Abstract
Dermatoses are essentially caused by infection or free radical aggression, immunoallergic disorders, or can be secondary to general diseases. Management of dermatoses by modern medicine is complex and costly, and the development of alternative treatments is urgent. Opilia amentacea Roxb. is a woody climber plant traditionally used in Burkina Faso for treatment of bad skin diseases. This study was carried out to evaluate the antimicrobial and antioxidant activities of extracts of O. amentacea and to characterize potent fractions. The antimicrobial activity was determined using the disc diffusion and microdilution methods, while antioxidant activity was assessed using the 2,2′-diphenyl-1-picrylhydrazyl radical (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS) and ferric reducing antioxidant power (FRAP) assays. The content of the plant extracts in polyphenols and flavonoids was also studied. The results revealed several secondary metabolites in the leaves, stems and root bark extracts of the plant, including sterols, triterpenes, and flavonoids and tannins, and a generally high total polyphenol and total flavonoid content. Dichloromethane fractions of leaves (FDFe) and stem barks (FDET) exhibited the best antioxidant activity and were the most active on Gram-positive bacilli. Hexane leaves (FHFe) and hexane root bark (FHER) fractions exhibited the best antifungal activity against Candida tropicalis. High correlation (R2 = 0.932) was found between the total flavonoid content of extracts and ferric-reducing antioxidant power. In view of these results, the present study describes O. amentacea as a potential source of antibacterial, antifungal and antioxidant agents and justifies the traditional uses of the plant as an anti-dermatosis plant.
1. Introduction
Dermatoses are a complex of diseases that manifest in the target organs, namely the skin, mucous membranes, and dander. Their prevalence has significantly increased in developing countries [1] because of promoting factors such as humidity, the aging of the general population, the increase in the number of bedridden patients, endocrine pathologies, and iatrogenic (transplant) and infectious (HIV/AIDS) immunosuppression, and the emergence of multi-resistant pathogens [2,3]. A study in Senegal reported that infectious dermatoses were responsible for 64.7% of dermatological emergencies in the dermatology department of the regional hospital of Thiès [4].
Dermatoses can occur in several contexts, and are due either to external aggression (microorganism, free radicals), immuno-allergic disorders, or secondary to general diseases such as endocrine diseases (diabetes, thyroid pathologies), liver diseases and vitamin deficiencies [5,6].
The most common infectious dermatoses are caused by Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli, Pseudomonas aeruginosa, and/or fungal strains such as Candida albicans and Candida tropicalis [1]. About one-third of the 126 cases of surgical-site secondary infection were due to S. aureus (39; 31%), followed by Escherichia coli (29; 23%), and Pseudomonas aeruginosa (12; 95%) according to a study carried out in Niger [7].
Nowadays, modern medicine encounters various inconveniences such as antibiotic resistance, high costs of active molecules, and difficulties for the most vulnerable groups in accessing treatments for microbial diseases due to the remoteness or absence of health services [8]. For this reason, many people use medicinal plants to treat infectious diseases [9].
Moreover, plants are important for pharmacological research and drug development, not only when bioactive molecules are used directly as therapeutic agents, but also as starting materials for drug synthesis or as models for pharmacologically active compounds [10].
Herbal preparations active on dermatosis should provide one or more of the following therapeutic activities: antiseptic, anti-inflammatory, antioxidant, healing, immunostimulant or hemostatic.
Numerous studies have shown that plant extracts possess biological properties such as immune system induction [11], anti-inflammatory [12], antioxidant [13], antimicrobial [14], antimutagenic and anticancer [15] properties. According to the literature, secondary metabolites such as polyphenols [16], triterpenes [17], and flavonoids [14] have antimicrobial, anti-inflammatory, antioxidant, healing, and fibroblast proliferation stimulation properties.
Therefore, the evaluation of the biological properties of medicinal plants claiming antimicrobial properties is attracting attention nowadays [18].
Opilia amentacea (Syn. Opilia celtidifolia) is one of the medicinal plants of the Burkinabe pharmacopeia used in the treatment of infectious dermatoses [19].
The genus Opilia (Opiliaceae) includes two species, namely O. amentacea and O. campestris. Opilia amentacea Roxb. (O. amentacea) is a West African woody climber plant, a heavily branched shrub or tree up to 10 m. O. amentacea grows in fringing forests and savannahs, often on anthills [20]. It is widespread from Senegal to Nigeria, and is dispersed over the drier parts of tropical Africa [11].
O. amentacea has been subjected to many investigations, including ethnobotanical, chemical, and pharmacological studies.
Ethnopharmacological surveys on O. amentacea have shown that West African traditional healers have used this medicinal plant widely to cure a various ailments. The leaves and roots of O. amentacea are mainly recommended by traditional healers in West Africa for the treatment of dermatoses, malaria, wounds, abdominal pain, internal worms, headaches, and fever [11]. Root powder is indicated for constipation, jaundice, liver cirrhosis and anorexia. The root decoction is purgative and diuretic. A leaf and root decoction is used against edema, leprosy and meningitis [21]. It is also used for healing wounds and ulcers [22]. In Mali, leaves are used against jaundice [23] and hepatitis [24]. In Benin, the stem barks of O. amentacea are widely used by traditional healers in the treatment of hepatitis B and C [25].
In Burkina Faso, O. amentacea is used in the country’s west for malaria treatment and the country’s center for skin diseases, hence the name “waagsalga”, referring to skin diseases in the local Moore language [19].
Because of the frequent and widespread use of medicinal plants in primary health care, many investigations have been carried out to scientifically validate the recipes of traditional medicine.
A screening for phytochemicals reported the presence of saponosides, coumarins, steroids, tannins, polyphenols, flavonoids, alkaloids and active polysaccharides [26,27] in the plant. Furthermore, previous pharmacological studies revealed that a saponin fraction of methanol extracts of the stem bark possess interesting biological properties, including intestinal antispasmodic, uterine stimulant, hypotensive and depression of the coronary outflow [28]. The polysaccharide fractions of the aqueous extracts of the leaves of O. amentacea showed a strong human complement fixing activity in vitro and causes hemolysis of 50% of sheep erythrocytes at about 0.9 μg/mL [29]. In another study, the pectic polysaccharides (Oc50A1.I.A) stimulated mouse protection against S. pneumoniae serotype 6B infection by inducing cytokine and chemokine production, which ensure protection against the bacteria [30]. Many studies have reported the antiplasmodial potency of O. amentacea. Chloroquine-resistant P. falciparum strains have been found to be effectively inhibited by dichloromethane extracts (IC50 = 4.01 and 2.08 µg/mL) [31,32], and moderately inhibited by ethyl acetate and dichloromethane extracts of the root bark (IC50 = 11 μg/mL) [33] and a crude alkaloid extract (IC50 = 6.9 µg/mL) [32].
With respect to anti-inflammatory potency, previous study has shown that the ethyl acetate fractions of O. celtidifolia possess membrane stabilization effect by inhibiting hypotonicity induced lyses of the RBC membrane meaning that the extract may also stabilize the lysosomal membrane [34], thus preventing the release of the lysosomal constituents of activated neutrophils such as bactericidal enzymes and proteases. These fractions also have proteinase inhibitory activity and lipoxygenase inhibitory activity at various concentrations [34].
In our preliminary investigation, the antimicrobial properties of aqueous and organic extracts (96° ethanol, 80° hydro-ethanol, n-hexane) of O. amentacea leaves, stems and root barks on American type culture collection (ATCC) reference strains showed an antibacterial activity. This study was undertaken to perform a more thorough chemical and biological investigation of the plant.
2. Materials and Methods
2.1. Plant Materials
The leaves, stem and root barks of O. amentacea Roxb. were harvested in January 2020 at Kombissiri, a city located in south-central Burkina Faso, about 42 km from Ouagadougou. The samples were identified and authenticated by mister Koura from the National Center for Scientific and Technological Research (CNRST), Ouagadougou, Burkina Faso, and a specimen was deposited in the herbarium under reference number 8730. The plant materials were washed thoroughly with water and dried in the MEPHATRA/PH department drying room at 25° under ventilation for about 4 weeks. Dry samples were later powdered with a mechanical grinder. The powders were stored in freezer bags away from light at room temperature for further use.
2.2. Microbial Strains
The antimicrobial effects were investigated on seven ATCC microbial strains, including two Gram-negative bacilli (Escherichia coli ATCC 25922; Pseudomonas aeruginosa ATCC 27653), three Gram-positive cocci (Staphylococcus aureus ATCC 25923; Streptococcus pyogenes ATCC 19615; Streptococcus agalactiae ATCC 13813) and two fungal strains (Candida albicans ATCC 90028; Candida tropicalis ATCC 750). These microbial strains were purchased from LGC standard distributor ATCC in South Africa.
2.3. Culture Media
Mueller Hinton agar (MH) (Liofilchem, lot: 032720504) was used for the isolation and susceptibility testing of S. aureus and Gram-negative bacilli (E. coli and Pseudomonas aeruginosa). Chocolate agar (GC Medium: Liofilchem, lot: 071320502) + Isovitalex (GC+IVx) were used for isolation and antibiogram of streptococci (S. pyogenes and S. agalactiae).
Sabouraud agar was used for the isolation and susceptibility testing of fungi strains (C. albicans and C. tropicalis).
MH (Liofilchem, lot: 011822506), Sabouraud (Liofilchem, lot: 07280501) and Brain Heart Broth (BHI) (Liofilchem, lot: 110420503) were used for Minimum Inhibitory Concentration (MIC) determination.
2.4. Chemicals and Standards
All the solvents used were of analytical quality. Ethanol, n-hexane and ethyl acetate were purchased from CARLO ERBA (Val de Reuil Cedex, France). 1-butanol and dichloromethane were purchased from Honeywell Specialty Chemicals (Seelze GmbH, Seelze, Germany). Dimethyl sulfoxide (DMSO) (≥99, 7%), vanillin (≥99%), sodium carbonate, ferric chloride, aluminum chloride (≥98%), 1,3,5-Triphenyltetrazolium Chloride (TTC), DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS (2,2′-azino-bis(3-ethylbenzothiazoline)-6-sulfonate), and Folin–Ciocalteu were purchased from Sigma-Aldrich (Laborchemikalien GmbH, Seelze, Germany). The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was purchased from EMD (Millipore Corp MW 414.3, Lot 3727633; Burlington, MA, USA). All the following standards were acquired from Sigma-Aldrich (St. Louis, MO, USA): Gallic acid, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), catechin, quercetin and ascorbic acid. Water was of Milli-Q-quality.
2.5. Plant Extract Preparation
One hundred grams of each sample powder were macerated at room temperature (25 ± 2 °C) for 24 h with 500 mL of ethanol 96°. The mixture was agitated in enclosed flasks using a stirrer (Modal T25 Digital Ultra Turrax) during maceration and filtered using a Buchner funnel and Whatman No. 5 filter paper. The filtrate was concentrated under decreased pressure with an evaporator (Heidolph Rotacool, Allemagne Type: Laborota 4003) at 40 °C. The concentrated extract was dried at 40 °C in an oven and the dry residue was weighed and stored at +4 °C until use.
Fractionation
The ethanolic extract was fractionated by liquid partition using organic solvents of increasing polarity: hexane, dichloromethane, ethyl acetate, and butanol and separatory funnel. Initially, 5 g of each crude ethanol extract was dissolved in 25 mL of distilled water and extracted with 25 mL of organic solvent (hexane, dichloromethane, ethyl acetate, and butanol successively). The experiment was performed three times for each solvent. The total organic phase was collected and concentrated at 40 °C using a rotary evaporator. Finally, the aqueous fraction was frozen and lyophilized. The dried fractions were stored at 4 °C.
2.6. Phytochemical Screening
Phytochemical screening was performed on the extracts using standard protocols and Thin-Layer Chromatography (TLC).
2.6.1. Characterization Reactions in Tubes
Crude ethanol extracts of O. amentacea were analyzed for the presence of secondary metabolites using standard procedures as described by Ciulei (1982) [35].
The principle is based on the ability of functional groups of these compounds to react with specific chemical reagents to give characteristic reactions.
Preparation of Ethanolic Extract Solutions
Crude ethanolic extract (1 g) was dissolved in 5 mL of ethanol 96% and 50 mL of distilled water was added. A part of the mixture at 20 mg/mL was hydrolyzed in an acid medium according to the following procedure:
Hydrolysis of Extract Solutions
First, 30 mL of the mixture (20 mg/mL) was mixed with 15 mL of 10% chlorohydric acid in a 100 mL ground-neck flask containing a few glass beads. The flask’s content was brought to boil under reflux for 30 min. After cooling, the hydrolyzed extract was extracted using liquid/liquid partitioning with 2 × 10 mL dichloromethane in a 100 mL separator funnel. The collected organic phases were dried on anhydrous sodium sulfate, filtered on Whatman n°5 paper, and concentrated under reduced pressure in the rotary evaporator.
The concentrated organic extracts were dispatched in test tubes to characterize O-heteroside compounds such as steroidal and triterpene glycosides, flavone, anthracene, coumarine derivatives and cardenolides through their genins.
The non-hydrolyzed solutions of ethanolic extract were used to screen phenolic compounds, reducing compounds, saponosides, alkaloid salts and anthocyanins.
Screening for Tannins
First, 1 mL of the ethanolic extract solution was diluted with 1 mL of distilled water in a test tube. After homogenization, two drops of a 2% ferric chloride solution were added to the content of the test tube. The appearance of a bluish-black or intense green color indicated the presence of tannins [26].
Screening for Flavonoids (Shibata or Cyanidin Reaction)
First, 1 mL of ethanolic extract solution was placed in a test tube containing some magnesium clippings. Then, 0.5 mL of concentrated hydrochloric acid (37%) was added. The appearance of a red or orange color after bubbling of the mixture indicated the presence of flavonols and flavanones, respectively [36].
Screening for Leucoanthocyans
First, 1 mL of ethanolic extract solution was mixed with 0.5 mL of hydrochloric butanol solution (Bate Smith reagent) over a bath of boiling water. The immediate appearance of a red color indicated a positive test for leucoanthocyans [31].
Screening for Anthracenosides/Anthraquinones (Bornträger Reaction)
First, 1 mL of the hydrolyzed organic extract was evaporated in a water bath. The dry residue was dissolved with 0.5 mL of dichloromethane (DCM). Then, 0.5 mL of a 25% ammonia solution was added. The mixture was shaken vigorously and allowed to stand. The formation of a cherry-red color in the aqueous phase indicated the presence of anthracenosides/anthraquinones [26].
Screening for Sterols and Terpenoids (Liebermann Burchard Reaction)
First, 1 mL of the hydrolyzed organic extract was evaporated in a water bath. The residue was dissolved in 0.5 mL of dichloromethane (DCM) and 0.5 mL of acetic anhydride.
The mixture was homogenized, followed by carefully adding 1 mL of concentrated sulfuric acid. The formation at the interface of the two liquids of a reddish-brown or reddish-purple ring with a greenish or purplish supernatant was a positive indication for the presence of steroids and terpenoids [36].
Screening for Coumarins and Derivatives (Feigl Reaction)
First, 1 mL of the hydrolyzed organic extract was evaporated in a water bath. The residue was dissolved in 1 mL of hot distilled water, shaken, and dispatched into two non-fluorescent hemolysis tubes (T and E). Distilled water (0.5 mL) was added to the T-tube (negative control), and 0.5 mL of a 10% ammonia solution was added to the E-tube (sample). The contents of both tubes (T and E) were observed under ultraviolet radiation at 254 nm and 365 nm. Intense greenish or bluish fluorescence in the E tube indicated the presence of coumarins and derivatives [36].
Screening for Saponosides by the Foam Test
First, 2 mL of ethanolic extract solution was mixed with 2 mL of distilled water in 16 mm diameter test tubes. The tubes were capped with parafilm and then shaken vigorously for 5 min. The development of a foam column with a height of at least 1 cm that persisted for 5 min indicated the presence of saponosides [36].
Screening for Cardiotonic Heterosides
First, 1 mL of the hydrolyzed organic extract was evaporated in a water bath. The residue was dissolved in 1 mL of 50% ethanolic solution and dispatched into two non-fluorescent hemolysis tubes (T1 and T2). Then, 0.5 mL of Baljet reagent was put into T1 and 0.5 mL of Kedde reagent was put into T2. Then, 0.5 mL of a 1N NaOH solution was added to both tubes, which were shaken and left to stand. The appearance of an orange (T1) and purplish (T2) color indicated the presence of cardiotonic heterosides [31].
Screening for Reducing Compounds
To the ethanolic extract solution, diluted 1/2 with distilled water, was added 1 mL of Fehling’s reagent (I + II). The mixture was heated over a boiling water bath. The appearance of a brick-red precipitate indicated the presence of reducing compounds [36].
Screening for Alkaloids
First, 10 mL of alcoholic extract solution was placed in a beaker and concentrated on a hot plate. The residual aqueous solution was diluted with 10 mL of alkalinized distilled water (pH = 8–9) with NaOH solution. The alkaline extract solution was extracted by liquid/liquid partitioning with 2 × 10 mL of DCM in a separatory funnel.
The organic phases were evaporated to dryness. The dry residue (alkaloid bases) was triturated with 0.5 mL of a 2% hydrochloric acid solution (alkaloid salts). The acid solution was dispatched into two watch glasses (V1 and V2). To the content of V1 were added two drops of Draggendorf’s reagent (potassium iodobismuthite), and to V2, two drops of Mayer’s reagent (potassium mercuri-ioduro). The appearance of precipitates of red or orange-yellow (V1) and whitish-yellow (V2) color indicated the presence of alkaloids [36].
Thin-Layer Chromatography (TLC)
The phytochemical analysis of O. amentacea hydroethanolic extracts of leaves (OCFe); stem bark (OCET) and root bark (OCER) was performed in 20 cm × 20 cm silica gel 60 F254 TLC plates (Macherey-Nagel, Düren, Germany). Extracts dissolved in 96 °C ethanol at the concentration of 10 mg/mL were used for thin-layer chromatographic analysis. The mobile phase used consisted of ethyl acetate, methanol, and distilled water (77; 13; 10). The prepared mobile phase was placed in TLC migration chambers. Sample solutions were applied onto the TLC plates using the capillary microtubes. Typically, 10 µL volumes of samples were applied. The distance between deposit points was 1 cm. The distances from the bottom edge were 1.5 cm, and they were 1 cm from the side edges. Plates were dried after development using a hairdryer and immersed vertically in the migration chambers containing mobile phases. The migration was performed over a path of 8 cm at 0.5 cm from the superior edge. After migration, the TLC plates were dried at room temperature.
The dried TLC plates were observed under white light and ultraviolet light at 254 nm and 365 nm. Secondary metabolites on the plates were revealed using 10% ferric chloride (FeCl3) for tannins, vanillin sulfuric acid for steroidal and triterpenic glycosides, anisaldehyde sulfuric acid for saponosides, and Neu’s reagent for flavonoids groups. The spraying of particular chemical reagents revealed the investigated secondary metabolite groups before observation.
2.6.2. Quantitative Phytochemical Assessment
Determination of Total Phenolics: Folin–Ciocalteu Method
The total phenolic contents (TPCs) of the O. amentacea ethanol crude extracts (OCFe, OCET, OCER) were determined using the Folin–Ciocalteu spectrophotometric method according to a method previously described by Koala et al. [37], with slight modifications. Phosphomolybdate reagent and sodium tungstate were reduced during phenol oxidation in alkaline medium to a mixture of tungsten blue and molybdenum.
Briefly, 25 μL of diluted sample solution (1 mg/mL) was mixed with 125 μL of diluted Folin–Ciocalteu reagent (FCR) solution (0.2 N). After incubation at room temperature for 5 min, 100 μL of a saturated sodium carbonate solution (7.5% in water) was added to the mixture. After 60 min in the dark and at 37 °C, the absorbance of the resulting blue hue was measured using a SHIMADZU UV-Vis spectrophotometer at 760 nm. Using the calibration curve equation Y = 0.0516x + 0.0673, R2 = 0.9936, the phenolic content of plant extracts was calculated. The data are reported as the equivalent of milligrams of gallic acid (GAE) per gram of dry weight. Each measurement was performed in triplicate (n = 3).
Determination of Total Flavonoids
The determination of the total flavonoid contents (TFCs) in the O. amentacea extracts was performed using the aluminum trichloride method with quercetin as a reference according to a method previously described by Koala et al. [37], with slight modifications. The method evaluates all compounds reacting with aluminum chloride (AlCl3). Briefly, crude ethanolic extract was exhaustively dissolved in methanol at room temperature at 1 mg/mL and filtered. Quercetin was used as standard, and a calibration curve consisted of concentrations ranging from 0.001 to 0.5 mg/mL of a quercetin solution. Then, 100 µL of the sample solution was mixed with 100 µL of a 2% methanol solution of aluminum trichloride (AlCl3). After incubation for1 h at room temperature, the absorbance of the supernatant was measured at 415 nm using a spectrophotometer. The TFC of the extract was obtained with respect to the absorbance read from the standard curve equation y = 0.0151x + 0.0593, R2 = 0.9992. The total flavonoid contents are expressed as mg quercetin equivalents per gram of dry weight. All determinations were performed in triplicate (n = 3).
2.7. Biological Properties
2.7.1. In Vitro Antimicrobial Assay
The in vitro antimicrobial activity of the fractions was assessed using the agar diffusion method to determine the inhibition zones and the microdilution method to determine the MIC and the minimum bactericidal concentration (MBC).
Disc Method in Agar Medium
The antimicrobial activity of the fractions on the reference strains was determined using the disk diffusion agar medium technique, according to a previous method [38]. Petri dishes containing 20 mL of appropriate agar medium were inoculated with an 18–24 h culture of bacterial strains from the nutrient broth. The inoculum was adjusted to about 108 UFC/mL with sterile saline solution.
Fractions were solubilized in DMSO at 100 mg/mL. Twenty-five microliters of each sample were applied separately to sterile filter paper disks (Whatman No 1; 6 mm in diameter) and placed on the surface of the inoculated medium.
Antibiotics (ciprofloxacin 5 µg, erythromycin 15 µg, and nystatin 100 IU) were used as a positive control, and diluted DMSO was used as a negative control. A swab-inoculated petri dish was used as a control for bacterial growth. Inoculated Petri dishes were placed in an oven under conditions according to the incubation conditions of the inoculated strains (Table 1). All tests were performed in triplicate.

Table 1.
Incubation conditions of the used microbial strains.
Microdilution Method
The MICs were determined using a 96-well microplate with samples having an inhibition zone ≥ 10 mm according to a method described previously [38]. Fresh colonies of each test strain were inoculated in sterile MH, sabouraud and Brain Heart Infusion (BHI) broths, and incubated according to the conditions of each strain. The overnight culture was appropriately diluted in broth to obtain viable counts of approximately 108 UFC/mL. Diluted solutions (0.025–25 mg/mL) of each sample were prepared from stock solutions of extract. To each microplate well, 100 µL of each diluted sample was added to 100 µL of inoculum prepared from each strain tested. DMSO 1/5 was used as a negative control. A growth control (inoculum alone) for each strain was also included in the test.
The microplates, covered by their lids were packed in plastic bags and incubated (5% CO2, 37 °C) for 24–48 h. Then, 25 µL of Triphenyltetrazolium Chloride (TTC) or MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) solution at 2 mg/mL was added to each well, and the plate was incubated at 37 °C. The indicator solution changed from clear to pink (TTC) to indicate bacterial activity, or from yellow to purple or dark blue (MTT) in the presence of fungal activity, while it remained clear when microbial growth was inhibited.
The MIC was defined as the lowest extract concentration at which visible growth of the test strain was inhibited. The minimum bactericidal concentration (MBC) was determined by spreading 0.1 mL of clear tubes that did not show any visible growth on appropriate agar plates and incubating according to the required conditions.
MBC was defined as the lowest extract concentration at which 99.99% of the test bacteria in the inoculum were killed. This corresponds to less than 100 CFU per 10 μL of well contents at concentrations below the MIC.
The tests were performed in triplicate on the same microplate for each sample, and the experiment was repeated three times.
Interpreting Antimicrobial Test Results
The sensitivity of the microbial strains to the fractions was interpreted on the basis of the criteria established by Ponce et al. [39], and their efficacy appreciated on the basis of the scale proposed by Kuete [40].
The BMC/MIC ratio was used to distinguish [41]:
- -
- Bactericidal extracts: BMC/MIC ≤ 2;
- -
- Bacteriostatic extracts: BMC/MIC > 2;
- -
- Extracts with an inhibition diameter ≥ 10 mm (selected for more investigation).
When the BMC/MIC ratio was ≥32, the strain was considered tolerant toward the extract.
2.7.2. Antioxidant Activity Assays
ABTS Radical Cation Scavenging Activity
The antioxidant activity using the ABTS radical cation (2,20-azino-bis(3-ethylbenzothiazoline)-6-Opisulphonic acid)) was assessed according to the Trolox equivalent antioxidant capacity (TEAC) assay, as previously described [42]. In brief, the ABTS radical cation (ABTS•+) solution was prepared by mixing 19.2 mg of ABTS and 3.12 mg of potassium persulphate into 5 mL of distilled water. The mixture was incubated at room temperature in the dark for 16 h. The ABTS•+ solution was then diluted with 80% ethanol (4.5 mL ABTS•+ in 220 mL ethanol). Then, 20 µL aliquots of samples in varying amounts were added to 200 µL of ABTS•+ solution and mixed thoroughly. The reaction mixture was allowed to stand at room temperature for 30 min, and the absorbance at 734 nm was immediately recorded using a spectrophotometer (SHIMADZU). A standard curve was obtained using Trolox standard solution at various concentrations in 80% ethanol. The calculation used to determine radical scavenging activity was as follows:
I% = [(Abs A0 − Abs A1)/AbsA0] × 100; where Abs A0 is the absorbance of analytical blank and Abs A1 is the absorbance in the presence of the extract solution.
The IC50 (concentration causing 50% inhibition) was determined graphically using a linear calibration curve by plotting the extract concentrations versus the associated scavenging action.
The absorbance of the reaction samples was compared to that of the Trolox standard, and the results are expressed as Trolox equivalents (TE) per gram of dry lyophilized extract (mg TE/g dw extract).
DPPH Radical Scavenging Activity
The antioxidant activity of O. amentacea extracts was evaluated using the 2,2-diphenyl-1-picrylhydrazyl radical scavenging method [37]. The process is based on the capacity of plant extracts to scavenge the DPPH radical (DPPH•) compared to Trolox in a dose–response curve. The DPPH radical absorbs visible light at a maximum wavelength of 517 nm and disappears upon reduction by an antioxidant compound [37].
In brief, 20 µL aliquots of samples in varying amounts were added to 200 µL of a DPPH–methanol solution. The 0.10 mM DPPH• solution was created by dissolving 4 milligrams of DPPH• in 100 milliliters of methanol. For the blank sample, 20 µL of methanol was added to 200 µL of DPPH•. After 30 min of incubation at room temperature in the dark, the spectrophotometric absorbance at 517 nm was measured (SHIMADZU). The calculation used to determine radical scavenging activity was as follows:
I% = [(Abs A0 − Abs A1)/AbsA0] × 100; where Abs A0 is the absorbance of analytical blank and Abs A1 is the absorbance in the presence of the extract solution.
The IC50 (concentration causing 50% inhibition) was determined graphically using a linear calibration curve by plotting the extract concentrations versus the associated scavenging action.
FRAP Assay
The ferric reducing antioxidant power (FRAP) assay was performed according to the method of Paré et al. [43], with slight modifications. In brief, 0.5 mL of various concentrations of sample extracts were mixed with 1.25 mL of 200 mmol/L sodium phosphate buffer (pH 6.6) and 1.25 mL of 1% potassium ferricyanide. The mixture was shaken vigorously and incubated at 50 °C for 30 min. After incubation, 1.25 mL of 10% trichloroacetic acid (w/v) was added, and then the mixture was centrifuged at 2000 rpm in a refrigerated centrifuge for 10 min. The upper layer (0.625 mL) was mixed with 0.625 mL of deionized water and 0.625 mL of 1% ferric chloride. The absorbance was measured using a spectrophotometer at 700 nm. Ascorbic acid (200–0.4 μg/mL; R2 = 0.99996) was used as the standard curve. The reduced compound (antioxidant) concentration in the extract is expressed as the molar equivalent of ascorbic acid (EAA) /g in the dry extract according to the following formula:
C = reducing compound concentration (mol EAA/g of dry extracts); c = sample concentration; D = dilution factor of the stock extract solution; M = molar mass of ascorbic acid (176 g/mol); Ci = concentration of the stock extract solution.
2.8. Statistical Analysis
Data are expressed as mean ± standard deviation (SD) of three experiments (n = 3). The results were analyzed using GraphPad Prism 5. One-way ANOVA was applied for the comparison of in vitro antioxidant activities of the extracts. The level of significance was considered to be p ≤ 0.05 for correlation studies and p ≤ 0.01 for in vitro studies.
3. Results and Discussion
3.1. Phytochemical Screening
3.1.1. Qualitative Phytochemical Screening
The results of the qualitative phytochemical screening of the crude ethanol extracts of O. amentacea (OCFe, OCET, OCER) are summarized in Table 2 and Figure 1. Secondary metabolites such as steroidal and triterpenes glycosides (saponosides), tannins, reducing compounds, anthraquinones, flavonoids, coumarins and its derivatives were detected in the extracts (Table 2). Leaves and stem barks of O. amentacea were found to be richer in secondary metabolites than roots. Phytochemicals like anthocyanins, alkaloids and cardenolides were not detected.

Table 2.
Phytochemical groups identified in O. amentacea extracts.
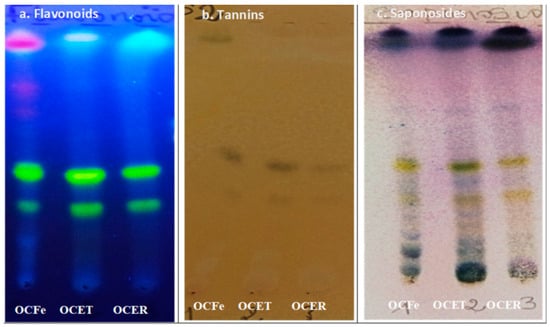
Figure 1.
Chromatogram of extracts from O. amentacea. OCFE: ethanol 96% extracts of O. amentacea leaves; OCET: ethanol 96% extracts of O. amentacea stem barks; OCER: ethanol 96% extracts of O. amentacea root barks.
The TLC analysis of the crude ethanol extract confirmed the presence of sterols, triterpenes, flavonoids and tannins.
The results of the phytochemical screening partly corroborate those of previous studies on O. amentacea or O. celtidifolia [27,44,45,46,47]. Secondary metabolites are bioactive compounds involved in the biological properties of plants [37] and their accumulation in O. amentacea crude ethanol extract could explain their medicinal properties. The detected chemical groups, including flavonoids, tannins, phenols, sterols, terpenes and reducing compounds, have been regularly reported in O. celtidifolia by several studies, despite the extraction process and solvent used [27,44,45,46,47]. Polyphenolic compounds are known for their diversity of biological properties [48], which could explain the possible antioxidant and antimicrobial effects of our plant extract. The flavonoid-rich fractions of O. celtidifolia showed antioxydant activity and moderate antihyperglycemia, antilipidemia, and antihypertensive properties [45]. Many sterols and saponins have been detected in the leaves and stem bark extracts of the plant [44,47]. Saponosides are well known for their ability to confer to the plant properties for decreasing the permeability of blood capillaries and reinforcing their resistance [27]. Aqueous extract from O. celtidifolia leaves, consisting only of tannins and saponins, possesses hepatoprotective effect against ethanol-induced hepatocellular injury [46]. Reducing compounds and polysaccharides were detected in almost all phytochemical screenings of the plant. The polysaccharide fractions from the leaves of O. amentacea possess immunomodulatory effects such as complement-fixing activity and the induction of a dose-dependent release of nitrite oxide by macrophages [29]. They can have an indirect antimicrobial effect by inducing the production of a range of cytokines and chemokines, ensuring protection against S. pneumoniae serotype 6B infection.
In contrast, alkaloids, which have regularly been reported in O. celtidifolia [22,26,47], were not identified in our extracts. This variation in chemical composition could be explained by the harvesting and drying conditions of the leaves, the climate of the harvesting area, and the chemical composition of the soil [27].
In addition to qualitative phytochemistry, a quantitative analysis of the total phenol and total flavonoid contents was carried out, as these two chemical groups represent major secondary metabolites with well-established pharmacological properties.
3.1.2. Quantitative Phytochemical Assessment
The quantitative assessment of the phytochemical constituents is summarized in Figure 2.
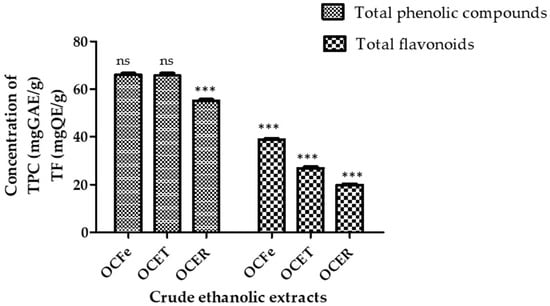
Figure 2.
Content of total phenolic compounds and total flavonoids. ns: not significant (p > 0.05); ***: p < 0.0001. Total phenolic compounds (TPC): OcFe (65.92 ± 0.93 mg GAE/g DWE); OCET (65.71 ± 0.57 mg GAE/g DWE); OCER (55.08 ± 0. 45 mg GAE/g DWE). Total flavonoids (TF): OcFe (38.8 ± 1.05 QE/g DWE); OCET (26.81 ± 0.19 QWE/g DE); OCER (19.54 ± 0.9 mg QE/g DWE).
The total phenolic content (TPC) of the ethanol extract of O. amentacea using the Folin–Ciocalteu reagent ranged from 65.92 ± 0.93 for leaves to 65.71 ± 0.57 for stem bark and 55.08 ± 0. 45 mg gallic acid equivalents/g dry weight of extract. Leaf extract showed the highest total content of phenolic compounds (65.92 ± 0.93 GAE/gDWE), followed by the stem bark (65.71 ± 0.57 GAE/gDWE) and the root bark (55.08 ± 0.45 GAE/gDWE). It has previously been recognized that the phenol content of any plant depends on intrinsic (genetic, extraction condition) and extrinsic (environment, handling and development stage) factors [49,50]. Plant polyphenols represent a large class of biologically active secondary metabolites [51]. Unfortunately, the Folin–Ciocalteu method does not give information on the different classes in addition to the quantity of phenolic compounds. A similar observation was reported by Konaté et al. [45] regarding ethyl acetate extracts, although our extracts possess a higher content. The polyphenol contents of the ethyl acetate fractions of O. celtidifolia were, in decreasing order, 62.01 ± 0.62 milligrams of gallic acid equivalents per gram (mg GAE/g) for leaves, 53.23 ± 0.18 mg GAE/g for leafy stems and 39.45 ± 0.01 mg GAE/g for roots.
In the same way, the total flavonoid contents of the ethyl acetate fraction of O.celtidifolia leaves, leafy stems and roots were 28.32 ± 0.01, 23.62 ± 0.68 and 5.31 ± 0.54 mg rutin equivalent per gram (mg RE/g), respectively [34]. This confirms the richness of O. amentacea in total polyphenols and flavonoids.
The total flavonoid contents (TFCs) showed a similar trend, ranging from 38.8 ± 1.05 for leaves to 26.81 ± 0.19 for stem bark and 19.54 ± 0.9 mg quercetin equivalents/g dry extract for root bark. As for the total phenolic content, the total flavonoid contents were ranked in the following order: root bark < stem bark < leaves. Higher contents of total polyphenols in aqueous leafy stems (89.712 mg GAE/g extract) and similar levels of total flavonoids (37.040 mg QAE/g extract) have previously been reported in Beninese samples [27]. Flavonoids are one of the largest classes of small molecular secondary metabolites produced in different parts of the plant [14]. They display a wide range of pharmacological effects that are beneficial for human health, which include, among others, antimicrobial activity, antioxidant activity, and anti-inflammatory and anticancer activities [13,14]. It has been reported that light intensity, temperature and altitude could influence the biosynthesis of flavonoids [52]. Their presence in our extracts could explain their possible antioxidant and antimicrobial effects.
3.2. Antimicrobial Activity
Sixteen fractions of O. amentacea were screened for antimicrobial activity against a panel of Gram-positive cocci, Gram-negative bacilli, and fungi. Table 3 and Table 4 present the results of the active fractions (seven in total). The hexane fractions of leaves and root bark, along with the dichloromethane fractions, showed the best inhibitory activities. The most susceptible bacteria in this study were Gram-positive cocci. The most apolar fractions were the most active (4/5). Using the disc diffusion method, the highest inhibitory effect was obtained with dichloromethane fractions (FDFe, FDET, and FDER) against Gram-positive cocci (S. aureus; S. pyogenes S. agalactiae) with inhibition diameters ranging from 12 to 15 mm on a standardized inoculum (108 CFU/mL). Their antimicrobial efficacy, with MICs ranging from 0.96 to 4.64 mg/mL, was very weak [40].

Table 3.
Antimicrobial activity of O. amentacea fractions determined by agar disc diffusion (Ø mm).

Table 4.
Antimicrobial parameters (MIC, MBC) of active extracts (n = 3).
No effect of the extracts was found on Gram-negative bacilli (E. coli and P. aeruginosa).
Furthermore, moderate fungicidal activity was found against C. tropicalis for both hexane leaf (FHFe) and hexane root bark (FHER) fractions, with an absence of growth at 0.23 mg/mL and 0.43 mg/mL, respectively. However, no effect was found on C. albicans.
As illustrated in Table 3 and Table 4, seven fractions of extracts demonstrated significant activity against selected microbial strains. The hexane fractions of leaves (FHFe) and roots (FHER) showed good antimicrobial activity. The hexane fractions of leaves and roots bark showed moderate fungicidal activity against C. tropicalis, with MICs of 0.23 and 0.43 mg/mL, respectively. These findings are consistent with a previous report that O. amentacea possesses antimicrobial activity [26].
Owolabi et al. [26] reported crude chloroform extracts of O. celtidifolia to possess moderate antibacterial activity against Bacillus cereus, E. coli, and P.aeruginosa (MIC = 156, 78, and 156 μg/mL, respectively) and a weak antibacterial activity against S. aureus (MIC = 625 µg/mL). Chloroform extracts of O. amentacea leaves were reported to possess a potent antifungal activity against Botrytis cinerea and Aspergillus niger, with respective MIC values of 0.156 μg/mL and 1.250 mg/mL [26].
The current results are also consistent with preliminary findings showing significant inhibitory activity of ethanolic extracts of the plant on S. pyogenes and S. agalactiae strains (MIC = 30 µg/mL and 240 µg/mL) [38]. Moreover, the decocted leaves and stem bark extracts showed almost the same inhibitory activity on C. albicans (21.67 and 24.33 mm) to nystatin (100 UI). On the other hand, our results differ from those reported in India, where the antibacterial potency of ethanol extract of the leaves on the diarrheal pathogens E. coli ATCC 25922, Salmonella enterica typhimurium, and Shigella dysentriae was revealed [47].
The phytochemical profile of O. amentacea consists mainly of polysaccharides, polyphenols, including flavonoids, saponosides, coumarins, reducing compounds and tannins such as gallotannins and ellagitannins [22,27,44]. This activity of the extract fractions would be due to the presence of chemical families identified as possessing antimicrobial properties, such as polyphenols, flavonoids, tannins, sterols and triterpenes and saponosides.
It can be found from the literature that phenolic components of herbal extracts are crucial secondary metabolites responsible for antimicrobial properties. The structure–activity relationship of phenol has been proven for p-hydroxy benzoic acid, and different functional groups with ester side chains have demonstrated effective antibacterial effects [53]. Several antimicrobial mechanisms of phenolic components have been described, among which are their interaction with bacterial proteins and cell wall structures, damage to cytoplasmic membranes, decrease in membrane fluidity, inhibition of nucleic acid synthesis and cell wall synthesis, and inhibition of energetic metabolism [51,54]. Among the many potential targets, the bacterial cell wall seems to be the primary molecular target for the antimicrobial action of most polyphenols. Gram-positive bacteria seem to be more susceptible to phenolic compounds, unlike Gram-negative bacteria, which seem to be protected by their outer membrane, which acts as a barrier, reducing the uptake of phenolic compounds [55].
Flavonoids are also more effective against different microbial strains [13]. Natural polyphenolics are distinguished by their core flavan, making them important components with a variety of pharmacological applications [53]. More specifically, flavonoid glycosides and lignans were isolated from an O. amentacea ethanolic extract of leaves. Flavonoid glycosides are well known to possess a broad-spectrum antimicrobial activity on the multi-drug-resistant Vibrio cholerae strains S. aureus, C. albicans and Cryptococcus neoformans [56]; the sugar moiety is an important factor determining their bioavailability [57]. The antimicrobial activity of flavonoid glycosides is a result of their ability to disrupt the permeability barrier of microbial membrane structures. However, structural features such as the presence of an aromatic ring, the sugar moiety, or the numbers of hydroxyl and methoxyl groups are essential for the compound’s antimicrobial properties [56]. Data on the biological properties of O. amentacea, and in particular its antimicrobial activity, are scarce, making it impossible to elucidate the contribution of each chemical group detected. Elsewhere, previous studies on another plant revealed tannins as having a more pronounced antimicrobial activity against Gram-positive bacilli, with the mechanism of this activity being attributable to their strong affinity for iron as well as to the inactivation of membrane-bound proteins [58].
Additionally, the potent fungicidal activity against C. tropicalis of fractions of the n-hexane leaf extract (MIC value of 0. 23 mg/mL) and the n-hexane root bark extract (MIC value of 0. 43 mg/mL) might be attributed to the presence of non-polar compounds such as terpenoids, as has previously been reported [50]. In light of this claim, the biological activity profile observed could be due either to an individual active molecule present in each crude extract, or to the synergistic effect of several molecules.
3.3. Antioxidant Activity
Ethanol (96°) extracts and fractions of O. amentacea were investigated for antioxidant activity using three assays to explore different aspects of antioxidant activity. The results of the free radical scavenging effect by DPPH and ABTS•+ assay are summarized in Table 5, while the ferric reducing power (FRAP) results are presented in Figure 3. The antioxidant potency, which is inversely proportional to the IC50 value, is more important when very small concentrations are required to scavenge half of the radicals, with higher FRAP values indicating better ferric reducing power [59].

Table 5.
Free radical scavenging activity of ABTS and DPPH.
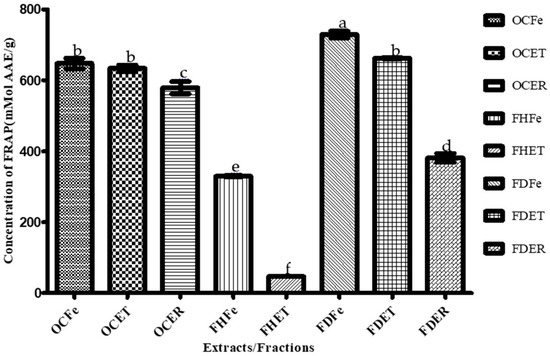
Figure 3.
Ferric reducing antioxidant power (FRAP) of O. amentacea extracts/fractions. Bars with different superscript (a–f) letters are significantly (p < 0.0001) different from one another; bars with the same superscript (b) letter are not significantly (p > 0.05) different.
The IC50 values obtained during the ABTS experiment ranged from 23.87 to 88.40 µg/mL for all extracts and fractions, indicating low antioxidant activity compared to Trolox (IC50 = 3.78 μg/mL). The hexane fractions of leaves (FHFe), stem barks (FHET) and root barks (FHER) were devoid of ABTS free radical scavenging activity. IC50 ranged from 853.68 for FHET to 1000 µg/mL for both FHFe and FHER. For DPPH radical scavenging activity, while only the dichloromethane fractions from leaves (FDFe) (IC50 = 463.33 µg/mL) and stem barks (FDET) (IC50 = 383.33 µg/mL) showed the lowest free radical scavenging values. The other extracts and fractions showed little to no DPPH activity (IC50 = 715.88–1000 µg/mL). Compared to the positive control Trolox, the free radical scavenging power of both the ABTS and DPPH tests is clearly very low (p < 0.0001). Our results are in agreement with those of studies carried out to investigate the antioxidant properties of O. amentacea [27,60], in which the authors reported ethanol 70% extract of leaves or aqueous extract of leaves of O. amentacea to possess a weak to moderate free radical scavenging power (IC50 = 250 μg/mL [60] and IC50 = 0.292 mg/mL, respectively) [27]. However, a strong antioxidant potential, revealed by hydrophilic and lipophilic assay, has been reported for the flavonoid-rich fractions of the leaves of O. amentacea (IC50 = 10 µg/mL) [45]. DPPH assay was used to estimate the ability of the sample to scavenge free radicals, which are species capable of causing damage to natural macromolecules, such as nucleic acids, polysaccharides and lipids [45].
A negative correlation, meaning that there was no relationship between total phenolic content of extracts/fractions and DPPH (R2 = −0.458) and ABTS (R2 = −0.534) scavenging activity, was observed (Table 5); however, extracts were found to be relatively rich in phenolic compounds. These results do not support the statement that the antioxidant activity of the plant extracts is correlated with the total phenolic content [61].
Dichloromethane fractions of the leaves and stem bark of O. amentacea showed the highest ferric reducing antioxidant power (FRAP) (741.15 and 662.84 µMol, respectively), followed by crude ethanolic extracts.
Iron reducing power translates into the electron donating property of the extracts, which have the ability to break the free radical chain by donating electrons, therefore decreasing the oxidative damage through the reduction of transition metals [16]. The efficiency of iron reduction implies the presence in the extracts of reducing mediators such as flavonoids, which are the main electron donors [62]. In the course of our experiment, a high correlation (R2 = 0.932) (Table 6) was found between total flavonoid content of extracts/fractions and ferric reducing antioxidant power. Several concurring studies have shown a reciprocal correlation between phenolic and flavonoid contents and antioxidant activity on the basis of DPPH, ABTS and FRAP assays. In our study, we noticed non-significant correlations between total flavonoids and the ABTS (p = −0.959) and DPPH (p = −0.931) tests, and between the FRAP and ABTS (p = −0.798) and the FRAP and DPPH (p = −630) tests. This observation could suggest that the reducing power exhibited by the different fractions is not only a function of total phenolic compounds, but of the presence of other constituents with antioxidant potential [63]. According to Magid et al., two lignans (compounds 10 and 11) were responsible for the antioxidant properties of ethanolic extracts of leaves of O. amentacea. The two lignans isolated from the ethanolic extract of leaves were identified as 5,5-dimethoxylariciresinol- 4-O-β-D-glucopyranoside (10) and (+) syringaresinol-4′-O-β-D-glucopyranoside, also named eleutheroside E1 (11) [60]. Compounds (10 and 11) demonstrated a good inhibitory effect via DPPH assay (IC50 = 85.1 and 42.1 μM, respectively). In the same study, the authors revealed that the two lignans (10–11) exhibit bi-functionality, having not just antioxidant activity, but also a tyrosinase inhibitory effect. In other studies, compound 11 has been reported to possess antioxidant activity (with IC50 = 104.2, 18 and 73.5 μg/mL being obtained via DPPH, ABTS and FRAP assays, respectively, in [64], and IC50 = 37.03 being obtained via DPPH assay in [65]). According to Meng et al. [66], radical scavenging activity depends on the conjugation between the B- and C-rings, which is affected by the structure, the number, and positions of hydroxyl groups, and the structural groups. This assumption was confirmed by “Załuski’s eleutheroside hypothesis”, in which it is suggested that the interaction of potential antioxidants with DPPH* depends on their structural conformation [65]. According to this hypothesis, the antioxidant mechanism of eleutheroside E1 is based on the complexation of DPPH* molecules with their aryl radicals. During this reaction, the aryl radicals of eleutheroside E1 (E1*) and DPPHH are created. Next, the aryl radicals (E1*) are complexed with other DPPH* molecules. Additionally, the aryl radicals can be stabilized by the presence of methoxy groups in the aromatic ring, which increases its antioxidative action. Based on this knowledge, it can be confirmed that O. amentacea possesses antioxidant properties that can only be valorized by purifying the molecules of interest.

Table 6.
Correlation between antioxidant activity and the total phenol and total flavonoid contents.
The dichloromethane fractions of the leaves and stem bark of the plant combined the highest FRAP values (741.15 and 662.84 mMol AAE/g) and the lowest IC50 for both the DPPH (463.33 and 383.33 µg/mL, respectively) and ABTS (88.40 and 36.85 µg/mL) assays, thus representing promising antioxidant candidates. These fractions were also the best antibacterial extracts. These fractions are still a mixture of several compounds, and this might explain their potency. Nevertheless, the isolation of appropriate molecules will make it possible to achieve good results. The hexane fractions, although lacking antioxidant activity, were the best antifungal fractions. A combination of these fractions could provide an effective broad-spectrum prototype against these susceptible microbial species.
4. Conclusions
The results of the present study indicate that Opilia amentacea Roxb. possesses numerous chemical compounds, including flavonoids, coumarins, and tannins, non-polar compounds like sterols and triterpenes, and reducing compounds. O. amentacea was found to be rich in polyphenols and flavonoids. Dichloromethane and hexane fractions have antibacterial and antifungal activities on Gram-positive cocci and C. tropicalis, respectively. The antimicrobial properties of plant extracts are nowadays well documented. These results indicate that the plant may be used for the treatment of infectious dermatoses caused by S. aureus, S. pyogenes, S. agalactiae and C. tropicalis species, but also for the treatment of non-infectious dermatoses as a result of the antioxidant activity of the extracts and the richness of the plant in flavonoids, which are known to be anti-inflammatory compounds. The polysaccharide fraction isolated from O. amentacea possesses immunomodulatory properties that could enhance the biological properties of the plant when used in vivo. Further pharmacological studies, including with respect to the inflammatory effect, toxicity and molecule isolation, are necessary.
Author Contributions
Conceptualization, O.Y., M.T.-C. and A.H.; methodology, B.R.H.M.-B., S.Y., B.Y., M.K. and N.O.; software, O.Y.; validation, M.T.-C., A.H. and B.Y.; formal analysis, B.R.H.M.-B., O.Y., B.Y. and T.K.T.; investigation, N.O.; resources, J.B.G.Y., E.K. and H.T.; data curation, O.Y.; writing—original draft preparation, O.Y.; writing—review and editing, S.Y., R.B., M.T.-C. and A.H.; supervision, M.T.-C. and A.H.; project administration, M.T.-C.; funding acquisition, M.T.-C. and R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fonds National de la Recherche et de l’Innovation pour le Développement (FONRID) under reference number AAP Rapide Covid19_mala infect_1-14 FONRID.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are reported in the manuscript.
Acknowledgments
The authors thank the National Laboratory of Public Health (LNSP) staff, the Laboratory of Biochemistry and Applied Chemistry (LABIOCA)/University Joseph KI-ZERBO of Ouagadougou and the Institute of Health Sciences Research (IRSS) through the Department of Medicine and Traditional Pharmacopoeia/Pharmacy (MEPHATRA/PH) and the Clinical Research Unit of Nanoro (CRUN). We thanks also Johannes Novak for his valuable contribution to the verification of the English content.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kandil, A. Prévalence Des Dermatoses En Médecine Communautaire à Marrakech. Ph.D. Thesis, Université Cadi Ayyad, Marrakech, Morocco, 2021. [Google Scholar]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida Glabrata, Candida Parapsilosis and Candida Tropicalis: Biology, Epidemiology, Pathogenicity and Antifungal Resistance. FEMS Microbiol. Rev. 2012, 36, 288–305. [Google Scholar] [CrossRef]
- Esposito, S.; Noviello, S.; Leone, S. Epidemiology and Microbiology of Skin and Soft Tissue Infections. Curr. Opin. Infect. Dis. 2016, 29, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Dione, H.; Bammo, M.; Lawson, A.T.D.; Seck, F.; Dioussé, P.; Guèye, N.; Faye, F.A.; Touré, P.S. Les Urgences Dermatologiques à l’hôpital Régional de Thiès/Sénégal: Une Série de 240 Cas. Rev. Afr. Med. Interne 2018, 5, 11–14. [Google Scholar]
- Villeneuve, D. Quelle Est l’épidémiologie de La Dermatoporose Dans Une Population de Médecine Générale En Île de France; Université Paris Descartes: Paris, France, 2017. [Google Scholar]
- Borda, L.J.; Louis, S.J.; Fethiere, M.; Dure, D.; Morrison, B.W. Prevalence of Skin Disease in Urban Haiti: A Cross-Sectional Study. Dermatology 2019, 235, 495–500. [Google Scholar] [CrossRef]
- Abdoulaye, O.; Laouali Harouna Amadou, M.; Amadou, O.; Adakal, O.; Magagi Larwanou, H.; Boubou, L.; Oumarou, D.; Abdoulaye, M.; Mamadou, S. Aspects Épidémiologiques et Bactériologiques Des Infections Du Site Opératoire (ISO) Dans Les Services de Chirurgie à l’Hôpital National de Niamey (HNN). Pan Afr. Med. J. 2018, 8688, 1–5. [Google Scholar] [CrossRef]
- Githiori, J.B.; Höglund, J.; Waller, P.J.; Baker, R.L. Anthelmintic Activity of Preparations Derived from Myrsine africana and Rapanea melanophloeos against the Nematode Parasite, Haemonchus contortus, of Sheep. J. Ethnopharmacol. 2002, 80, 187–191. [Google Scholar] [CrossRef]
- Saad, B.; Azaizeh, H.; Abu-Hijleh, G.; Said, O. Safety of Traditional Arab Herbal Medicine. Evid.-Based Complement. Altern. Med. 2006, 3, 433–439. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Grønhaug, T.E.; Ghildyal, P.; Barsett, H.; Michaelsen, T.E.; Morris, G.; Diallo, D.; Inngjerdingen, M.; Paulsen, B.S. Bioactive Arabinogalactans from the Leaves of Opilia celtidifolia Endl. Ex Walp. (Opiliaceae). Glycobiology 2010, 20, 1654–1664. [Google Scholar] [CrossRef]
- Hoffmann, J.; Gendrisch, F.; Schempp, C.M.; Wölfle, U. New Herbal Biomedicines for the Topical Treatment of Dermatological Disorders. Biomedicines 2020, 8, 27. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, P.; Baquero, L.P.; Larrota, H.R. Flavonoids: Potential Therapeutic Agents by Their Antioxidant Capacity. In Bioactive Compounds: Health Benefits and Potential Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 265–288. ISBN 9780128147757. [Google Scholar]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Zaid, H.; Silbermann, M.; Ben-Arye, E.; Saad, B. Greco-Arab and Islamic Herbal-Derived Anticancer Modalities: From Tradition to Molecular Mechanisms. Evid.-Based Complement. Altern. Med. 2012, 2012, 349040. [Google Scholar] [CrossRef] [PubMed]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef] [PubMed]
- Sawadogo, W.R.; Lompo, M.; Guissou, I.P.; Nacoulma, O.G. Dosage Des Triterpènes et Steroïdes de Dicliptera Verticillata et Évaluation de Leur Activité Anti-Inflammatoire Topique. Med. Afr. Noire 2008, 4, 55–62. [Google Scholar]
- Bhat, S.G. Medicinal Plants and Its Pharmacological Values. In Natural Medicinal Plants; Intechopen: London, UK, 2021; Volume 34, pp. 57–67. [Google Scholar] [CrossRef]
- Nacoulma, O.G. Plantes Médicinales et Pratiques Médicinales Traditionnelles: Cas Du Plateau Central; Université de Ouagadougou: Ouagadougou, Burkina Faso, 1996. [Google Scholar]
- Le, C.T.; Liu, B.; Barrett, R.L.; Lu, L.M.; Wen, J.; Chen, Z.D. Phylogeny and a New Tribal Classification of Opiliaceae (Santalales) Based on Molecular and Morphological Evidence. J. Syst. Evol. 2018, 56, 56–66. [Google Scholar] [CrossRef]
- Malgras, D. Arbres et Arbustes Guérisseurs Des Savanes Maliennes; Karthala et ACCT: Paris, France, 1992; ISBN 2865373770. [Google Scholar]
- Togola, A.; Karabinta, K.; Denou, A.; Haidara, M.; Sanogo, R.; Diallo, D. Effet Protecteur Des Feuilles de Opilia celtidifolia Contre l’ulcère Induit Par l’éthanol Chez Le Rat. Int. J. Biol. Chem. Sci. 2014, 8, 2416. [Google Scholar] [CrossRef][Green Version]
- Makan, S. Etude de l’activité Appétissante Du Décocté de Feuilles de Opilia celtidifolia Guill.et Perr (Opiliaceae) Chez Les Rats. Ph.D. Thesis, Université des Sciences, des Techniques et des Technologies de Bamako, Bamako, Mali, 2012; pp. 1–85. [Google Scholar]
- Sombie, E.N.; Tibiri, A.; N’do, J.Y.-P.; Traore, T.K.; Ouedraogo, N.; Hilou, A.; Guissou, P.I.; Nacoulma, O.G. Ethnobotanical Study and Antioxidant Activity of Anti-Hepatitis Plants Extracts of the COMOE Province, Burkina Faso. Int. J. Biol. Chem. Sci. 2018, 12, 1308. [Google Scholar] [CrossRef]
- Guinnin, F.; Sacramento, T.; Sezan, A.; Ategbo, J. Etude Ethnobotanique Des Plantes Médicinales Utilisées Dans Le Traitement Traditionnel Des Hépatites Virales B et C Dans Quelques Départements Du Bénin. Int. J. Biol. Chem. Sci. 2015, 9, 1354. [Google Scholar] [CrossRef][Green Version]
- Owolabi, M.S.; Omowonuola, A.A.; Lawal, O.A.; Dosoky, N.S.; Collins, J.T.; Ogungbe, I.V.; Setzer, W.N. Phytochemical and Bioactivity Screening of Six Nigerian Medicinal Plants. J. Pharmacogn. Phytochem. 2017, 6, 1430–1437. [Google Scholar]
- Bienvenu, G.; Morel, D.E.; Fidèle, A.M.; Huguette, A.B.; Toklo, P.M.; Djidénou, A.; Salomé, K.D.S.; Eléonore, L.Y.C.; Joachim, D. Phytochemical Study, Antioxidant and Anticonvulsant Activities of Aqueous Extract of Leaves of Opilia celtidifolia (Guill. Et Perr.) Endl. Ex Walp. Opiliaceae, from Benin. OSR J. Pharm. Biol. Sci. 2022, 17, 43–55. [Google Scholar]
- Shihata, I.M.; El-Gendi, A.Y.I.; Abd El–Malik, M.M. Pharmacochemical Studies on Saponin Fraction of Opilia celtidifolia. Planta Med. 1977, 31, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Togola, A.; Inngjerdingen, M.; Diallo, D.; Barsett, H.; Rolstad, B.; Michaelsen, T.E.; Smestad, B. Polysaccharides with Complement Fixing and Macrophage Stimulation Activity from Opilia celtidifolia, Isolation and Partial Characterisation. J. Ethno-Pharmacol. 2008, 115, 423–431. [Google Scholar] [CrossRef]
- Inngjerdingen, K.T.; Langerud, B.K.; Rasmussen, H.; Olsen, T.K.; Austarheim, I.; Grønhaug, T.E.; Aaberge, I.S.; Diallo, D.; Paulsen, B.S.; Michaelsen, T.E. Pectic Polysaccharides Isolated from Malian Medicinal Plants Protect against Streptococcus pneumoniae in a Mouse Pneumococcal Infection Model. Scand. J. Immunol. 2013, 77, 372–388. [Google Scholar] [CrossRef] [PubMed]
- Sangaré, D. Etude de La Prise En Charge Du Paludisme Par Les Thérapeutes Traditionnels Dans Les Aires de Santé de Kendie (Bandiagara) et de Finkolo AC (Sikasso); Université de Bamako: Bamako, Mali, 2003. [Google Scholar]
- Sanon, S.; Gansane, A.; Ouattara, L.P.; Traore, A.; Ouedraogo, I.N.; Tiono, A.; Taramelli, D.; Basilico, N.; Sirima, S.B. In Vitro Antiplasmodial and Cytotoxic Properties of Some Medicinal Plants from Western Burkina Faso. Afr. J. Lab. Med. 2013, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Traore, M. Phytochemical Studies of Plants Used as Antimalarials in Burkina Faso; University of Copenhagen: Copenhagen, Denmark, 2008. [Google Scholar]
- Konaté, K.; Arsène, M.; Yomalan, K.; Sytar, O.; Souza, A.; Brestic, M.; Dicko, M.H. In Vitro Antioxidant and Anti-Inflammatory Profiles of Bioactive Fraction from Opilia celtidifolia (Guill. & Perr.) Endl. Ex Walp (Opiliaceae). World J. Pharm. Res. 2019, 8, 141–156. [Google Scholar]
- Ciulei, I. Methodology for Analysis of Vegetable Drugs. Practical Manual on the Industrial Utilisation of Medicinal and Aromatic Plants; Faculty of Pharmacy: Bucharest, Romania, 1982. [Google Scholar]
- Kouliga, K.B.; Serge, Y.R.; Ollo, D.; Sibidou, Y.; Magloire, N.H.; Georges Anicet, O.; Bosco, O.J.; Coulibaly Maminata, T. In Vivo Antimalarial Activity, Safety and Phytochemical Screening of Canthium Multiflorum (Schumach. &Thonn.) Hiern (Rubiaceae). J Med. Plants Res. 2020, 10, 196–204. [Google Scholar]
- Koala, M.; Kaboré, B.; Rimwagna Ouedraogo, C.W.; Belemnaba, L.; Nitiema, M.; Compaoré, S.; Ouedraogo, S.; Ouedraogo, N.; Dabiré, C.M.; Kini, F.B.; et al. High-Performance Thin-Layer Chromatography Phytochemical Profiling, Antioxidant Activities, and Acute Toxicity of Leaves Extracts of Lannea velutina A. Rich. J. Med. Chem. Sci. 2023, 6, 410–423. [Google Scholar] [CrossRef]
- Youl, O.; Yougbare, S.; Lompo, P.; Yaro, B.; Tahita, C.M.; Tinto, H.; Hilou, A.; Traore/Coulibaly, M. Preliminary Screening of the Antimicrobial Activity of Nine Medicinal Plant Species from Burkina Faso. J. Med. Plants Res. 2021, 15, 522–530. [Google Scholar] [CrossRef]
- Ponce, A.G.; Fritz, R.; Del Valle, C.; Roura, S.I. Antimicrobial Activity of Essential Oils on the Native Microflora of Organic Swiss Chard. LWT Food Sci. Technol. 2003, 36, 679–684. [Google Scholar] [CrossRef]
- Kuete, V. Potential of Cameroonian Plants and Derived Products against Microbial Infections: A Review. Planta Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef]
- Marmonier, A. Introduction Aux Techniques d’étude Des Antibiotiques. Bacteriol. Med. Tech. Usuelles 1990, 1, 227–236. [Google Scholar]
- Bance, A.; Sourabié, S.; Compaoré, S.; Compaoré, E.; Belem-Kabre, W.L.M.E.; Ouedraogo, V.; Rouamba, A.; Ouedraogo, N.; Kiendrebeogo, M. Therapeutic Properties of Aqueous Extracts of Leaves and Stems Bark of Prosopis africana (Guill. & Perr.) Taub. (Fabaceae) Used in the Management of Dental Caries. J. Drug Deliv. Ther. 2021, 11, 108–114. [Google Scholar] [CrossRef]
- Paré, D.; N’do, J.Y.P.; Hilou, A. Phytochemical and Biological Investigation of 5 Bioactive Fractions of Caralluma acutangula, a Medicinal Plant Used in Traditional Medicine in Northern of Burkina Faso. GSC Biol. Pharm. Sci. 2020, 11, 81–91. [Google Scholar] [CrossRef]
- Koumare, B.; Diallo, D.; Sanogo, R.; Diarra, B. Study of Phytochemistry and Appetizing Activity of Decocted Leaves of Opilia celtidifolia Guill. et Perr. (OPILIACEAE) in Rats. EasyChair, 2020; preprint. [Google Scholar]
- Konaté, K.; Yomalan, K.; Sytar, O.; Zerbo, P.; Brestic, M.; Patrick, V.D.; Gagniuc, P.; Barro, N. Free Radicals Scavenging Capacity, Antidiabetic and Antihypertensive Activities of Flavonoid-Rich Fractions from Leaves of Trichilia emetica and Opilia amentacea in an Animal Model of Type 2 Diabetes Mellitus. Evid.-Based Complement. Altern. Med. 2014, 2014, 867075. [Google Scholar] [CrossRef]
- Amang, A.P.; Kodji, E.; Mezui, C.; Baane, M.P.; Siwe, G.T.; Kuissu, T.M.; Emakoua, J.; Tan, P.V. Hepatoprotective Effects of Aqueous Extract of Opilia celtidifolia (Opiliaceae) Leaves against Ethanol-Induced Liver Damage in Rats. Evid.-Based Complement. Altern. Med. 2020, 2020, 6297475. [Google Scholar] [CrossRef]
- Bhuvaneswari, D.S.; Subashini, G.; Renugadevi, B. Antimicrobial Activity and Phytochemical Analysis of Opilia amentaceae Medicinal Plant Extracts. J. Emerg. Technol. Innov. Res. 2019, 6, 346–354. [Google Scholar]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Medini, F.; Fellah, H.; Ksouri, R.; Abdelly, C. Total Phenolic, Flavonoid and Tannin Contents and Antioxidant and Antimicrobial Activities of Organic Extracts of Shoots of the Plant. J. Taiba Univ. Sci. 2014, 8, 216–224. [Google Scholar] [CrossRef]
- Tadić, V.; Oliva, A.; Božović, M.; Cipolla, A.; De Angelis, M.; Vullo, V.; Garzoli, S.; Ragno, R. Chemical and Antimicrobial Analyses of Sideritis romana L. Subsp. purpurea (Tal. Ex Benth.) Heywood, an Endemic of the Western Balkan. Molecules 2017, 22, 1395. [Google Scholar] [CrossRef]
- Slobodníková, L.; Fialová, S.; Rendeková, K.; Kováč, J.; Mučaji, P. Antibiofilm Activity of Plant Polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L.; Määttä-Riihinen, K.; Kärenlampi, S.; Hohtola, A. Activation of Flavonoid Biosynthesis by Solar Radiation in Bilberry (Vaccinium myrtillus L.) Leaves. Planta 2004, 218, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Dubale, S.; Kebebe, D.; Zeynudin, A.; Abdissa, N.; Suleman, S. Phytochemical Screening and Antimicrobial Activity Evaluation of Selected Medicinal Plants in Ethiopia. J. Exp. Pharmacol. 2023, 15, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, R.; Ibrahim, S.A. Natural Products as Antimicrobial Agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Habbal, O.; Hasson, S.S.; El-Hag, A.H.; Al-Mahrooqi, Z.; Al-Hashmi, N.; Al-Bimani, Z.; Al-Balushi, M.S.; Al-Jabri, A.A. Antibacterial Activity of Lawsonia Inermis Linn (Henna) against Pseudomonas Aeruginosa. Asian Pac. J. Trop. Biomed. 2011, 1, 173–176. [Google Scholar] [CrossRef]
- Tagousop, C.N.; Tamokou, J.D.D.; Ekom, S.E.; Ngnokam, D.; Voutquenne-Nazabadioko, L. Antimicrobial Activities of Flavonoid Glycosides from Graptophyllum Grandulosum and Their Mechanism of Antibacterial Action. BMC Complement. Altern. Med. 2018, 18, 252. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef]
- Skenderidis, P.; Leontopoulos, S.; Petrotos, K.; Mitsagga, C.; Giavasis, I. The in Vitro and in Vivo Synergistic Antimicrobial Activity Assessment of Vacuum Microwave Assisted Aqueous Extracts from Pomegranate and Avocado Fruit Peels and Avocado Seeds Based on a Mixtures Design Model. Plants 2021, 10, 1757. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary Polyphenols, Oxidative Stress and Antioxidant and Anti-Inflammatory Effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Magid, A.A.; Abdellah, A.; Pecher, V.; Pasquier, L.; Harakat, D.; Voutquenne-Nazabadioko, L. Flavonol Glycosides and Lignans from the Leaves of Opilia amentacea. Phytochem. Lett. 2017, 21, 84–89. [Google Scholar] [CrossRef]
- Parikh, B.; Patel, V.H. Quantification of Phenolic Compounds and Antioxidant Capacity of an Underutilized Indian Fruit: Rayan [Manilkara Hexandra (Roxb.) Dubard]. Food Sci. Hum. Wellness 2017, 6, 10–19. [Google Scholar] [CrossRef]
- Kada, S. Recherche D’extraits de Plantes Médicinales Doués d’activités Biologiques; Université Ferhat Abbas Sétif 1: Setif, Algeria, 2018. [Google Scholar]
- Beddou, M.F. Etude Phytochimique et Activités Biologiques de Deux Plantes Médicinales Sahariennes Rumex Vesicarius L. et Anvillea Radiata Coss. & Dur; Université Abou Bekr Belkaid: Tlemcen, Algeria, 2015. [Google Scholar]
- Huang, S.W.; Qiao, J.W.; Sun, X.; Gao, P.Y.; Li, L.Z.; Liu, Q.B.; Sun, B.; Wu, D.L.; Song, S.J. Secoiridoids and Lignans from the Leaves of Diospyros kaki Thunb. with Antioxidant and Neuroprotective Activities. J. Funct. Foods 2016, 24, 183–195. [Google Scholar] [CrossRef]
- Załuski, D.; Kuźniewski, R.; Janeczko, Z. HPTLC-Profiling of Eleutherosides, Mechanism of Antioxidative Action of Eleutheroside E1, the PAMPA Test with LC/MS Detection and the Structure–Activity Relationship. Saudi J. Biol. Sci. 2018, 25, 520–528. [Google Scholar] [CrossRef]
- Meng, Q.; Yang, Z.; Jie, G.; Gao, Y.; Zhang, X.; Li, W.; Li, B.; Tu, Y. Evaluation of Antioxidant Activity of Tea Polyphenols by a Quantum Chemistry Calculation Method—PM6. J. Food Nutr. Res. 2014, 2, 965–972. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).