Effect of 2-Aminoethoxydiphenyl Borate (2-APB) on Heart Rate and Relation with Suppressed Calcium-Activated Potassium Channels: Larval Drosophila Model
Abstract
Highlights
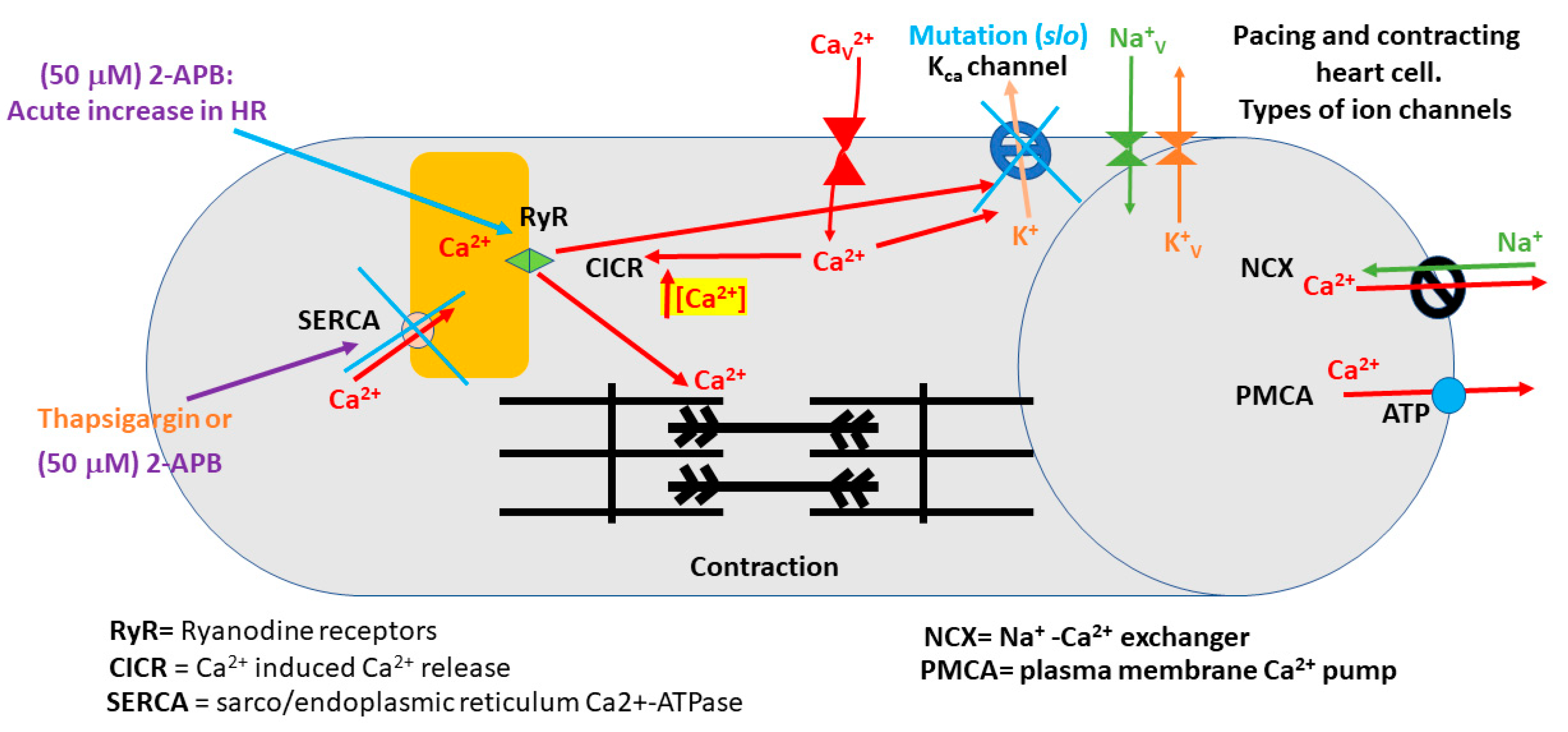
- 2-APB slowed heart rate at a high concentration (>10 µM) in the slo mutant line.
- The slo strain and CS strain both increased heart rate when exposed to 5-HT.
- The slo strain and CS strain both increased heart rate when exposed to 5-HT following 2-APB incubation.
- No differences were measured between the use of thapsigargin or 2-APB on heart rate with exposure to 5-HT between the slo and CS strain.
Abstract
1. Introduction
2. Methods
2.1. Animals
2.2. Dissection and Procedures
2.3. Statistical Methods
3. Results
3.1. Effect of 2-APB on Heart Rate in CS and Slo Strains
3.2. Effect of 2-APB and 5-HT on Heart Rate in CS and Slo
3.3. Effect of Thapsigargin and 5-HT on Heart Rate in CS and Slo
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olvera Lopez, E.; Ballard, B.D.; Jan, A. Cardiovascular Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK535419/ (accessed on 1 May 2023).
- Waller, B.F.; Gering, L.E.; Branyas, N.A.; Slack, J.D. Anatomy, histology, and pathology of the cardiac conduction system—Part III. Clin. Cardiol. 1993, 16, 436–442. [Google Scholar] [CrossRef]
- Marks, A.R. Calcium cycling proteins and heart failure: Mechanisms and therapeutics. J. Clin. Investig. 2013, 123, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Hadri, L.; Hajjar, R.J. Calcium cycling proteins and their association with heart failure. Clin. Pharmacol. Ther. 2011, 90, 620–624. [Google Scholar] [CrossRef]
- Landstrom, A.P.; Dobrev, D.; Wehrens, X.H.T. Calcium Signaling and Cardiac Arrhythmias. Circ. Res. 2017, 120, 1969–1993. [Google Scholar] [CrossRef] [PubMed]
- Lesnefsky, E.J.; Chen, Q.; Tandler, B.; Hoppel, C.L. Mitochondrial dysfunction and myocardial ischemia-reperfusion implications for novel therapies. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 535–565. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Shi, L.; Chen, X.; Cui, C.; Huang, J.; Chen, B.; Hall, D.D.; Pan, Z.; Lu, M.; et al. Integrin β1D deficiency-mediated RyR2 dysfunction contributes to catecholamine-sensitive ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy. Circulation 2020, 141, 1477–1493. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.L.; Bai, Y.L.; Cai, B.Z. Calcium-activated potassium channels: Potential target for cardiovascular diseases. Adv. Protein Chem. Struct. Biol. 2016, 104, 233–261. [Google Scholar]
- Weisbrod, D. Small and intermediate calcium activated potassium channels in the heart: Role and strategies in the treatment of cardiovascular diseases. Front Physiol. 2020, 11, 590534. [Google Scholar] [CrossRef]
- Pineda, S.; Nikolova-Krstevski, V.; Leimena, C.; Atkinson, A.J.; Altekoester, A.K.; Cox, C.D.; Jacoby, A.; Huttner, I.G.; Ju, Y.K.; Soka, M.; et al. Conserved Role of the large conductance calcium-activated potassium channel, KCa1.1, in sinus node function and arrhythmia risk. Circ. Genom. Precis. Med. 2021, 14, e003144. [Google Scholar] [CrossRef]
- Bailey, C.S.; Moldenhauer, H.J.; Park, S.M.; Keros, S.; Meredith, A.L. KCNMA1-linked channelopathy. J. Gen. Physiol. 2019, 151, 1173–1189. [Google Scholar] [CrossRef]
- Frankenreiter, S.; Bednarczyk, P.; Kniess, A.; Bork, N.I.; Straubinger, J.; Koprowski, P.; Wrzosek, A.; Mohr, E.; Logan, A.; Murphy, M.P.; et al. cGMP-elevating compounds and ischemic conditioning provide cardioprotection against ischemia and reperfusion injury via cardiomyocyte-specific BK channels. Circulation 2017, 136, 2337–2355. [Google Scholar] [CrossRef]
- Imlach, W.L.; Finch, S.C.; Miller, J.H.; Meredith, A.L.; Dalziel, J.E. A role for BK channels in heart rate regulation in rodents. PLoS ONE 2010, 5, e8698. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.H.; Wu, Y.; Gao, Z.; Anderson, M.E.; Dalziel, J.E.; Meredith, A.L. BK channels regulate sinoatrial node firing rate and cardiac pacing in vivo. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1327–H1338. [Google Scholar] [PubMed]
- Patel, N.H.; Johannesen, J.; Shah, K.; Goswami, S.K.; Patel, N.J.; Ponnalagu, D.; Kohut, A.R.; Singh, H. Inhibition of BKCa negatively alters cardiovascular function. Physiol. Rep. 2018, 6, e13748. [Google Scholar] [PubMed]
- Taghli-Lamallem, O.; Plantié, E.; Jagla, K. Drosophila in the heart of understanding cardiac diseases: Modeling channelopathies and cardiomyopathies in the fruitfly. J. Cardiovasc. Dev. Dis. 2016, 3, 7. [Google Scholar] [CrossRef]
- Souidi, A.; Jagla, K. Drosophila heart as a model for cardiac development and diseases. Cells 2021, 10, 3078. [Google Scholar] [CrossRef]
- Chen, Y.F.; Lin, P.C.; Yeh, Y.M.; Chen, L.H.; Shen, M.R. Store-operated Ca2+ entry in tumor progression: From molecular mechanisms to clinical implications. Cancers 2019, 11, 899. [Google Scholar] [CrossRef]
- Schild, A.; Bhardwaj, R.; Wenger, N.; Tscherrig, D.; Kandasamy, P.; Dernič, J.; Baur, R.; Peinelt, C.; Hediger, M.A.; Lochner, M. synthesis and pharmacological characterization of 2-aminoethyl diphenylborinate (2-APB) derivatives for inhibition of store-operated calcium entry (SOCE) in MDA-MB-231 breast cancer cells. Int. J. Mol. Sci. 2020, 21, 5604. [Google Scholar] [CrossRef]
- Pan, J.; Wei, Y.; Ni, L.; Li, X.; Deng, Y.; Xu, B.; Yang, T.; Sun, J.; Liu, W. Unbalanced ER-mitochondrial calcium homeostasis promotes mitochondrial dysfunction and associated apoptotic pathways activation in methylmercury exposed rat cortical neurons. J. Biochem. Mol. Toxicol. 2022, 36, e23136. [Google Scholar] [CrossRef]
- Ma, H.T.; Venkatachalam, K.; Parys, J.B.; Gill, D.L. Modification of store-operated channel coupling and inositol trisphosphate receptor function by 2-aminoethoxydiphenyl borate in DT40 lymphocytes. J. Biol. Chem. 2002, 277, 6915–6922. [Google Scholar]
- Bilmen, J.G.; Wootton, L.L.; Godfrey, R.E.; Smart, O.S.; Michelangeli, F. Inhibition of SERCA Ca2+ pumps by 2-aminoethoxydiphenyl borate (2-APB): 2-APB reduces both Ca2+ binding anphosphoryl transfer from ATP, by interfering with the pathway leading to the Ca2+-binding sites. Eur. J. Biochem. 2002, 269, 3678–3687. [Google Scholar] [CrossRef] [PubMed]
- Bootman, M.D.; Collins, T.J.; Mackenzie, L.; Roderick, H.L.; Berridge, M.J.; Peppiatt, C.M. 2-Aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002, 16, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Kanaji, T.; Nakade, S.; Kanno, T.; Mikoshiba, K. 2APB, 2-aminoethoxydiphenylborate, a membrane-penetrable modulator of ins(l,4,5) P3-induced Ca2+ release. J. Biochem. 1997, 122, 498–505. [Google Scholar] [CrossRef]
- Hu, H.Z.; Gu, Q.; Wang, C.; Colton, C.K.; Tang, J.; Kinoshita-Kawada, M.; Lee, L.Y.; Wood, J.D.; Zhu, M.X. 2-Aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J. Biol. Chem. 2004, 279, 35741–35748. [Google Scholar] [CrossRef]
- Chokshi, R.; Fruasaha, P.; Kozak, J.A. 2-aminoethyl diphenyl borinate (2-APB) inhibits TRPM7 channels through an intracellular acidification mechanism. Channels 2012, 6, 362–369. [Google Scholar] [CrossRef]
- Dixon, R.E.; Britton, F.C.; Baker, S.A.; Hennig, G.W.; Rollings, C.M.; Sanders, K.M.; Ward, S.M. Electrical slow waves in the mouse oviduct are dependent on extracellular and intracellular calcium sources. Am. J. Physiol. Cell Physiol. 2011, 301, C1458–C1469. [Google Scholar] [CrossRef]
- Morgan, J.P. Abnormal intracellular modulation of calcium as a major cause of cardiac contractile dysfunction. N. Engl. J. Med. 1991, 325, 625–632. [Google Scholar] [CrossRef]
- Chien, K.R. Stress pathways and heart failure. Cell 1999, 98, 555–558. [Google Scholar] [CrossRef]
- Hove-Madsen, L.; Llach, A.; Bayes-Genís, A.; Roura, S.; Font, E.R.; Arís, A.; Cinca, J. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation 2004, 110, 1358–1363. [Google Scholar] [CrossRef]
- Dorn, G.W.; Maack, C. SR and mitochondria: Calcium cross-talk between kissing cousins. J. Mol. Cell. Cardiol. 2013, 55, 42–49. [Google Scholar] [CrossRef]
- Williams, G.S.; Boyman, L.; Lederer, W.J. Mitochondrial calcium and the regulation of metabolism in the heart. J. Mol. Cell Cardiol. 2015, 78, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Bier, E.; Bodmer, R. Drosophila, an emerging model for cardiac disease. Gene 2004, 342, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cammarato, A.; Ahrens, C.H.; Alayari, N.N.; Qeli, E.; Rucker, J.; Reedy, M.C.; Zmasek, C.M.; Gucek, M.; Cole, R.N.; Van Eyk, J.E.; et al. A mighty small heart: The cardiac proteome of adult Drosophila melanogaster. PLoS ONE 2011, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Ocorr, K.; Reeves, N.L.; Wessells, R.J.; Fink, M.; Chen, H.S.V.; Akasaka, T.; Yasuda, S.; Metzger, J.M.; Giles, W.; Posakony, J.W.; et al. KCNQ potassium channel mutations cause cardiac arrhythmias in Drosophila that mimic the effects of aging. Proc. Natl. Acad. Sci. USA 2007, 104, 3943–3948. [Google Scholar] [CrossRef]
- Wolf, M.J.; Amrein, H.; Izatt, J.A.; Choma, M.A.; Reedy, M.C.; Rockman, H.A. Drosophila as a model for the identification of genes causing adult human heart disease. Proc. Natl. Acad. Sci. USA 2006, 103, 1394–1399. [Google Scholar] [CrossRef]
- Perrin, L.; Röder, L. Pathologies et vieillissement cardiaque–Les leçons d’un tout petit cœur [Cardiac pathologies and aging: Lessons from a tiny heart]. Med. Sci. 2016, 32, 470–477. (In French) [Google Scholar]
- Hüser, J.; Blatter, L.A.; Lipsius, S.L. Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells. J. Physiol. 2000, 524, 415–422. [Google Scholar] [CrossRef]
- Subramani, S.; Subbanna, P.K. Calcium-transporters in myocardial cells. Indian J. Physiol. Pharmcol. 2006, 50, 99–113. [Google Scholar]
- Opthof, T. Embryological development of pacemaker hierarchy and membrane currents related to the function of the adult sinus node: Implications for autonomic modulation of biopacemakers. Med. Biol. Eng. Comput. 2007, 45, 119–132. [Google Scholar] [CrossRef]
- Gu, G.G.; Singh, S. Pharmacological analysis of heartbeat in Drosophila. J. Neurobiol. 1995, 28, 269–280. [Google Scholar] [CrossRef]
- Bers, D.M. Cardiac excitation-contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef]
- Philipson, K.D.; Nicoll, D.A. Sodium-calcium exchange: A molecular perspective. Annu. Rev. Physiol. 2000, 62, 111–133. [Google Scholar] [CrossRef]
- Kapur, N.; Banach, K. Inositol-1,4,5-trisphosphate-mediated spontaneous activity in mouse embryonic stem cell-derived cardiomyocytes. J. Physiol. 2007, 581, 1113–1127. [Google Scholar] [CrossRef] [PubMed]
- Sanders, L.; Rakovic, S.; Lowe, M.; Mattick, P.A.; Terrar, D.A. Fundamental importance of Na+–Ca2+ exchange for the pacemaking mechanism in guinea-pig sino-atrial node. J. Physiol. 2006, 571, 639–649. [Google Scholar] [CrossRef]
- Vinogradova, T.M.; Maltsev, V.A.; Bogdanov, K.Y.; Lyashkov, A.; Lakatta, E.G. Rhythmic Ca2+ oscillations drive sinoatrial nodal cell pacemaker function to make the heart tick. Ann. N. Y. Acad. Sci. 2005, 1047, 138–156. [Google Scholar] [CrossRef]
- Maltsev, V.A.; Lakatta, E.G. Dynamic interactions of an intracellular Ca2+ clock and membrane ion channel clock underlie robust initiation and regulation of cardiac pacemaker function. Cardiovasc. Res. 2008, 77, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Salkoff, L.; Butler, A.; Ferreira, G.; Santi, C.; Wei, A. High-conductance potassium channels of the SLO family. Nat. Rev. Neurosci. 2006, 7, 921–931. [Google Scholar] [CrossRef]
- Atkinson, N.S.; Robertson, G.A.; Ganetzky, B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science 1991, 253, 551–555. [Google Scholar] [CrossRef]
- Elkins, T.; Ganetzky, B.; Wu, C.-F. A Drosophila mutation that eliminates a calcium-dependent potassium current. Proc. Natl. Acad. Sci. USA 1986, 83, 8415–8419. [Google Scholar] [CrossRef]
- Schopperle, W.M.; Holmqvist, M.H.; Zhou, Y.; Wang, J.; Wang, Z.; Griffith, L.C.; Keselman, I.; Kusinitz, F.; Dagan, D.; Levitan, I.B. Slob, a novel protein that interacts with the Slowpoke calcium-dependent potassium channel. Neuron 1998, 20, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Pineda, S. 2016. The KCNMA1 Drosophila Ortholog Slowpoke in Drosophila melanogaster Cardiac Function and Human Disease. UC San Diego. ProQuest ID: Pineda_ucsd_0033D_15898. Merritt ID: ark:/13030/m5pw17cr. Available online: https://escholarship.org/uc/item/62z6w2j6 (accessed on 16 May 2023).
- Brenner, R.; Chen, Q.H.; Vilaythong, A.; Toney, G.M.; Noebels, J.L.; Aldrich, R.W. BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat. Neurosci. 2005, 8, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Majeed, Z.R.; Stacy, A.; Cooper, R.L. Pharmacological identification of serotonin receptor subtypes on Drosophila larval heart. J. Comp. Physiol. B 2014, 184, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Cooper, R.L. Direct influence of serotonin on the larval heart of Drosophila melanogaster. J. Comp. Physiol. B 2006, 176, 349–357. [Google Scholar] [CrossRef]
- De Castro, C.; Titlow, J.; Majeed, Z.R.; Cooper, R.L. Analysis of various physiological salines for heart rate, CNS function, and synaptic transmission at neuromuscular junctions in Drosophila melanogaster larvae. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2014, 200, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Dowse, H.; Ringo, J.; Power, J.; Johnson, E.; Kinney, K.; White, L. A congenital heart defect in Drosophila caused by an action potential mutation. J. Neurogenet. 1995, 10, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.; Ringo, J.; Dowse, H. Modulation of Drosophila heartbeat by neurotransmitters. J. Comp. Physiol. B 1997, 167, 89–97. [Google Scholar] [CrossRef]
- Desai-Shah, M.; Papoy, A.R.; Ward, M.; Cooper, R.L. Roles of the Sarcoplasmic/Endoplasmic reticulum Ca2+-ATPase, plasma membrane Ca2+-ATPase and Na+/Ca2+ exchanger in regulation of heart rate in larval Drosophila. Open Physiol. J. 2010, 3, 16–36. [Google Scholar] [CrossRef]
- Johnstone, A.F.M.; Cooper, R.L. Direct innervation of the Drosophila melanogaster larval aorta. Brain Res. 2006, 1083, 159–163. [Google Scholar] [CrossRef]
- Dulcis, D.; Levine, R.B. Innervation of the heart of the adult fruit fly, Drosophila melanogaster. J. Comp. Neurol. 2003, 27, 560–578. [Google Scholar] [CrossRef]
- Johnson, E.; Ringo, J.; Dowse, H. Native and heterologous neuropeptides are cardioactive in Drosophila melanogaster. J. Insect Physiol. 2000, 46, 1229–1236. [Google Scholar] [CrossRef]
- Nichols, R.; Kaminski, S.; Walling, E.; Zornik, E. Regulating the activity of a cardioacceleratory peptide. Peptides. 1999, 20, 1153–1158. [Google Scholar] [CrossRef]
- Zornik, E.; Paisley, K.; Nichols, R. Neural transmitters and a peptide modulate Drosophila heart rate. Peptides 1999, 20, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Titlow, J.S.; Rufer, J.; King, K.; Cooper, R.L. Pharmacological analysis of dopamine modulation in the Drosophila melanogaster larval heart. Physiol. Rep. 2013, 1, e00020. [Google Scholar] [CrossRef] [PubMed]
- Majeed, Z.R.; Nichols, C.D.; Cooper, R.L. 5-HT stimulation of heart rate in Drosophila does not act through cAMP as revealed by pharmacogenetics. J. Appl. Physiol. 2013, 115, 1656–1665. [Google Scholar] [CrossRef]
- Malloy, C.; Sifers, J.; Mikos, A.; Samadi, A.; Omar, A.; Hermanns, C.; Cooper, R.L. Using optogenetics to assess neuroendocrine modulation of heart rate in Drosophila melanogaster larvae. J. Comp. Physiol. A 2017, 203, 791–806. [Google Scholar] [CrossRef]
- Anyagaligbo, O.; Bernard, J.; Greenhalgh, A.; Cooper, R.L. The effects of bacterial endotoxin (LPS) on cardiac function in a medicinal blow fly (Phaenicia sericata) and a fruit fly (Drosophila melanogaster). Comp. Biochem. Physiol. C 2019, 217, 15–24. [Google Scholar] [CrossRef]
- Cooper, A.S.; Rymond, K.E.; Ward, M.A.; Bocook, E.L.; Cooper, R.L. Monitoring heart function in larval Drosophila melanogaster for physiological studies. J. Vis. Exp. 2009, 33, 1596. [Google Scholar] [CrossRef]
- Stewart, B.A.; Atwood, H.L.; Renger, J.J.; Wang, J.; Wu, C.F. Improved stability of Drosophila larval neuromuscular preparation in haemolymph-like physiological solutions. J. Comp. Physiol. A 1994, 175, 179–191. [Google Scholar] [CrossRef]
- De Castro, C.; Titlow, J.S.; Majeed, Z.R.; Malloy, C.; King, K.E.; Cooper, R.L. Chemical and mechanical factors required for maintaining cardiac rhythm in Drosophila melanogaster larva. J. Entomol. 2019, 16, 62–73. [Google Scholar] [CrossRef]
- Prakriya, M.; Lewis, R.S. Store-operated calcium channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef]
- Prakriya, M.; Lewis, R.S. Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP 3 receptors. J. Physiol. 2001, 536, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Freichel, M.; Schweig, U.; Stauffenberger, S.; Freise, D.; Schorb, W.; Flockerzi, V. Store-operated cation channels in the heart and cells of the cardiovascular system. Cell. Physiol. Biochem. 1999, 9, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Morihara, H.; Obana, M.; Tanaka, S.; Kawakatsu, I.; Tsuchiyama, D.; Mori, S.; Suizu, H.; Ishida, A.; Kimura, R.; Tsuchimochi, I.; et al. 2-aminoethoxydiphenyl borate provides an anti-oxidative effect and mediates cardioprotection during ischemia reperfusion in mice. PLoS ONE 2017, 12, e0189948. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, S.; Suzuki, A.Z.; Bauer, P.O.; Ebisui, E.; Mikoshiba, K. 2-Aminoethyl diphenylborinate (2-APB) analogues: Regulation of Ca2+ signaling. Biochem. Biophys. Res. Commun. 2013, 441, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Diver, J.M.; Sage, S.O.; Rosado, J.A. The inositol trisphosphate receptor antagonist 2-aminoethoxydiphenylborate (2-APB) blocks Ca2+ entry chan-nels in human platelets: Cautions for its use in studying Ca2+ influx. Cell Calcium 2001, 30, 323–329. [Google Scholar] [CrossRef]
- Chen, G.L.; Zeng, B.; Eastmond, S.; Elsenussi, S.E.; Boa, A.N.; Xu, S.Z. Pharmacological comparison of novel synthetic fenamate analogues with econazole and 2-APB on the inhibition of TRPM2 channels. Br. J. Pharmacol. 2012, 167, 1232–1243. [Google Scholar] [CrossRef]
- Kirby, M.S.; Sagara, Y.; Gaa, S.; Inesi, G.; Lederer, W.J.; Rogers, T.B. Thapsigargin inhibits contraction and Ca2+ transient in cardiac cells by specific inhibition of the sarcoplasmic reticulum Ca2+ pump. J. Biol. Chem. 1992, 267, 12545–12551. [Google Scholar] [CrossRef]
- Lytton, J.; Westlin, M.; Hanley, M.R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J. Biol. Chem. 1991, 266, 17067–17071. [Google Scholar] [CrossRef]
- Goto, J.; Suzuki, A.Z.; Ozaki, S.; Matsumoto, N.; Nakamura, T.; Ebisui, E.; Fleig, A.; Penner, R.; Mikoshiba, K. Two novel 2-aminoethyl diphenylborinate (2-APB) analogues differentially activate and inhibit store-operated Ca2+ entry via STIM proteins. Cell Calcium 2010, 47, 1–10. [Google Scholar] [CrossRef]
- Lindner, M.; Böhle, T.; Beuckelmann, D.J. Ca2+-handling in heart failure—A review focusing on Ca2+ sparks. Basic Res. Cardiol. 2002, 97 (Suppl. S1), I79–I82. [Google Scholar] [CrossRef]
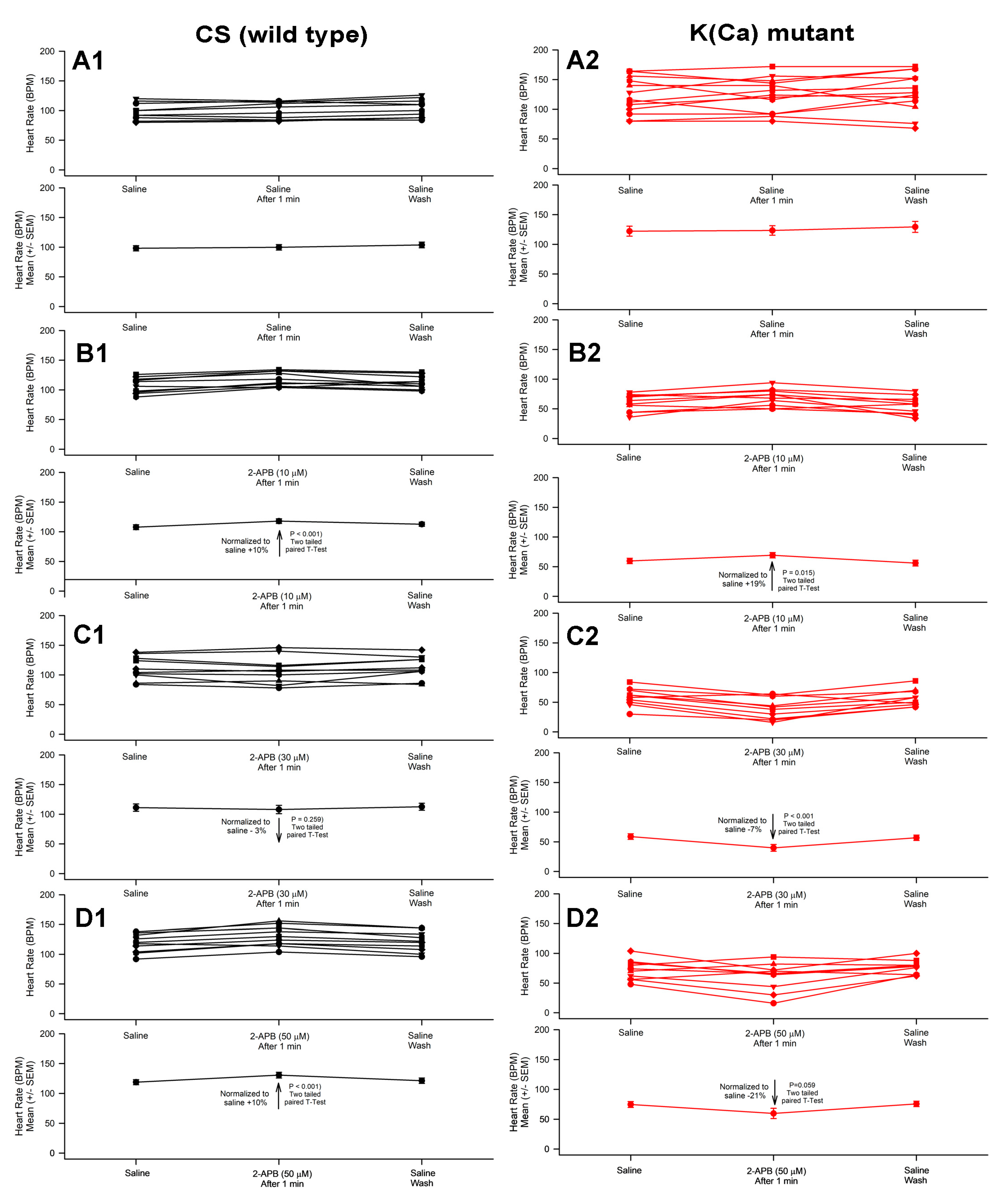
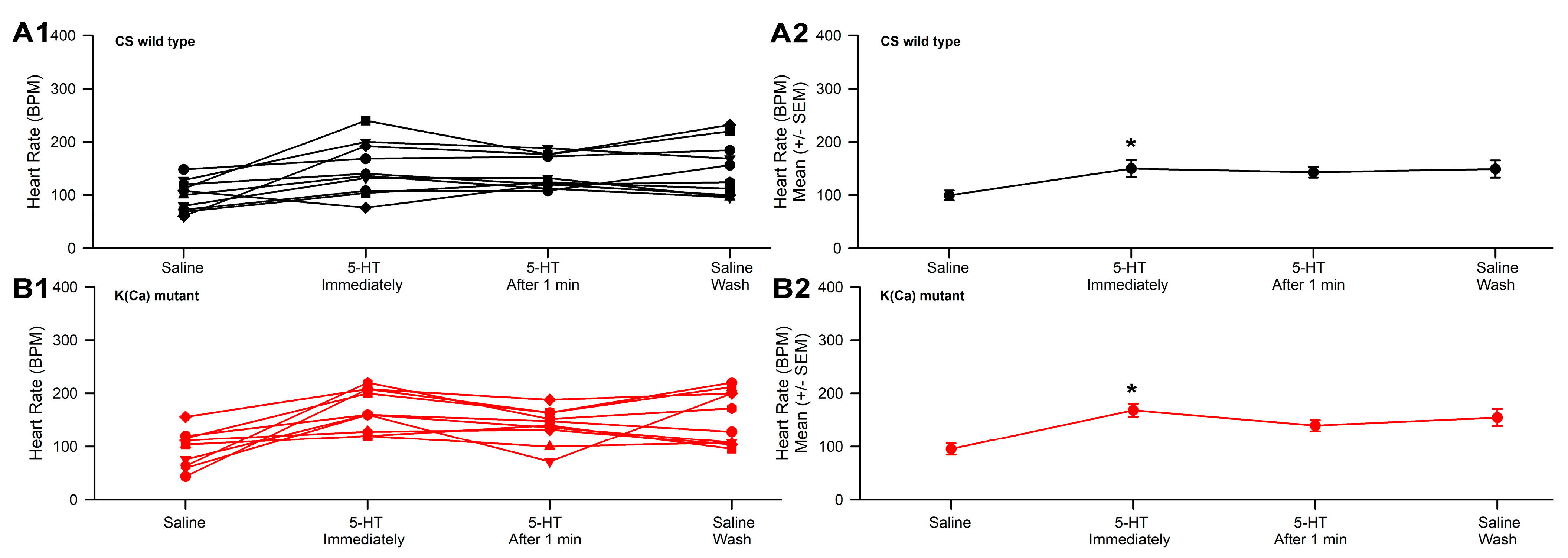
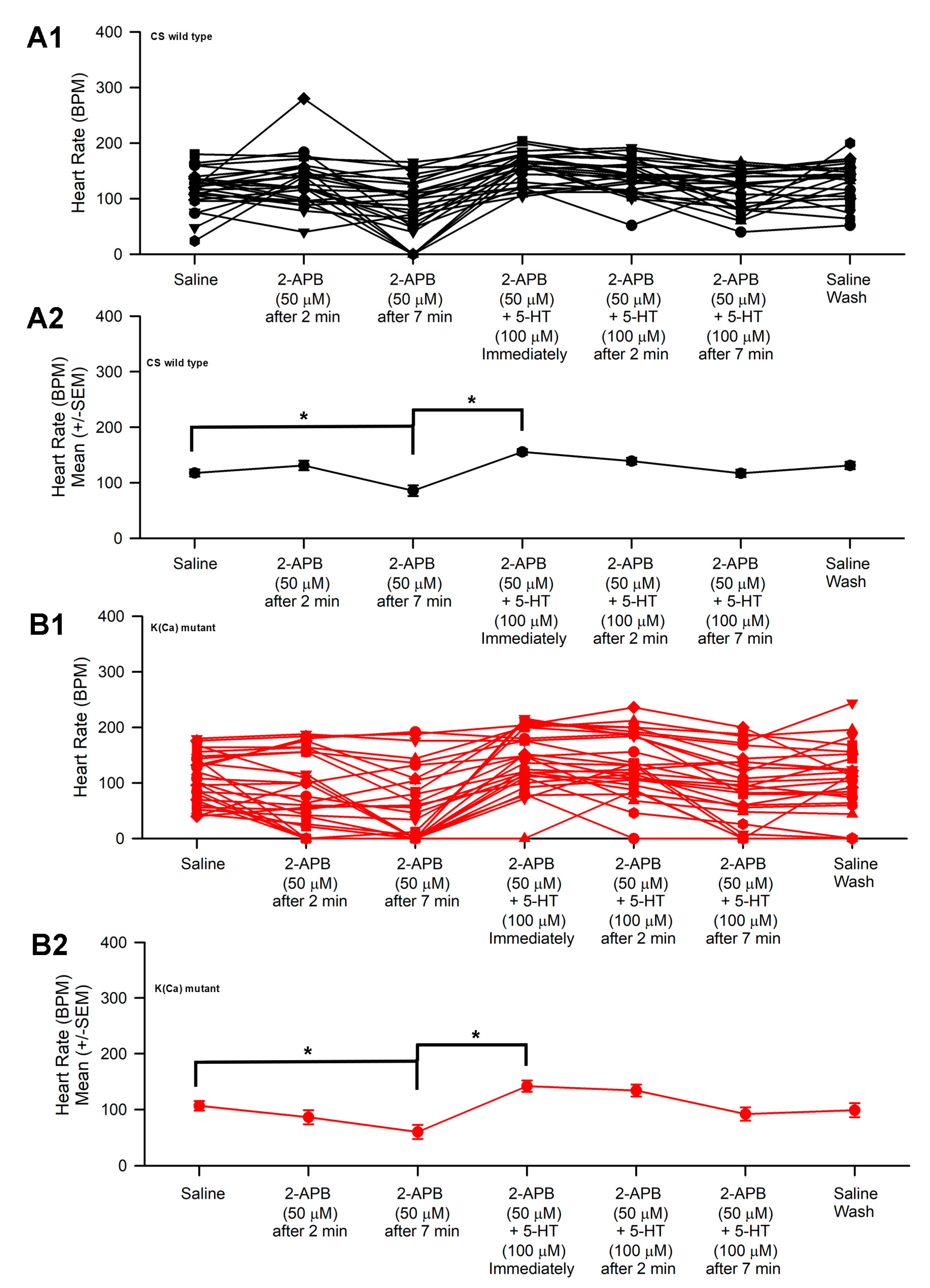
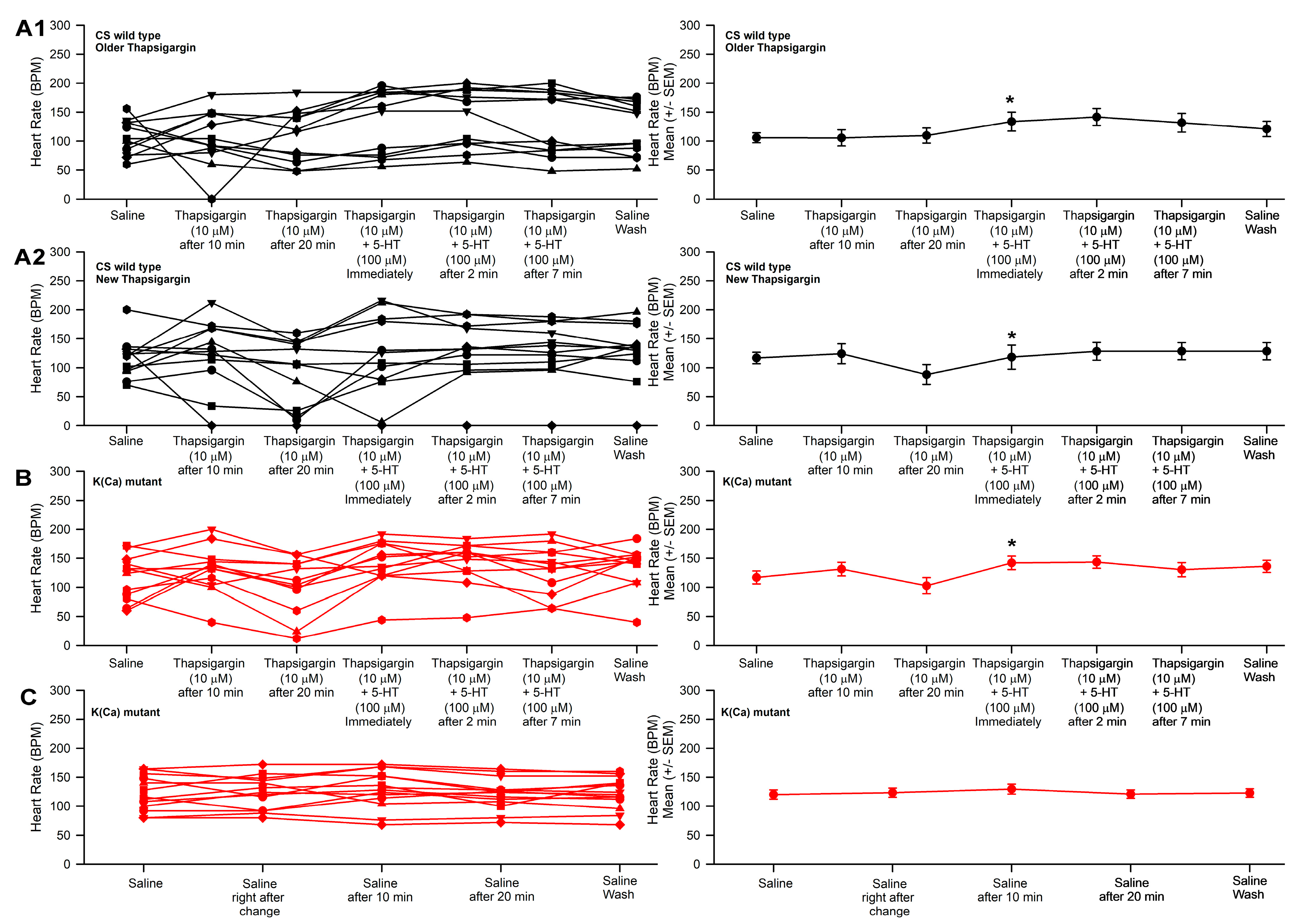
| 2-ABP | 2-APB 50 µM | Thapsigargin 10 µM | ||||
| 10 µM | 30 µM | 50 µM | 5-HT 100 µM | 5-HT 100 µM | 5-HT 100 µM | |
| CS | ↑ | ↓ | ↑ | ↑ | ↑ | ↑ |
| Slo | ↑ | ↓ | ↓ | ↑ | ↑ | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hensley, N.; Elliott, E.R.; Abul-Khoudoud, M.O.; Cooper, R.L. Effect of 2-Aminoethoxydiphenyl Borate (2-APB) on Heart Rate and Relation with Suppressed Calcium-Activated Potassium Channels: Larval Drosophila Model. Appl. Biosci. 2023, 2, 236-248. https://doi.org/10.3390/applbiosci2020017
Hensley N, Elliott ER, Abul-Khoudoud MO, Cooper RL. Effect of 2-Aminoethoxydiphenyl Borate (2-APB) on Heart Rate and Relation with Suppressed Calcium-Activated Potassium Channels: Larval Drosophila Model. Applied Biosciences. 2023; 2(2):236-248. https://doi.org/10.3390/applbiosci2020017
Chicago/Turabian StyleHensley, Nicole, Elizabeth R. Elliott, Maya O. Abul-Khoudoud, and Robin L. Cooper. 2023. "Effect of 2-Aminoethoxydiphenyl Borate (2-APB) on Heart Rate and Relation with Suppressed Calcium-Activated Potassium Channels: Larval Drosophila Model" Applied Biosciences 2, no. 2: 236-248. https://doi.org/10.3390/applbiosci2020017
APA StyleHensley, N., Elliott, E. R., Abul-Khoudoud, M. O., & Cooper, R. L. (2023). Effect of 2-Aminoethoxydiphenyl Borate (2-APB) on Heart Rate and Relation with Suppressed Calcium-Activated Potassium Channels: Larval Drosophila Model. Applied Biosciences, 2(2), 236-248. https://doi.org/10.3390/applbiosci2020017







