Deciphering the Role of the Nucleus Accumbens Shell Area on Spatial Memory Deficits Induced by Neuropathic Pain in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Surgical Procedures
2.2.1. Electrical Lesion of the NAcSh
2.2.2. Neuropathic Pain Model
2.3. Experimental Design and Behavioral Procedures
2.3.1. Spatial Alternation Task
2.3.2. Evaluation of Peripheral Mechanical Pain Responses
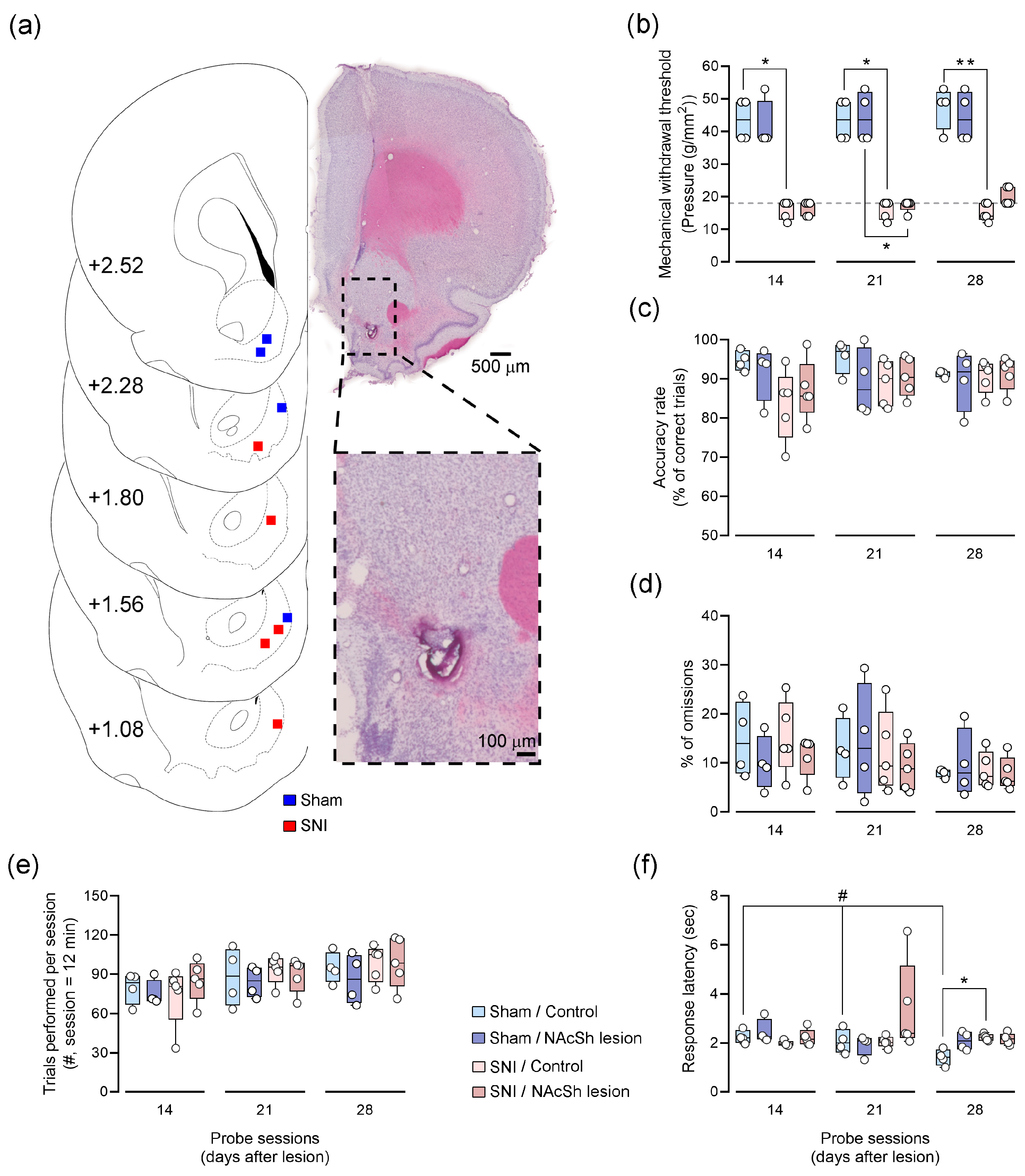
2.3.3. Anatomical and Histological Validation
2.4. Data Analysis, Representations, and Statistics
3. Results
3.1. Spatial Alternation Task Learning Phase
3.2. Impact of NAcSh Lesion on Spatial Memory and Nociception
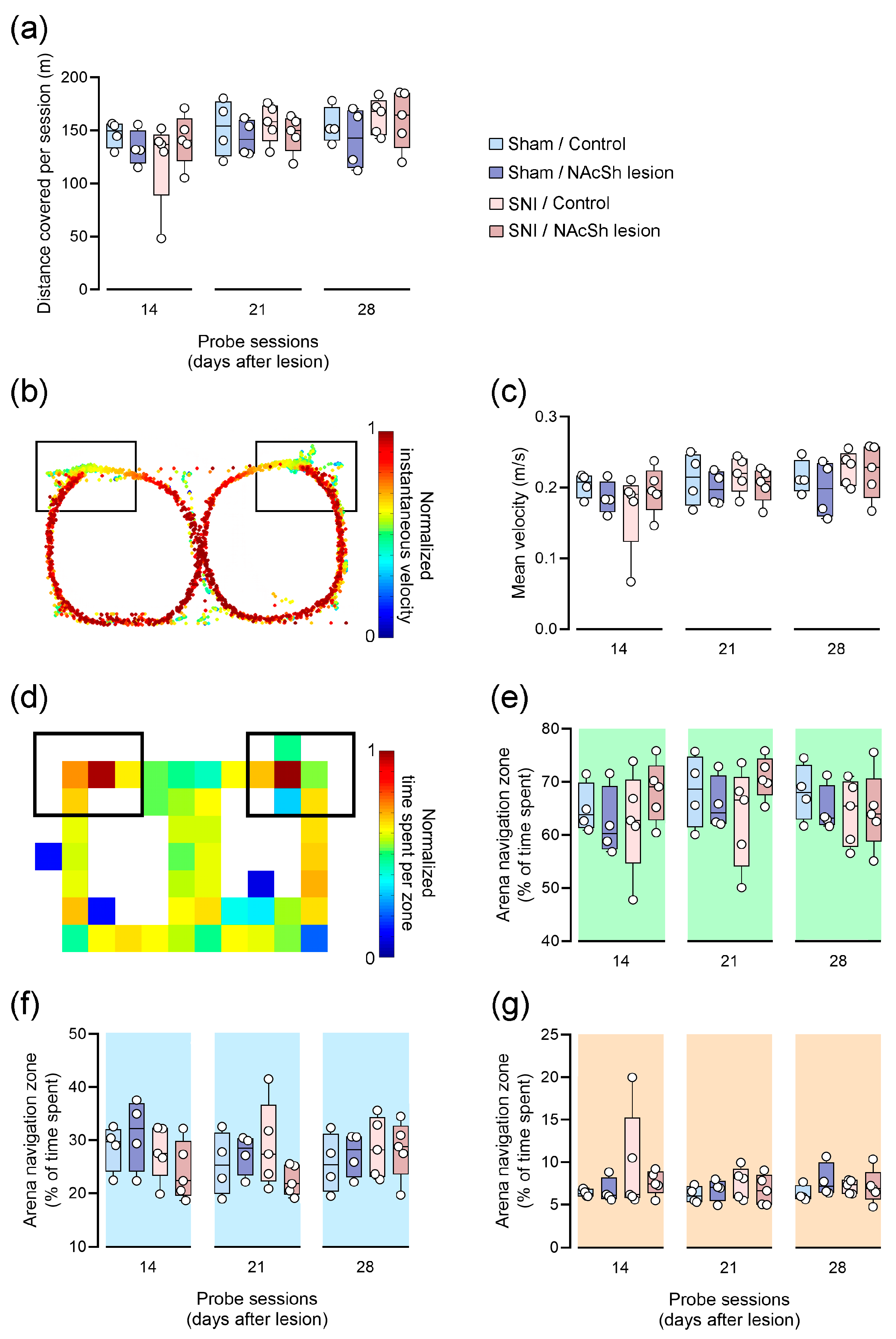
3.3. Impact of NAcSh Lesion on Motor Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- West, E.A.; Moschak, T.M.; Carelli, R.M. Distinct Functional Microcircuits in the Nucleus Accumbens Underlying Goal-Directed Decision-Making. In Goal-Directed Decision Making; Academic Press: Cambridge, MA, USA, 2018; pp. 199–219. [Google Scholar]
- Salgado, S.; Kaplitt, M.G. The nucleus accumbens: A comprehensive review. Ster. Funct. Neurosurg. 2015, 93, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Makary, M.M.; Polosecki, P.; Cecchi, G.A.; DeAraujo, I.E.; Barron, D.S.; Constable, T.R.; Whang, P.G.; Thomas, D.A.; Mowafi, H.; Small, D.M.; et al. Loss of nucleus accumbens low-frequency fluctuations is a signature of chronic pain. Proc. Natl. Acad. Sci. USA 2020, 117, 10015–10023. [Google Scholar] [CrossRef] [PubMed]
- Becerra, L.; Borsook, D. Signal valence in the nucleus accumbens to pain onset and offset. Eur. J. Pain 2008, 12, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Becerra, L.; Breiter, H.C.; Wise, R.; Gonzalez, R.; Borsook, D. Reward circuitry activation by noxious thermal stimuli. Neuron 2001, 32, 927–946. [Google Scholar] [CrossRef] [PubMed]
- Beyeler, A.; Eckhardt, C.A.; Tye, K.M. Deciphering memory function with optogenetics. Prog. Mol. Biol. Transl. Sci. 2014, 122, 341–390. [Google Scholar] [PubMed]
- Ito, R.; Robbins, T.W.; Pennartz, C.M.; Everitt, B.J. Functional Interaction between the hippocampus and nucleus accumbens shell is necessary for the acquisition of appetitive spatial context conditioning. J. Neurosci. 2008, 28, 6950–6959. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Hayen, A. Opposing roles of nucleus accumbens core and shell dopamine in the modulation of limbic information processing. J. Neurosci. 2011, 31, 6001–6007. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Beaver, J.; Halcomb, C.J.; Jasnow, A.M. Dissociable roles of the nucleus accumbens core and shell subregions in the expression and extinction of conditioned fear. Neurobiol. Stress 2021, 15, 100365. [Google Scholar] [CrossRef] [PubMed]
- Del Arco, A.; Mora, F. Prefrontal cortex–nucleus accumbens interaction: In vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacol. Biochem. Behav. 2008, 90, 226–235. [Google Scholar] [CrossRef]
- Baliki, M.N.; Petre, B.; Torbey, S.; Herrmann, K.M.; Huang, L.; Schnitzer, T.J.; Fields, H.L.; Apkarian, A.V. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat. Neurosci. 2012, 15, 1117–1119. [Google Scholar] [CrossRef]
- Baliki, M.N.; Mansour, A.R.; Baria, A.T.; Apkarian, A.V. Functional reorganization of the default mode network across chronic pain conditions. PLoS ONE 2014, 9, e106133. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-C.; Pollema-Mays, S.L.; Centeno, M.V.; Procissi, D.; Contini, M.; Baria, A.T.; Martina, M.; Apkarian, A.V. Role of nucleus accumbens in neuropathic pain: Linked multi-scale evidence in the rat transitioning to neuropathic pain. Pain 2014, 155, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Becker, S.; Schweinhardt, P.; Cahill, C. Mesolimbic dopamine signaling in acute and chronic pain: Implications for motivation, analgesia, and addiction. Pain 2016, 157, 1194. [Google Scholar] [CrossRef] [PubMed]
- Sato, D.; Narita, M.; Hamada, Y.; Mori, T.; Tanaka, K.; Tamura, H.; Yamanaka, A.; Matsui, R.; Watanabe, D.; Suda, Y.; et al. Relief of neuropathic pain by cell-specific manipulation of nucleus accumbens dopamine D1- and D2-receptor-expressing neurons. Mol. Brain 2022, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Centeno, M.V.; Berger, S.; Wu, Y.; Na, X.; Liu, X.; Kondapalli, J.; Apkarian, A.V.; Martina, M.; Surmeier, D.J. The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain. Nat. Neurosci. 2016, 19, 220–222. [Google Scholar] [CrossRef]
- Jarcho, J.M.; Mayer, E.A.; Jiang, Z.K.; Feier, N.A.; London, E.D. Pain, affective symptoms, and cognitive deficits in patients with cerebral dopamine dysfunction. Pain 2012, 153, 744–754. [Google Scholar] [CrossRef]
- Cardoso-Cruz, H.; Laranjeira, I.; Monteiro, C.; Galhardo, V. Altered prefrontal-striatal theta-band oscillatory dynamics underlie working memory deficits in neuropathic pain rats. Eur. J. Pain 2022, 26, 1546–1568. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Boudier-Revéret, M.; Choo, Y.J.; Chang, M.C. Association between chronic pain and alterations in the mesolimbic dopaminergic system. Brain Sci. 2020, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Ploeger, G.E.; BSpruijt, M.; Cools, A.R. Spatial localization in the Morris water maze in rats: Acquisition is affected by intra-accumbens injections of the dopaminergic antagonist haloperidol. Behav. Neurosci. 1994, 108, 927. [Google Scholar] [CrossRef]
- Klein, S.; Hadamitzky, M.; Koch, M.; Schwabe, K. Role of glutamate receptors in nucleus accumbens core and shell in spatial behaviour of rats. Neuroscience 2004, 128, 229–238. [Google Scholar] [CrossRef]
- Baliki, M.N.; Chang, P.C.; Baria, A.T.; Centeno, M.V.; Apkarian, A.V. Resting-state functional reorganization of the rat limbic system following neuropathic injury. Sci. Rep. 2014, 4, srep06186. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Decosterd, I.; Woolf, C.J. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain 2000, 87, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Cruz, H.; Lima, D.; Galhardo, V. Impaired spatial memory performance in a rat model of neuropathic pain is associated with reduced hippocampus–prefrontal cortex connectivity. J. Neurosci. 2013, 33, 2465–2480. [Google Scholar] [CrossRef]
- Aguiar, P.; Mendonça, L.; Galhardo, V. OpenControl: A free opensource software for video tracking and automated control of behavioral mazes. J. Neurosci. Methods 2007, 166, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Cardoso-Cruz, H.; Lima, D.; Galhardo, V. Instability of spatial encoding by CA1 hippocampal place cells after peripheral nerve injury. Eur. J. Neurosci. 2011, 33, 2255–2264. [Google Scholar] [CrossRef]
- Haber, S.N. Corticostriatal circuitry. Dialogues Clin. Neurosci. 2016, 18, 7–21. [Google Scholar] [CrossRef]
- Jongen-Rêlo, A.L.; Kaufmann, S.; Feldon, J. A differential involvement of the shell and core subterritories of the nucleus accumbens of the rats in memory processes. Behav. Neurosci. 2003, 117, 150. [Google Scholar] [CrossRef] [PubMed]
- Gal, G.; Joel, D.; Gusak, O.; Feldon, J.; Weiner, I. The effects of electrolytic lesion to the shell subterritory of the nucleus accumbens on delayed non-matching-to-sample and four-arm baited eight-arm radial-maze tasks. Behav. Neurosci. 1997, 111, 92–103. [Google Scholar] [CrossRef]
- Kerfoot, E.C.; Williams, C.L. Contributions of the Nucleus Accumbens Shell in Mediating the Enhancement in Memory Following Noradrenergic Activation of Either the Amygdala or Hippocampus. Front. Pharmacol. 2018, 9, 47. [Google Scholar] [CrossRef]
- Maldonado-Irizarry, C.S.; Kelley, A.E. Excitatory amino acid receptors within nucleus accumbens subregions differentially mediate spatial learning in the rat. Behav. Pharmacol. 1995, 6, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.-J.; Liu, Y.; Zhou, L.-J.; Li, W.; Zhong, Y.; Pang, R.-P.; Xin, W.-J.; Wei, X.-H.; Wang, J.; Zhu, H.-Q.; et al. Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of tnf-α in rodents. Neuropsychopharmacology 2011, 36, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, J.; Hu, Y.; Wang, Y.; Li, W. Amitriptyline rather than lornoxicam ameliorates neuropathic pain-induced deficits in abilities of spatial learning and memory. Eur. J. Anaesthesiol. 2010, 27, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Leite-Almeida, H.; Cerqueira, J.J.; Wei, H.; Ribeiro-Costa, N.; Anjos-Martins, H.; Sousa, N.; Pertovaara, A.; Almeida, A. Differential effects of left/right neuropathy on rats’ anxiety and cognitive behavior. Pain 2012, 153, 2218–2225. [Google Scholar] [CrossRef] [PubMed]
- Guida, F.; De Gregorio, D.; Palazzo, E.; Ricciardi, F.; Boccella, S.; Belardo, C.; Iannotta, M.; Infantino, R.; Formato, F.; Marabese, I.; et al. Behavioral, Biochemical and Electrophysiological Changes in Spared Nerve Injury Model of Neuropathic Pain. Int. J. Mol. Sci. 2020, 21, 3396. [Google Scholar] [CrossRef] [PubMed]
- Thifault, S.; Krémarik, P.; Lalonde, R. Effects of bilateral electrolytic lesions of the medial nucleus accumbens on exploration and spatial learning. Arch. Physiol. Biochem. 1998, 106, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, C.J.; Stins, J.F.; Pont, M.; Kerckhoff, F.; Beek, P.J. Effects of attention on the control of locomotion in individuals with chronic low back pain. J. Neuroeng. Rehabil. 2008, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Borsook, D. Pain and motor system plasticity. Pain 2007, 132, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Dourado, M.; Cardoso-Cruz, H.; Monteiro, C.; Galhardo, V. Effect of Motor Impairment on Analgesic Efficacy of Dopamine D2/3 Receptors in a Rat Model of Neuropathy. J. Exp. Neurosci. 2016, 10, 51–57. [Google Scholar] [CrossRef]
- Cardoso-Cruz, H.; Dourado, M.; Monteiro, C.; Matos, M.R.; Galhardo, V. Activation of dopaminergic D2/D3 receptors modulates dorsoventral connectivity in the hippocampus and reverses the impairment of working memory after nerve injury. J. Neurosci. 2014, 34, 5861–5873. [Google Scholar] [CrossRef]
- Cardoso-Cruz, H.; Sousa, M.; Vieira, J.B.; Lima, D.; Galhardo, V. Prefrontal cortex and mediodorsal thalamus reduced connectivity is associated with spatial working memory impairment in rats with inflammatory pain. Pain 2013, 154, 2397–2406. [Google Scholar] [CrossRef] [PubMed]
- Apkarian, A.V.; Baliki, M.N.; Farmer, M.A. Predicting transition to chronic pain. Curr. Opin. Neurol. 2013, 26, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Manders, T.R.; Eberle, S.E.; Su, C.; D’Amour, J.; Yang, R.; Lin, H.Y.; Deisseroth, K.; Froemke, R.C.; Wang, J. Activation of corticostriatal circuitry relieves chronic neuropathic pain. J. Neurosci. 2015, 35, 5247–5259. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.; Lin, H.H.; Zhou, H.; Dale, J.; Liu, K.; Wang, J. Corticostriatal Regulation of Acute Pain. Front. Cell. Neurosci. 2017, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Navratilova, E.; Xie, J.Y.; Meske, D.; Qu, C.; Morimura, K.; Okun, A.; Arakawa, N.; Ossipov, M.; Fields, H.L.; Porreca, F. Endogenous opioid activity in the anterior cingulate cortex is required for relief of pain. J. Neurosci. 2015, 35, 7264–7271. [Google Scholar] [CrossRef] [PubMed]
- Fields, H.L.; Margolis, E.B. Understanding opioid reward. Trends Neurosci. 2015, 38, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Gusain, C.; Bhatt, B.; Lal, R.; Bishnoi, M. Sex-specific effects of sucrose withdrawal on anxiety-like behavior and neuroimmune response. Neuropharmacology 2024, 249, 109868. [Google Scholar] [CrossRef] [PubMed]
- Douton, J.E.; Carelli, R.M. Unraveling Sex Differences in Affect Processing: Unique Oscillatory Signaling Dynamics in the Infralimbic Cortex and Nucleus Accumbens Shell. Biol. Psychiatry Glob. Open Sci. 2023, 4, 354–362. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerqueira-Nunes, M.; Monteiro, C.; Galhardo, V.; Cardoso-Cruz, H. Deciphering the Role of the Nucleus Accumbens Shell Area on Spatial Memory Deficits Induced by Neuropathic Pain in Rats. Appl. Biosci. 2024, 3, 283-295. https://doi.org/10.3390/applbiosci3020019
Cerqueira-Nunes M, Monteiro C, Galhardo V, Cardoso-Cruz H. Deciphering the Role of the Nucleus Accumbens Shell Area on Spatial Memory Deficits Induced by Neuropathic Pain in Rats. Applied Biosciences. 2024; 3(2):283-295. https://doi.org/10.3390/applbiosci3020019
Chicago/Turabian StyleCerqueira-Nunes, Mariana, Clara Monteiro, Vasco Galhardo, and Helder Cardoso-Cruz. 2024. "Deciphering the Role of the Nucleus Accumbens Shell Area on Spatial Memory Deficits Induced by Neuropathic Pain in Rats" Applied Biosciences 3, no. 2: 283-295. https://doi.org/10.3390/applbiosci3020019
APA StyleCerqueira-Nunes, M., Monteiro, C., Galhardo, V., & Cardoso-Cruz, H. (2024). Deciphering the Role of the Nucleus Accumbens Shell Area on Spatial Memory Deficits Induced by Neuropathic Pain in Rats. Applied Biosciences, 3(2), 283-295. https://doi.org/10.3390/applbiosci3020019







