Sustainable Pulse Proteins: Physical, Chemical and Fermentative Modifications
Abstract
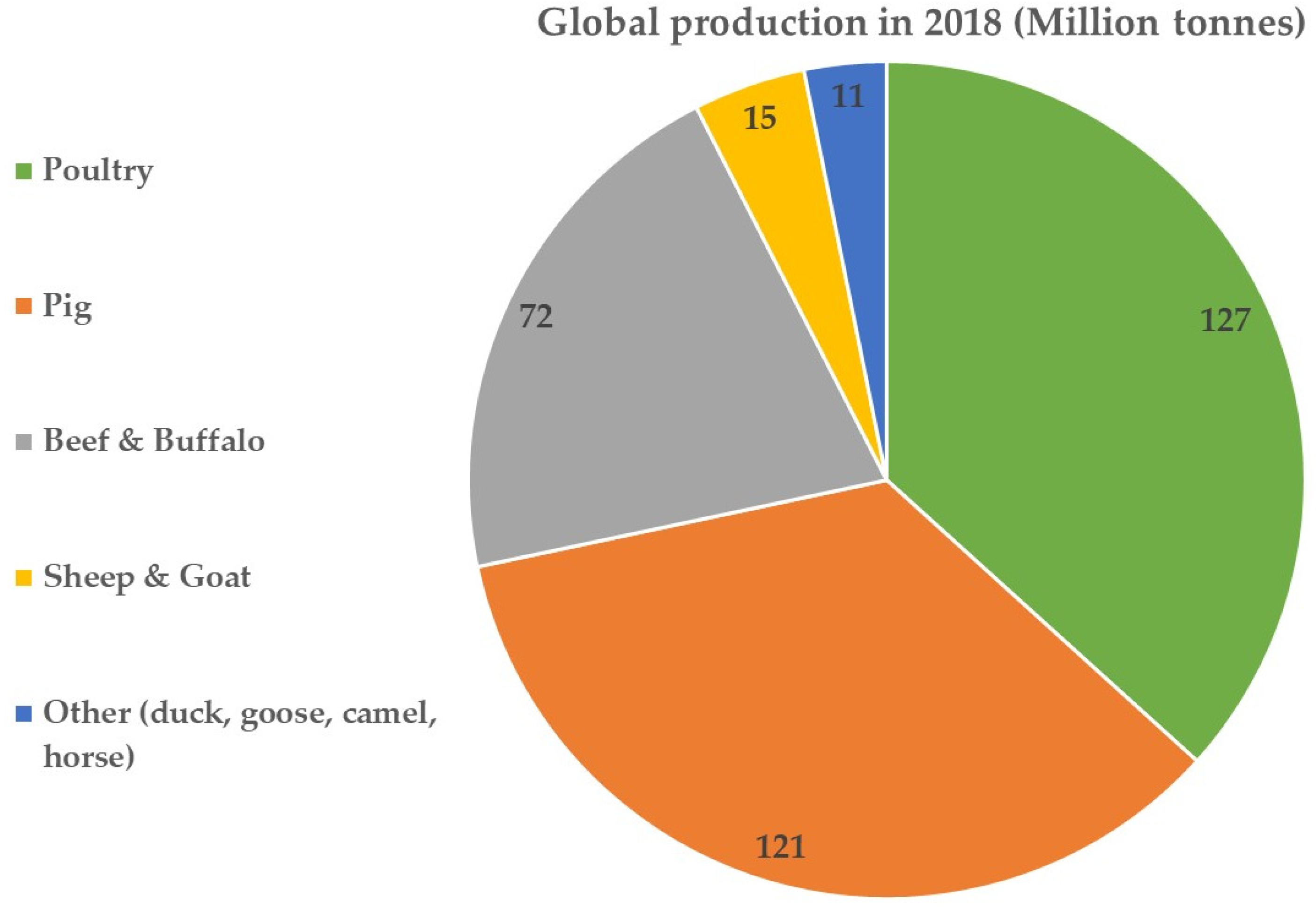
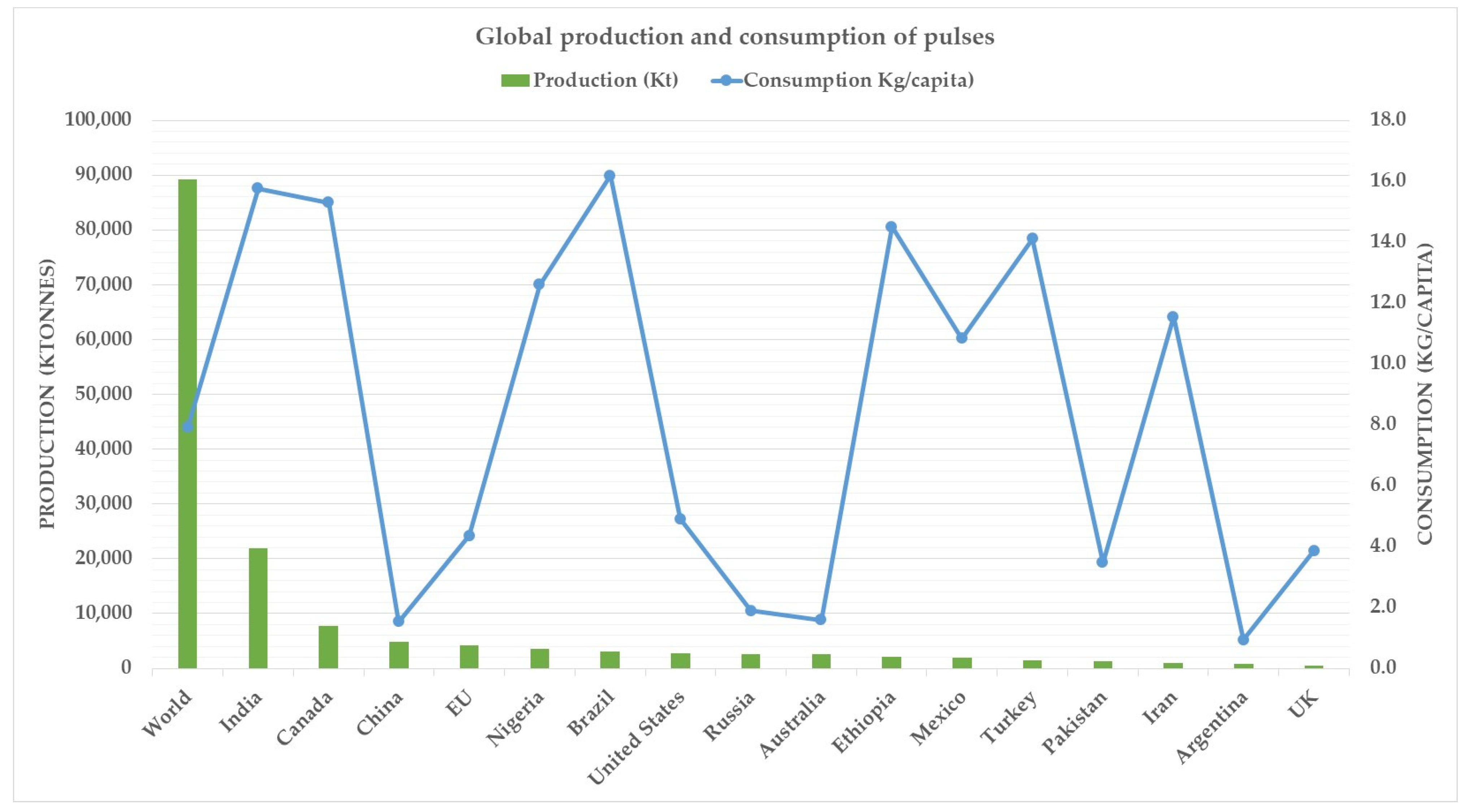
1. Introduction
2. Pulse Proteins in the Alternative Protein Space and Sustainability
2.1. Advances in Fractionation of Pulse Proteins
2.2. Pulse Protein Modification for Modulating Structure and Enhancing Functionality
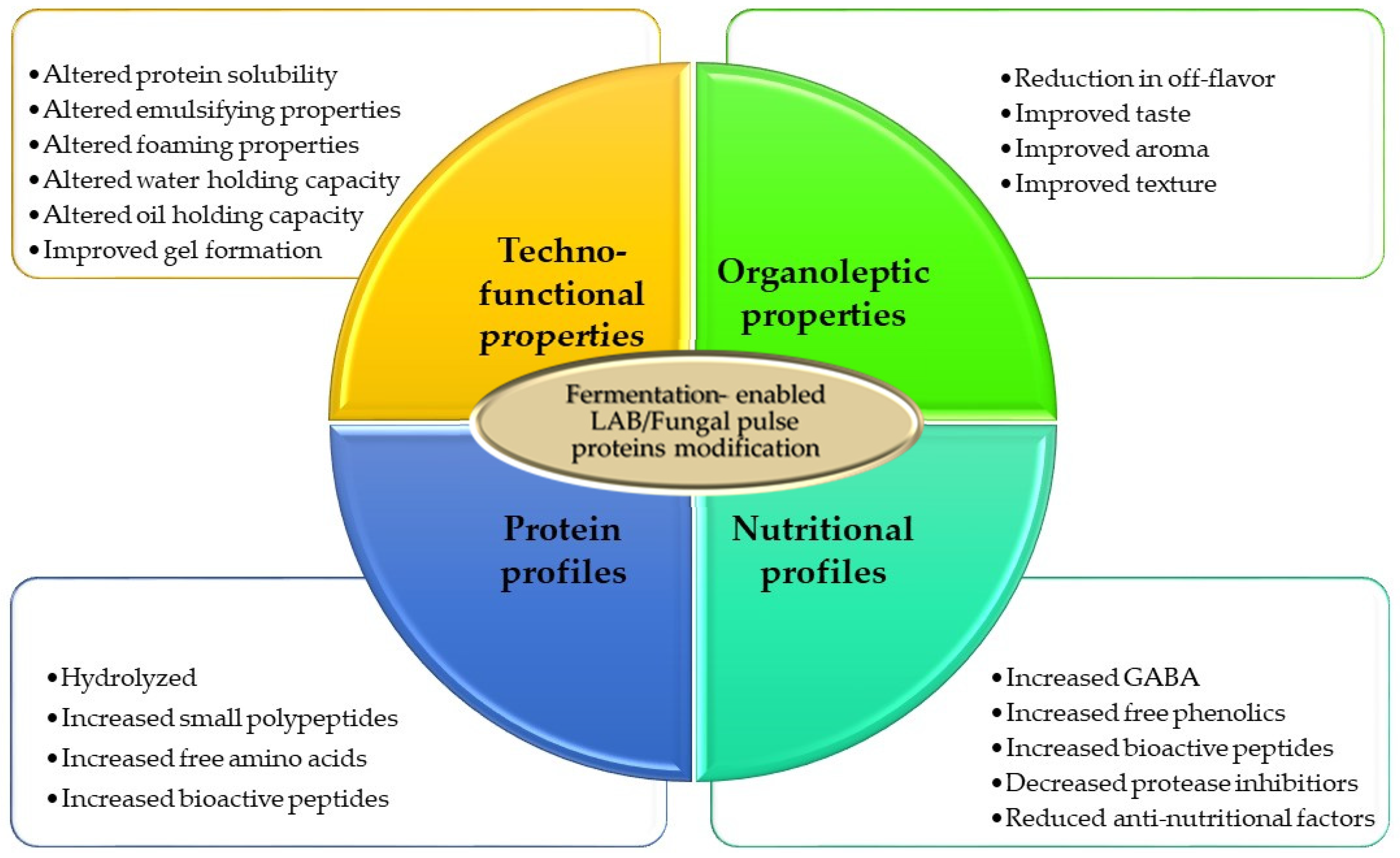
3. Fermentation-Based Pulse Protein Valorization
3.1. Lactic Acid Bacteria (LAB) Fermentation of Pulse Proteins
3.2. Fungal Fermentation of Pulse Proteins
3.3. Mixed Culture Fermentation of Pulse Proteins
4. Scale-Up Challenges in Pulse Protein Fermentation and Implementation of Sustainable Practices
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bunn, H.T.; Ezzo, J.A. Hunting and Scavenging by Plio-Pleistocene Hominids: Nutritional Constraints, Archaeological Patterns, and Behavioural Implications. J. Archaeol. Sci. 1993, 20, 365–398. [Google Scholar] [CrossRef]
- TheWorldCounts. The World Counts—Impact through Awareness. Available online: https://www.theworldcounts.com/challenges/consumption/foods-and-beverages/world-consumption-of-meat (accessed on 26 May 2024).
- FAO. The State of World Fisheries and Aquaculture. 2022. Available online: https://www.fao.org/3/cc0461en/online/sofia/2022/world-fisheries-aquaculture.html (accessed on 22 May 2024).
- Espinosa-Marrón, A.; Adams, K.; Sinno, L.; Cantu-Aldana, A.; Tamez, M.; Marrero, A.; Bhupathiraju, S.N.; Mattei, J. Environmental Impact of Animal-Based Food Production and the Feasibility of a Shift Toward Sustainable Plant-Based Diets in the United States. Front. Sustain. 2022, 3, 841106. [Google Scholar] [CrossRef]
- Ercin, A.E.; Aldaya, M.M.; Hoekstra, A.Y. The water footprint of soy milk and soy burger and equivalent animal products. Ecol. Indic. 2012, 18, 392–402. [Google Scholar] [CrossRef]
- Xu, X.; Lan, Y. A comparative study on carbon footprints between plant- and animal-based foods in China. J. Clean. Prod. 2016, 112, 2581–2592. [Google Scholar] [CrossRef]
- Poore, J.; Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I.C.F.R. Adding Molecules to Food, Pros and Cons: A Review on Synthetic and Natural Food Additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399. [Google Scholar] [CrossRef] [PubMed]
- McClements, D. Future Foods: How Modern Science Is Transforming the Way We Eat; Copernicus: Cham, Switzerland, 2019; p. 366. [Google Scholar]
- WHO. Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 22 May 2024).
- McClements, D.J.; Grossmann, L. A brief review of the science behind the design of healthy and sustainable plant-based foods. npj Sci. Food 2021, 5, 17. [Google Scholar] [CrossRef]
- Al-Heeti, A. Whole Foods CEO Says Plant-Based ‘Meat’ Is Unhealthy. Available online: https://www.cnet.com/health/nutrition/whole-foods-ceo-says-plant-based-meat-is-unhealthy/ (accessed on 5 September 2023).
- Bryant, C.J. Plant-based animal product alternatives are healthier and more environmentally sustainable than animal products. Future Foods 2022, 6, 100174. [Google Scholar] [CrossRef]
- OECD; FAO. Table C.46—Pulses Projections: Production and Food Consumption. In OECD-FAO Agricultural Outlook 2021–2030; OECD Publishing: Paris, France, 2021. [Google Scholar] [CrossRef]
- Brun, P.; Camacho, M.; Perea, F.; Rubio, M.J.; Rodríguez-Navarro, D.N. Characterization of Spanish chickpea genotypes (Cicer arietinum L.): Proximate, mineral, and phenolic compounds composition. Eur. Food Res. Technol. 2024, 250, 1007–1016. [Google Scholar] [CrossRef]
- Johnson, N.; Boatwright, J.L.; Bridges, W.; Thavarajah, P.; Kumar, S.; Thavarajah, D. Targeted improvement of plant-based protein: Genome-wide association mapping of a lentil (Lens culinaris Medik.) diversity panel. Plants People Planet 2024, 6, 640–655. [Google Scholar] [CrossRef]
- Carvalho, M.; Carnide, V.; Sobreira, C.; Castro, I.; Coutinho, J.; Barros, A.; Rosa, E. Cowpea Immature Pods and Grains Evaluation: An Opportunity for Different Food Sources. Plants 2022, 11, 2079. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, D.; Vurro, F.; Santamaria, M.; Garzon, R.; Rosell, C.M.; Summo, C.; Pasqualone, A. Effect of dry-fractionated pea protein on the physicochemical properties and the nutritional features of gluten-free focaccia flat bread. LWT 2023, 182, 114873. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Wan, Z.; Jha, A.B.; Gali, K.K.; Warkentin, T.D.; House, J.D. Influence of different amino acid scoring patterns on the protein quality of field peas. J. Food Compos. Anal. 2024, 127, 105938. [Google Scholar] [CrossRef]
- Locali-Pereira, A.R.; Caruso, Í.P.; Rabesona, H.; Laurent, S.; Meynier, A.; Kermarrec, A.; Birault, L.; Geairon, A.; Le Gall, S.; Thoulouze, L.; et al. Pre-treatment effects on the composition and functionalities of pigeon pea seed ingredients. Food Hydrocoll. 2024, 152, 109923. [Google Scholar] [CrossRef]
- Dnyaneshwar Patil, N.; Bains, A.; Kaur, S.; Yadav, R.; Ali, N.; Patil, S.; Goksen, G.; Chawla, P. Influence of dual succinylation and ultrasonication modification on the amino acid content, structural and functional properties of Chickpea (Cicer arietinum L.) protein concentrate. Food Chem. 2024, 445, 138671. [Google Scholar] [CrossRef] [PubMed]
- Matysek, J.; Baier, A.; Kalla-Bertholdt, A.-M.; Grebenteuch, S.; Rohn, S.; Rauh, C. Effect of ultrasound and fibre enrichment on aroma profile and texture characteristics of pea protein-based yoghurt alternatives. Innov. Food Sci. Emerg. Technol. 2024, 93, 103610. [Google Scholar] [CrossRef]
- Greulich, O.; Duedahl-Olesen, L.; Mikkelsen, M.S.; Smedsgaard, J.; Bang-Berthelsen, C.H. Fourier Transform Infrared Spectroscopy Tracking of Fermentation of Oat and Pea Bases for Yoghurt-Type Products. Fermentation 2024, 10, 189. [Google Scholar] [CrossRef]
- FIAL. Protein Market: Size of the Prize Analysis for Australia. Available online: https://www.fial.com.au/blogs/post/protein-market-size-of-the-prize-analysis-for-australia (accessed on 13 September 2023).
- van Zanten, H.H.E.; Mollenhorst, H.; Klootwijk, C.W.; van Middelaar, C.E.; de Boer, I.J.M. Global food supply: Land use efficiency of livestock systems. Int. J. Life Cycle Assess. 2016, 21, 747–758. [Google Scholar] [CrossRef]
- van der Spiegel, M.; Noordam, M.Y.; van der Fels-Klerx, H.J. Safety of Novel Protein Sources (Insects, Microalgae, Seaweed, Duckweed, and Rapeseed) and Legislative Aspects for Their Application in Food and Feed Production. Compr. Rev. Food Sci. Food Saf. 2013, 12, 662–678. [Google Scholar] [CrossRef]
- Borlaug, N.E. Feeding a world of 10 billion people: The miracle ahead. In Vitro Cell. Dev. Biol.-Plant 2002, 38, 221–228. [Google Scholar] [CrossRef]
- Boland, M.J.; Rae, A.N.; Vereijken, J.M.; Meuwissen, M.P.M.; Fischer, A.R.H.; van Boekel, M.A.J.S.; Rutherfurd, S.M.; Gruppen, H.; Moughan, P.J.; Hendriks, W.H. The future supply of animal-derived protein for human consumption. Trends Food Sci. Technol. 2013, 29, 62–73. [Google Scholar] [CrossRef]
- Onwezen, M.C.; Bouwman, E.P.; Reinders, M.J.; Dagevos, H. A systematic review on consumer acceptance of alternative proteins: Pulses, algae, insects, plant-based meat alternatives, and cultured meat. Appetite 2021, 159, 105058. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.; de Moura, A.P.; Cunha, L.M. Increasing Pulse Consumption to Improve Human Health and Food Security and to Mitigate Climate Change. In Climate Change-Resilient Agriculture and Agroforestry: Ecosystem Services and Sustainability; Castro, P., Azul, A.M., Leal Filho, W., Azeiteiro, U.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 21–35. [Google Scholar]
- Chaudhary, A.; Tremorin, D. Nutritional and Environmental Sustainability of Lentil Reformulated Beef Burger. Sustainability 2020, 12, 6712. [Google Scholar] [CrossRef]
- MacWilliam, S.; Parker, D.; Marinangeli, C.P.F.; Trémorin, D. A meta-analysis approach to examining the greenhouse gas implications of including dry peas (Pisum sativum L.) and lentils (Lens culinaris M.) in crop rotations in western Canada. Agric. Syst. 2018, 166, 101–110. [Google Scholar] [CrossRef]
- AGT. History of Pulses. Available online: http://www.agtfoods.com/about-pulses/history-of-pulses.html (accessed on 30 March 2024).
- Balasubramanian, P. Pulse Crops. Available online: https://www.thecanadianencyclopedia.ca/en/article/pulse-crops (accessed on 13 September 2023).
- Pelgrom, P.J.M.; Wang, J.; Boom, R.M.; Schutyser, M.A.I. Pre- and post-treatment enhance the protein enrichment from milling and air classification of legumes. J. Food Eng. 2015, 155, 53–61. [Google Scholar] [CrossRef]
- Fernando, S. Production of protein-rich pulse ingredients through dry fractionation: A review. LWT 2021, 141, 110961. [Google Scholar] [CrossRef]
- Xing, Q.; Utami, D.P.; Demattey, M.B.; Kyriakopoulou, K.; de Wit, M.; Boom, R.M.; Schutyser, M.A.I. A two-step air classification and electrostatic separation process for protein enrichment of starch-containing legumes. Innov. Food Sci. Emerg. Technol. 2020, 66, 102480. [Google Scholar] [CrossRef]
- Li, L.; Yuan, T.Z.; Setia, R.; Raja, R.B.; Zhang, B.; Ai, Y. Characteristics of pea, lentil and faba bean starches isolated from air-classified flours in comparison with commercial starches. Food Chem. 2019, 276, 599–607. [Google Scholar] [CrossRef]
- Allotey, D.K.; Kwofie, E.M.; Adewale, P.; Lam, E.; Ngadi, M. A meta-analysis of pulse-protein extraction technologies: Impact on recovery and purity. J. Food Eng. 2022, 327, 111048. [Google Scholar] [CrossRef]
- Dumoulin, L.; Jacquet, N.; Malumba, P.; Richel, A.; Blecker, C. Dry and wet fractionation of plant proteins: How a hybrid process increases yield and impacts nutritional value of faba beans proteins. Innov. Food Sci. Emerg. Technol. 2021, 72, 102747. [Google Scholar] [CrossRef]
- Schlangen, M.; Taghian Dinani, S.; Schutyser, M.A.I.; van der Goot, A.J. Dry fractionation to produce functional fractions from mung bean, yellow pea and cowpea flour. Innov. Food Sci. Emerg. Technol. 2022, 78, 103018. [Google Scholar] [CrossRef]
- Chang, L.; Lan, Y.; Bandillo, N.; Ohm, J.-B.; Chen, B.; Rao, J. Plant proteins from green pea and chickpea: Extraction, fractionation, structural characterization and functional properties. Food Hydrocoll. 2022, 123, 107165. [Google Scholar] [CrossRef]
- Tanger, C.; Engel, J.; Kulozik, U. Influence of extraction conditions on the conformational alteration of pea protein extracted from pea flour. Food Hydrocoll. 2020, 107, 105949. [Google Scholar] [CrossRef]
- Higa, F.A.; Boyd, L.; Sopiwnyk, E.; Nickerson, M.T. Effect of particle size, flour:water ratio and type of pulse on the physicochemical and functional properties of wet protein extraction. Cereal Chem. 2022, 99, 1049–1062. [Google Scholar] [CrossRef]
- Gao, Z.; Shen, P.; Lan, Y.; Cui, L.; Ohm, J.-B.; Chen, B.; Rao, J. Effect of alkaline extraction pH on structure properties, solubility, and beany flavor of yellow pea protein isolate. Food Res. Int. 2020, 131, 109045. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.G.; Speranza, P.; Kurozawa, L.E.; Kawazoe Sato, A.C. Lentil protein: Impact of different extraction methods on structural and functional properties. Heliyon 2022, 8, e11775. [Google Scholar] [CrossRef] [PubMed]
- Möller, A.C.; Li, J.; van der Goot, A.J.; van der Padt, A. A water-only process to fractionate yellow peas into its constituents. Innov. Food Sci. Emerg. Technol. 2022, 75, 102894. [Google Scholar] [CrossRef]
- Yue, J.; Zhu, Z.; Yi, J.; Lan, Y.; Chen, B.; Rao, J. Structure and functionality of oat protein extracted by choline chloride–dihydric alcohol deep eutectic solvent and its water binary mixtures. Food Hydrocoll. 2021, 112, 106330. [Google Scholar] [CrossRef]
- Bowen, H.; Durrani, R.; Delavault, A.; Durand, E.; Chenyu, J.; Yiyang, L.; Lili, S.; Jian, S.; Weiwei, H.; Fei, G. Application of deep eutectic solvents in protein extraction and purification. Front. Chem. 2022, 10, 912411. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Wang, X.; Liu, W. Application of Deep Eutectic Solvents in Food Analysis: A Review. Molecules 2019, 24, 4594. [Google Scholar] [CrossRef] [PubMed]
- De Santis, M.A.; Rinaldi, M.; Menga, V.; Codianni, P.; Giuzio, L.; Fares, C.; Flagella, Z. Influence of Organic and Conventional Farming on Grain Yield and Protein Composition of Chickpea Genotypes. Agronomy 2021, 11, 191. [Google Scholar] [CrossRef]
- Asen, N.D.; Aluko, R.E. Physicochemical and Functional Properties of Membrane-Fractionated Heat-Induced Pea Protein Aggregates. Front. Nutr. 2022, 9, 852225. [Google Scholar] [CrossRef] [PubMed]
- Mahdavian Mehr, H.; Koocheki, A. Effect of atmospheric cold plasma on structure, interfacial and emulsifying properties of Grass pea (Lathyrus sativus L.) protein isolate. Food Hydrocoll. 2020, 106, 105899. [Google Scholar] [CrossRef]
- Basak, S.; Annapure, U.S. Recent trends in the application of cold plasma for the modification of plant proteins—A review. Future Foods 2022, 5, 100119. [Google Scholar] [CrossRef]
- Maria Medeiros Theóphilo Galvão, A.; Lamy Rasera, M.; de Figueiredo Furtado, G.; Grossi Bovi Karatay, G.; Tavares, G.M.; Dupas Hubinger, M. Lentil protein isolate (Lens culinaris) subjected to ultrasound treatment combined or not with heat-treatment: Structural characterization and ability to stabilize high internal phase emulsions. Food Res. Int. 2024, 183, 114212. [Google Scholar] [CrossRef] [PubMed]
- Asen, N.D.; Aluko, R.E. Effect of Heat Treatment on Yellow Field Pea (Pisum sativum) Protein Concentrate Coupled with Membrane Ultrafiltration on Emulsification Properties of the Isolated >50 kDa Proteins. Membranes 2023, 13, 767. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuan, J.-j.; Zhang, Y.-r.; Chen, X.; Wang, J.-l.; Chen, B.; Li, K.; Bai, Y.-h. Unraveling the effect of combined heat and high-pressure homogenization treatment on the improvement of chickpea protein solubility from the perspectives of colloidal state change and structural characteristic modification. Food Chem. 2024, 442, 138470. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.A.-O.X.; Arnal, M.; Barat, J.M.; Talens, P.A.-O. Effect of Cooking on Protein Digestion and Antioxidant Activity of Different Legume Pastes. Foods 2020, 10, 47. [Google Scholar] [CrossRef]
- Nasrollahzadeh, F.; Alexi, N.; Skov, K.B.; Roman, L.; Sfyra, K.; Martinez, M.M. Texture profiling of muscle meat benchmarks and plant-based analogues: An instrumental and sensory design approach with focus on correlations. Food Hydrocoll. 2024, 151, 109829. [Google Scholar] [CrossRef]
- Hooper, S.D.; Bassett, A.; Wiesinger, J.A.; Glahn, R.P.; Cichy, K.A. Extrusion and drying temperatures enhance sensory profile and iron bioavailability of dry bean pasta. Food Chem. Adv. 2023, 3, 100422. [Google Scholar] [CrossRef]
- Silvestre-De-León, R.; Espinosa-Ramírez, J.; Pérez-Carrillo, E.; Serna-Saldívar, S.O. Extruded chickpea flour sequentially treated with alcalase and α-amylase produces dry instant beverage powders with enhanced yield and nutritional properties. Int. J. Food Sci. Technol. 2021, 56, 5178–5189. [Google Scholar] [CrossRef]
- Santhosh, R.; Babu, D.M.; Thakur, R.; Nath, D.; Hoque, M.; Gaikwad, K.K.; Ahmed, J.; Sarkar, P. Effect of atmospheric cold plasma treatment on structural, thermal, and mechanical properties of pea protein isolate edible films. Sustain. Chem. Pharm. 2024, 37, 101398. [Google Scholar] [CrossRef]
- Rout, S.; Srivastav, P.P. Modification of soy protein isolate and pea protein isolate by high voltage dielectric barrier discharge (DBD) atmospheric cold plasma: Comparative study on structural, rheological and techno-functional characteristics. Food Chem. 2024, 447, 138914. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, G.; Flamminii, F.; Faieta, M.; Prete, R.; Di Michele, A.; Pittia, P.; Di Mattia, C.D. High pressure homogenization to boost the technological functionality of native pea proteins. Curr. Res. Food Sci. 2023, 6, 100499. [Google Scholar] [CrossRef] [PubMed]
- Yaver, E.; Bilgiçli, N. Effect of ultrasound-accelerated debittering method on total alkaloid and total carotenoid content of lupin seeds (Lupinus albus L.) and storage stability of thermally treated lupin flours. J. Food Meas. Charact. 2023, 17, 3378–3389. [Google Scholar] [CrossRef]
- Osemwota, E.C.; Alashi, A.A.-O.; Aluko, R.A.-O. Comparative Study of the Structural and Functional Properties of Membrane-Isolated and Isoelectric pH Precipitated Green Lentil Seed Protein Isolates. Membranes 2021, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Zha, F.; Dong, S.; Rao, J.; Chen, B. The structural modification of pea protein concentrate with gum Arabic by controlled Maillard reaction enhances its functional properties and flavor attributes. Food Hydrocoll. 2019, 92, 30–40. [Google Scholar] [CrossRef]
- Mamilla, R.K.; Mishra, V.K. Effect of germination on antioxidant and ACE inhibitory activities of legumes. LWT 2017, 75, 51–58. [Google Scholar] [CrossRef]
- Karabulut, G.; Yildiz, S.; Karaca, A.C.; Yemiş, O. Ultrasound and enzyme-pretreated extraction for the valorization of pea pod proteins. J. Food Process Eng. 2023, 46, e14452. [Google Scholar] [CrossRef]
- Massmann, C.M.; Berhow, M.; Gibbons, W.R.; Karki, B. The effects of fungal bioprocessing on air-classified pea protein concentrates. LWT 2022, 154, 112686. [Google Scholar] [CrossRef]
- Valtonen, A.; Aisala, H.; Nisov, A.; Nikinmaa, M.; Honkapää, K.; Sozer, N. Synergistic use of fermentation and extrusion processing to design plant protein-based sausages. LWT 2023, 184, 115067. [Google Scholar] [CrossRef]
- Rämö, S.; Kahala, M.; Joutsjoki, V. Aflatoxin B1 Binding by Lactic Acid Bacteria in Protein-Rich Plant Material Fermentation. Appl. Sci. 2022, 12, 12769. [Google Scholar] [CrossRef]
- Ferawati, F.; Zahari, I.; Barman, M.; Hefni, M.; Ahlström, C.; Witthöft, C.; Östbring, K. High-Moisture Meat Analogues Produced from Yellow Pea and Faba Bean Protein Isolates/Concentrate: Effect of Raw Material Composition and Extrusion Parameters on Texture Properties. Foods 2021, 10, 843. [Google Scholar] [CrossRef] [PubMed]
- Blandino, M.; Bresciani, A.; Locatelli, M.; Loscalzo, M.; Travaglia, F.; Vanara, F.; Marti, A. Pulse type and extrusion conditions affect phenolic profile and physical properties of extruded products. Food Chem. 2023, 403, 134369. [Google Scholar] [CrossRef]
- Martin, A.; Schmidt, V.; Osen, R.; Bez, J.; Ortner, E.; Mittermaier, S. Texture, sensory properties and functionality of extruded snacks from pulses and pseudocereal proteins. J. Sci. Food Agric. 2022, 102, 5011–5021. [Google Scholar] [CrossRef] [PubMed]
- Pennells, J.; Bless, I.; Juliano, P.; Ying, D. Extrusion Processing of Biomass By-Products for Sustainable Food Production. In From Biomass to Biobased Products; Jacob-Lopes, E., Zepka, Q.L., Dias, R.R., Eds.; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Zha, F.; Rao, J.; Chen, B. Modification of pulse proteins for improved functionality and flavor profile: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3036–3060. [Google Scholar] [CrossRef]
- Charoensuk, D.; Brannan, R.G.; Chanasattru, W.; Chaiyasit, W. Physicochemical and emulsifying properties of mung bean protein isolate as influenced by succinylation. Int. J. Food Prop. 2018, 21, 1633–1645. [Google Scholar] [CrossRef]
- Shen, Y.; Hong, S.; Singh, G.; Koppel, K.; Li, Y. Improving functional properties of pea protein through “green” modifications using enzymes and polysaccharides. Food Chem. 2022, 385, 132687. [Google Scholar] [CrossRef]
- Boukid, F.; Ganeshan, S.; Wang, Y.; Tülbek, M.Ç.; Nickerson, M.T. Bioengineered Enzymes and Precision Fermentation in the Food Industry. Int. J. Mol. Sci. 2023, 24, 10156. [Google Scholar] [CrossRef]
- Fang, L.; Xiang, H.; Sun-Waterhouse, D.; Cui, C.; Lin, J. Enhancing the Usability of Pea Protein Isolate in Food Applications through Modifying Its Structural and Sensory Properties via Deamidation by Glutaminase. J. Agric. Food Chem. 2020, 68, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Marengo, M.; Carpen, A.; Bonomi, F.; Casiraghi, M.C.; Meroni, E.; Quaglia, L.; Iametti, S.; Pagani, M.A.; Marti, A. Macromolecular and Micronutrient Profiles of Sprouted Chickpeas to Be Used for Integrating Cereal-Based Food. Cereal Chem. 2017, 94, 82–88. [Google Scholar] [CrossRef]
- Avezum, L.; Rondet, E.; Mestres, C.; Achir, N.; Madode, Y.; Gibert, O.; Lefevre, C.; Hemery, Y.; Verdeil, J.-L.; Rajjou, L. Improving the nutritional quality of pulses via germination. Food Rev. Int. 2022, 39, 6011–6044. [Google Scholar] [CrossRef]
- Atudorei, D.; Stroe, S.-G.; Codină, G.G. Impact of Germination on the Microstructural and Physicochemical Properties of Different Legume Types. Plants 2021, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Song, L.; Feng, S.; Liu, Y.; He, G.; Yioe, Y.; Liu, S.Q.; Huang, D. Germination Dramatically Increases Isoflavonoid Content and Diversity in Chickpea (Cicer arietinum L.) Seeds. J. Agric. Food Chem. 2012, 60, 8606–8615. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Jin, Z.; Simsek, S.; Hall, C.; Rao, J.; Chen, B. Effect of germination on the chemical composition, thermal, pasting, and moisture sorption properties of flours from chickpea, lentil, and yellow pea. Food Chem. 2019, 295, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Swanson, G.; Chen, B.; Xu, M. Sensory profile of pulse-based high moisture meat analogs: A study on the complex effect of germination and extrusion processing. Food Chem. 2023, 426, 136585. [Google Scholar] [CrossRef] [PubMed]
- Burlingame, B.; Dernini, S.; Charrondiere, U.; Stadlmayr, B.; Mondovi, S.; Dop, M. Biodiversity and Sustainable Diets; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011; Available online: https://hdl.handle.net/10568/104606 (accessed on 27 May 2024).
- Steinkraus, K.H. Lactic acid fermentation in the production of foods from vegetables, cereals and legumes. Antonie Van Leeuwenhoek 1983, 49, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Cui, C.; Ruan, Z. Fermentation-enabled wellness foods: A fresh perspective. Food Sci. Hum. Wellness 2019, 8, 203–243. [Google Scholar] [CrossRef]
- Wuyts, S.; Van Beeck, W.; Allonsius, C.N.; van den Broek, M.F.L.; Lebeer, S. Applications of plant-based fermented foods and their microbes. Curr. Opin. Biotechnol. 2020, 61, 45–52. [Google Scholar] [CrossRef]
- Ahmad, M.I.; Farooq, S.; Alhamoud, Y.; Li, C.; Zhang, H. A review on mycoprotein: History, nutritional composition, production methods, and health benefits. Trends Food Sci. Technol. 2022, 121, 14–29. [Google Scholar] [CrossRef]
- Rastogi, Y.R.; Thakur, R.; Thakur, P.; Mittal, A.; Chakrabarti, S.; Siwal, S.S.; Thakur, V.K.; Saini, R.V.; Saini, A.K. Food fermentation—Significance to public health and sustainability challenges of modern diet and food systems. Int. J. Food Microbiol. 2022, 371, 109666. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.A.; Berovič, M.; Krieger, N. Solid-State Fermentation Bioreactor Fundamentals: Introduction and Overview. In Solid-State Fermentation Bioreactors: Fundamentals of Design and Operation; Mitchell, D.A., Berovič, M., Krieger, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–12. [Google Scholar]
- Berovic, M. Cultivation of Medicinal Mushroom Biomass by Solid-State Bioprocessing in Bioreactors. In Solid State Fermentation: Research and Industrial Applications; Steudler, S., Werner, A., Cheng, J.J., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–25. [Google Scholar]
- Verni, M.; Pontonio, E.; Montemurro, M.; Rizzello, C.G. Fermentation as Strategy for Improving Nutritional, Functional, Technological, and Sensory Properties of Legumes. In Legumes Research; Jose, C.J.-L., Alfonso, C., Eds.; IntechOpen: Rijeka, Croatia, 2022; Chapter 13. [Google Scholar]
- Klupsaite, D.; Juodeikiene, G.; Zadeike, D.; Bartkiene, E.; Maknickiene, Z.; Liutkute, G. The influence of lactic acid fermentation on functional properties of narrow-leaved lupine protein as functional additive for higher value wheat bread. LWT 2017, 75, 180–186. [Google Scholar] [CrossRef]
- Kumar, Y.; Basu, S.; Goswami, D.; Devi, M.; Shivhare, U.S.; Vishwakarma, R.K. Anti-nutritional compounds in pulses: Implications and alleviation methods. Legume Sci. 2022, 4, e111. [Google Scholar] [CrossRef]
- Coda, R.; Melama, L.; Rizzello, C.G.; Curiel, J.A.; Sibakov, J.; Holopainen, U.; Pulkkinen, M.; Sozer, N. Effect of air classification and fermentation by Lactobacillus plantarum VTT E-133328 on faba bean (Vicia faba L.) flour nutritional properties. Int. J. Food Microbiol. 2015, 193, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Romero-Espinoza, A.M.; Serna-Saldivar, S.O.; Vintimilla-Alvarez, M.C.; Briones-García, M.; Lazo-Vélez, M.A. Effects of fermentation with probiotics on anti-nutritional factors and proximate composition of lupin (Lupinus mutabilis sweet). LWT 2020, 130, 109658. [Google Scholar] [CrossRef]
- Chandra-Hioe, M.V.; Wong, C.H.M.; Arcot, J. The Potential Use of Fermented Chickpea and Faba Bean Flour as Food Ingredients. Plant Foods Hum. Nutr. 2016, 71, 90–95. [Google Scholar] [CrossRef]
- Curiel, J.A.; Coda, R.; Centomani, I.; Summo, C.; Gobbetti, M.; Rizzello, C.G. Exploitation of the nutritional and functional characteristics of traditional Italian legumes: The potential of sourdough fermentation. Int. J. Food Microbiol. 2015, 196, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Dekker, S.; Kyriakopoulou, K.; Boom, R.M.; Smid, E.J.; Schutyser, M.A.I. Enhanced nutritional value of chickpea protein concentrate by dry separation and solid state fermentation. Innov. Food Sci. Emerg. Technol. 2020, 59, 102269. [Google Scholar] [CrossRef]
- Limón, R.I.; Peñas, E.; Torino, M.I.; Martínez-Villaluenga, C.; Dueñas, M.; Frias, J. Fermentation enhances the content of bioactive compounds in kidney bean extracts. Food Chem. 2015, 172, 343–352. [Google Scholar] [CrossRef]
- Rui, X.; Wen, D.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enrichment of ACE inhibitory peptides in navy bean (Phaseolus vulgaris) using lactic acid bacteria. Food Funct. 2014, 6, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Emkani, M.; Oliete, B.; Saurel, R. Effect of Lactic Acid Fermentation on Legume Protein Properties, a Review. Fermentation 2022, 8, 244. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Hernández-Ledesma, B.; Fernández-Tomé, S.; Curiel, J.A.; Pinto, D.; Marzani, B.; Coda, R.; Gobbetti, M. Italian legumes: Effect of sourdough fermentation on lunasin-like polypeptides. Microb. Cell Factories 2015, 14, 168. [Google Scholar] [CrossRef]
- Valdez-González, F.; Gutiérrez-Dorado, R.; Hernández-Llamas, A.; García-Ulloa, M.; Sánchez-Magaña, L.; Cuevas-Rodríguez, B.; Rodríguez-González, H. Bioprocessing of common beans in diets for tilapia: In vivo digestibility and antinutritional factors. J. Sci. Food Agric. 2017, 97, 4087–4093. [Google Scholar] [CrossRef]
- Sánchez-García, J.; Asensio Grau, A.; García-Hernández, J.; Heredia, A.; Andrés, A. Nutritional and antioxidant changes in lentils and quinoa through fungal solid-state fermentation with Pleurotus ostreatus. Bioresour. Bioprocess. 2022, 9, 51. [Google Scholar] [CrossRef]
- Olanipekun, B.F.; Otunola, E.T.; Oyelade, O.J. Effect of fermentation on antinutritional factors and in vitro protein digestibility of Bambara nut (Voandzeia subterranean L.). Food Sci. Qual. Manag 2015, 39. Available online: https://api.semanticscholar.org/CorpusID:82228002 (accessed on 27 May 2024).
- Toor, B.S.; Kaur, A.; Kaur, J. Fermentation of legumes with Rhizopus oligosporus: Effect on physicochemical, functional and microstructural properties. Int. J. Food Sci. Technol. 2022, 57, 1763–1772. [Google Scholar] [CrossRef]
- Souza Filho, P.F.; Nair, R.B.; Andersson, D.; Lennartsson, P.R.; Taherzadeh, M.J. Vegan-mycoprotein concentrate from pea-processing industry byproduct using edible filamentous fungi. Fungal Biol. Biotechnol. 2018, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Finnigan, T.J.A.; Wall, B.T.; Wilde, P.J.; Stephens, F.B.; Taylor, S.L.; Freedman, M.R. Mycoprotein: The Future of Nutritious Nonmeat Protein, a Symposium Review. Curr. Dev. Nutr. 2019, 3, nzz021. [Google Scholar] [CrossRef]
- Clark, A.J.; Soni, B.K.; Sharkey, B.; Acree, T.; Lavin, E.; Bailey, H.M.; Stein, H.H.; Han, A.; Elie, M.; Nadal, M. Shiitake mycelium fermentation improves digestibility, nutritional value, flavor and functionality of plant proteins. LWT 2022, 156, 113065. [Google Scholar] [CrossRef]
- Soni, B.K.; Kelly, B.J.; Langan, J.P.; Davis, H.; Hahn, A.D. Methods for the Production and Use of Myceliated High Protein Food Compositions. U.S. Patent 20170295837A1, 3 July 2018. Available online: https://patents.google.com/patent/US20170295837A1 (accessed on 27 May 2024).
- Sieuwerts, S.; de Bok Frank, A.M.; Hugenholtz, J.; van Hylckama Vlieg Johan, E.T. Unraveling Microbial Interactions in Food Fermentations: From Classical to Genomics Approaches. Appl. Environ. Microbiol. 2008, 74, 4997–5007. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, J.G.; Rinker, D.C. The genomics of microbial domestication in the fermented food environment. Curr. Opin. Genet. Dev. 2015, 35, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.L.; Klaenhammer, T.R. Genomic Evolution of Domesticated Microorganisms. Annu. Rev. Food Sci. Technol. 2010, 1, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Steensels, J.; Verstrepen, K.J. Taming Wild Yeast: Potential of Conventional and Nonconventional Yeasts in Industrial Fermentations. Annu. Rev. Microbiol. 2014, 68, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Schindler, S.; Zelena, K.; Krings, U.; Bez, J.; Eisner, P.; Berger, R.G. Improvement of the Aroma of Pea (Pisum sativum) Protein Extracts by Lactic Acid Fermentation. Food Biotechnol. 2012, 26, 58–74. [Google Scholar] [CrossRef]
- Yousseef, M.; Lafarge, C.; Valentin, D.; Lubbers, S.; Husson, F. Fermentation of cow milk and/or pea milk mixtures by different starter cultures: Physico-chemical and sensorial properties. LWT—Food Sci. Technol. 2016, 69, 430–437. [Google Scholar] [CrossRef]
- Ben-Harb, S.; Saint-Eve, A.; Panouillé, M.; Souchon, I.; Bonnarme, P.; Dugat-Bony, E.; Irlinger, F. Design of microbial consortia for the fermentation of pea-protein-enriched emulsions. Int. J. Food Microbiol. 2019, 293, 124–136. [Google Scholar] [CrossRef] [PubMed]
- El Youssef, C.; Bonnarme, P.; Fraud, S.; Péron, A.-C.; Helinck, S.; Landaud, S. Sensory Improvement of a Pea Protein-Based Product Using Microbial Co-Cultures of Lactic Acid Bacteria and Yeasts. Foods 2020, 9, 349. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, S.; Kim, S.H.; Vujanovic, V. Scaling-up production of plant endophytes in bioreactors: Concepts, challenges and perspectives. Bioresour. Bioprocess. 2021, 8, 63. [Google Scholar] [CrossRef]
- Pedrosa, M.M.; Guillamón, E.; Arribas, C. Autoclaved and Extruded Legumes as a Source of Bioactive Phytochemicals: A Review. Foods 2021, 10, 379. [Google Scholar] [CrossRef]
- Kårlund, A.; Gómez-Gallego, C.; Korhonen, J.; Palo-oja, O.-M.; El-Nezami, H.; Kolehmainen, M. Harnessing Microbes for Sustainable Development: Food Fermentation as a Tool for Improving the Nutritional Quality of Alternative Protein Sources. Nutrients 2020, 12, 1020. [Google Scholar] [CrossRef] [PubMed]
- del Cueto, A.G.; Martinez, W.H.; Frampton, V.L. Heat Effects on Peas, Effect of Autoclaving on the Basic Amino Acids and Proteins of the Chick Pea. J. Agric. Food Chem. 1960, 8, 331–332. [Google Scholar] [CrossRef]
- Srihara, P.; Alexander, J.C. Protein Quality of Raw and Autoclaved Plant Protein Blends. Can. Inst. Food Sci. Technol. J. 1983, 16, 63–67. [Google Scholar] [CrossRef]
- Khattab, R.Y.; Arntfield, S.D.; Nyachoti, C.M. Nutritional quality of legume seeds as affected by some physical treatments, Part 1: Protein quality evaluation. LWT—Food Sci. Technol. 2009, 42, 1107–1112. [Google Scholar] [CrossRef]
- Donzella, S.; Fumagalli, A.; Arioli, S.; Pellegrino, L.; D’Incecco, P.; Molinari, F.; Speranza, G.; Ubiali, D.; Robescu, M.S.; Compagno, C. Recycling Food Waste and Saving Water: Optimization of the Fermentation Processes from Cheese Whey Permeate to Yeast Oil. Fermentation 2022, 8, 341. [Google Scholar] [CrossRef]

| Approaches | Techniques | Examples | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Physical | Thermal | Conventional heating | Produces soluble protein aggregates and improves solubility and water holding capacity. | Could promote protein aggregation and reduce solubility. | [56,57] |
| Conventional heating and high-pressure homogenization | Improves protein solubility from reinforced disulphide crosslinking, which introduces steric hindrance. | [58] | |||
| Conventional heating, high-pressure and microwave | Releases peptides and amino acids, enhances digestibility and antioxidant properties. | [59] | |||
| Extrusion cooking | Improves digestibility and functional properties and lowers or eliminates antinutrients. Cost effective means of producing ready-to-eat snacks and meat analogues from pulses. | Increased denaturation, aggregate formation and low solubility. | [60,61,62] | ||
| Cold plasma | Increases heat stability and improves solubility and protein digestibility. | Reduction in α-helical structures leads to increased aggregation. | [63,64] | ||
| Pressure | High-pressure homogenization | Improves solubility and water and oil holding capacity due to structural unfolding. | Emulsifying capacity and interfacial tension is not enhanced. | [65] | |
| Ultrasound | Ultrasound and heat | Sustainable removal of alkaloid compounds. | [66] | ||
| Ultrafiltration | Membrane ultrafiltration | Green and non-inversive method; no residues or byproducts are formed. | Membrane fouling and time-consuming. | [67] | |
| Chemical | Conjugation | Maillard reaction | Solubility is enhanced and beany flavour is masked | [68] | |
| Biological | Germination | Germination for 6–18 h | Protein content and crude fibre increase, while fat content decreases after 18 h; improves mineral availability during optimal germination period; germination enhances antioxidant properties. | Optimal germination period varies for each cultivar. | [69] |
| Enzyme | Ultrasound and enzyme assistance | Higher protein extraction yield. | High cost of enzymes. | [70] | |
| Fermentation | Fungal fermentation | Enhances total phenolics, solubility and fibre content | Proliferation of harmful microbes and toxins; process controls could be a challenge. | [71] | |
| Lactic acid fermentation | Reduces beany flavour and improves the texture of a plant-based sausage product. | [72,73] |
| SSF | SmF |
|---|---|
| Low moisture conditions required for efficient production of certain products. | Versatile for the production of a wide array of products from diverse microorganisms including bacteria, yeasts and fungi. |
| Medium can be simple substrates such as grains or organic residues. | Culture medium generally contains highly refined substrates, making it more expensive. Complex ingredients need to be processed to extract and solubilize nutrients. |
| Low water availability reduces growth of contaminants. | High water activity may lead to contamination but, with proper operational diligence, can be obviated. |
| Growth rate and yield can be low, but the use of concentrated media and smaller fermenters leads to higher volumetric productivity. | Media components are generally in diluted form in large volumes, which can lower volumetric productivity. |
| Highly concentrated fermented products can be obtained using high concentrations of substrates. | High concentrations of substrates can affect rheology within the fermentation system and a feeding substrate may be necessary. |
| Lower pressure in fermenters implies less power for aeration. A large surface area of substrate particles makes for easier gas transfer. | High air pressure is required for dissolved oxygen (DO) maintenance. Limitations in the gas transfer rate from gas to liquid can be slow and affect microbial growth. |
| Mixing within substrate particles is not achievable and reduced nutrient diffusion can limit growth. Difficult to monitor growth kinetics. | High agitation is possible to maintain aeration and even temperature. Nutrient uptake by microorganisms is not limiting. Well-established growth kinetics monitoring systems. |
| Difficulty in dissipating metabolic heat generated during microbial growth can lead to overheating. | Temperature control and uniformity is consistent due to fluidity of the medium. |
| Online measurements for biomass, pH and DO are difficult and thus limit process control. Substrate feeding during fermentation is also challenging. | Online systems for real-time monitoring are readily available. Substrate feeding is easy to set up and control. |
| Downstream processing is less cumbersome due to reduced water content. | Large volumes of water make downstream processing challenging for dewatering, and thus more expensive. |
| No liquid waste generated. | Large volumes of liquid waste are generated. |
| Scale-up is attainable and transferable to SmF to some extent. | Scaling-up fungal fermentation may be challenging due to increased broth viscosity, thereby limiting oxygen transfer rate and preventing uniform temperature distribution. |
| Fungal mycelium fermentation is less labour-intensive to conduct. | High operational demand and labour-intensive. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganeshan, S.; Asen, N.; Wang, Y.; Tülbek, M.Ç.; Nickerson, M.T. Sustainable Pulse Proteins: Physical, Chemical and Fermentative Modifications. Appl. Biosci. 2024, 3, 263-282. https://doi.org/10.3390/applbiosci3020018
Ganeshan S, Asen N, Wang Y, Tülbek MÇ, Nickerson MT. Sustainable Pulse Proteins: Physical, Chemical and Fermentative Modifications. Applied Biosciences. 2024; 3(2):263-282. https://doi.org/10.3390/applbiosci3020018
Chicago/Turabian StyleGaneshan, Seedhabadee, Nancy Asen, Yingxin Wang, Mehmet Ç. Tülbek, and Michael T. Nickerson. 2024. "Sustainable Pulse Proteins: Physical, Chemical and Fermentative Modifications" Applied Biosciences 3, no. 2: 263-282. https://doi.org/10.3390/applbiosci3020018
APA StyleGaneshan, S., Asen, N., Wang, Y., Tülbek, M. Ç., & Nickerson, M. T. (2024). Sustainable Pulse Proteins: Physical, Chemical and Fermentative Modifications. Applied Biosciences, 3(2), 263-282. https://doi.org/10.3390/applbiosci3020018









