241. [Google Scholar] [CrossRef]
- Kleinbauer, I.; Dullinger, S.; Peterseil, J.; Essl, F. Climate change might drive the invasive tree Robinia pseudacacia into nature reserves and endangered habitats. Biol. Conserv. 2010, 143, 382–390. [Google Scholar] [CrossRef]
- Šibíková, M.; Jarolímek, I.; Hegedüšová, K.; Májeková, J.; Mikulová, K.; Slabejová, D.; Medvecká, J. Effect of planting alien Robinia pseudoacacia trees on homogenization of Central European Forest vegetation. Sci. Total Environ. 2019, 687, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Slabejová, D.; Bacigál, T.; Hegedüšová, K.; Májeková, J.; Medvecká, J.; Mikulová, K.; Jarolímek, I. Comparison of the understory vegetation of native forests and adjacent Robinia pseudoacacia plantations in the Carpathian-Pannonian region. For. Ecol. Manag. 2019, 439, 8–40. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Wu, C.; Linghu, G.; Zhu, H.; Khamphilavong, K.; Li, M.; Zhou, X.; Ma, G.; Kang, Y. Far-reaching effects on soil properties and underground microbial ecosystem after the introduction of black locusts in forest. Front. Ecol. Evol. 2023, 11, 1210498. [Google Scholar] [CrossRef]
- Wohlgemuth, T.; Gossner, M.M.; Campagnaro, T.; Marchante, H.; Van Loo, M.; Vacchiano, G.; Castro-Díez, P.; Dobrowolska, D.; Gazda, A.; Keren, S.; et al. Impact of non-native tree species in Europe on soil properties and biodiversity: A review. Neobiota 2022, 78, 45–69. [Google Scholar] [CrossRef]
- Campagnaro, T.; Nascimbene, J.; Tasinazzo, S.; Trentanovi, G.; Sitzia, T. Exploring patterns, drivers and structure of plant community composition in alien Robinia pseudoacacia secondary woodlands. iForest 2018, 11, 586–593. [Google Scholar] [CrossRef]
- Order of the Ministry of the Republic of Lithuania 2016 Nowember 28 No. D1-810 “Dėl Invazinių Lietuvoje Organizmų Rūšių Sąrašo Patvirtinimo ir dėl kai Kurių Aplinkos Ministro Įsakymų Pripažinimo Netekusiais Galios”. Available online: https://e-seimas.lrs.lt/portal/legalAct/lt/TAD/TAIS.239692/asr (accessed on 25 February 2025).
- Puchałka, R.; Dyderski, M.K.; Vítková, M.; Sádlo, J.; Klisz, M.; Netsvetov, M.; Jagodziński, A.M. Black locust (Robinia pseudoacacia L.) range contraction and expansion in Europe under changing climate. Glob. Change Biol. 2021, 27, 1587–1600. [Google Scholar] [CrossRef]
- Menkis, A.; Marčiulynas, A.; Gedminas, A.; Lynikienė, J.; Povilaitienė, A. High-Throughput sequencing reveals drastic changes in fungal communities in the phyllosphere of Norway spruce (Picea abies) following invasion of the spruce bud scale (Physokermes piceae). Microb. Ecol. 2015, 70, 904–911. [Google Scholar] [CrossRef]
- Lian, C.; Hogetsu, T. Development of microsatellite markers in black locust (Robinia pseudoacacia) using a dual-supression-PCR technique. Mol. Ecol. Notes 2002, 2, 211–213. [Google Scholar] [CrossRef]
- Kurokochi, H.; Hogetsu, T. Fine-scale initiation of non-native Robinia pseudoacacia riparian forests along the Chikumagawa River in central Japan. J. Ecol. Environ. 2014, 37, 21–29. [Google Scholar] [CrossRef]
- From Data to Discovery—Faster. Geneious Puts Industry-Leading Bioinformaticsw, Molecular Biology and Antibody Discovery Tools Directly into Researchers Hands, Streamlining Sequence Analysis and Insights. Available online: http://www.geneious.com (accessed on 25 March 2025).
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Gallagher, J.; Lordan, C.; Hughes, G.M.; Jonasson, J.P.; Carlsson, J. Microsatellites obtained using high throughput sequencing and a novel microsatellite genotyping method reveals population genetic structure in Norway Lobster, Nephrops norvegicus. J. Sea Res. 2022, 179, 102139. [Google Scholar] [CrossRef]
- Excoffier, L.; Smouse, P.E.; Quattro, J.M. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitocondrial DNA restriction sites. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef]
- Mengoni, A.; Bazzicalupo, M. The statistical treatment of data and the analysis of molecular variance (AMOVA) in molecular microbial ecology. Ann. Microbiol. 2002, 52, 95–101. [Google Scholar]
- Nei, M. Genetic distance between populations. Am. Nat. 1972, 106, 283–392. [Google Scholar] [CrossRef]
- Nei, M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 1978, 89, 583–590. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Aoki, Y. Statistical and probabilistic bases of forensic DNA testing. J. Iwate Med. Assoc. 2002, 54, 81–94. [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 4. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 14 June 2025).
- Sneath, P.H.A.; Sokal, R.R. Numerical Taxonomy: The Principles and Practice of Numerical Classification; WF Freeman & Co.: San Francisco, CA, USA, 1973; 573p. [Google Scholar]
- Suzuki, R.; Shimodaira, H. An application of multiscale bootstrap resampling to hierarchical clustering of microarray data: How accurate are these clusters? In Proceedings of the 2004 Fifteenth International Conference on Genome Informatics, Yokohama, Japan, 13–15 December 2004; Available online: https://www.researchgate.net/publication/228851295_An_Application_of_Multiscale_Bootstrap_Resampling_to_Hierarchical_Clustering_of_Microarray_Data_How_Accurate_are_these_Clusters (accessed on 14 June 2025).
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Earl, D.A.; von Holdt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Szyp-Borowska, I.; Zawadzka, A.; Wojda, T.; Klisz, M. Analysis of the genetic diversity and population structures of black locust (Robinia pseudoacacia L.) stands in Poland based on simple sequence repeat markers. Folia For. Pol. 2023, 65, 187–198. [Google Scholar] [CrossRef]
- Surless, E.; Hamrickj, L.; Bongartenb, C. Mating systems in open-pollinated families of black locust (Robinia Pseudoacacia). Silvae Genet. 1990, 39, 35–40. [Google Scholar]
- Dooley, T. Lessons learned from eleven years of prescribed fire at the Albany Pine Bush Preserve. In Using fire to Control Invasive Plants: What’s New, What Works in the Northeast?—Proceedings of a Workshop; Bennett, P., Dibble, A.C., Patterson, W.A., Eds.; Urban Forestry Center: Portsmouth, NH, USA, 2003; pp. 7–10. [Google Scholar]
- Elliott, K.J.; Boring, L.R.; Swank, W.T. Changes in vegetation structure and diversity after grass-to-forest succession in a southern Appalachian watershed. Am. Midl. Nat. 1998, 140, 219–232. [Google Scholar] [CrossRef]
- Rice, S.K.; Westerman, B.; Federici, R. Impacts of the exotic, nitrogen-fixing black locust (Robinia pseudoacacia) on nitrogen-cycling in a pine–oak ecosystem. Plant Ecol. 2004, 174, 97–107. [Google Scholar] [CrossRef]
- Sterrett, J.P.; Chappell, W.E. The effect of auxin on suckering in black locust. Weeds 1967, 15, 323–326. [Google Scholar] [CrossRef]
- Böhm, C.H.; Quinkenstein, A.; Freese, D. Yield prediction of young black locust (Robinia pseudoacacia L.) plantations for woody biomass production using allometric relations. Ann. For. Res. 2011, 54, 215–227. Available online: https://www.afrjournal.org/index.php/afr/article/view/91/137 (accessed on 19 June 2025).
- Straker, K.C.; Quinn, L.D.; Voigt, T.B.; Lee, D.K.; Kling, G.J. Black locust as a bioenergy feedstock: A review. BioEnergy Res. 2015, 8, 1117–1135. [Google Scholar] [CrossRef]
- Olson, D.F. Robinia L.; locust. In Seeds of Woody Plants in the United States; Forest Service, United States Department of Agriculture: Washington, DC, USA, 1974; pp. 728–731. [Google Scholar]
- Roach, A.B. Black locust (Robinia pseudoacacia L.). In Silvics of Forest Trees of the United States; Fowells, H.A., Ed.; Agriculture Handbook 271; comp. U.S. Department of Agriculture: Washington, DC, USA, 1965; pp. 642–648. [Google Scholar]
- Petit, R.J.; Hampe, A. Some evolutionary consequences of being a tree. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 187–214. [Google Scholar] [CrossRef]
- Guo, Q.; Cao, S.; Dong, L.; Li, X.; Zhang, J.; Zhang, Y.; Zhang, Z.; Sun, Y.; Long, C.; Fan, Y.; et al. Genetic diversity and population structure of Robinia pseudoacacia from six improved variety bases in China as revealed by simple sequence repeat markers. J. For. Res. 2022, 33, 611–621. [Google Scholar] [CrossRef]
- Chang, C.S.; Hamrickj, L.; Bongarten, C. Genetic structure of natural populations of black locust (Robinia pseudoacacia L.) at Coweeta, North Carolina. J. Plant Res. 1998, 111, 17–24. [Google Scholar] [CrossRef]
- Zhang, K.; Shen, Z.; Yang, X.; Ma, L.; Duan, J.; Li, Y. Spatial patterns in different stages of regeneration after clear-cutting of a black locust forest in central China. Forests 2019, 10, 1066. [Google Scholar] [CrossRef]
- Kunakh, O.; Ivanko, I.; Holoborodko, K.; Zhukov, O. A spontaneous spread of black locust (Robinia pseudoacacia L.): The importance of seed and vegetative reproduction. Folia Oecologica 2024, 51, 120–135. [Google Scholar] [CrossRef]
- Gyokusen, K.; Iijima, Y.; Yahata, H. Spatial distribution and morphological features of root sprouts in niseakashia (Robinia pseudoacacia) growing under a coastal black pine forest. Bull. Kyushu Univ. For. 1991, 54, 13–28. [Google Scholar]
- Negreros-Castillo, P.; Hall, R.B. Sprouting capability of 17 tropical tree species after overstory removal in Quintana Roo, Mexico. For. Ecol. Manag. 2000, 126, 399–403. [Google Scholar] [CrossRef]
- Keim, R.F.; Chambers, J.L.; Hughes, M.S.; Dimov, L.D.; Conner, W.H.; Shaffer, G.P.; Gardiner, E.S.; Day, J.W. Long-term success of stump sprouts in high-graded baldcypress–water tupelo swamps in the Mississippi delta. For. Ecol. Manag. 2006, 234, 24–33. [Google Scholar] [CrossRef]
- Guo, Q.; Li, X.Y.; Yang, S.H.; Yang, Z.H.; Sun, Y.H.; Zhang, J.T.; Cao, S.; Dong, L.; Uddin, S.; Li, Y. Evaluation of the genetic diversity and differentiation of black locust (Robinia pseudoacacia L.) based on genomic and expressed sequence tag-simple sequence repeats. Int. J. Mol. Sci. 2018, 19, 2492. [Google Scholar] [CrossRef] [PubMed]
- Von der Lippe, M.; Kowarik, I. Do cities export biodiversity? Traffic as dispersal vector across urban-rural gradients. Divers. Distrib. 2008, 14, 8–25. [Google Scholar] [CrossRef]

| Locus | Primer Sequence | Fluorescent Label | Repeat Motif | Fragment Size in bp |
|---|---|---|---|---|
| Rops 02 | F: CAGAACTGTGGAGAATAATTCTGAACCG | 6-Fam | (AC)13(AT)4 | 102–138 |
| R: CGCCATCTGTTAGTTTGTTGC | ||||
| Rops 05 | F: TGGTGATTAAGTCGCAAGGTG | Ned | (AC)2GC(AC)7 | 114–146 |
| R: GTTGTGACTTGTACGTAAGTC | ||||
| Rops 06 | F: CTAAGGAGGTGCTGACCCTC | Pet | (GT)3ACA(GT)11 | 112–146 |
| R: TTAATCTGTGATGGGACACTG | ||||
| Rops 08 | F: TTCTGAGGAAGGGTTCCGTGG | Vic | (CA)8TA(CA)3 | 190–206 |
| R: GTTAAAGCAACAGGCACATGG |
| Plot | N | Na | Ne | Ho | He | F |
|---|---|---|---|---|---|---|
| Vaišvydava 1 | 99 | 8.75 ± 1.11 | 4.16 ± 0.79 | 0.80 ± 0.10 | 0.72 ± 0.06 | −0.09 ± 0.06 |
| Vaišvydava 2 | 49 | 6.00 ± 0.82 | 2.90 ± 0.25 | 0.80 ± 0.09 | 0.65 ± 0.03 | −0.23 ± 0.15 |
| Vaišvydava 3 | 76 | 6.00 ± 0.71 | 3.19 ± 0.17 | 0.92 ± 0.03 | 0.68 ± 0.02 | −0.35 ± 0.08 |
| Vaišvydava 4 | 45 | 7.00 ± 0.41 | 4.07 ± 0.26 | 0.86 ± 0.05 | 0.75 ± 0.02 | −0.14 ± 0.05 |
| Juodkrantė 5 | 110 | 6.75 ± 0.75 | 3.96 ± 0.34 | 0.78 ± 0.13 | 0.74 ± 0.02 | −0.03 ± 0.15 |
| In total | 75.8 ± 6.0 | 6.9 ± 0.39 | 3.66 ± 0.21 | 0.83 ± 0.04 | 0.71 ± 0.02 | −0.17 ± 0.05 |
| Plot | Plot Size, ha | No. of Individuals | No. of Unique Genotypes | No. of Genets with Only One Ramet | Proportion of Clonal Plants in the Plot % | Average Gene Size |
|---|---|---|---|---|---|---|
| Vaišvydava 1 | 5.5 | 99 | 45 | 29 | 70 | 4.37 |
| Vaišvydava 2 | 1.0 | 49 | 21 | 11 | 77 | 3.8 |
| Vaišvydava 3 | 1.0 | 76 | 11 | 4 | 94 | 10.28 |
| Vaišvydava 4 | 1.0 | 45 | 23 | 14 | 68 | 3.44 |
| Juodkrantė 5 | 3.0 | 110 | 19 | 8 | 92 | 9.27 |
| In total | 11.5 | 379 | 119 | 66 | 83 | 5.9 |
| Vaišvydava 1 | - | 0.181 *** (0.135 ***) | 0.190 *** (0.096 ***) | 0.109 *** (0.057 ***) | 0.132 *** (0.075 ***) |
| Vaišvydava 2 | 0.705 | - | 0.180 *** (0.031 *) | 0.180 *** (0.129 ***) | 0.167 *** (0.116 ***) |
| Vaišvydava 3 | 0.846 | 0.604 | - | 0.133 *** (0.070 ***) | 0.153 *** (0.053 **) |
| Vaišvydava 4 | 0.454 | 0.750 | 0.512 | - | 0.139 *** (0.091 ***) |
| Juodkrantė 5 | 0.569 | 0.657 | 0.624 | 0.694 | - |
| Plot | Vaišvydava 1 | Vaišvydava 2 | Vaišvydava 3 | Vaišvydava 4 | Juodkrantė 5 |
| Source of Variation | Degrees of Freedom (df) | Sum of Squares | Components of Variation | Percentage of Diversity | Fixation Index | p Value |
|---|---|---|---|---|---|---|
| Among research plots | 4 (4) | 160.478 (33.376) | 0.264 (0.154) | 14% (9%) | 0.155 (0.091) | 0.001 (0.001) |
| Within research plots | 379 (119) | 623.5 (193) | 1.645 (1.622) | 86% (91%) | - | - |
| Total | 383 (123) | 783.977 (226.376) | 1.909 (1.775) | 100% (100%) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Černulienė, S.; Verbylaitė, R.; Stakėnas, V. Evolution of the Genetic Diversity and Spatial Distribution of Self-Establishing Black Locust (Robinia Pseudoacacia L.) Stands. Appl. Biosci. 2025, 4, 33. https://doi.org/10.3390/applbiosci4030033
Černulienė S, Verbylaitė R, Stakėnas V. Evolution of the Genetic Diversity and Spatial Distribution of Self-Establishing Black Locust (Robinia Pseudoacacia L.) Stands. Applied Biosciences. 2025; 4(3):33. https://doi.org/10.3390/applbiosci4030033
Chicago/Turabian StyleČernulienė, Sinilga, Rita Verbylaitė, and Vidas Stakėnas. 2025. "Evolution of the Genetic Diversity and Spatial Distribution of Self-Establishing Black Locust (Robinia Pseudoacacia L.) Stands" Applied Biosciences 4, no. 3: 33. https://doi.org/10.3390/applbiosci4030033
APA StyleČernulienė, S., Verbylaitė, R., & Stakėnas, V. (2025). Evolution of the Genetic Diversity and Spatial Distribution of Self-Establishing Black Locust (Robinia Pseudoacacia L.) Stands. Applied Biosciences, 4(3), 33. https://doi.org/10.3390/applbiosci4030033






