Exploring the Cellular Interactions of Flavonoids with Similar Structures in Cells Overexpressing the 70 kDa Human Heat Shock Protein
Abstract
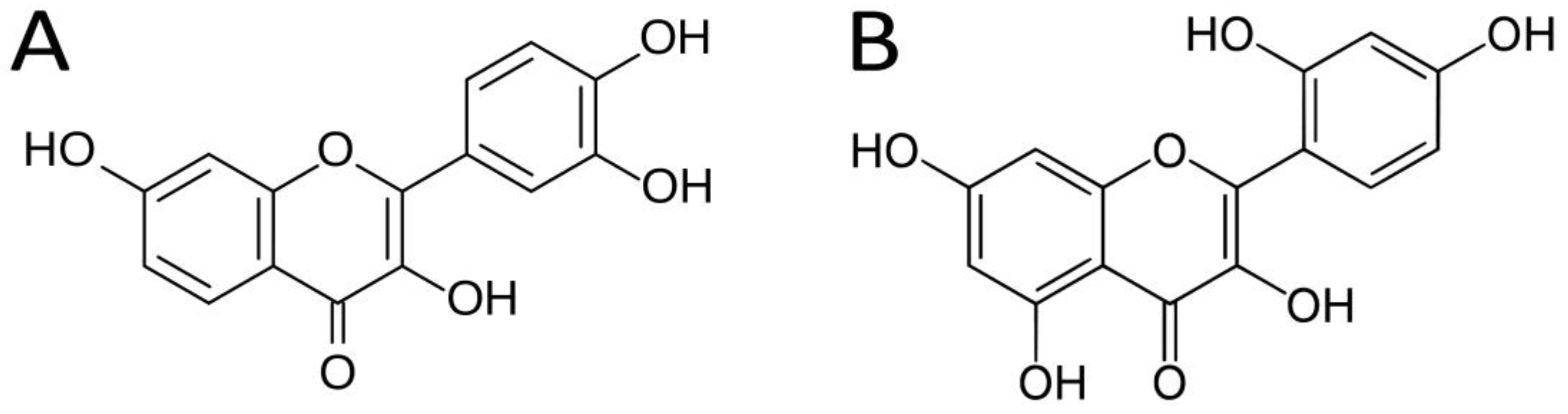
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Lines
2.3. Cell Viability Assay
2.4. Clonogenic Assay
2.5. Determination of Reactive Oxygen Species (ROS) Formation
2.6. Detection of Apoptosis
2.7. Cell Cycle Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Cytotoxicity of Fisetin and Morin against NIH/3T3 and Tg/Tg Cells
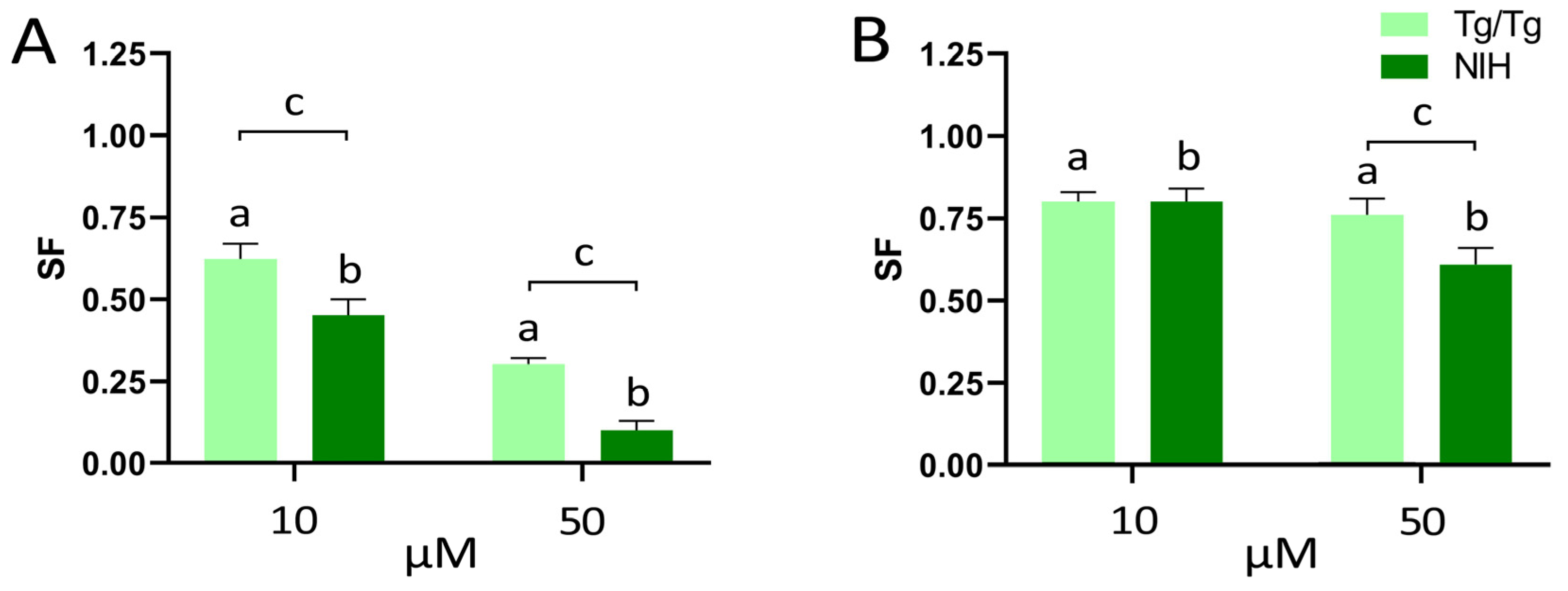
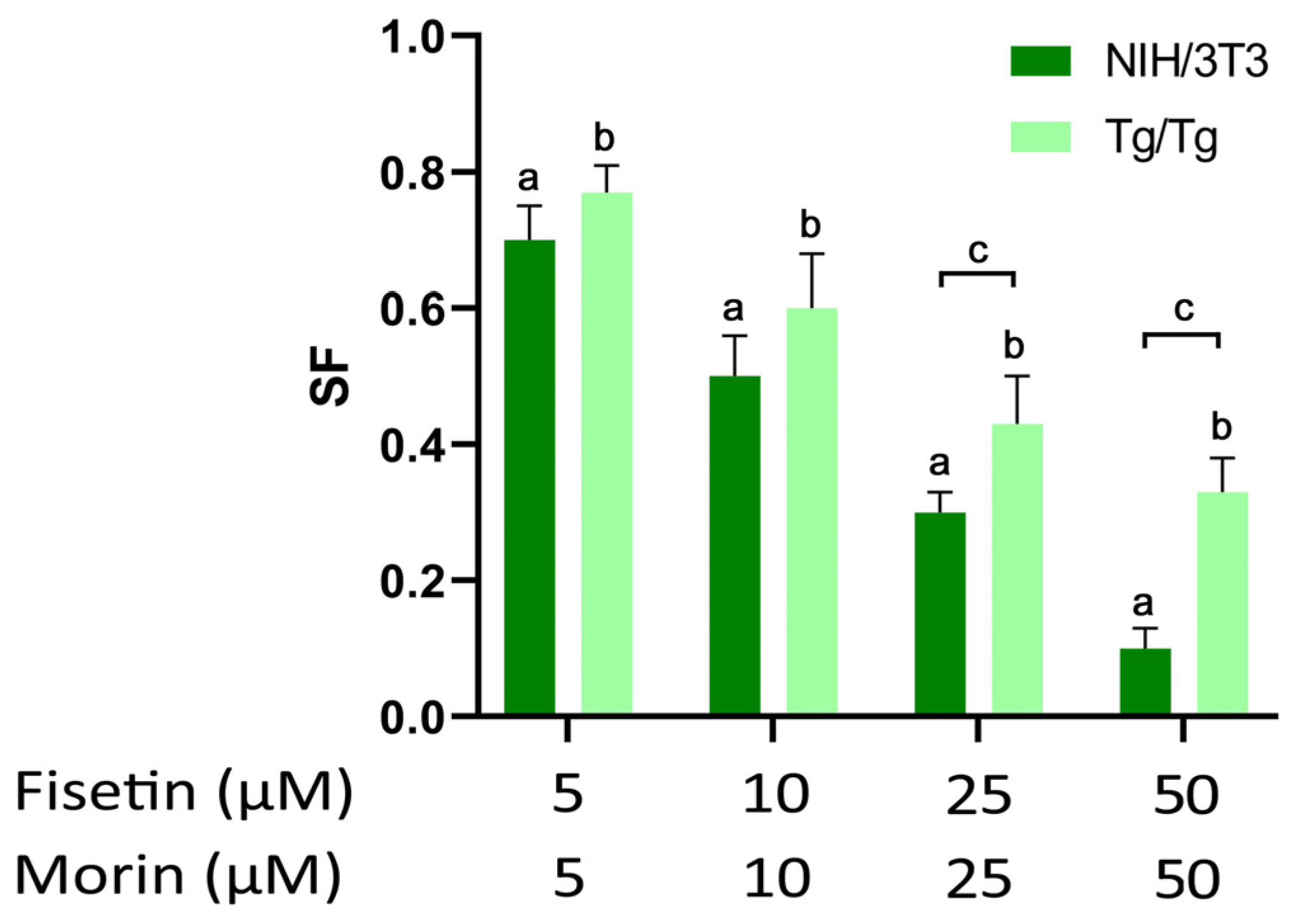
3.2. Long-Term Survival of NIH/3T3 and Tg/Tg Cells after Exposure to Fisetin and Morin
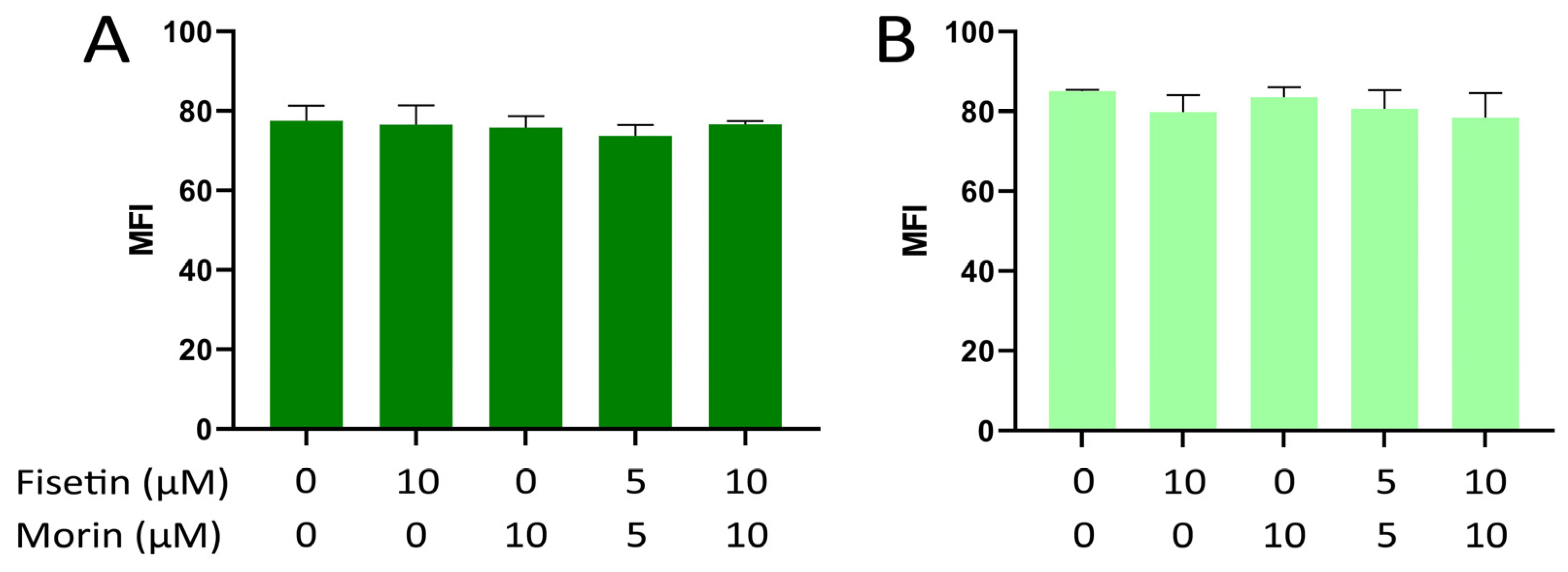
3.3. Intracellular ROS Formation in NIH/3T3 and Tg/Tg Cells
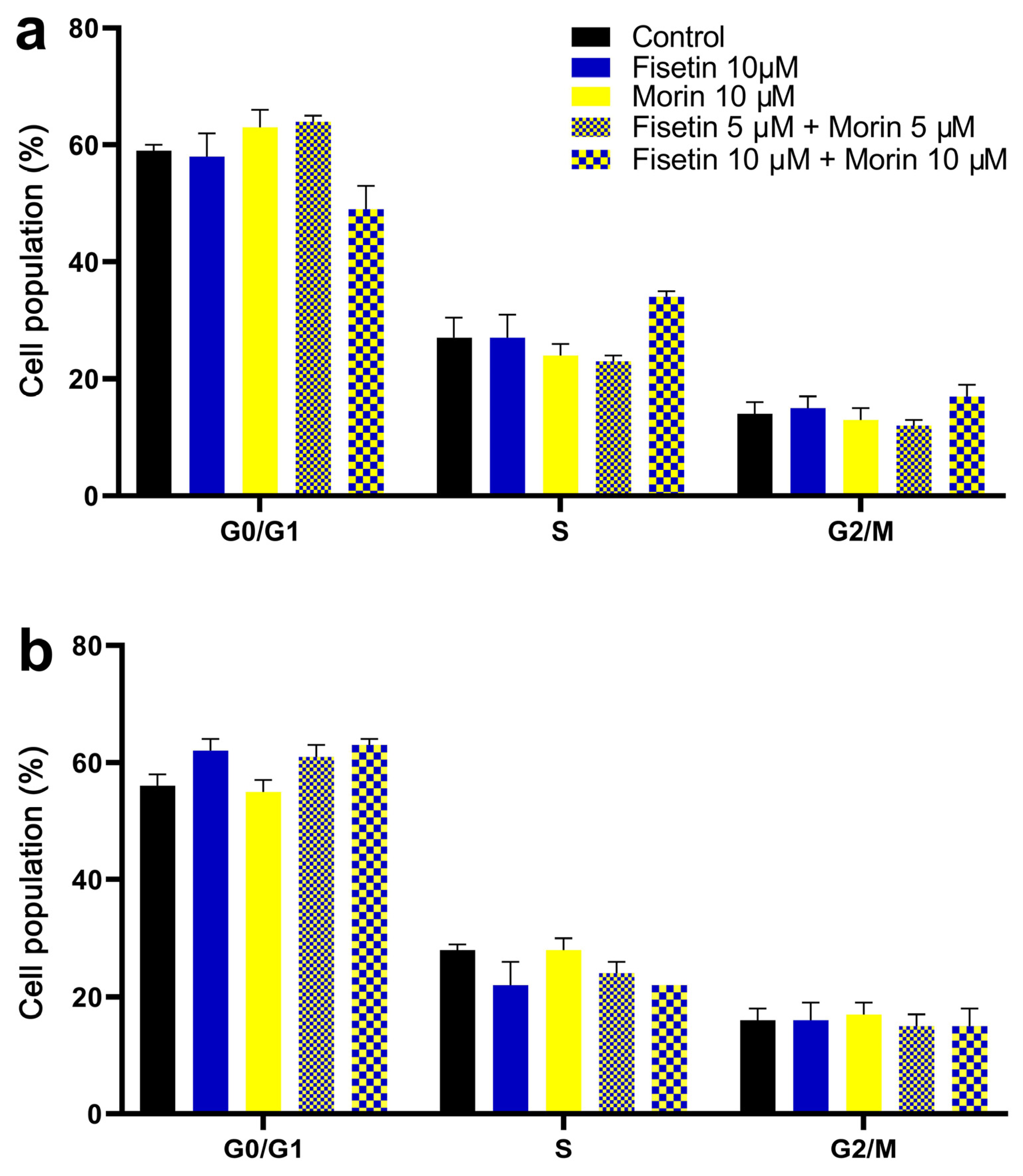
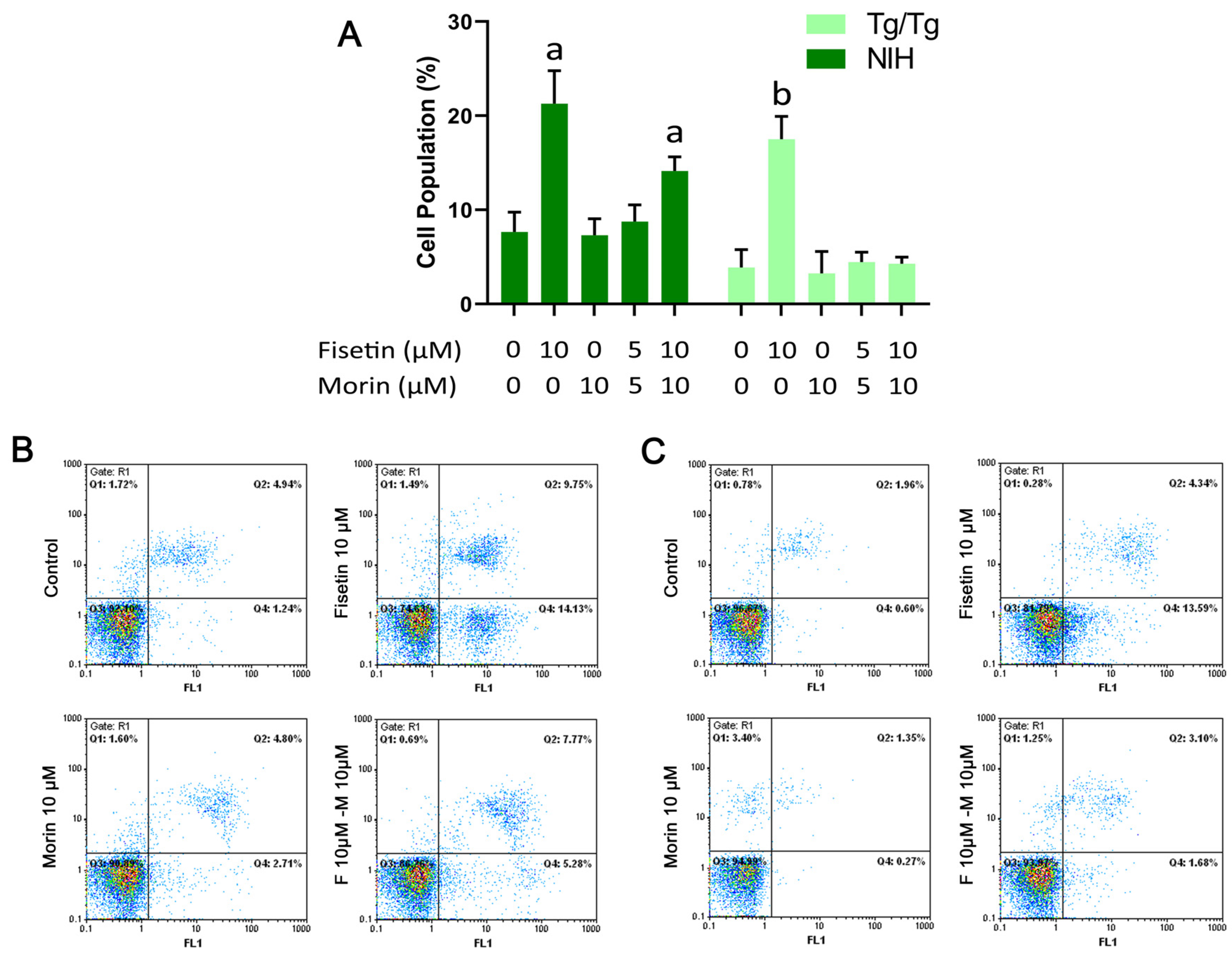
3.4. Cell Cycle Analysis and Induction of Apoptosis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hernández-Rodríguez, P.; Baquero, L.P.; Larrota, H.R. Flavonoids. In Bioactive Compounds; Campos, M.R.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 265–288. [Google Scholar]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Futur. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef]
- Safe, S.; Jayaraman, A.; Chapkin, R.S.; Howard, M.; Mohankumar, K.; Shrestha, R. Flavonoids: Structure–function and mechanisms of action and opportunities for drug development. Toxicol. Res. 2021, 37, 147–162. [Google Scholar] [CrossRef]
- Shomali, A.; Das, S.; Arif, N.; Sarraf, M.; Zahra, N.; Yadav, V.; Aliniaeifard, S.; Chauhan, D.K.; Hasanuzzaman, M. Diverse physiological roles of flavonoids in plant environmental stress responses and tolerance. Plants 2022, 11, 3158. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mussallem, M.Q. Flavonoids as potential anti-inflammatory molecules: A review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Dhiman, A.; Nanda, A.; Ahmad, S. A quest for staunch effects of flavonoids: Utopian protection against hepatic ailments. Arab. J. Chem. 2016, 9, S1813–S1823. [Google Scholar] [CrossRef]
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, Ș.C.; Răchișan, A.L.; Negrean, V.; Perné, M.-G.; Donca, V.I.; Alexescu, T.-G.; Para, I.; et al. The effects of flavonoids in cardiovascular diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef] [PubMed]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of biosynthesis, biological activity, and current extraction techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, P.; Jagadeesan, R.; Sekaran, S.; Dhanasekaran, A.; Vimalraj, S. Flavonoids: Classification, function, and molecular mechanisms involved in bone remodeling. Front. Endocrinol. 2021, 12, 779638. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K. Metabolic syndrome: Preventive effects of dietary flavonoids. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 60, pp. 1–28. [Google Scholar]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A dietary antioxidant for health promotion. Antioxid. Redox. Signal. 2013, 19, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Sharma, A.; Sak, K.; Tuli, H.S.; Buttar, H.S.; Bishayee, A. Fisetin: A bioactive phytochemical with potential for cancer prevention and pharmacotherapy. Life Sci. 2018, 194, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Pawar, A.; Mahadik, K.; Bothiraja, C. Emerging novel drug delivery strategies for bioactive flavonol fisetin in biomedicine. Biomed. Pharmacother. 2018, 106, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, A.F.; Alizadeh, M. Antioxidant properties of the flavonoid fisetin: An updated review of in vivo and in vitro studies. Trends Food Sci. Technol. 2017, 70, 34–44. [Google Scholar] [CrossRef]
- Solairaja, S.; Andrabi, M.Q.; Dunna, N.R.; Venkatabalasubramanian, S. Overview of Morin and its complementary role as an adjuvant for anticancer agents. Nutr. Cancer 2021, 73, 927–942. [Google Scholar] [CrossRef]
- Deriabina, A.; Prutskij, T.; Castillo Trejo, L.; Sanchez Gutierrez, M.P.; Gonzalez Jimenez, E. Experimental and theoretical study of fluorescent properties of Morin. Molecules 2022, 27, 4965. [Google Scholar] [CrossRef]
- Rajput, S.A.; Wang, X.-Q.; Yan, H.-C. Morin hydrate: A comprehensive review on novel natural dietary bioactive compound with versatile biological and pharmacological potential. Biomed. Pharmacother. 2021, 138, 111511. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef]
- Mayer, M.P.; Bukau, B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005, 62, 670–684. [Google Scholar] [CrossRef]
- Ran, R.; Lu, A.; Zhang, L.; Tang, Y.; Zhu, H.; Xu, H.; Feng, Y.; Han, C.; Zhou, G.; Rigby, A.C.; et al. Hsp70 promotes TNF-mediated apoptosis by binding IKKγ and impairing NF-κB survival signaling. Genes. Dev. 2004, 18, 1466–1481. [Google Scholar] [CrossRef]
- Beere, H.M.; Wolf, B.B.; Cain, K.; Mosser, D.D.; Mahboubi, A.; Kuwana, T.; Tailor, P.; Morimoto, R.I.; Cohen, G.M.; Green, D.R. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2000, 2, 469–475. [Google Scholar] [CrossRef]
- Craig, E.A. Hsp70 at the membrane: Driving protein translocation. BMC Biol. 2018, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Hutagalung, R.A.; Reinhart, S.E.; Rosmalena, R.; Nurbaya, S.; Eldafira, E.; Prasasty, V.D. Flavonoid derivatives as potential inhibitors for human Hsp70 using in silico approaches. Eur. Chem. Bull. 2023, 12, 1535–1541. [Google Scholar]
- Kim, J.Y.; Barua, S.; Huang, M.Y.; Park, J.; Yenari, M.A.; Lee, J.E. Heat Shock Protein 70 (HSP70) Induction: Chaperonotherapy for Neuroprotection after Brain Injury. Cells 2020, 9, 2020. [Google Scholar] [CrossRef] [PubMed]
- Naka, K.K.; Vezyraki, P.; Kalaitzakis, A.; Zerikiotis, S.; Michalis, L.; Angelidis, C. Hsp70 regulates doxorubicin-mediated heart failure in Hsp70-transgenic mice. Cell Stress Chaperones 2014, 19, 853–864. [Google Scholar] [CrossRef]
- Zygouri, P.; Athinodorou, A.M.; Spyrou, K.; Simos, Y.V.; Subrati, M.; Asimakopoulos, G.; Vasilopoulos, K.C.; Vezyraki, P.; Peschos, D.; Tsamis, K.; et al. Oxidized-Multiwalled Carbon Nanotubes as Non-Toxic Nanocarriers for Hydroxytyrosol Delivery in Cells. Nanomaterials 2023, 13, 714. [Google Scholar] [CrossRef]
- Papanikolaou, E.; Simos, Y.V.; Spyrou, K.; Patila, M.; Alatzoglou, C.; Tsamis, K.; Vezyraki, P.; Stamatis, H.; Gournis, D.P.; Peschos, D.; et al. Does Green Exfoliation of Graphene Produce More Biocompatible Structures? Pharmaceutics 2023, 15, 993. [Google Scholar] [CrossRef] [PubMed]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Geissmann, Q. OpenCFU, a new free and open-source software to count cell colonies and other circular objects. PLoS ONE 2013, 8, e54072. [Google Scholar] [CrossRef]
- Qaed, E.; Al-Hamyari, B.; Al-Maamari, A.; Qaid, A.; Alademy, H.; Almoiliqy, M.; Munyemana, J.C.; Al-Nusaif, M.; Alafifi, J.; Alyafeai, E.; et al. Fisetin’s promising antitumor effects: Uncovering mechanisms and targeting for future therapies. Glob. Med. Genet. 2023, 10, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Touil, Y.S.; Seguin, J.; Scherman, D.; Chabot, G.G. Improved antiangiogenic and antitumour activity of the combination of the natural flavonoid fisetin and cyclophosphamide in Lewis lung carcinoma-bearing mice. Cancer Chemother. Pharmacol. 2011, 68, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Fotsis, T.; Pepper, M.S.; Aktas, E.; Breit, S.; Rasku, S.; Adlercreutz, H.; Wähälä, K.; Montesano, R.; Schweigerer, L. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997, 57, 2916–2921. [Google Scholar] [PubMed]
- Lee, W.-R.; Shen, S.-C.; Lin, H.-Y.; Hou, W.-C.; Yang, L.-L.; Chen, Y.-C. Wogonin and fisetin induce apoptosis in human promyeloleukemic cells, accompanied by a decrease of reactive oxygen species, and activation of caspase 3 and Ca2+-dependent endonuclease. Biochem. Pharmacol. 2002, 63, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Sabarwal, A.; Agarwal, R.; Singh, R.P. Fisetin inhibits cellular proliferation and induces mitochondria-dependent apoptosis in human gastric cancer cells. Mol. Carcinog. 2017, 56, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Wenzel, U.; Daniel, H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur. J. Nutr. 1999, 38, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, W.I.; Kim, S.Y.; Cho, S.W.; Nam, H.S.; Lee, S.H.; Cho, M.K. Flavonoid morin inhibits proliferation and induces apoptosis of melanoma cells by regulating reactive oxygen species, Sp1 and Mcl-1. Arch. Pharm. Res. 2019, 42, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, Y. Morin inhibits ovarian cancer growth through the inhibition of NF-κB signaling pathway. Anti-Cancer Agents Med. Chem. 2020, 19, 2243–2250. [Google Scholar] [CrossRef]
- Shin, S.-S.; Won, S.Y.; Noh, D.-H.; Hwang, B.; Kim, W.-J.; Moon, S.-K. Morin inhibits proliferation, migration, and invasion of bladder cancer EJ cells via modulation of signaling pathways, cell cycle regulators, and transcription factor-mediated MMP-9 expression. Drug Dev. Res. 2017, 78, 81–90. [Google Scholar] [CrossRef]
- Yao, D.; Cui, H.; Zhou, S.; Guo, L. Morin inhibited lung cancer cells viability, growth, and migration by suppressing miR-135b and inducing its target CCNG2. Tumor Biol. 2017, 39, 101042831771243. [Google Scholar] [CrossRef]
- Lotfizadeh, R.; Sepehri, H.; Attari, F.; Delphi, L. Flavonoid calycopterin induces apoptosis in human prostate cancer cells in-vitro. Iran. J. Pharm. Res. 2020, 19, 391–401. [Google Scholar] [PubMed]
- Samuel, T.; Fadlalla, K.; Turner, T.; Yehualaeshet, T.E. The flavonoid quercetin transiently inhibits the activity of taxol and nocodazole through interference with the cell cycle. Nutr. Cancer 2010, 62, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Intuyod, K.; Priprem, A.; Pairojkul, C.; Hahnvajanawong, C.; Vaeteewoottacharn, K.; Pinlaor, P.; Pinlaor, S. Anthocyanin complex exerts anti-cholangiocarcinoma activities and improves the efficacy of drug treatment in a gemcitabine-resistant cell line. Int. J. Oncol. 2018, 52, 1715–1726. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.O.; de Bianchi, S.E.; Figueiró, F.; Heimfarth, L.; Moresco, K.S.; Gonçalves, R.M.; Hoppe, J.B.; Klein, C.P.; Salbego, C.G.; Gelain, D.P.; et al. Anticancer activity of flavonoids isolated from Achyrocline satureioides in gliomas cell lines. Toxicol. Vitr. 2018, 51, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef] [PubMed]
- Dholaria, M. Flavonoids and its anticancer activity for ROS induced breast cancer. In Handbook of Oxidative Stress in Cancer: Therapeutic Aspects; Chakraborti, S., Ed.; Springer: Singapore, 2021; Volume 1, pp. 305–326. [Google Scholar]
- Xi, X.; Wang, J.; Qin, Y.; You, Y.; Huang, W.; Zhan, J. The biphasic effect of flavonoids on oxidative stress and cell proliferation in breast cancer cells. Antioxidants 2022, 11, 622. [Google Scholar] [CrossRef]
- Kocyigit, A.; Guler, E.M.; Dikilitas, M. Role of antioxidant phytochemicals in prevention, formation and treatment of cancer. In Reactive Oxygen Species (ROS) in Living Cells; Filip, C., Albu, E., Eds.; InTech: Rijeka, Croatia, 2018. [Google Scholar]
- Rodius, S.; de Klein, N.; Jeanty, C.; Sánchez-Iranzo, H.; Crespo, I.; Ibberson, M.; Xenarios, I.; Dittmar, G.; Mercader, N.; Niclou, S.P.; et al. Fisetin protects against cardiac cell death through reduction of ROS production and caspases activity. Sci. Rep. 2020, 10, 2896. [Google Scholar] [CrossRef]
- Dai, X.; Kuang, Q.; Sun, Y.; Xu, M.; Zhu, L.; Ge, C.; Tan, J.; Wang, B. Fisetin represses oxidative stress and mitochondrial dysfunction in NAFLD through suppressing GRP78-mediated endoplasmic reticulum (ER) stress. J. Funct. Foods 2022, 90, 104954. [Google Scholar] [CrossRef]
- Bachewal, P.; Gundu, C.; Yerra, V.G.; Kalvala, A.K.; Areti, A.; Kumar, A. Morin exerts neuroprotection via attenuation of ROS induced oxidative damage and neuroinflammation in experimental diabetic neuropathy. BioFactors 2018, 44, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kang, K.A.; Piao, M.J.; Maeng, Y.H.; Lee, K.H.; Chang, W.Y.; You, H.J.; Kim, J.S.; Kang, S.S.; Hyun, J.W. Cellular protection of morin against the oxidative stress induced by hydrogen peroxide. Chem.-Biol. Interact. 2009, 177, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yao, F.; Li, K.; Zhang, L.; Yin, G.; Du, M.; Wu, B. Fisetin regulates astrocyte migration and proliferation in vitro. Int. J. Mol. Med. 2017, 39, 783–790. [Google Scholar] [CrossRef]
- Pal, H.C.; Sharma, S.; Elmets, C.A.; Athar, M.; Afaq, F. Fisetin inhibits growth, induces G2/M arrest and apoptosis of human epidermoid carcinoma A431 cells: Role of mitochondrial membrane potential disruption and consequent caspases activation. Exp. Dermatol. 2013, 22, 470–475. [Google Scholar] [CrossRef]
- Adan, A.; Baran, Y. The pleiotropic effects of fisetin and hesperetin on human acute promyelocytic leukemia cells are mediated through apoptosis, cell cycle arrest, and alterations in signaling networks. Tumor Biol. 2015, 36, 8973–8984. [Google Scholar] [CrossRef] [PubMed]
- Afroze, N.; Pramodh, S.; Shafarin, J.; Bajbouj, K.; Hamad, M.; Sundaram, M.K.; Haque, S.; Hussain, A. Fisetin deters cell proliferation, induces apoptosis, alleviates oxidative stress and inflammation in human cancer cells, HeLa. Int. J. Mol. Sci. 2022, 23, 1707. [Google Scholar] [CrossRef]
- Kim, J.A.; Lee, S.; Kim, D.-E.; Kim, M.; Kwon, B.-M.; Han, D.C. Fisetin, a dietary flavonoid, induces apoptosis of cancer cells by inhibiting HSF1 activity through blocking its binding to the hsp70 promoter. Carcinogenesis 2015, 36, 696–706. [Google Scholar] [CrossRef]
- Hyun, H.-B.; Lee, W.S.; Go, S.-I.; Nagappan, A.; Park, C.; Han, M.H.O.; Hong, S.U.H.; Kim, G.; Kim, G.I.Y.; Cheong, J.; et al. The flavonoid morin from Moraceae induces apoptosis by modulation of Bcl-2 family members and Fas receptor in HCT 116 cells. Int. J. Oncol. 2015, 46, 2670–2678. [Google Scholar] [CrossRef] [PubMed]
- Malgorzata, O.T.; Wianowska, D. Antioxidant Properties of Selected Flavonoids in Binary Mixtures—Considerations on Myricetin, Kaempferol and Quercetin. Int. J. Mol. Sci. 2023, 24, 10070. [Google Scholar]
- Nie, Z.-Y.; Yang, L.; Liu, X.-J.; Yang, Z.; Yang, G.-S.; Zhou, J.; Qin, Y.; Yu, J.; Jiang, L.-L.; Wen, J.-K.; et al. Morin inhibits proliferation and induces apoptosis by modulating the miR-188-5p/PTEN/AKT regulatory pathway in CML cells. Mol. Cancer Ther. 2019, 18, 2296–2307. [Google Scholar] [CrossRef]
- Vasaikar, S.V.; Ghosh, S.; Narain, P.; Basu, A.; Gomes, J. HSP70 mediates survival in apoptotic cells—Boolean network prediction and experimental validation. Front. Cell. Neurosci. 2015, 9, 319. [Google Scholar] [CrossRef]
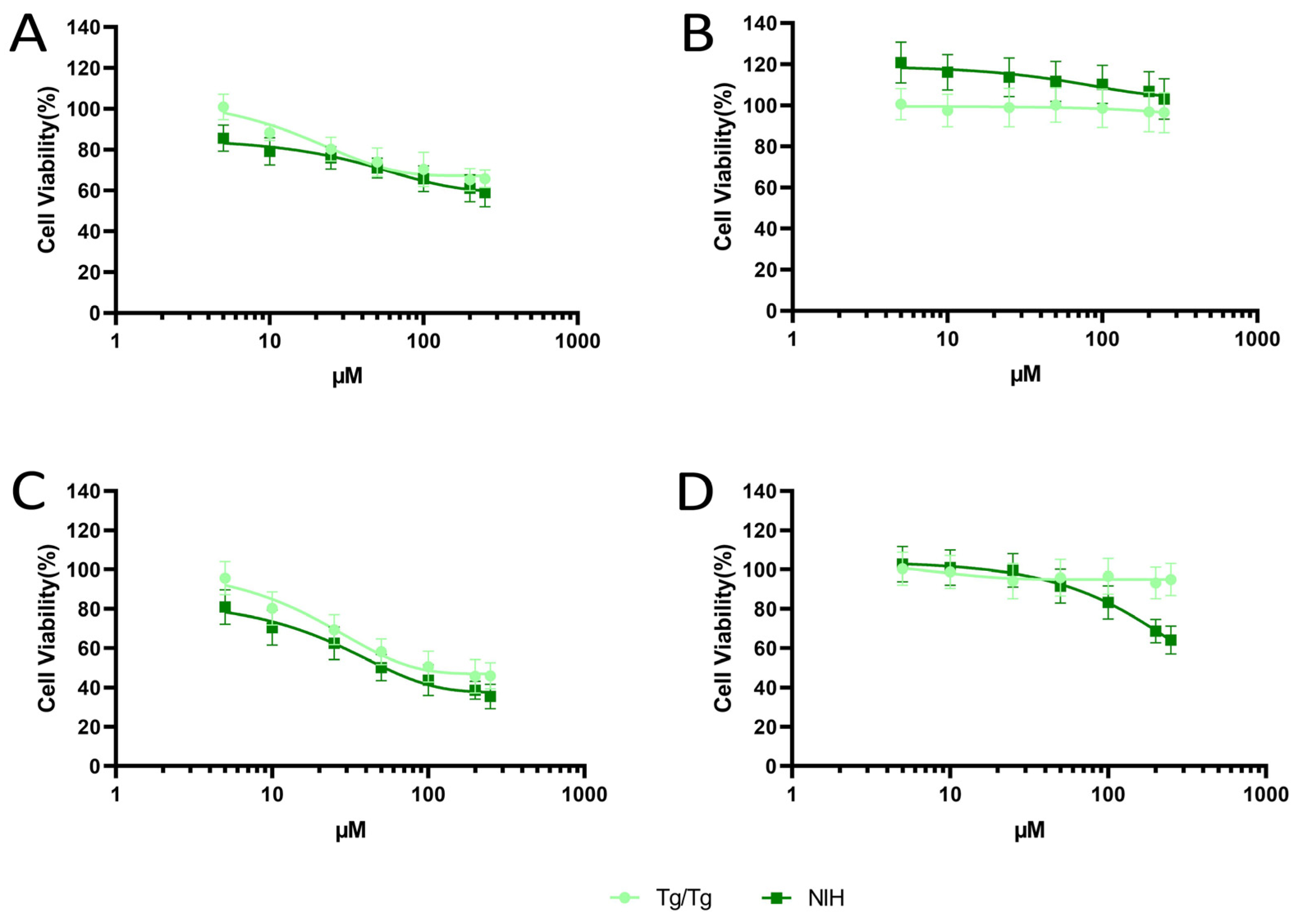
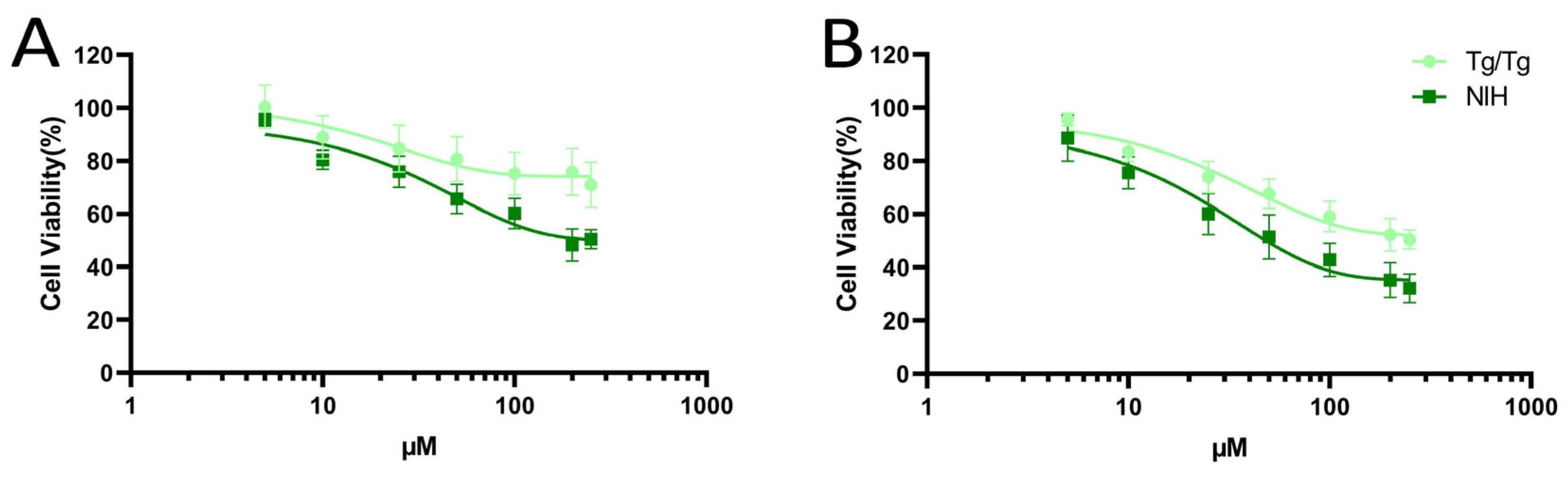
| NIH/3T3 | Tg/Tg | ||
|---|---|---|---|
| Fisetin | 24 h | >250 μM | >250 μM |
| 48 h | 55 ± 6 μM | 82 ± 5 μM | |
| Morin | 24 h | >250 μM | >250 μM |
| 48 h | >250 μM | >250 μM | |
| Fisetin/Morin 1:1 | 24 h | >250 μM | >250 μM |
| 48 h | 48 ± 7 μM | >250 μM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papa, G.; Simos, Y.V.; Athinodorou, A.-M.; Tsamis, K.I.; Peschos, D.; Angelidis, C.; Pappas, P.; Vezyraki, P. Exploring the Cellular Interactions of Flavonoids with Similar Structures in Cells Overexpressing the 70 kDa Human Heat Shock Protein. Appl. Biosci. 2024, 3, 137-150. https://doi.org/10.3390/applbiosci3010009
Papa G, Simos YV, Athinodorou A-M, Tsamis KI, Peschos D, Angelidis C, Pappas P, Vezyraki P. Exploring the Cellular Interactions of Flavonoids with Similar Structures in Cells Overexpressing the 70 kDa Human Heat Shock Protein. Applied Biosciences. 2024; 3(1):137-150. https://doi.org/10.3390/applbiosci3010009
Chicago/Turabian StylePapa, Garyfallia, Yannis V. Simos, Antrea-Maria Athinodorou, Konstantinos I. Tsamis, Dimitrios Peschos, Charalampos Angelidis, Periklis Pappas, and Patra Vezyraki. 2024. "Exploring the Cellular Interactions of Flavonoids with Similar Structures in Cells Overexpressing the 70 kDa Human Heat Shock Protein" Applied Biosciences 3, no. 1: 137-150. https://doi.org/10.3390/applbiosci3010009
APA StylePapa, G., Simos, Y. V., Athinodorou, A.-M., Tsamis, K. I., Peschos, D., Angelidis, C., Pappas, P., & Vezyraki, P. (2024). Exploring the Cellular Interactions of Flavonoids with Similar Structures in Cells Overexpressing the 70 kDa Human Heat Shock Protein. Applied Biosciences, 3(1), 137-150. https://doi.org/10.3390/applbiosci3010009







