Direct Expression of CPT1a Enables a High Throughput Platform for the Discovery of CPT1a Modulators
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
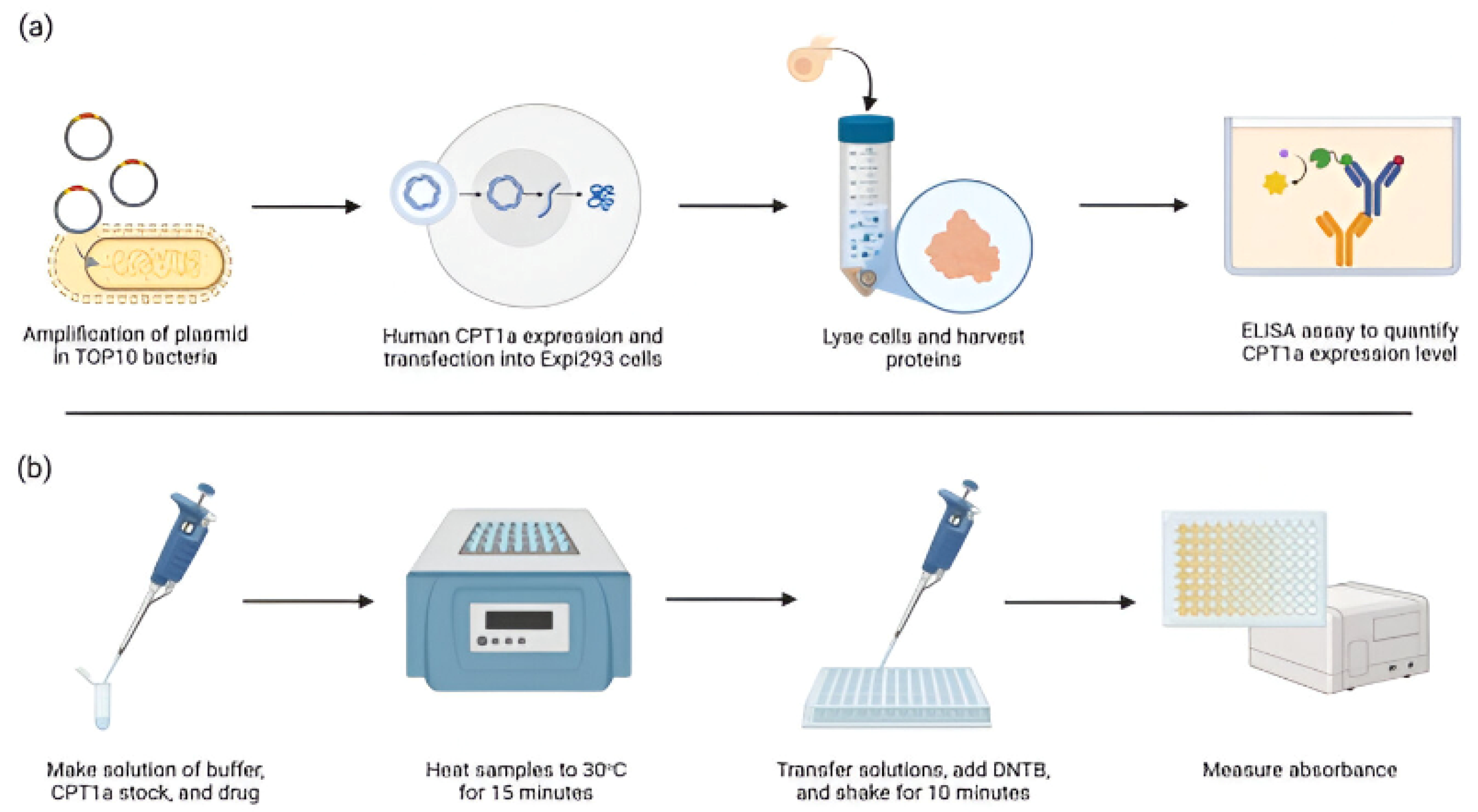
2.2. Human CPT1a Enzyme Expression
2.3. Immunoassay to Quantify CPT1a
2.4. CPT Enzyme Activity Assay and Inhibitor Sensitivity Assay
2.5. High-Throughput Screening of a Small Molecule Library
2.6. Data Analysis
3. Results
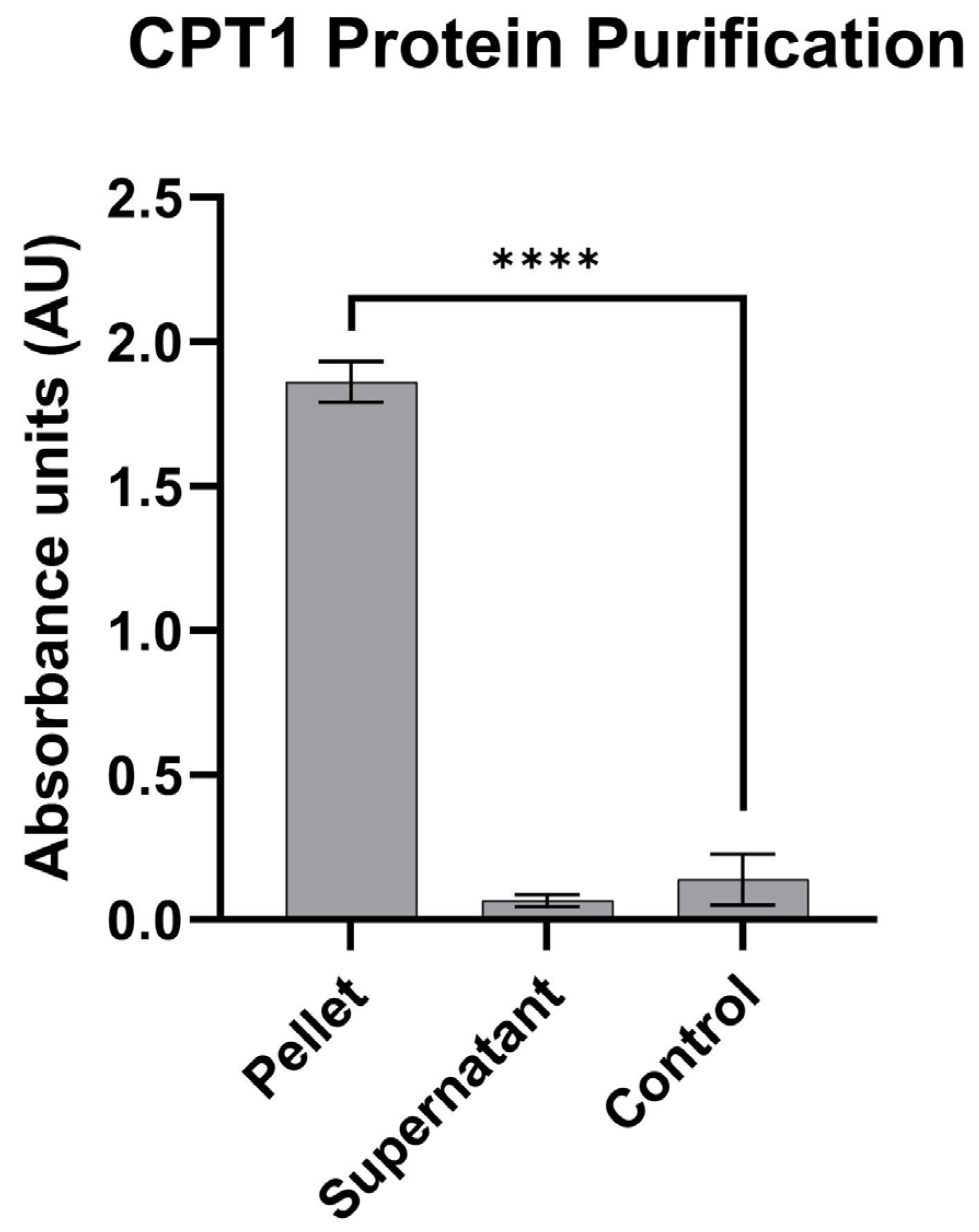
3.1. Human CPT1a Enzyme Expression and Protein Isolation
3.2. CPT1a Immunogenicity Assay
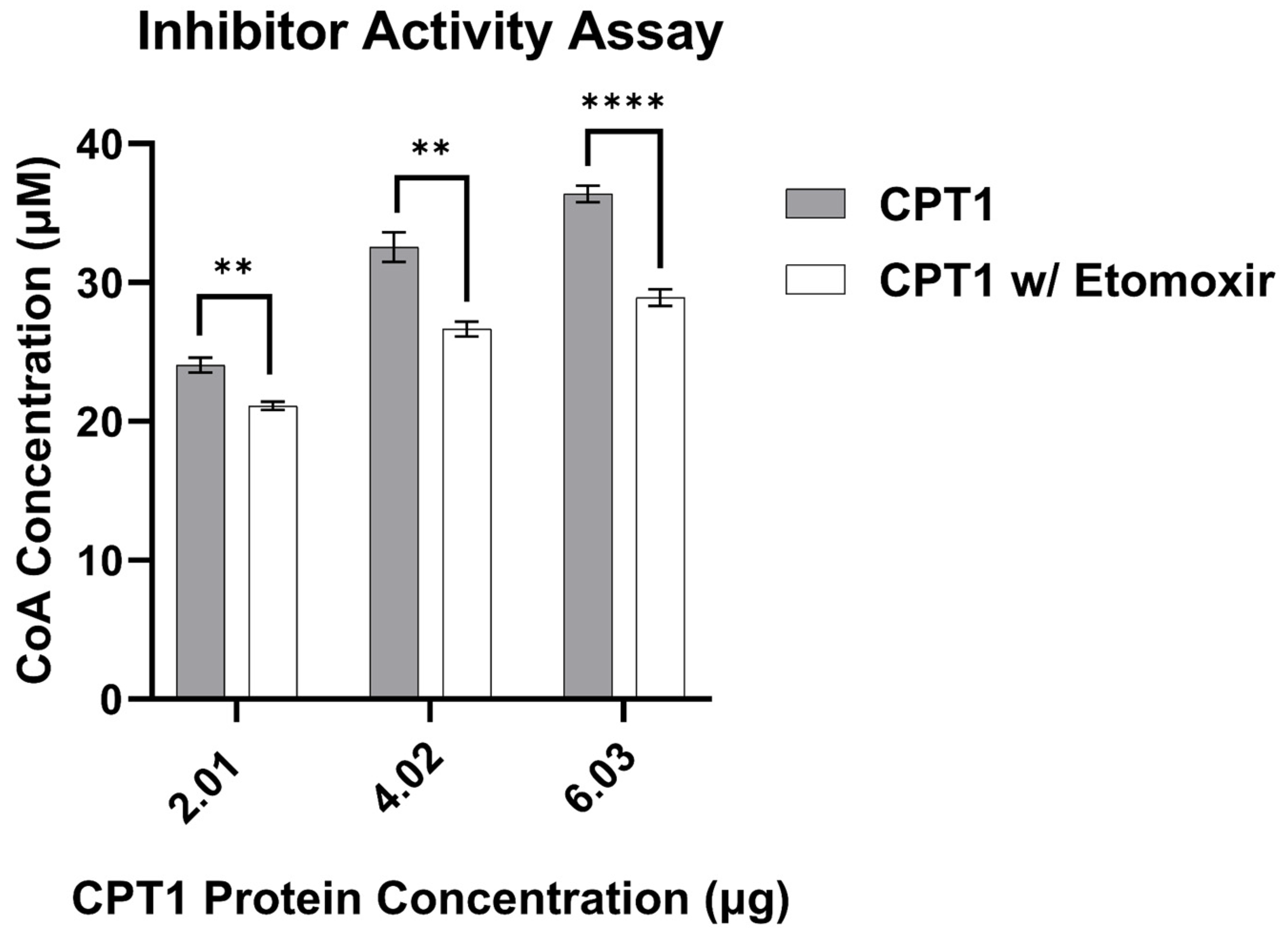
3.3. CPT1 Enzyme Activity Assay Validation
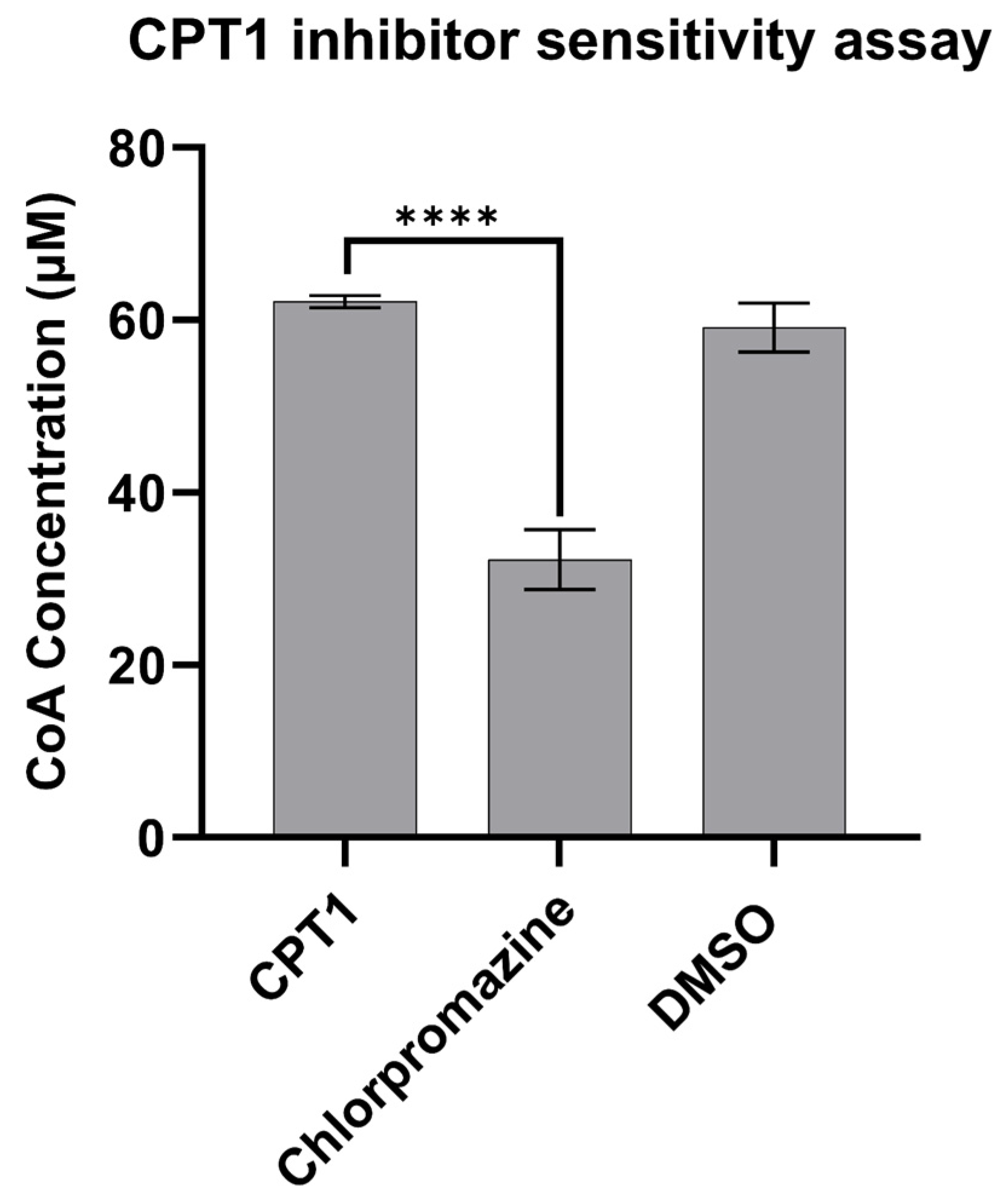
3.4. CPT1 Enzyme Inhibitor Sensitivity Assay
3.5. Application and Performance Qualification to Screen Small Molecule Compounds
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, Y.; Temkin, S.M.; Hawkridge, A.M.; Guo, C.; Wang, W.; Wang, X.-Y.; Fang, X. Fatty Acid Oxidation: An Emerging Facet of Metabolic Transformation in Cancer. Cancer Lett. 2018, 435, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiang, H.; Lu, Y.; Wu, T.; Ji, G. The Role and Therapeutic Implication of CPTs in Fatty Acid Oxidation and Cancers Progression. Am. J. Cancer Res. 2021, 11, 2477–2494. [Google Scholar] [PubMed]
- Roden, M.; Bernroider, E. Hepatic Glucose Metabolism in Humans—Its Role in Health and Disease. Best. Pract. Res. Clin. Endocrinol. Metab. 2003, 17, 365–383. [Google Scholar] [CrossRef]
- Park, E.A.; Mynatt, R.L.; Cook, G.A.; Kashfi, K. Insulin Regulates Enzyme Activity, Malonyl-CoA Sensitivity and MRNA Abundance of Hepatic Carnitine Palmitoyltransferase-I. Biochem. J. 1995, 310, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Guo, M.; Yang, F.; Han, T.; Du, W.; Zhao, F.; Li, J.; Li, W.; Feng, Y.; Wang, S.; et al. Association of CPT1A Gene Polymorphism with the Risk of Gestational Diabetes Mellitus: A Case-Control Study. J. Assist. Reprod. Genet. 2021, 38, 1861–1869. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, J.; Li, A.; Liu, C.; Shu, G.; Yin, G. The Role of the CPT Family in Cancer: Searching for New Therapeutic Strategies. Biol. 2024, 13, 892. [Google Scholar] [CrossRef]
- Sadeghi, R.N.; Karami-Tehrani, F.; Salami, S. Targeting Prostate Cancer Cell Metabolism: Impact of Hexokinase and CPT-1 Enzymes. Tumor Biol. 2015, 36, 2893–2905. [Google Scholar] [CrossRef]
- Thupari, J.N.; Pinn, M.L.; Kuhajda, F.P. Fatty Acid Synthase Inhibition in Human Breast Cancer Cells Leads to Malonyl-CoA-Induced Inhibition of Fatty Acid Oxidation and Cytotoxicity. Biochem. Biophys. Res. Commun. 2001, 285, 217–223. [Google Scholar] [CrossRef]
- Joshi, M.; Kim, J.; D’Alessandro, A.; Monk, E.; Bruce, K.; Elajaili, H.; Nozik-Grayck, E.; Goodspeed, A.; Costello, J.C.; Schlaepfer, I.R. CPT1A Over-Expression Increases Reactive Oxygen Species in the Mitochondria and Promotes Antioxidant Defenses in Prostate Cancer. Cancers 2020, 12, 3431. [Google Scholar] [CrossRef]
- Soler-Vázquez, M.C.; del Mar Romero, M.; Todorcevic, M.; Delgado, K.; Calatayud, C.; Benitez-Amaro, A.; La Chica Lhoëst, M.T.; Mera, P.; Zagmutt, S.; Bastías-Pérez, M.; et al. Implantation of CPT1AM-Expressing Adipocytes Reduces Obesity and Glucose Intolerance in Mice. Metab. Eng. 2023, 77, 256–272. [Google Scholar] [CrossRef]
- Orellana-Gavaldà, J.M.; Herrero, L.; Malandrino, M.I.; Pañeda, A.; Sol Rodríguez-Peña, M.; Petry, H.; Asins, G.; Van Deventer, S.; Hegardt, F.G.; Serra, D. Molecular Therapy for Obesity and Diabetes Based on a Long-Term Increase in Hepatic Fatty-Acid Oxidation §Δ. Hepatology 2011, 53, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Calle, P.; Muñoz, A.; Sola, A.; Hotter, G. CPT1a Gene Expression Reverses the Inflammatory and Anti-Phagocytic Effect of 7-Ketocholesterol in RAW264.7 Macrophages. Lipids Health Dis. 2019, 18, 215. [Google Scholar] [CrossRef] [PubMed]
- Malandrino, M.I.; Fucho, R.; Weber, M.; Calderon-Dominguez, M.; Mir, J.F.; Valcarcel, L.; Escoté, X.; Gómez-Serrano, M.; Peral, B.; Salvadó, L.; et al. Enhanced Fatty Acid Oxidation in Adipocytes and Macrophages Reduces Lipid-Induced Triglyceride Accumulation and Inflammation. Am. J. Physiol.-Endocrinol. Metab. 2015, 308, E756–E769. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, K.; Liao, X.; Hu, H.; Chen, L.; Meng, L.; Gao, W.; Li, Q. Carnitine Palmitoyltransferase System: A New Target for Anti-Inflammatory and Anticancer Therapy? Front. Pharmacol. 2021, 12, 760581. [Google Scholar] [CrossRef]
- Jogl, G.; Hsiao, Y.; Tong, L. Structure and Function of Carnitine Acyltransferases. Ann. N. Y. Acad. Sci. 2004, 1033, 17–29. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Gandour, R.D.; van der Leij, F.R. Molecular Enzymology of Carnitine Transfer and Transport. Biochim. Biophys. Acta (BBA)—Protein Struct. Mol. Enzymol. 2001, 1546, 21–43. [Google Scholar] [CrossRef]
- Kim, C.S.; Hoppel, C.L. Carnitine Palmitoyltransferase Activity in the Rabbit Choroid Plexus: Its Possible Function in Fatty Acid Metabolism and Transport. Neurosci. Lett. 1992, 140, 13–15. [Google Scholar] [CrossRef]
- Liang, K. Mitochondrial CPT1A: Insights into Structure, Function, and Basis for Drug Development. Front. Pharmacol. 2023, 14, 1160440. [Google Scholar] [CrossRef]
- Bonnefont, J. Carnitine Palmitoyltransferases 1 and 2: Biochemical, Molecular and Medical Aspects. Mol. Asp. Med. 2004, 25, 495–520. [Google Scholar] [CrossRef]
- Schlaepfer, I.R.; Joshi, M. CPT1A-Mediated Fat Oxidation, Mechanisms, and Therapeutic Potential. Endocrinology 2020, 161, bqz046. [Google Scholar] [CrossRef]
- Foster, D.W. Malonyl-CoA: The Regulator of Fatty Acid Synthesis and Oxidation. J. Clin. Investig. 2012, 122, 1958–1959. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.A. Differences in the Sensitivity of Carnitine Palmitoyltransferase to Inhibition by Malonyl-CoA Are Due to Differences in Ki Values. J. Biol. Chem. 1984, 259, 12030–12033. [Google Scholar] [CrossRef] [PubMed]
- Warfel, J.D.; Vandanmagsar, B.; Dubuisson, O.S.; Hodgeson, S.M.; Elks, C.M.; Ravussin, E.; Mynatt, R.L. Examination of Carnitine Palmitoyltransferase 1 Abundance in White Adipose Tissue: Implications in Obesity Research. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R816–R820. [Google Scholar] [CrossRef]
- Rufer, A.C.; Thoma, R.; Benz, J.; Stihle, M.; Gsell, B.; De Roo, E.; Banner, D.W.; Mueller, F.; Chomienne, O.; Hennig, M. The Crystal Structure of Carnitine Palmitoyltransferase 2 and Implications for Diabetes Treatment. Structure 2006, 14, 713–723. [Google Scholar] [CrossRef]
- Hsiao, Y.-S.; Jogl, G.; Esser, V.; Tong, L. Crystal Structure of Rat Carnitine Palmitoyltransferase II (CPT-II). Biochem. Biophys. Res. Commun. 2006, 346, 974–980. [Google Scholar] [CrossRef][Green Version]
- Rodríguez-Rodríguez, R.; Fosch, A.; Garcia-Chica, J.; Zagmutt, S.; Casals, N. Targeting Carnitine Palmitoyltransferase 1 Isoforms in the Hypothalamus: A Promising Strategy to Regulate Energy Balance. J. Neuroendocrinol. 2023, 35, e13234. [Google Scholar] [CrossRef]
- Brown, N.F.; Weis, B.C.; Husti, J.E.; Foster, D.W.; McGarry, J.D. Mitochondrial Carnitine Palmitoyltransferase I Isoform Switching in the Developing Rat Heart. J. Biol. Chem. 1995, 270, 8952–8957. [Google Scholar] [CrossRef]
- Price, N.T.; van der Leij, F.R.; Jackson, V.N.; Corstorphine, C.G.; Thomson, R.; Sorensen, A.; Zammit, V.A. A Novel Brain-Expressed Protein Related to Carnitine Palmitoyltransferase I. Genomics 2002, 80, 433–442. [Google Scholar] [CrossRef]
- Shetty, S.S.; Kumari, S. Fatty Acids and Their Role in Type-2 Diabetes (Review). Exp. Ther. Med. 2021, 22, 706. [Google Scholar] [CrossRef]
- Chen, M.; Huang, J. The Expanded Role of Fatty Acid Metabolism in Cancer: New Aspects and Targets. Precis. Clin. Med. 2019, 2, 183–191. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Reprogramming of Fatty Acid Metabolism in Cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar] [CrossRef]
- Wakil, S.J.; Abu-Elheiga, L.A. Fatty Acid Metabolism: Target for Metabolic Syndrome. J. Lipid Res. 2009, 50, S138–S143. [Google Scholar] [CrossRef]
- Massart, J.; Begriche, K.; Buron, N.; Porceddu, M.; Borgne-Sanchez, A.; Fromenty, B. Drug-Induced Inhibition of Mitochondrial Fatty Acid Oxidation and Steatosis. Curr. Pathobiol. Rep. 2013, 1, 147–157. [Google Scholar] [CrossRef]
- Mørkholt, A.S.; Oklinski, M.K.; Larsen, A.; Bockermann, R.; Issazadeh-Navikas, S.; Nieland, J.G.K.; Kwon, T.-H.; Corthals, A.; Nielsen, S.; Nieland, J.D.V. Pharmacological Inhibition of Carnitine Palmitoyl Transferase 1 Inhibits and Reverses Experimental Autoimmune Encephalitis in Rodents. PLoS ONE 2020, 15, e0234493. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Wang, H.; Xu, Q.; Pan, Y.; Jiang, Z.; Li, S.; Qu, Y.; Hu, Y.; Wu, H.; Wang, X. A Novel CPT1A Covalent Inhibitor Modulates Fatty Acid Oxidation and CPT1A-VDAC1 Axis with Therapeutic Potential for Colorectal Cancer. Redox Biol. 2023, 68, 102959. [Google Scholar] [CrossRef] [PubMed]
- McGuffee, R.M.; McCommis, K.S.; Ford, D.A. Etomoxir: An Old Dog with New Tricks. J. Lipid Res. 2024, 65, 100604. [Google Scholar] [CrossRef]
- Ratheiser, K.; Schneeweif, B.; Waldhausl, W.; Nowotny, P. Inhibition by Etomoxir of Carnitine Palmitoyltransferase I Reduces Hepatic Glucose Production and Plasma Lipids in Non-Insulin-Dependent Diabetes Mellitus. Metabolism 1991, 40, 1185–1190. [Google Scholar] [CrossRef]
- Bristow, M. Etomoxir: A New Approach to Treatment of Chronic Heart Failure. Lancet 2000, 356, 1621–1622. [Google Scholar] [CrossRef]
- Holubarsch, C.J.F.; Rohrbach, M.; Karrasch, M.; Boehm, E.; Polonski, L.; Ponikowski, P.; Rhein, S. A Double-Blind Randomized Multicentre Clinical Trial to Evaluate the Efficacy and Safety of Two Doses of Etomoxir in Comparison with Placebo in Patients with Moderate Congestive Heart Failure: The ERGO (Etomoxir for the Recovery of Glucose Oxidation) Study. Clin. Sci. 2007, 113, 205–212. [Google Scholar] [CrossRef]
- Conti, R.; Mannucci, E.; Pessotto, P.; Tassoni, E.; Carminati, P.; Giannessi, F.; Arduini, A. Selective Reversible Inhibition of Liver Carnitine Palmitoyl-Transferase 1 by Teglicar Reduces Gluconeogenesis and Improves Glucose Homeostasis. Diabetes 2011, 60, 644–651. [Google Scholar] [CrossRef]
- Giannessi, F.; Pessotto, P.; Tassoni, E.; Chiodi, P.; Conti, R.; De Angelis, F.; Dell’Uomo, N.; Catini, R.; Deias, R.; Tinti, M.O.; et al. Discovery of a Long-Chain Carbamoyl Aminocarnitine Derivative, a Reversible Carnitine Palmitoyltransferase Inhibitor with Antiketotic and Antidiabetic Activity. J. Med. Chem. 2003, 46, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Dai, J.-Y. Progress of Potential Drugs Targeted in Lipid Metabolism Research. Front. Pharmacol. 2022, 13, 1067652. [Google Scholar] [CrossRef] [PubMed]
- Gugiatti, E.; Tenca, C.; Ravera, S.; Fabbi, M.; Ghiotto, F.; Mazzarello, A.N.; Bagnara, D.; Reverberi, D.; Zarcone, D.; Cutrona, G.; et al. A Reversible Carnitine Palmitoyltransferase (CPT1) Inhibitor Offsets the Proliferation of Chronic Lymphocytic Leukemia Cells. Haematologica 2018, 103, e531–e536. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, M.R.; Mirabilii, S.; Allegretti, M.; Licchetta, R.; Calarco, A.; Torrisi, M.R.; Foà, R.; Nicolai, R.; Peluso, G.; Tafuri, A. Targeting the Leukemia Cell Metabolism by the CPT1a Inhibition: Functional Preclinical Effects in Leukemias. Blood 2015, 126, 1925–1929. [Google Scholar] [CrossRef]
- Ashrafian, H.; Horowitz, J.D.; Frenneaux, M.P. Perhexiline. Cardiovasc. Drug Rev. 2007, 25, 76–97. [Google Scholar] [CrossRef]
- Dhakal, B.; Tomita, Y.; Drew, P.; Price, T.; Maddern, G.; Smith, E.; Fenix, K. Perhexiline: Old Drug, New Tricks? A Summary of Its Anti-Cancer Effects. Molecules 2023, 28, 3624. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Unger, S.A.; Horowitz, J.D. Inhibition of Carnitine Palmitoyltransferase-1 in Rat Heart and Liver by Perhexiline and Amiodarone. Biochem. Pharmacol. 1996, 52, 273–280. [Google Scholar] [CrossRef]
- Kargman, S.; Wong, E.; Greig, G.M.; Falgueyret, J.-P.; Cromlish, W.; Ethier, D.; Yergey, J.A.; Riendeau, D.; Evans, J.F.; Kennedy, B.; et al. Mechanism of Selective Inhibition of Human Prostaglandin G/H Synthase-1 and -2 in Intact Cells. Biochem. Pharmacol. 1996, 52, 1113–1125. [Google Scholar] [CrossRef]
- Wang, X.J.; Song, Z.G.; Jiao, H.C.; Lin, H. Dexamethasone Facilitates Lipid Accumulation in Chicken Skeletal Muscle. Stress 2012, 15, 443–456. [Google Scholar] [CrossRef]
- Xu, L.; Xia, H.; Ni, D.; Hu, Y.; Liu, J.; Qin, Y.; Zhou, Q.; Yi, Q.; Xie, Y. High-Dose Dexamethasone Manipulates the Tumor Microenvironment and Internal Metabolic Pathways in Anti-Tumor Progression. Int. J. Mol. Sci. 2020, 21, 1846. [Google Scholar] [CrossRef]
- Ceccarelli, S.M.; Chomienne, O.; Gubler, M.; Arduini, A. Carnitine Palmitoyltransferase (CPT) Modulators: A Medicinal Chemistry Perspective on 35 Years of Research. J. Med. Chem. 2011, 54, 3109–3152. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhu, X.; Yu, W.; Zhang, Y.; Yang, K.; Liu, Z.; Qiao, X.; Song, Y. A Mini Review of Small-Molecule Inhibitors Targeting Palmitoyltransferases. Eur. J. Med. Chem. Rep. 2022, 5, 100041. [Google Scholar] [CrossRef]
- Sabbah, H.N. Biologic Rationale for the Use of Beta-Blockers in the Treatment of Heart Failure. Heart Fail. Rev. 2004, 9, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Dhillon, P.; Wambolt, R.; Parsons, H.; Brownsey, R.; Allard, M.F.; McNeill, J.H. Metoprolol Improves Cardiac Function and Modulates Cardiac Metabolism in the Streptozotocin-Diabetic Rat. Am. J. Physiol.-Heart Circ. Physiol. 2008, 294, H1609–H1620. [Google Scholar] [CrossRef]
- Hamdan, M.; Urien, S.; Le Louet, H.; Tillement, J.-P.; Morin, D. Inhibition of Mitochondrial Carnitine Palmitoyltransferase-1 by a Trimetazidine Derivative, S-15176. Pharmacol. Res. 2001, 44, 99–104. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Liu, C.; Wang, Y.; Zhang, W.; Xing, Y. Trimetazidine and L-carnitine Prevent Heart Aging and Cardiac Metabolic Impairment in Rats via Regulating Cardiac Metabolic Substrates. Exp. Gerontol. 2019, 119, 120–127. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Horowitz, J.D. Effect of Trimetazidine on Carnitine Palmitoyltransferase-1 in the Rat Heart. Cardiovasc. Drugs Ther. 1998, 12, 359–363. [Google Scholar] [CrossRef]
- Stephens, T.W.; Higgins, A.J.; Cook, G.A.; Harris, R.A. Two Mechanisms Produce Tissue-Specific Inhibition of Fatty Acid Oxidation by Oxfenicine. Biochem. J. 1985, 227, 651–660. [Google Scholar] [CrossRef]
- Maarman, G.; Marais, E.; Lochner, A.; du Toit, E.F. Effect of Chronic CPT-1 Inhibition on Myocardial Ischemia-Reperfusion Injury (I/R) in a Model of Diet-Induced Obesity. Cardiovasc. Drugs Ther. 2012, 26, 205–216. [Google Scholar] [CrossRef]
- Bielefeld, D.; Vary, T.; Neely, J. Inhibition of Carnitine Palmitoyl-CoA Transferase Activity and Fatty Acid Oxidation by Lactate and Oxfenicine in Cardiac Muscle. J. Mol. Cell Cardiol. 1985, 17, 619–625. [Google Scholar] [CrossRef]
- Cambridge, G.; Stern, C.M. The Uptake of Tritium-Labelled Carnitine by Monolayer Cultures of Human Fetal Muscle and Its Potential as a Label in Cytotoxicity Studies. Clin. Exp. Immunol. 1981, 44, 211–219. [Google Scholar] [PubMed]
- Guzmán, M.; Geelen, M.J.H. Activity of Carnitine Palmitoyltransferase in Mitochondrial Outer Membranes and Peroxisomes in Digitonin-Permeabilized Hepatocytes. Selective Modulation of Mitochondrial Enzyme Activity by Okadaic Acid. Biochem. J. 1992, 287, 487–492. [Google Scholar] [CrossRef] [PubMed]
- McGarry, J.D.; Leatherman, G.F.; Foster, D.W. Carnitine Palmitoyltransferase I. The Site of Inhibition of Hepatic Fatty Acid Oxidation by Malonyl-CoA. J. Biol. Chem. 1978, 253, 4128–4136. [Google Scholar] [CrossRef]
- Bruce, C.R.; Brolin, C.; Turner, N.; Cleasby, M.E.; van der Leij, F.R.; Cooney, G.J.; Kraegen, E.W. Overexpression of Carnitine Palmitoyltransferase I in Skeletal Muscle in Vivo Increases Fatty Acid Oxidation and Reduces Triacylglycerol Esterification. Am. J. Physiol.-Endocrinol. Metab. 2007, 292, E1231–E1237. [Google Scholar] [CrossRef]
- McGarry, J.D.; Mills, S.E.; Long, C.S.; Foster, D.W. Observations on the Affinity for Carnitine, and Malonyl-CoA Sensitivity, of Carnitine Palmitoyltransferase I in Animal and Human Tissues. Demonstration of the Presence of Malonyl-CoA in Non-Hepatic Tissues of the Rat. Biochem. J. 1983, 214, 21–28. [Google Scholar] [CrossRef]
- Bruce, C.R.; Hoy, A.J.; Turner, N.; Watt, M.J.; Allen, T.L.; Carpenter, K.; Cooney, G.J.; Febbraio, M.A.; Kraegen, E.W. Overexpression of Carnitine Palmitoyltransferase-1 in Skeletal Muscle Is Sufficient to Enhance Fatty Acid Oxidation and Improve High-Fat Diet-Induced Insulin Resistance. Diabetes 2009, 58, 550–558. [Google Scholar] [CrossRef]
- Kim, S.-W.; Roh, J.; Park, C.-S. Immunohistochemistry for Pathologists: Protocols, Pitfalls, and Tips. J. Pathol. Transl. Med. 2016, 50, 411–418. [Google Scholar] [CrossRef]
- Draper, J.M.; Smith, C.D. Palmitoyl Acyltransferase Assays and Inhibitors (Review). Mol. Membr. Biol. 2009, 26, 5–13. [Google Scholar] [CrossRef]
- Bieber, L.L.; Abraham, T.; Helmrath, T. A Rapid Spectrophotometric Assay for Carnitine Palmitoyltransferase. Anal. Biochem. 1972, 50, 509–518. [Google Scholar] [CrossRef]
- Hassett, R.P.; Crockett, E.L. Endpoint Fluorometric Assays for Determining Activities of Carnitine Palmitoyltransferase and Citrate Synthase. Anal. Biochem. 2000, 287, 176–179. [Google Scholar] [CrossRef]
- Tang, M.; Dong, X.; Xiao, L.; Tan, Z.; Luo, X.; Yang, L.; Li, W.; Shi, F.; Li, Y.; Zhao, L.; et al. CPT1A-Mediated Fatty Acid Oxidation Promotes Cell Proliferation via Nucleoside Metabolism in Nasopharyngeal Carcinoma. Cell Death Dis. 2022, 13, 331. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Xiao, L.; Tang, M.; Bai, F.; Li, J.; Li, L.; Shi, F.; Li, N.; Li, Y.; Du, Q.; et al. Targeting CPT1A-Mediated Fatty Acid Oxidation Sensitizes Nasopharyngeal Carcinoma to Radiation Therapy. Theranostics 2018, 8, 2329–2347. [Google Scholar] [CrossRef] [PubMed]
- Faye, A.; Borthwick, K.; Esnous, C.; Price, N.T.; Gobin, S.; Jackson, V.N.; Zammit, V.A.; Girard, J.; Prip-Buus, C. Demonstration of N- and C-Terminal Domain Intramolecular Interactions in Rat Liver Carnitine Palmitoyltransferase 1 That Determine Its Degree of Malonyl-CoA Sensitivity. Biochem. J. 2005, 387 Pt 1, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Pucci, S.; Zonetti, M.J.; Fisco, T.; Polidoro, C.; Bocchinfuso, G.; Palleschi, A.; Novelli, G.; Spagnoli, L.G.; Mazzarelli, P. Carnitine Palmitoyl Transferase-1A (CPT1A): A New Tumor Specific Target in Human Breast Cancer. Oncotarget 2016, 7, 19982–19996. [Google Scholar] [CrossRef] [PubMed]
- Zierz, S.; Neumann-Schmidt, S. Inhibition of Carnitine Palmitoyltransferase (CPT) by Chlorpromazine in Muscle of Patients with CPT Deficiency. J. Neurol. 1989, 236, 251–252. [Google Scholar] [CrossRef]
- Chen, Z.; Cui, F.; Meng, L.; Chen, G.; Li, Z.; Ma, Z.; Woldegiorgis, G. Carnitine Palmitoyltransferase Inhibitor in Diabetes. J. Mol. Genet. Med. 2016, 10, 1747-0862. [Google Scholar] [CrossRef]
- Liao, P.-C.; Bergamini, C.; Fato, R.; Pon, L.A.; Pallotti, F. Isolation of Mitochondria from Cells and Tissues. Methods Cell Biol. 2020, 155, 3–31. [Google Scholar] [CrossRef]
- Raud, B.; Roy, D.G.; Divakaruni, A.S.; Tarasenko, T.N.; Franke, R.; Ma, E.H.; Samborska, B.; Hsieh, W.Y.; Wong, A.H.; Stüve, P.; et al. Etomoxir Actions on Regulatory and Memory T Cells Are Independent of Cpt1a-Mediated Fatty Acid Oxidation. Cell Metab. 2018, 28, 504–515. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Tran, T.; Wong, A.; Wang, L.; Annaluru, P.; Sreekanth, V.; Murthy, S.; Munjeti, L.; Park, T.; Bhat, U.; et al. Direct Expression of CPT1a Enables a High Throughput Platform for the Discovery of CPT1a Modulators. Appl. Biosci. 2025, 4, 25. https://doi.org/10.3390/applbiosci4020025
Chen J, Tran T, Wong A, Wang L, Annaluru P, Sreekanth V, Murthy S, Munjeti L, Park T, Bhat U, et al. Direct Expression of CPT1a Enables a High Throughput Platform for the Discovery of CPT1a Modulators. Applied Biosciences. 2025; 4(2):25. https://doi.org/10.3390/applbiosci4020025
Chicago/Turabian StyleChen, Jason, Tuyen Tran, Anthony Wong, Luofei Wang, Pranavi Annaluru, Vibha Sreekanth, Samika Murthy, Laasya Munjeti, Tanya Park, Utkarsh Bhat, and et al. 2025. "Direct Expression of CPT1a Enables a High Throughput Platform for the Discovery of CPT1a Modulators" Applied Biosciences 4, no. 2: 25. https://doi.org/10.3390/applbiosci4020025
APA StyleChen, J., Tran, T., Wong, A., Wang, L., Annaluru, P., Sreekanth, V., Murthy, S., Munjeti, L., Park, T., Bhat, U., Leong, G., Li, Y., Chen, S., Kong, N., Raval, R., Xie, Y., Somani, S., Bhambhani, A. M., Zhu, Z., ... Chen, Z. (2025). Direct Expression of CPT1a Enables a High Throughput Platform for the Discovery of CPT1a Modulators. Applied Biosciences, 4(2), 25. https://doi.org/10.3390/applbiosci4020025






