When Gut Hormones Influence Brain Function in Depression
Abstract
1. Introduction
2. Literature Search Method
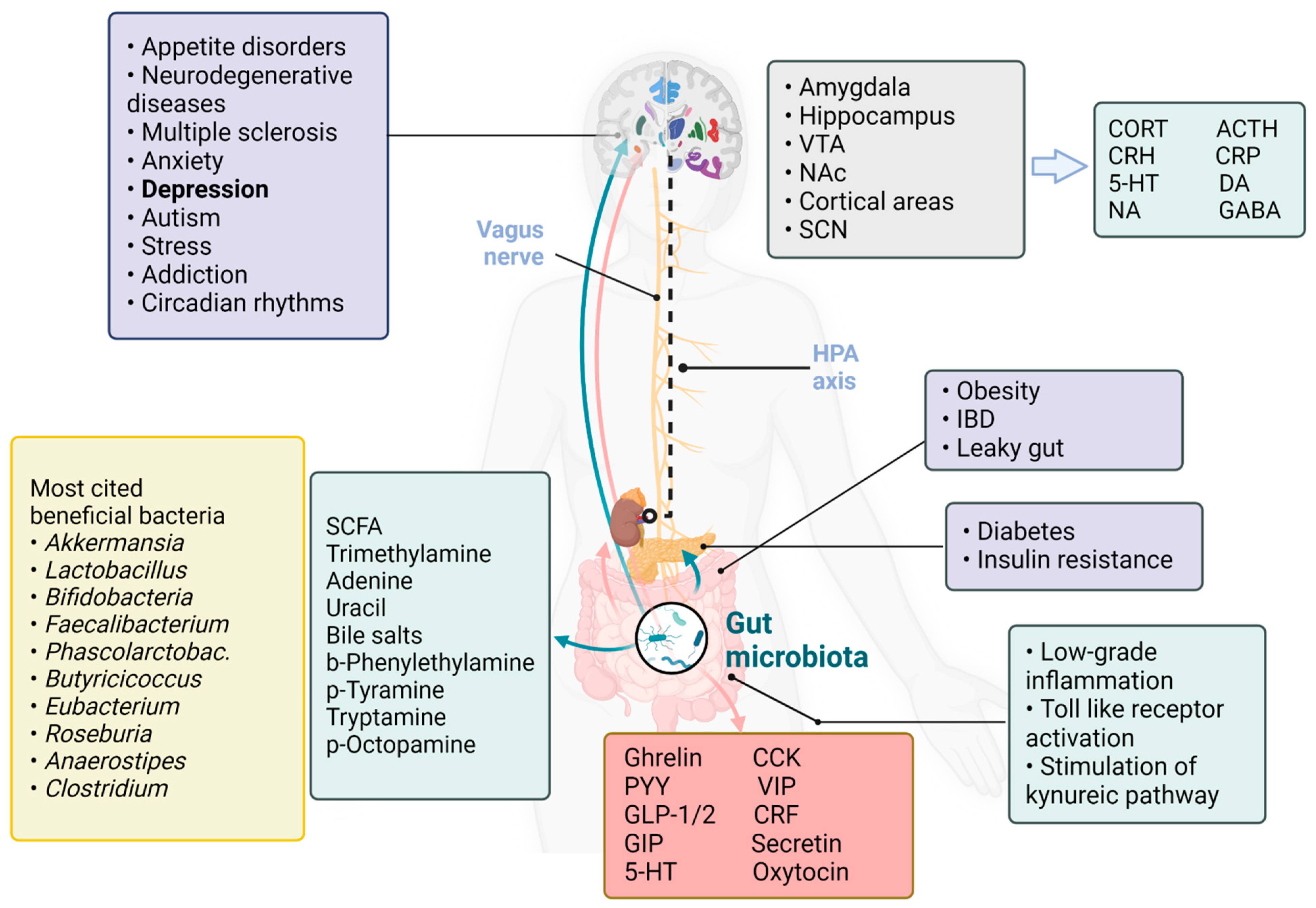
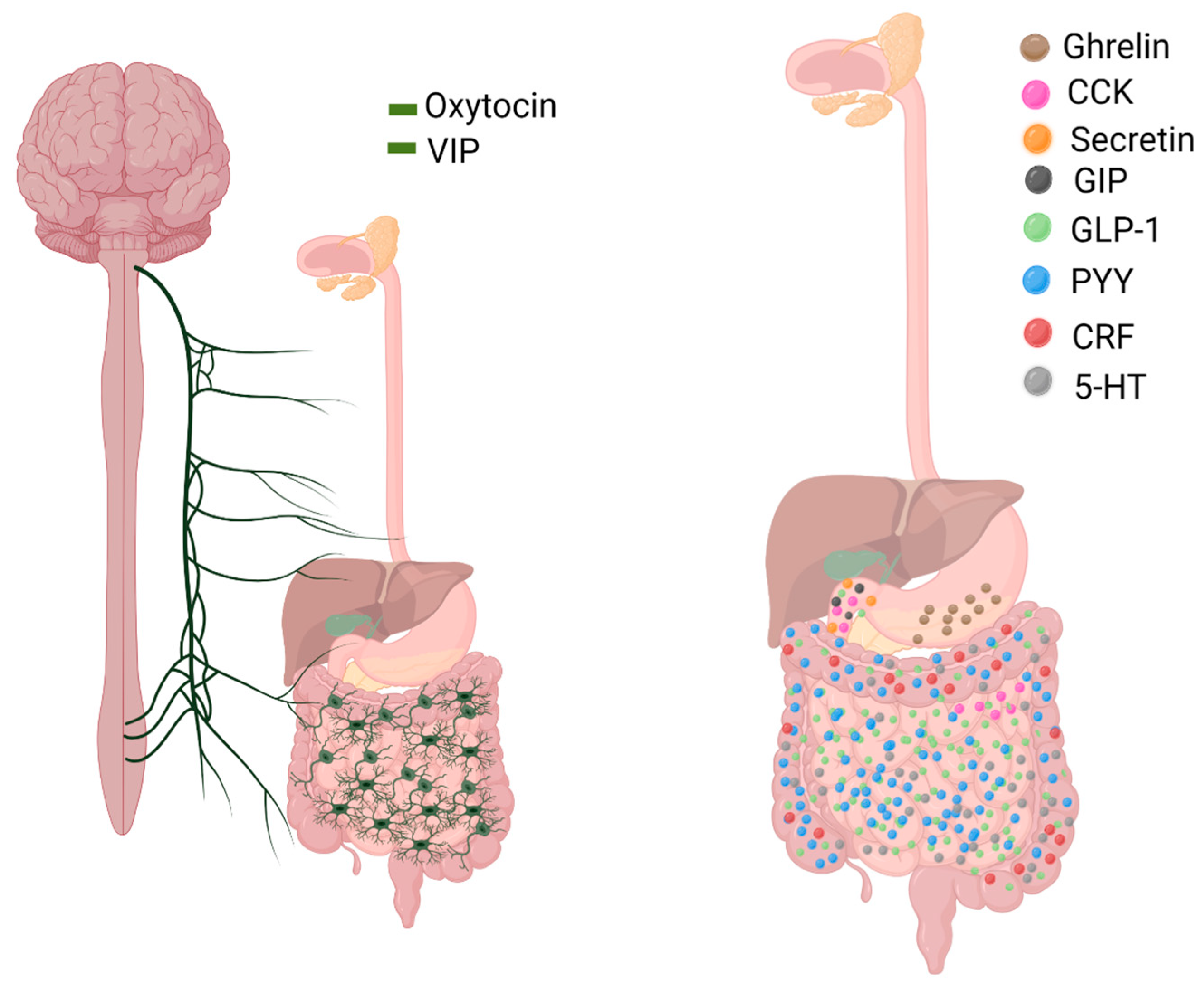
3. Gut Hormones
3.1. Ghrelin
3.2. Cholecystokinin (CCK)
3.3. Glucagon-like Peptide (GLP-1, GLP-2)
3.4. Peptide YY (PYY)
3.5. Vasoactive Intestinal Polypeptide (VIP)
4. Depression, Gut Microbiome and Hormones
4.1. Gut Microbiome, Depression and Nutrition
4.2. Gut Microbiome, Depression and Pro/Prebiotics
5. Circadian Rhythms, Gut Microbiome and Hormones
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-HT | Serotonin | HFD | High-Fat Diet |
| ABT | Antibiotic Treatment | HPA axis | Hypothalamic–Pituitary–Adrenal Axis |
| ACTH | Adrenocorticotropic Hormone | IBS | Irritable Bowel Syndrome |
| AS | Asperger’s Syndrome | IFN-γ | Interferon Gamma |
| BMI | Body Mass Index | IL | Interleukin |
| CCK | Cholecystokinin | MDD | Major Depressive Disorder |
| CNS | Central Nervous System | NA | Noradrenaline |
| CORT | Cortisol (or Corticosterone) | NAc | Nucleus Accumbens |
| CRF | Corticotropin-Releasing Factor | NPY | Neuropeptide Y |
| CRH | Corticotropin-Releasing Hormone | OCT | p-Octopamine |
| CRP | C-Reactive Protein | PEA | b-Phenylethylamine |
| DA | Dopamine | PP | Pancreatic Polypeptide |
| DSM-V | Diagnostic and Statistical Manual of Mental Disorders V | PPD | Postpartum Depression |
| EDs | Eating Disorders | PYY | Peptide YY |
| EE cells | Enteroendocrine cells | REM | Rapid Eye Movement |
| ENS | Enteric Nervous System | SCFA | Short-Chain Fat Acids |
| EV | Extracellular Vesicles | SCN | Suprachiasmatic Nucleus |
| FT | Faecal Transplant | SPF | Specific Pathogen-Free |
| GABA | Gamma-Aminobutyric Acid | TRP | Tryptamine |
| GF | Germ-Free | TYR | p-Tyramine |
| GIP | Glucose-Dependent Insulinotropic Polypeptide | VIP | Vasoactive Intestinal Polypeptide |
| GLP-1/2 | Glucagon-Like Peptide 1 and 2 | VTA | Ventral Tegmental Area |
| GR | Glucocorticoid Receptor |
References
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef]
- Qi, X.-R.; Zhang, L. The Potential Role of Gut Peptide Hormones in Autism Spectrum Disorder. Front. Cell. Neurosci. 2020, 14, 73. [Google Scholar] [CrossRef]
- Wong, M.-L.; Inserra, A.; Lewis, M.D.; Mastronardi, C.A.; Leong, L.; Choo, J.; Kentish, S.; Xie, P.; Morrison, M.; Wesselingh, S.L.; et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol. Psychiatry 2016, 21, 797–805. [Google Scholar] [CrossRef]
- Fan, X.; Deng, H.; Qiu, J.; Ji, H.; Shen, X. Antibiotics-induced depression in mice via the microbiota-gut-brain axis. J. Affect. Disord. 2022, 318, 152–158. [Google Scholar] [CrossRef]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef]
- Rode, J.; Carlman, H.M.T.E.; König, J.; Hutchinson, A.N.; Thunberg, P.; Persson, J.; Brummer, R.J. Multi-Strain Probiotic Mixture Affects Brain Morphology and Resting State Brain Function in Healthy Subjects: An RCT. Cells 2022, 11, 2922. [Google Scholar] [CrossRef]
- Tan, A.H.; Chong, C.W.; Lim, S.; Yap, I.K.S.; Teh, C.S.J.; Loke, M.F.; Song, S.; Tan, J.Y.; Ang, B.H.; Tan, Y.Q.; et al. Gut Microbial Ecosystem in Parkinson Disease: New Clinicobiological Insights from Multi-Omics. Ann. Neurol. 2021, 89, 546–559. [Google Scholar] [CrossRef]
- Bi, M.; Feng, L.; He, J.; Liu, C.; Wang, Y.; Jiang, H.; Liu, S.-J. Emerging insights between gut microbiome dysbiosis and Parkinson’s disease: Pathogenic and clinical relevance. Ageing Res. Rev. 2022, 82, 101759. [Google Scholar] [CrossRef]
- Jiang, C.; Li, G.; Huang, P.; Liu, Z.; Zhao, B. The Gut Microbiota and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 58, 1–15. [Google Scholar] [CrossRef]
- Bahari-Javan, S.; Varbanov, H.; Halder, R.; Benito, E.; Kaurani, L.; Burkhardt, S.; Anderson-Schmidt, H.; Anghelescu, I.; Budde, M.; Stilling, R.M.; et al. HDAC1 links early life stress to schizophrenia-like phenotypes. Proc. Natl. Acad. Sci. USA 2017, 114, E4686–E4694. [Google Scholar] [CrossRef]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 2017, 15, 36–59. [Google Scholar] [CrossRef]
- Luczynski, P.; McVey Neufeld, K.-A.; Oriach, C.S.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Growing up in a Bubble: Using Germ-Free Animals to Assess the Influence of the Gut Microbiota on Brain and Behavior. Int. J. Neuropsychopharmacol. 2016, 19, pyw020. [Google Scholar] [CrossRef]
- Fröhlich, E.E.; Farzi, A.; Mayerhofer, R.; Reichmann, F.; Jačan, A.; Wagner, B.; Zinser, E.; Bordag, N.; Magnes, C.; Fröhlich, E.; et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav. Immun. 2016, 56, 140–155. [Google Scholar] [CrossRef]
- Crumeyrolle-Arias, M.; Jaglin, M.; Bruneau, A.; Vancassel, S.; Cardona, A.; Daugé, V.; Naudon, L.; Rabot, S. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology 2014, 42, 207–217. [Google Scholar] [CrossRef]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef]
- Tanelian, A.; Nankova, B.; Miari, M.; Nahvi, R.J.; Sabban, E.L. Resilience or susceptibility to traumatic stress: Potential influence of the microbiome. Neurobiol. Stress 2022, 19, 100461. [Google Scholar] [CrossRef]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal flora and gastrointestinal status in children with autism—Comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef]
- McElhanon, B.O.; McCracken, C.; Karpen, S.; Sharp, W.G. Gastrointestinal Symptoms in Autism Spectrum Disorder: A Meta-analysis. Pediatrics 2014, 133, 872–883. [Google Scholar] [CrossRef]
- Tan, A.H.; Mahadeva, S.; Marras, C.; Thalha, A.M.; Kiew, C.K.; Yeat, C.M.; Ng, S.W.; Ang, S.P.; Chow, S.K.; Loke, M.F.; et al. Helicobacter pylori infection is associated with worse severity of Parkinson’s disease. Park. Relat. Disord. 2015, 21, 221–225. [Google Scholar] [CrossRef]
- Saji, N.; Niida, S.; Murotani, K.; Hisada, T.; Tsuduki, T.; Sugimoto, T.; Kimura, A.; Toba, K.; Sakurai, T. Analysis of the relationship between the gut microbiome and dementia: A cross-sectional study conducted in Japan. Sci. Rep. 2019, 9, 1008. [Google Scholar] [CrossRef]
- Gorard, D.A.; Gomborone, J.E.; Libby, G.W.; Farthing, M.J. Intestinal transit in anxiety and depression. Gut 1996, 39, 551–555. [Google Scholar] [CrossRef]
- Fitzgerald, P.; Eugene, M.C.; Clarke, G.; Scully, P.; Barry, S.; Eamonn, M.M.Q.; Shanahan, F.; Cryan, J.; Timothy, G.D. Tryptophan catabolism in females with irritable bowel syndrome: Relationship to interferon-gamma, severity of symptoms and psychiatric co-morbidity. Neurogastroenterol. Motil. 2008, 20, 1291–1297. [Google Scholar] [CrossRef]
- Goldbacher, E.M.; Matthews, K.A. Are psychological characteristics related to risk of the metabolic syndrome? A review of the literature. Ann. Behav. Med. 2007, 34, 240–252. [Google Scholar] [CrossRef]
- Hawkins, M.A.; Stewart, J.C. Do negative emotional factors have independent associations with excess adiposity? J. Psychosom. Res. 2012, 73, 243–250. [Google Scholar] [CrossRef]
- Gowey, M.A.; Khodneva, Y.; Tison, S.E.; Carson, A.P.; Cherrington, A.L.; Howard, V.J.; Safford, M.M.; Dutton, G.R. Depressive symptoms, perceived stress, and metabolic health: The REGARDS study. Int. J. Obes. 2018, 43, 615–632. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Gwak, M.-G.; Chang, S.-Y. Gut-Brain Connection: Microbiome, Gut Barrier, and Environmental Sensors. Immune Netw. 2021, 21, e20. [Google Scholar] [CrossRef]
- Martin, A.M.; Sun, E.W.; Rogers, G.B.; Keating, D.J. The Influence of the Gut Microbiome on Host Metabolism through the Regulation of Gut Hormone Release. Front. Physiol. 2019, 10, 428. [Google Scholar] [CrossRef]
- Lund, M.L.; Egerod, K.L.; Engelstoft, M.S.; Dmytriyeva, O.; Theodorsson, E.; Patel, B.A.; Schwartz, T.W. Enterochromaffin 5-HT cells—A major target for GLP-1 and gut microbial metabolites. Mol. Metab. 2018, 11, 70–83. [Google Scholar] [CrossRef]
- Köves, K.; Kausz, M.; Reser, D.; Horváth, K. What may be the anatomical basis that secretin can improve the mental functions in autism? Regul. Pept. 2002, 109, 167–172. [Google Scholar] [CrossRef]
- Holst, J.J. On the Physiology of GIP and GLP-1. Horm. Metab. Res. 2004, 36, 747–754. [Google Scholar] [CrossRef]
- Welch, M.G.; Margolis, K.G.; Li, Z.; Gershon, M.D.; Jurek, B.; Neumann, I.D.; Vittorio, J.; Talavera, M.; Gluck, K.; Iuga, A.; et al. Oxytocin regulates gastrointestinal motility, inflammation, macromolecular permeability, and mucosal maintenance in mice. Am. J. Physiol. Liver Physiol. 2014, 307, G848–G862. [Google Scholar] [CrossRef]
- Iwasaki, M.; Akiba, Y.; Kaunitz, J.D. Recent advances in vasoactive intestinal peptide physiology and pathophysiology: Focus on the gastrointestinal system. F1000Research 2019, 8, 1629. [Google Scholar] [CrossRef]
- Xu, X.; Chen, R.; Zhan, G.; Wang, D.; Tan, X.; Xu, H. Enterochromaffin Cells: Sentinels to Gut Microbiota in Hyperalgesia? Front. Cell. Infect. Microbiol. 2021, 11, 760076. [Google Scholar] [CrossRef]
- Latorre, R.; Sternini, C.; de Giorgio, R.; Greenwood-Van Meerveld, B. Enteroendocrine cells: A review of their role in brain-gut communication. Neurogastroenterol. Motil. 2016, 28, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Psichas, A.; Reimann, F.; Gribble, F.M. Gut chemosensing mechanisms. J. Clin. Investig. 2015, 125, 908–917. [Google Scholar] [CrossRef]
- Masule, M.V.; Rathod, S.; Agrawal, Y.; Patil, C.R.; Nakhate, K.T.; Ojha, S.; Goyal, S.N.; Mahajan, U.B. Ghrelin mediated regulation of neurosynaptic transmitters in depressive disorders. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100113. [Google Scholar] [CrossRef]
- Ferrini, F.; Salio, C.; Lossi, L.; Merighi, A. Ghrelin in Central Neurons. Curr. Neuropharmacol. 2009, 7, 37–49. [Google Scholar] [CrossRef]
- Maury, E. Off the Clock: From Circadian Disruption to Metabolic Disease. Int. J. Mol. Sci. 2019, 20, 1597. [Google Scholar] [CrossRef]
- Hamamah, S.; Covasa, M. Gut Microbiota Restores Central Neuropeptide Deficits in Germ-Free Mice. Int. J. Mol. Sci. 2022, 23, 11756. [Google Scholar] [CrossRef]
- Khosravi, Y.; Seow, S.W.; Amoyo, A.A.; Chiow, K.H.; Tan, T.L.; Wong, W.Y.; Poh, Q.H.; Sentosa, I.M.D.; Bunte, R.M.; Pettersson, S.; et al. Helicobacter pylori infection can affect energy modulating hormones and body weight in germ free mice. Sci. Rep. 2015, 5, srep08731. [Google Scholar] [CrossRef] [PubMed]
- Fetissov, S.O.; Averina, O.V.; Danilenko, V.N. Neuropeptides in the microbiota-brain axis and feeding behavior in autism spectrum disorder. Nutrition 2019, 61, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Leeuwendaal, N.K.; Cryan, J.F.; Schellekens, H. Gut peptides and the microbiome: Focus on ghrelin. Curr. Opin. Endocrinol. Diabetes 2021, 28, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Horne, R.; Foster, J.A. Metabolic and Microbiota Measures as Peripheral Biomarkers in Major Depressive Disorder. Front. Psychiatry 2018, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Sonali, S.; Ray, B.; Tousif, H.A.; Rathipriya, A.G.; Sunanda, T.; Mahalakshmi, A.M.; Rungratanawanich, W.; Essa, M.M.; Qoronfleh, M.W.; Chidambaram, S.B.; et al. Mechanistic Insights into the Link between Gut Dysbiosis and Major Depression: An Extensive Review. Cells 2022, 11, 1362. [Google Scholar] [CrossRef]
- Chu, C.-Q.; Yu, L.-L.; Chen, W.; Tian, F.-W.; Zhai, Q.-X. Dietary patterns affect Parkinson’s disease via the microbiota-gut-brain axis. Trends Food Sci. Technol. 2021, 116, 90–101. [Google Scholar] [CrossRef]
- Westfall, S.; Lomis, N.; Kahouli, I.; Dia, S.Y.; Singh, S.P.; Prakash, S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell. Mol. Life Sci. 2017, 74, 3769–3787. [Google Scholar] [CrossRef]
- de JR De-Paula, V.; Forlenza, A.S.; Forlenza, O.V. Relevance of gutmicrobiota in cognition, behaviour and Alzheimer’s disease. Pharmacol. Res. 2018, 136, 29–34. [Google Scholar] [CrossRef]
- Zeng, Q.; Ou, L.; Wang, W.; Guo, D.-Y. Gastrin, Cholecystokinin, Signaling, and Biological Activities in Cellular Processes. Front. Endocrinol. 2020, 11, 112. [Google Scholar] [CrossRef]
- Degen, L.; Matzinger, D.; Drewe, J.; Beglinger, C. The effect of cholecystokinin in controlling appetite and food intake in humans. Peptides 2001, 22, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Del Bel, E.A.; Guimarães, F.S. Social isolation increases cholecystokinin mRNA in the central nervous system of rats. Neuroreport 1997, 8, 3597–3600. [Google Scholar] [CrossRef] [PubMed]
- Lang, U.E.; Borgwardt, S. Molecular Mechanisms of Depression: Perspectives on New Treatment Strategies. Cell. Physiol. Biochem. 2013, 31, 761–777. [Google Scholar] [CrossRef]
- Whissell, P.D.; Bang, J.Y.; Khan, I.; Xie, Y.-F.; Parfitt, G.M.; Grenon, M.; Plummer, N.W.; Jensen, P.; Bonin, R.P.; Kim, J.C. Selective Activation of Cholecystokinin-Expressing GABA (CCK-GABA) Neurons Enhances Memory and Cognition. Eneuro 2019, 6, ENEURO.0360-18.2019. [Google Scholar] [CrossRef] [PubMed]
- Skibicka, K.P.; Dickson, S.L. Enteroendocrine hormones—central effects on behavior. Curr. Opin. Pharmacol. 2013, 13, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Ballaz, S.J. Cholecystokinin-Mediated Neuromodulation of Anxiety and Schizophrenia: A “Dimmer-Switch” Hypothesis. Curr. Neuropharmacol. 2021, 19, 925–938. [Google Scholar] [CrossRef] [PubMed]
- Abramov, U.; Raud, S.; Kõks, S.; Innos, J.; Kurrikoff, K.; Matsui, T.; Vasar, E. Targeted mutation of CCK2 receptor gene antagonises behavioural changes induced by social isolation in female, but not in male mice. Behav. Brain Res. 2004, 155, 1–11. [Google Scholar] [CrossRef]
- Iourov, I.Y.; Vorsanova, S.G.; Voinova, V.Y.; Yurov, Y.B. 3p22.1p21.31 microdeletion identifies CCK as Asperger syndrome candidate gene and shows the way for therapeutic strategies in chromosome imbalances. Mol. Cytogenet. 2015, 8, 82. [Google Scholar] [CrossRef]
- Plagman, A.; Hoscheidt, S.; McLimans, K.E.; Klinedinst, B.; Pappas, C.; Anantharam, V.; Kanthasamy, A.; Willette, A.A. Cholecystokinin and Alzheimer’s disease: A biomarker of metabolic function, neural integrity, and cognitive performance. Neurobiol. Aging 2019, 76, 201–207. [Google Scholar] [CrossRef]
- Bogunovic, M.; Davé, S.H.; Tilstra, J.S.; Chang, D.T.W.; Harpaz, N.; Xiong, H.; Mayer, L.F.; Plevy, S.E. Enteroendocrine cells express functional Toll-like receptors. Am. J. Physiol. Liver Physiol. 2007, 292, G1770–G1783. [Google Scholar] [CrossRef]
- Duca, F.A.; Swartz, T.; Sakar, Y.; Covasa, M. Increased Oral Detection, but Decreased Intestinal Signaling for Fats in Mice Lacking Gut Microbiota. PLoS ONE 2012, 7, e39748. [Google Scholar] [CrossRef]
- Federico, A.; Dallio, M.; Tolone, S.; Gravina, A.G.; Patrone, V.; Romano, M.; Tuccillo, C.; Mozzillo, A.L.; Amoroso, V.; Misso, G.; et al. Gastrointestinal Hormones, Intestinal Microbiota and Metabolic Homeostasis in Obese Patients: Effect of Bariatric Surgery. In Vivo 2016, 30, 321–330. [Google Scholar] [PubMed]
- Janssen, P.; Rotondo, A.; Mulé, F.; Tack, J. Review article: A comparison of glucagon-like peptides 1 and 2. Aliment. Pharmacol. Ther. 2012, 37, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, S.; Myers, B.; Herman, J.P. Role of central glucagon-like peptide-1 in stress regulation. Physiol. Behav. 2013, 122, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Kinzig, K.P.; D’Alessio, D.A.; Herman, J.; Sakai, R.R.; Vahl, T.P.; Figueiredo, H.F.; Murphy, E.K.; Seeley, R. CNS Glucagon-Like Peptide-1 Receptors Mediate Endocrine and Anxiety Responses to Interoceptive and Psychogenic Stressors. J. Neurosci. 2003, 23, 6163–6170. [Google Scholar] [CrossRef]
- Zheng, H.; Reiner, D.J.; Hayes, M.R.; Rinaman, L. Chronic Suppression of Glucagon-Like Peptide-1 Receptor (GLP1R) mRNA Translation in the Rat Bed Nucleus of the Stria Terminalis Reduces Anxiety-Like Behavior and Stress-Induced Hypophagia, But Prolongs Stress-Induced Elevation of Plasma Corticosterone. J. Neurosci. 2019, 39, 2649–2663. [Google Scholar] [CrossRef]
- Diz-Chaves, Y.; Herrera-Pérez, S.; González-Matías, L.C.; Lamas, J.A.; Mallo, F. Glucagon-Like Peptide-1 (GLP-1) in the Integration of Neural and Endocrine Responses to Stress. Nutrients 2020, 12, 3304. [Google Scholar] [CrossRef]
- Hölscher, C. The incretin hormones glucagonlike peptide 1 and glucose-dependent insulinotropic polypeptide are neuroprotective in mouse models of Alzheimer’s disease. Alzheimer’s Dement. 2014, 10, 47–54. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Xu, Z.; Chen, S.; Yao, W.; Gao, X. GLP-1 receptor agonists downregulate aberrant GnT-III expression in Alzheimer’s disease models through the Akt/GSK-3β/β-catenin signaling. Neuropharmacology 2018, 131, 190–199. [Google Scholar] [CrossRef]
- Wang, L.; Zhan, X.; Wang, Z.; Ma, J.; Chang, X.; Zhu, X. Toll-like receptor 4 is necessary for glucose-dependent glucagon-like peptide-1 secretion in male mice. Biochem. Biophys. Res. Commun. 2019, 510, 104–109. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-Like Peptide-1 Secretion via the G-Protein-Coupled Receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Layden, B.T.; Angueira, A.R.; Brodsky, M.; Durai, V.; Lowe, W.L., Jr. Short chain fatty acids and their receptors: New metabolic targets. Transl. Res. 2013, 161, 131–140. [Google Scholar] [CrossRef]
- Chimerel, C.; Emery, E.; Summers, D.K.; Keyser, U.; Gribble, F.M.; Reimann, F. Bacterial Metabolite Indole Modulates Incretin Secretion from Intestinal Enteroendocrine L Cells. Cell Rep. 2014, 9, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Zhang, H.; Qi, J.; Hu, A.; Jiang, Q.; Hou, Y.; Feng, Q.; Ojo, O.; Wang, X. An Almond-Based Low Carbohydrate Diet Improves Depression and Glycometabolism in Patients with Type 2 Diabetes through Modulating Gut Microbiota and GLP-1: A Randomized Controlled Trial. Nutrients 2020, 12, 3036. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-J.; Li, J.-N.; Nie, Y.-Z. Gut hormones in microbiota-gut-brain cross-talk. Chin. Med. J. 2020, 133, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Lindner, D.; Stichel, J.; Beck-Sickinger, A.G. Molecular recognition of the NPY hormone family by their receptors. Nutrition 2008, 24, 907–917. [Google Scholar] [CrossRef]
- Verma, D.; Wood, J.; Lach, G.; Herzog, H.; Sperk, G.; Tasan, R. Hunger Promotes Fear Extinction by Activation of an Amygdala Microcircuit. Neuropsychopharmacology 2015, 41, 431–439. [Google Scholar] [CrossRef]
- Reichmann, F.; Holzer, P. Neuropeptide Y: A stressful review. Neuropeptides 2016, 55, 99–109. [Google Scholar] [CrossRef]
- Batterham, R.L.; Ffytche, D.H.; Rosenthal, J.M.; Zelaya, F.O.; Barker, G.J.; Withers, D.J.; Williams, S.C.R. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature 2007, 450, 106–109. [Google Scholar] [CrossRef]
- Painsipp, E.; Herzog, H.; Sperk, G.; Holzer, P. Sex-dependent control of murine emotional-affective behaviour in health and colitis by peptide YY and neuropeptide Y. Br. J. Pharmacol. 2011, 163, 1302–1314. [Google Scholar] [CrossRef]
- Stadlbauer, U.; Langhans, W.; Meyer, U. Administration of the Y2 Receptor Agonist PYY3-36 in Mice Induces Multiple Behavioral Changes Relevant to Schizophrenia. Neuropsychopharmacology 2013, 38, 2446–2455. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.M.; Mancano, G.; Kashofer, K.; Liebisch, G.; Farzi, A.; Zenz, G.; Claus, S.P.; Holzer, P. Anhedonia induced by high-fat diet in mice depends on gut microbiota and leptin. Nutr. Neurosci. 2020, 25, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Said, S.I. The discovery of VIP: Initially looked for in the lung, isolated from intestine, and identified as a neuropeptide. Peptides 2007, 28, 1620–1621. [Google Scholar] [CrossRef] [PubMed]
- Pozo, D.; Delgado, M.; Martínez, C.; Guerrero, J.M.; Leceta, J.; Gomariz, R.P.; Calvo, J.R. Immunobiology of vasoactive intestinal peptide (VIP). Immunol. Today 2000, 21, 7–11. [Google Scholar] [CrossRef]
- Vosko, A.M.; Schroeder, A.; Loh, D.; Colwell, C.S. Vasoactive intestinal peptide and the mammalian circadian system. Gen. Comp. Endocrinol. 2007, 152, 165–175. [Google Scholar] [CrossRef]
- Mosley, R.L.; Lu, Y.; Olson, K.E.; Machhi, J.; Yan, W.; Namminga, K.L.; Smith, J.R.; Shandler, S.J.; Gendelman, H.E. A Synthetic Agonist to Vasoactive Intestinal Peptide Receptor-2 Induces Regulatory T Cell Neuroprotective Activities in Models of Parkinson’s Disease. Front. Cell. Neurosci. 2019, 13, 421. [Google Scholar] [CrossRef]
- Olson, K.E.; Kosloski-Bilek, L.M.; Anderson, K.M.; Diggs, B.J.; Clark, B.E.; Gledhill, J.M.; Shandler, S.J.; Mosley, R.L.; Gendelman, H.E. Selective VIP Receptor Agonists Facilitate Immune Transformation for Dopaminergic Neuroprotection in MPTP-Intoxicated Mice. J. Neurosci. 2015, 35, 16463–16478. [Google Scholar] [CrossRef]
- Villanueva-Romero, R.; Gutiérrez-Cañas, I.; Carrión, M.; Pérez-García, S.; Seoane, I.V.; Martínez, C.; Gomariz, R.P.; Juarranz, Y. The Anti-Inflammatory Mediator, Vasoactive Intestinal Peptide, Modulates the Differentiation and Function of Th Subsets in Rheumatoid Arthritis. J. Immunol. Res. 2018, 2018, 6043710. [Google Scholar] [CrossRef]
- Paiva, I.H.R.; Duarte-Silva, E.; Peixoto, C.A. The role of prebiotics in cognition, anxiety, and depression. Eur. Neuropsychopharmacol. 2020, 34, 1–18. [Google Scholar] [CrossRef]
- Culpepper, L. Understanding the Burden of Depression. J. Clin. Psychiatry 2011, 72, e19. [Google Scholar] [CrossRef]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; De Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Hasler, G.; Drevets, W.C.; Manji, H.K.; Charney, D.S. Discovering Endophenotypes for Major Depression. Neuropsychopharmacology 2004, 29, 1765–1781. [Google Scholar] [CrossRef]
- Dean, J.; Keshavan, M. The neurobiology of depression: An integrated view. Asian J. Psychiatry 2017, 27, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Shin, C. The Microbiota-Gut-Brain Axis in Neuropsychiatric Disorders: Pathophysiological Mechanisms and Novel Treatments. Curr. Neuropharmacol. 2018, 16, 559–573. [Google Scholar] [CrossRef]
- Chen, J.-J.; Zheng, P.; Liu, Y.-Y.; Zhong, X.-G.; Wang, H.-Y.; Guo, Y.-J.; Xie, P. Sex differences in gut microbiota in patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ma, W.; Gu, X.; Zhao, L.; Jiang, J.; Xu, Y.; Zhang, L.; Zhou, M.; Yang, L. Metabolomic signatures and microbial community profiling of depressive rat model induced by adrenocorticotrophic hormone. J. Transl. Med. 2019, 17, 224. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017, 18, 2. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Mood by microbe: Towards clinical translation. Genome Med. 2016, 8, 36. [Google Scholar] [CrossRef]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef]
- Saleh, A.; Potter, G.G.; McQuoid, D.R.; Boyd, B.; Turner, R.; MacFall, J.R.; Taylor, W.D. Effects of early life stress on depression, cognitive performance and brain morphology. Psychol. Med. 2017, 47, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Collins, S.M.; Verdu, E.F. Microbes and the gut-brain axis. Neurogastroenterol. Motil. 2012, 24, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Padua, D.; Tillisch, K. Altered brain-gut axis in autism: Comorbidity or causative mechanisms? BioEssays 2014, 36, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Mazmanian, S.K. Control of Brain Development, Function, and Behavior by the Microbiome. Cell Host Microbe 2015, 17, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Makino, H.; Cetinyurek Yavuz, A.; Ben-Amor, K.; Roelofs, M.; Ishikawa, E.; Kubota, H.; Swinkels, S.; Sakai, T.; Oishi, K.; et al. Early-Life Events, Including Mode of Delivery and Type of Feeding, Siblings and Gender, Shape the Developing Gut Microbiota. PLoS ONE 2016, 11, e0158498. [Google Scholar] [CrossRef]
- Butel, M.-J.; Waligora-Dupriet, A.-J.; Wydau-Dematteis, S. The developing gut microbiota and its consequences for health. J. Dev. Orig. Health Dis. 2018, 9, 590–597. [Google Scholar] [CrossRef]
- Sterpu, I.; Fransson, E.; Hugerth, L.W.; Du, J.; Pereira, M.; Cheng, L.; Radu, S.A.; Calderón-Pérez, L.; Zha, Y.; Angelidou, P.; et al. No evidence for a placental microbiome in human pregnancies at term. Am. J. Obstet. Gynecol. 2021, 224, 296.e1–296.e23. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The Placenta Harbors a Unique Microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef]
- Collado, M.C.; Cernada, M.; Baüerl, C.; Vento, M.; Pérez-Martínez, G. Microbial ecology and host-microbiota interactions during early life stages. Gut Microbes 2012, 3, 352–365. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Azad, M.B.; Konya, T.; Maughan, H.; Guttman, D.S.; Field, C.J.; Chari, R.S.; Sears, M.R.; Becker, A.B.; Scott, J.A.; Kozyrskyj, A.L. Gut microbiota of healthy Canadian infants: Profiles by mode of delivery and infant diet at 4 months. Can. Med. Assoc. J. 2013, 185, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: A systematic review. BMC Gastroenterol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Guaraldi, F.; Salvatori, G. Effect of Breast and Formula Feeding on Gut Microbiota Shaping in Newborns. Front. Cell. Infect. Microbiol. 2012, 2, 94. [Google Scholar] [CrossRef] [PubMed]
- Jost, T.; Lacroix, C.; Braegger, C.; Chassard, C. Impact of human milk bacteria and oligosaccharides on neonatal gut microbiota establishment and gut health. Nutr. Rev. 2015, 73, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Oozeer, R.; van Limpt, K.; Ludwig, T.; Ben Amor, K.; Martin, R.; Wind, R.D.; Boehm, G.; Knol, J. Intestinal microbiology in early life: Specific prebiotics can have similar functionalities as human-milk oligosaccharides. Am. J. Clin. Nutr. 2013, 98, 561S–571S. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y. Healthy gut microbiota and long term health. Benef. Microbes 2015, 6, 173–179. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, C.; Yu, H.; Yang, Z. Fecal Microbiota Changes in Patients With Postpartum Depressive Disorder. Front. Cell. Infect. Microbiol. 2020, 10, 567268. [Google Scholar] [CrossRef]
- Ghaisas, S.; Maher, J.; Kanthasamy, A. Gut microbiome in health and disease: Linking the microbiome–gut–brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol. Ther. 2016, 158, 52–62. [Google Scholar] [CrossRef]
- Dinan, T.G.; Quigley, E.M.M.; Ahmed, S.M.M.; Scully, P.; O’Brien, S.; O’Mahony, L.; O’Mahony, S.; Shanahan, F.; Keeling, P.W.N. Hypothalamic-Pituitary-Gut Axis Dysregulation in Irritable Bowel Syndrome: Plasma Cytokines as a Potential Biomarker? Gastroenterology 2006, 130, 304–311. [Google Scholar] [CrossRef]
- Muscatello, M.R.A. Role of negative affects in pathophysiology and clinical expression of irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 7570–7586. [Google Scholar] [CrossRef] [PubMed]
- Mackos, A.R.; Maltz, R.; Bailey, M.T. The role of the commensal microbiota in adaptive and maladaptive stressor-induced immunomodulation. Horm. Behav. 2017, 88, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Perez, N.B.; Wright, F.; Vorderstrasse, A. A Microbial Relationship Between Irritable Bowel Syndrome and Depressive Symptoms. Biol. Res. Nurs. 2021, 23, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Lennon, G.; Balfe, Á.; Bambury, N.; Lavelle, A.; Maguire, A.; Docherty, N.G.; Coffey, J.C.; Winter, D.C.; Sheahan, K.; O’Connell, P.R. Correlations between colonic crypt mucin chemotype, inflammatory grade and Desulfovibrio species in ulcerative colitis. Color. Dis. 2014, 16, O161–O169. [Google Scholar] [CrossRef] [PubMed]
- Loubinoux, J.; Bronowicki, J.-P.; Pereira, I.A.; Mougenel, J.-L.; Le Faou, A.E. Sulfate-reducing bacteria in human feces and their association with inflammatory bowel diseases. FEMS Microbiol. Ecol. 2002, 40, 107–112. [Google Scholar] [CrossRef]
- Winter, E.S.; Bäumler, A.J. Dysbiosis in the inflamed intestine. Gut Microbes 2014, 5, 71–73. [Google Scholar] [CrossRef]
- Becker, C.; Zeau, B.; Rivat, C.; Blugeot, A.; Hamon, M.; Benoliel, J.-J. Repeated social defeat-induced depression-like behavioral and biological alterations in rats: Involvement of cholecystokinin. Mol. Psychiatry 2007, 13, 1079–1092. [Google Scholar] [CrossRef]
- Evans, J.M.; Morris, L.S.; Marchesi, J.R. The gut microbiome: The role of a virtual organ in the endocrinology of the host. J. Endocrinol. 2013, 218, R37–R47. [Google Scholar] [CrossRef] [PubMed]
- Petra, A.I.; Panagiotidou, S.; Hatziagelaki, E.; Stewart, J.M.; Conti, P.; Theoharides, T.C. Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders with Suspected Immune Dysregulation. Clin. Ther. 2015, 37, 984–995. [Google Scholar] [CrossRef]
- Kelly, J.; Borre, Y.; El Aidy, S.; Deane, J.; Patterson, E.; Kennedy, P.; Beers, S.; Scott, K.; Moloney, G.; Scott, L.; et al. P.4.001 Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. Eur. Neuropsychopharmacol. 2016, 26, S85–S86. [Google Scholar] [CrossRef]
- Al Loman, A.; Ju, L.-K. Inhibitory effects of arabitol on caries-associated microbiologic parameters of oral Streptococci and Lactobacilli. Arch. Oral Biol. 2015, 60, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Bartnicka, A.; Szachta, P.; Gałęcka, M. Faecal microbiota transplant—Prospects and safety. Pomeranian J. Life Sci. 2016, 61, 282–286. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.-J.; Fan, S.-H.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Settanni, C.R.; Ianiro, G.; Bibbò, S.; Cammarota, G.; Gasbarrini, A. Gut microbiota alteration and modulation in psychiatric disorders: Current evidence on fecal microbiota transplantation. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 109, 110258. [Google Scholar] [CrossRef]
- Maes, M.; Leonard, B.; Myint, A.; Kubera, M.; Verkerk, R. The new ‘5-HT’ hypothesis of depression: Cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 702–721. [Google Scholar] [CrossRef]
- Liu, T.; Song, X.; Khan, S.; Li, Y.; Guo, Z.; Li, C.; Wang, S.; Dong, W.; Liu, W.; Wang, B.; et al. The gut microbiota at the intersection of bile acids and intestinal carcinogenesis: An old story, yet mesmerizing. Int. J. Cancer 2019, 146, 1780–1790. [Google Scholar] [CrossRef]
- Hansson, C.; Alvarez-Crespo, M.; Taube, M.; Skibicka, K.P.; Schmidt, L.; Karlsson-Lindahl, L.; Egecioglu, E.; Nissbrandt, H.; Dickson, S.L. Influence of ghrelin on the central serotonergic signaling system in mice. Neuropharmacology 2014, 79, 498–505. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Carmassi, C.; Mucci, F.; Marazziti, D. Depression, Serotonin and Tryptophan. Curr. Pharm. Des. 2016, 22, 949–954. [Google Scholar] [CrossRef]
- Zhao, H.; Jin, K.; Jiang, C.; Pan, F.; Wu, J.; Luan, H.; Zhao, Z.; Chen, J.; Mou, T.; Wang, Z.; et al. A pilot exploration of multi-omics research of gut microbiome in major depressive disorders. Transl. Psychiatry 2022, 12, 8. [Google Scholar] [CrossRef]
- Harrington, L.; Srikanth, C.V.; Antony, R.; Rhee, S.J.; Mellor, A.L.; Shi, H.N.; Cherayil, B.J. Deficiency of Indoleamine 2,3-Dioxygenase Enhances Commensal-Induced Antibody Responses and Protects against Citrobacter rodentium-Induced Colitis. Infect. Immun. 2008, 76, 3045–3053. [Google Scholar] [CrossRef]
- Subba, R.; Sandhir, R.; Singh, S.P.; Mallick, B.N.; Mondal, A.C. Pathophysiology linking depression and type 2 diabetes: Psychotherapy, physical exercise, and fecal microbiome transplantation as damage control. Eur. J. Neurosci. 2021, 53, 2870–2900. [Google Scholar] [CrossRef] [PubMed]
- Penninx, B.W.J.H.; Lange, S.M.M. Metabolic syndrome in psychiatric patients: Overview, mechanisms, and implications. Dialog-Clin. Neurosci. 2018, 20, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Matta, J.; Hoertel, N.; Kesse-Guyot, E.; Plessz, M.; Wiernik, E.; Carette, C.; Czernichow, S.; Limosin, F.; Goldberg, M.; Zins, M.; et al. Diet and physical activity in the association between depression and metabolic syndrome: Constances study. J. Affect. Disord. 2019, 244, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Weltens, N.; Zhao, D.; Van Oudenhove, L. Where is the comfort in comfort foods? Mechanisms linking fat signaling, reward, and emotion. Neurogastroenterol. Motil. 2014, 26, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.L.; Li, Q.; Minhajuddin, A.; Czysz, A.H.; Coughlin, L.A.; Hussain, S.K.; Koh, A.Y.; Trivedi, M.H. Reduced anti-inflammatory gut microbiota are associated with depression and anhedonia. J. Affect. Disord. 2020, 266, 394–401. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Y.; Yan, Y.; Tian, S.; Zheng, D.; Leng, D.; Wang, C.; Jiao, J.; Wang, Z.; Bai, Y. Promising Treatment for Type 2 Diabetes: Fecal Microbiota Transplantation Reverses Insulin Resistance and Impaired Islets. Front. Cell. Infect. Microbiol. 2020, 9, 455. [Google Scholar] [CrossRef]
- Hashemi, Z.; Fouhse, J.; Im, H.S.; Chan, C.B.; Willing, B.P. Dietary Pea Fiber Supplementation Improves Glycemia and Induces Changes in the Composition of Gut Microbiota, Serum Short Chain Fatty Acid Profile and Expression of Mucins in Glucose Intolerant Rats. Nutrients 2017, 9, 1236. [Google Scholar] [CrossRef]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.J.H.; Zitman, F.G. Overweight, Obesity, and Depression. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef]
- Goodman, A. Neurobiology of addiction. Biochem. Pharmacol. 2008, 75, 266–322. [Google Scholar] [CrossRef]
- Schreiber, L.R.N.; Odlaug, B.L.; Grant, J.E. The overlap between binge eating disorder and substance use disorders: Diagnosis and neurobiology. J. Behav. Addict. 2013, 2, 191–198. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Bäckhed, F. Diet–microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Smitka, K.; Prochazkova, P.; Roubalova, R.; Dvorak, J.; Papezova, H.; Hill, M.; Pokorny, J.; Kittnar, O.; Bilej, M.; Tlaskalova-Hogenova, H. Current Aspects of the Role of Autoantibodies Directed Against Appetite-Regulating Hormones and the Gut Microbiome in Eating Disorders. Front. Endocrinol. 2021, 12, 613983. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut Microbiota: The Neglected Endocrine Organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Neuman, H.; Debelius, J.W.; Knight, R.; Koren, O. Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 2015, 39, 509–521. [Google Scholar] [CrossRef]
- Pimentel, G.D.; Micheletti, T.O.; Pace, F.; Rosa, J.C.; Santos, R.V.; Lira, F.S. Gut-central nervous system axis is a target for nutritional therapies. Nutr. J. 2012, 11, 22. [Google Scholar] [CrossRef]
- Sinno, M.H.; Rego, J.C.D.; Coëffier, M.; Bole-Feysot, C.; Ducrotté, P.; Gilbert, D.; Tron, F.; Costentin, J.; Hökfelt, T.; Déchelotte, P.; et al. Regulation of feeding and anxiety by α-MSH reactive autoantibodies. Psychoneuroendocrinology 2009, 34, 140–149. [Google Scholar] [CrossRef]
- Inui, A.; Chen, C.-Y.; Meguid, M. Microbiome, peptide autoantibodies, and eating disorders: A missing link between gut and brain. Nutrition 2015, 31, 544–545. [Google Scholar] [CrossRef]
- Coquerel, Q.; Sinno, M.H.; Boukhettala, N.; Coëffier, M.; Terashi, M.; Bole-Feysot, C.; Breuillé, D.; Déchelotte, P.; Fetissov, S.O. Intestinal inflammation influences α-MSH reactive autoantibodies: Relevance to food intake and body weight. Psychoneuroendocrinology 2012, 37, 94–106. [Google Scholar] [CrossRef]
- Terashi, M.; Asakawa, A.; Harada, T.; Ushikai, M.; Coquerel, Q.; Sinno, M.H.; Déchelotte, P.; Inui, A.; Fetissov, S.O. Ghrelin reactive autoantibodies in restrictive anorexia nervosa. Nutrition 2011, 27, 407–413. [Google Scholar] [CrossRef]
- Garcia, F.D.; Coquerel, Q.; Rego, J.-C.D.; Cravezic, A.; Bole-Feysot, C.; Kiive, E.; Déchelotte, P.; Harro, J.; Fetissov, S.O. Anti-neuropeptide Y plasma immunoglobulins in relation to mood and appetite in depressive disorder. Psychoneuroendocrinology 2012, 37, 1457–1467. [Google Scholar] [CrossRef]
- Million, M.; Angelakis, E.; Maraninchi, M.; Henry, M.; Giorgi, R.; Valero, R.; Vialettes, B.; Raoult, D. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int. J. Obes. 2013, 37, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Fetissov, S.O.; Hökfelt, T. On the origin of eating disorders: Altered signaling between gut microbiota, adaptive immunity and the brain melanocortin system regulating feeding behavior. Curr. Opin. Pharmacol. 2019, 48, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Gardini, F.; Özogul, Y.; Suzzi, G.; Tabanelli, G.; Özogul, F. Technological Factors Affecting Biogenic Amine Content in Foods: A Review. Front. Microbiol. 2016, 7, 1218. [Google Scholar] [CrossRef] [PubMed]
- Pessione, E.; Cirrincione, S. Bioactive Molecules Released in Food by Lactic Acid Bacteria: Encrypted Peptides and Biogenic Amines. Front. Microbiol. 2016, 7, 876. [Google Scholar] [CrossRef]
- Gainetdinov, R.R.; Hoener, M.C.; Berry, M.D. Trace Amines and Their Receptors. Pharmacol. Rev. 2018, 70, 549–620. [Google Scholar] [CrossRef]
- Gwilt, K.B.; González, D.P.; Olliffe, N.; Oller, H.; Hoffing, R.; Puzan, M.; El Aidy, S.; Miller, G.M. Actions of Trace Amines in the Brain-Gut-Microbiome Axis via Trace Amine-Associated Receptor-1 (TAAR1). Cell. Mol. Neurobiol. 2020, 40, 191–201. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Sittipo, P.; Choi, J.; Lee, S.; Lee, Y.K. The function of gut microbiota in immune-related neurological disorders: A review. J. Neuroinflamm. 2022, 19, 154. [Google Scholar] [CrossRef]
- Gao, K.; Pi, Y.; Mu, C.-L.; Peng, Y.; Huang, Z.; Zhu, W.-Y. Antibiotics-induced modulation of large intestinal microbiota altered aromatic amino acid profile and expression of neurotransmitters in the hypothalamus of piglets. J. Neurochem. 2018, 146, 219–234. [Google Scholar] [CrossRef]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef]
- Stenman, L.K.; Patterson, E.; Meunier, J.; Roman, F.J.; Lehtinen, M.J. Strain specific stress-modulating effects of candidate probiotics: A systematic screening in a mouse model of chronic restraint stress. Behav. Brain Res. 2020, 379, 112376. [Google Scholar] [CrossRef]
- Liang, S.; Wang, T.; Hu, X.; Luo, J.; Li, W.; Wu, X.; Duan, Y.; Jin, F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 2015, 310, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Karen, C.; Shyu, D.J.H.; Rajan, K.E. Lactobacillus paracasei Supplementation Prevents Early Life Stress-Induced Anxiety and Depressive-Like Behavior in Maternal Separation Model-Possible Involvement of Microbiota-Gut-Brain Axis in Differential Regulation of MicroRNA124a/132 and Glutamate Receptors. Front. Neurosci. 2021, 15, 719933. [Google Scholar] [CrossRef]
- Choi, J.; Kim, Y.-K.; Han, P.-L. Extracellular Vesicles Derived from Lactobacillus plantarum Increase BDNF Expression in Cultured Hippocampal Neurons and Produce Antidepressant-like Effects in Mice. Exp. Neurobiol. 2019, 28, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Genet. 2015, 13, 605–619. [Google Scholar] [CrossRef]
- Ding, Y.; Bu, F.; Chen, T.; Shi, G.; Yuan, X.; Feng, Z.; Duan, Z.; Wang, R.; Zhang, S.; Wang, Q.; et al. A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress–induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl. Microbiol. Biotechnol. 2021, 105, 8411–8426. [Google Scholar] [CrossRef] [PubMed]
- Tao, D.; Zhong, T.; Pang, W.; Li, X. Saccharomyces boulardii improves the behaviour and emotions of spastic cerebral palsy rats through the gut-brain axis pathway. BMC Neurosci. 2021, 22, 76. [Google Scholar] [CrossRef]
- Green, M.; Arora, K.; Prakash, S. Microbial Medicine: Prebiotic and Probiotic Functional Foods to Target Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 2890. [Google Scholar] [CrossRef] [PubMed]
- Savignac, H.M.; Corona, G.; Mills, H.; Chen, L.; Spencer, J.P.; Tzortzis, G.; Burnet, P.W. Prebiotic feeding elevates central brain derived neurotrophic factor, N-methyl-d-aspartate receptor subunits and d-serine. Neurochem. Int. 2013, 63, 756–764. [Google Scholar] [CrossRef]
- Tandon, D.; Haque, M.M.; Gote, M.; Jain, M.; Bhaduri, A.; Dubey, A.K.; Mande, S.S. A prospective randomized, double-blind, placebo-controlled, dose-response relationship study to investigate efficacy of fructo-oligosaccharides (FOS) on human gut microflora. Sci. Rep. 2019, 9, 5473. [Google Scholar] [CrossRef]
- Borges, N.A.; Stenvinkel, P.; Bergman, P.; Qureshi, A.R.; Lindholm, B.; Moraes, C.; Stockler-Pinto, M.B.; Mafra, D. Effects of Probiotic Supplementation on Trimethylamine-N-Oxide Plasma Levels in Hemodialysis Patients: A Pilot Study. Probiotics Antimicrob. Proteins 2019, 11, 648–654. [Google Scholar] [CrossRef]
- Wells, A.M.; Ridener, E.; Bourbonais, C.A.; Kim, W.; Pantazopoulos, H.; Carroll, F.I.; Kim, K.-S.; Cohen, B.M.; Carlezon, W.A. Effects of Chronic Social Defeat Stress on Sleep and Circadian Rhythms Are Mitigated by Kappa-Opioid Receptor Antagonism. J. Neurosci. 2017, 37, 7656–7668. [Google Scholar] [CrossRef]
- Ota, S.M.; Suchecki, D.; Meerlo, P. Chronic social defeat stress suppresses locomotor activity but does not affect the free-running circadian period of the activity rhythm in mice. Neurobiol. Sleep Circadian Rhythms 2018, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Martynhak, B.J.; Correia, D.; Morais, L.H.; Araujo, P.; Andersen, M.L.; Lima, M.M.; Louzada, F.M.; Andreatini, R. Neonatal exposure to constant light prevents anhedonia-like behavior induced by constant light exposure in adulthood. Behav. Brain Res. 2011, 222, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Martynhak, B.J.; Hogben, A.L.; Zanos, P.; Georgiou, P.; Andreatini, R.; Kitchen, I.; Archer, S.N.; von Schantz, M.; Bailey, A.; van der Veen, D.R. Transient anhedonia phenotype and altered circadian timing of behaviour during night-time dim light exposure in Per3−/− mice, but not wildtype mice. Sci. Rep. 2017, 7, 40399. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, N.; Spada, J.; Sander, C.; Riedel-Heller, S.G.; Hegerl, U. Circadian skin temperature rhythms, circadian activity rhythms and sleep in individuals with self-reported depressive symptoms. J. Psychiatr. Res. 2019, 117, 38–44. [Google Scholar] [CrossRef]
- Hori, H.; Koga, N.; Hidese, S.; Nagashima, A.; Kim, Y.; Higuchi, T.; Kunugi, H. 24-h activity rhythm and sleep in depressed outpatients. J. Psychiatr. Res. 2016, 77, 27–34. [Google Scholar] [CrossRef]
- Borgio, J.G.F.; Koga, C.M.T.; Matynhak, B.; Louzada, F.M. Impairment of sleep quality and quality of life in bimodal chronotype individuals. Chronobiol. Int. 2018, 35, 1179–1184. [Google Scholar] [CrossRef]
- Qu, Y.; Li, T.; Xie, Y.; Tao, S.; Yang, Y.; Zou, L.; Zhang, D.; Zhai, S.; Tao, F.; Wu, X. Association of chronotype, social jetlag, sleep duration and depressive symptoms in Chinese college students. J. Affect. Disord. 2023, 320, 735–741. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Korem, T.; Dohnalová, L.; Shapiro, H.; Jaitin, D.A.; David, E.; Winter, D.R.; Gury-BenAri, M.; Tatirovsky, E.; et al. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell 2016, 167, 1495–1510. [Google Scholar] [CrossRef]
- Liang, X.; Bushman, F.D.; FitzGerald, G.A. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc. Natl. Acad. Sci. USA 2015, 112, 10479–10484. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.-W.; Thompson, T.; Solmi, M.; Vieta, E.; Yang, F.-C.; Tseng, P.-T.; Hsu, C.-W.; Tu, Y.-K.; Yu, C.-L.; Tsai, C.-K.; et al. Variability and efficacy in treatment effects on manic symptoms with lithium, anticonvulsants, and antipsychotics in acute bipolar mania: A systematic review and meta-analysis. Eclinicalmedicine 2022, 54, 101690. [Google Scholar] [CrossRef] [PubMed]
- Hafen, T.; Wollnik, F. Effect of lithium carbonate on activity level and circadian period in different strains of rats. Pharmacol. Biochem. Behav. 1994, 49, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Hu, S.; Liu, S.; Tang, B.; Liu, Y.; Tang, L.; Lei, Y.; Zhong, L.; Yang, S.; He, S. Lithium carbonate alleviates colon inflammation through modulating gut microbiota and Treg cells in a GPR43-dependent manner. Pharmacol. Res. 2022, 175, 105992. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Song, J.; Wang, H.; Shi, F.; Zhou, N.; Jiang, J.; Xu, Y.; Zhang, L.; Yang, L.; Zhou, M. Chronic paradoxical sleep deprivation-induced depressionlike behavior, energy metabolism and microbial changes in rats. Life Sci. 2019, 225, 88–97. [Google Scholar] [CrossRef]
- Swanson, G.R.; Siskin, J.; Gorenz, A.; Shaikh, M.; Raeisi, S.; Fogg, L.; Forsyth, C.; Keshavarzian, A. Disrupted diurnal oscillation of gut-derived Short chain fatty acids in shift workers drinking alcohol: Possible mechanism for loss of resiliency of intestinal barrier in disrupted circadian host. Transl. Res. 2020, 221, 97–109. [Google Scholar] [CrossRef]
- Manchia, M.; Squassina, A.; Pisanu, C.; Congiu, D.; Garzilli, M.; Guiso, B.; Suprani, F.; Paribello, P.; Pulcinelli, V.; Iaselli, M.N.; et al. Investigating the relationship between melatonin levels, melatonin system, microbiota composition and bipolar disorder psychopathology across the different phases of the disease. Int. J. Bipolar Disord. 2019, 7, 27. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siba, I.P.; Martynhak, B.J.; Pereira, M. When Gut Hormones Influence Brain Function in Depression. Appl. Biosci. 2023, 2, 31-51. https://doi.org/10.3390/applbiosci2010005
Siba IP, Martynhak BJ, Pereira M. When Gut Hormones Influence Brain Function in Depression. Applied Biosciences. 2023; 2(1):31-51. https://doi.org/10.3390/applbiosci2010005
Chicago/Turabian StyleSiba, Isadora P., Bruno J. Martynhak, and Marcela Pereira. 2023. "When Gut Hormones Influence Brain Function in Depression" Applied Biosciences 2, no. 1: 31-51. https://doi.org/10.3390/applbiosci2010005
APA StyleSiba, I. P., Martynhak, B. J., & Pereira, M. (2023). When Gut Hormones Influence Brain Function in Depression. Applied Biosciences, 2(1), 31-51. https://doi.org/10.3390/applbiosci2010005






