Abstract
Microbiologically influenced corrosion (MIC) of metal alloys is promoted by biofilms formed on metal surfaces. In the marine environment, MIC causes serious metal infrastructure problems, which lead to significant economic losses. In this study, we used an enrichment culture approach to examine the bacterial community that grows on metal surface at levels below the detection limit as a preliminary study for developing guidelines to prevent biofilm formation. An enrichment culture approach was employed to analyze the bacterial community on metal surface without biofilms and corrosion. Genomic DNA was extracted from culture sample after incubation in the enrichment culture with a metal piece, and then the V3–V4 variable regions of the bacterial 16S rRNA gene were amplified using the extracted genomic DNA as the template. Subsequently, using a next-generation sequencing approach, the amplified V3–V4 regions were sequenced, and the bacterial community was analyzed using the QIIME 2 microbiome bioinformatics platform. Using this enrichment culture approach, more than 80 bacterial genera were detected with Sphingomonas bacteria exhibiting the highest relative abundance (44%). These results demonstrated that this method could be useful for bacterial community analysis for bacteria below detection limits, and will serve as a basis for the development of the guidelines.
1. Introduction
Microorganisms grow by adapting to environmental conditions. Biofilms are biological membranes enclosed in a matrix composed of polymeric substances produced by multiple species of microorganisms [1]. When microorganisms adhere to the metal surfaces of infrastructure in the marine environment such as bridges, offshore wind-power equipment and ocean-going vessels, biofilms form on the metal surfaces. The polymeric substance matrix of the biofilm protects the microorganisms within the biofilm from stress associated with chemicals and environmental changes [1]. Thus, a favorable environment for the growth of microorganisms is created within the biofilm [1]. When biofilms form over time on metal surfaces of infrastructure, the relative abundance of corrosion-causing microorganisms increases, ultimately leading to microbiologically influenced corrosion (MIC). Marine infrastructure components affected by MIC exhibit progressive metal degradation on the metal surfaces of the infrastructure, which reduces its useful lifetime. It is estimated that MIC is responsible for approximately 20% of metal corrosion affecting marine infrastructure components [2]. For example, in 2013, it was estimated that MIC causes economic losses of $2.5 trillion, which correspond to approximately 3.4% of the gross world product. MIC of the marine infrastructure is therefore a global problem.
High-throughput amplicon sequencing is an effective method for identifying the bacterial community that causes MIC. Sulfate-reducing and sulfur-oxidizing bacteria are the major causative organisms of corrosion for several metal alloys including aluminum alloy [3], carbon steel [4], stainless steel [5] and other engineering steel [6,7,8]. In some cases, iron-oxidizing, nitrate-reducing and manganese-oxidizing bacteria have been associated with MIC [9]. Those previous characterizations of the bacterial community were carried out on samples with corrosion. On the other hand, a bacterial community is changed significantly during the early stages of biofilm formation to MIC development. In other words, major bacterial species that act on biofilm formation and development as well as MIC are different. Thus, we hypothesized that guidelines to prevent biofilm formation on the metal surfaces of marine infrastructure could be established if the structure of the bacterial community on the metal surfaces could be characterized before biofilm formation. Moreover, the lifetime of marine infrastructure might be extended if the effective utilization of such guidelines could reduce the occurrence of MIC. However, no studies have examined the bacterial community before biofilm formation on metal surfaces of marine infrastructure because the number of bacteria in the community is too low, so it is difficult to prepare the sufficient genomic DNA required for high-throughput amplicon sequencing.
Enrichment culture methods are used to increase the abundance of specific bacterial species within the total bacterial population of an environmental sample, and they are different from environmental enrichment, which is used to elicit normal behavior in captive animals [10,11,12]. Although the culture condition needs to be set according to the purpose of the research, this method can be used to grow bacteria below the detection limit to a detectable level. Using an enrichment culture approach, previous studies have reported metal corrosion-causing bacteria or bacterial communities growing on steel [13] and stainless steel [14,15]. However, the nutritional conditions used in previous studies differed from the natural environment because organic acids, sugars or yeast extract were added as carbon sources to the enrichment culture media. Those media contained high concentrations of carbon sources compared to the natural environment, and many kinds of environmental bacteria cannot grow in those media due to their growths are inhibited by high carbon concentrations [16]. Thus, the results of previous studies may not accurately reflect the communities in the natural environment, as poor-nutrient media should be used for enrichment culture media to obtain results closer to the actual target environment.
Here we report a preliminary study for developing guidelines to prevent biofilm formation. Using a synthetic medium with low concentrations of carbonate as the single carbon source, an enrichment culture was performed with a metal piece without biofilm or corrosion and genomic DNA was extracted from the culture sample. Subsequently, we amplified the V3–V4 variable regions of the bacterial 16S rRNA gene (amplicon) using the extracted genomic DNA as a template and then analyzed the bacterial community based on high-throughput sequencing of the V3–V4 variable regions. As a result, more than 80 bacterial genera were detected by applying an enrichment culture approach, which demonstrated that this method could be useful in developing the guidelines.
2. Materials and Methods
2.1. Sampling and Enrichment Culture
Several kinds of metal pieces (approximately 2.0 cm2) consisting of stainless steel SUS304 and free of biofilm or corrosion were collected from a non-corroded area at the Akitsu Port, Hiroshima Prefecture, Japan. The temperature on the sampling day was approximately 20 °C, and there had been no precipitation for 1 week before sample collection. The collected metal pieces were each separated into a sterile tube, immediately placed in a cool box at 4 °C, and transported to our laboratory within 2 h.
To prevent contamination, flasks were autoclaved at 121 °C for 20 min and cooled to room temperature. Subsequently, each of the metal pieces were subjected to an enrichment culture for 24 h at 25 °C in a flask containing a synthetic medium (pH 7.0) consisting of 3.0 g/L NaCl, 1.7 g/L Na2HPO4·12H2O, 1.0 g/L K2CO3, 0.3 g/L KH2PO4, 0.1 g/L NH4Cl, 0.024 g/L MgSO4·7H2O and 0.0011 g/L CaCl2·2H2O. The growth of bacteria was assessed by monitoring the OD600 value in comparison with cell-free control samples using an Eppendorf BioSpectrometer (Eppendorf, Hamburg, Germany).
2.2. Genomic DNA Extraction and PCR Conditions
After genomic DNA was extracted from the culture sample using an illustraTM bacteria genomicPrep Mini Spin kit (GE Healthcare, Chicago, IL, USA), the concentration and purity were measured using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The concentration of genomic DNA extracted from the enrichment culture sample with the highest OD600 value was 143 ng/µL. Using genomic DNA as the template, the V3–V4 variable regions were amplified by KOD -Plus- Neo (TOYOBO) with the bacterial domain–specific primers 341F (5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′) [17] and 805R (5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3′) [18]. The PCR protocol for amplification of the V3–V4 variable regions entailed initial denaturation at 94 °C for 2 min, followed by 35 cycles of denaturation at 98 °C for 10 s, annealing at 55 °C for 30 s, and extension at 68 °C for 30 s, with a final extension at 68 °C for 5 min.
2.3. Sequencing Library Preparation, Sequencing, and Bioinformatics Analysis
Using a Wizard SV Gel and PCR Clean-Up System (Promega, Madison, WI, USA), the PCR products (approximately 450 bp) were purified. After libraries for high-throughput amplicon sequencing were prepared using a Nextera XT DNA Library Preparation Kit (Illumina, San Diego, CA, USA), the concentration of the sequencing libraries was determined using a Quanti Fluor™ dsDNA System (Promega). The sequencing of libraries was performed using a MiSeq sequencer (Illumina) with MiSeq Reagent Kit v3 (Illumina). The sequence data have been deposited in the DDBJ database under BioProject number PRJDB13529, BioSample number SAMD00469933 and DRA number DRA014101.
The analysis of the bacterial community was performed using the QIIME 2 microbiome bioinformatics platform [19]. After the quality of sequence data was confirmed using FastQC ver. 0.11.9 [20], the filtered sequence data were yielded. Subsequently, using the DADA2 algorithm [21], amplicon sequence variants (ASVs) were prepared based on the filtered sequence data. Using the Sliva taxonomic database ver. 138 [22,23], operation taxonomic units (OTUs) at 99% sequence identity were grouped based on the ASVs. The neighbor-joining tree based on a dataset consisting of more than 1% of the OTUs was constructed using MEGA 11 software [24].
3. Results and Discussion
When bacteria on metal surfaces are targeted for the analysis, the number of bacteria is too low for preparation of genomic DNA since high-throughput amplicon sequencing cannot be performed. Indeed, we could not extract genomic DNA from a non-cultured sample. Subsequently, to obtain a sufficient amount of bacteria for the preparation of genomic DNA while preventing deviation from the actual target environment, we used filter-sterilized seawater or artificial seawater as the enrichment culture media, but bacterial growth could not be confirmed. To increase the number of bacteria for preparation of genomic DNA, therefore, a synthetic medium containing low concentrations of nutrients was used for the enrichment culture. When this enrichment culture approach using the synthetic medium was performed for each of the metal pieces, the OD600 values increased from 0.08–0.14. As a control test, an enrichment culture was also performed without adding metal pieces, but no growth of the bacteria could be confirmed. Moreover, genomic DNA could be extracted from each of the culture samples, and the V3–V4 variable regions could be amplified using the genomic DNA as the template. Thus, genomic DNA extracted from the culture sample with the highest OD600 value was used for bacterial community analysis.
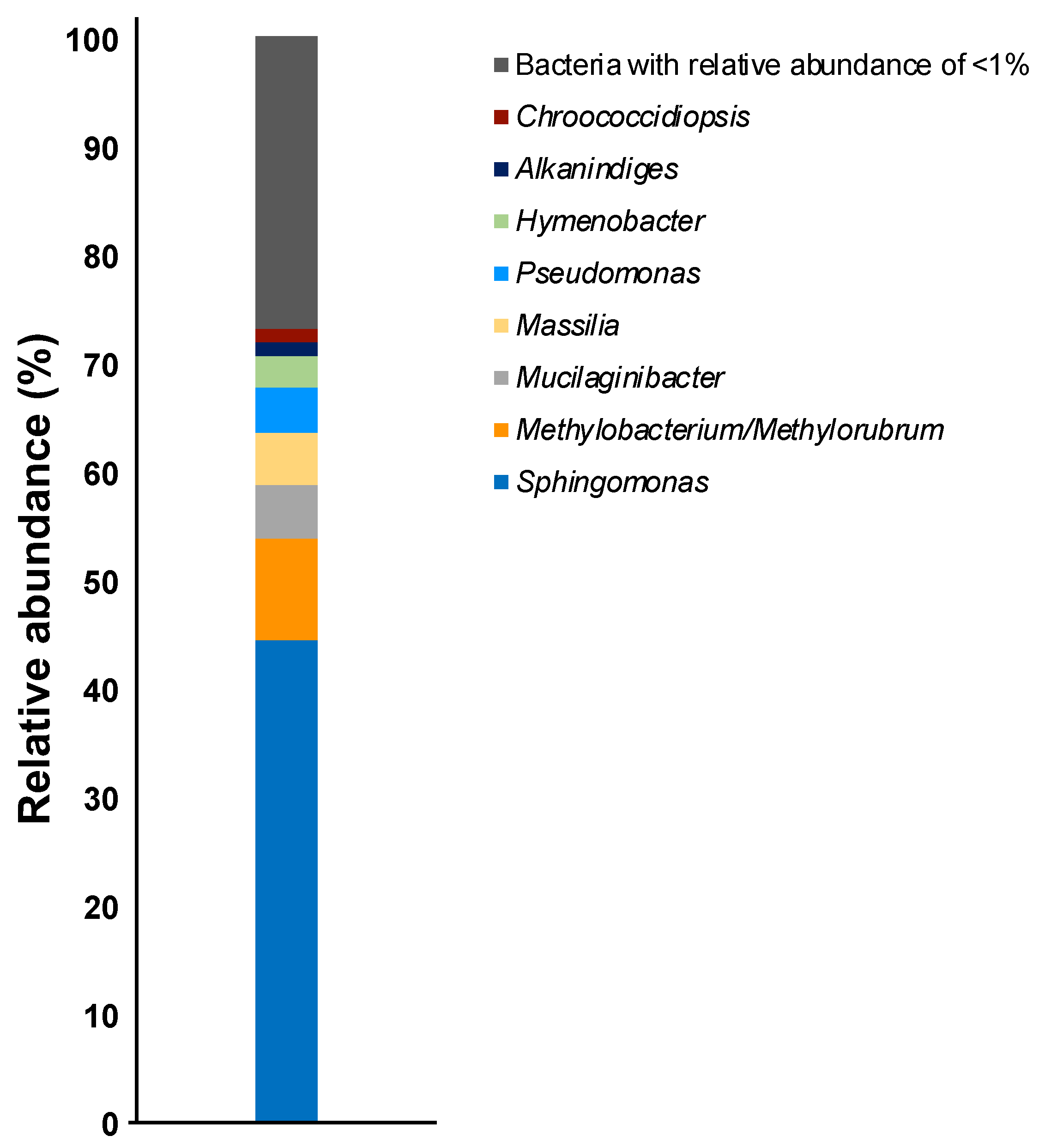
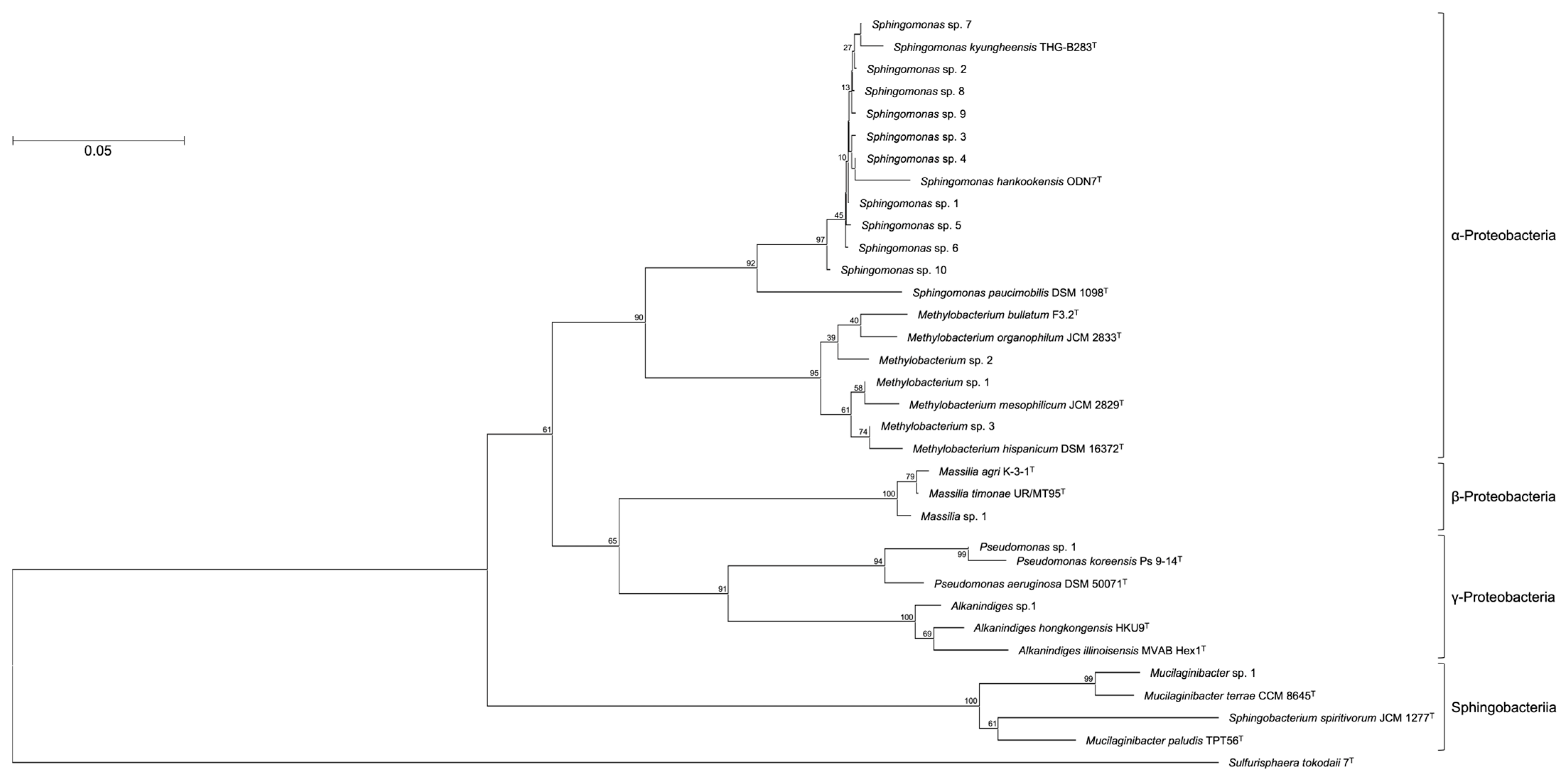
OTUs based on the analysis of genomic DNA from the cultured samples indicated the presence of more than 80 bacterial genera with the following genera present at >1% relative abundance: Sphingomonas (44%), Methylobacterium/Methylorubrum (9.4%), Mucilaginibacter (4.9%), Massilia (4.8%), Pseudomonas (4.1%), Hymenobacter (2.9%), Alkanindiges (1.3%), and Chroococcidiopsis (1.2%) (Figure 1). To confirm the phylogenetic relationship between these bacterial genera, a neighbor-joining tree based on the V3–V4 variable regions was constructed (Figure 2). The neighbor-joining tree included α-Proteobacteria such as Sphingomonas sp. and Methylobacterium sp., β-Proteobacteria such as Massilia sp., γ-Proteobacteria such as Pseudomonas sp. and Alkanindiges sp., and Sphingobacteria such as Mucilaginibacter sp. In particular, Sphingomonas sp. 1–10 formed a major cluster with S. kyungheensis THG-B283T and S. hankookensis ODN7T and exhibited a 97.6–99.1% sequence similarity to S. kyungheensis THG-B283T (Table 1). These results suggested that a variety of bacteria existed on the metal surface before biofilm formation, and biofilm formation may be promoted when the bacterial community changes in environmental conditions and the relative abundance of bacteria with biofilm-forming ability increases. In fact, our previous study suggested that the bacterial community changes significantly in biofilms before and after the occurrence of metal corrosion [25]. On the other hand, the proportion of bacteria with a relative abundance of <1% was about 15.4% (Supplementary Table S1), and the decrease in this proportion is considered to be one of the improvements for future study. The proportion could be improved by changing the bacterial domain-specific primer set. According to Bukin et al. [26], the V2–V3 variable regions of the 16S rRNA gene are also useful for the identification of bacterial genera and species in bacterial community analysis. The V4–V5 variable regions of the 16S rRNA gene have a higher resolution for the identification of an archaeal community [27]. Thus, to decrease the proportion of unclassified bacteria, we are going to use those primer sets for bacterial community analysis in our next study.
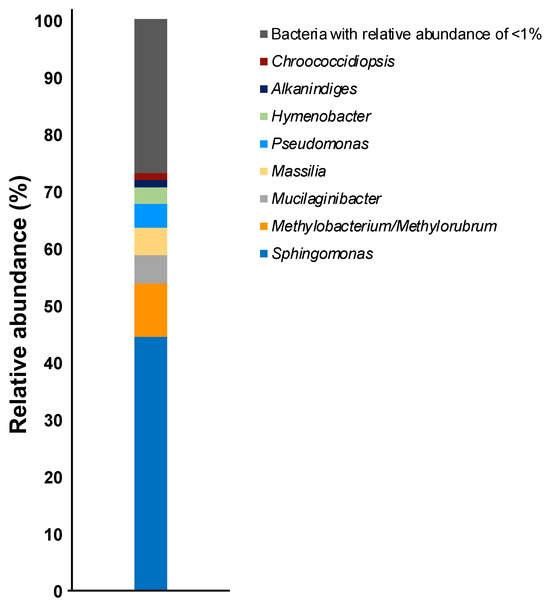
Figure 1.
Relative abundances of the bacterial communities in the culture sample at the genus level.
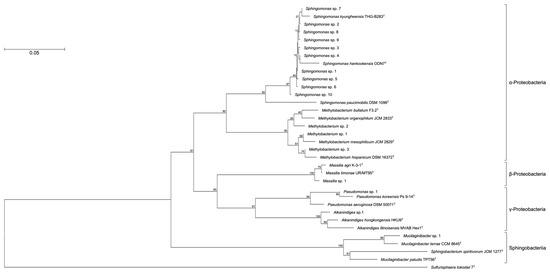
Figure 2.
Consensus bootstrap phylogenetic tree constructed from analysis of the 16S rRNA gene sequences showing the relationships between OTUs comprising more than 1% and the related type strains. Each OTU is indicated as its representative type strain. The tree was rooted using Sulfurisphaera tokodaii 7T as the outgroup. The bar indicates a 0.05% nucleotide substitution rate.

Table 1.
Bacteria with more than 1% of OTUs observed in the culture sample.
Sphingomonas bacteria are one species of the most abundant microorganisms in a variety of environments including freshwater, seawater and soils, due to the capacity of these bacteria to utilize a wide variety of organic compounds [28,29]. When Sphingomonas bacteria adhere to metal surfaces, microcolonies form and then biofilms develop over time [30]. Within developed biofilms in which Sphingomonas bacteria are the dominant species, an oxygen-free environment is created and the growth of other corrosive bacteria is accelerated, leading to MIC of the metal substrate [31]. In addition to Sphingomonas bacteria, other MIC-causing bacteria were observed such as Massilia and Pseudomonas bacteria (Figure 1). These bacteria reportedly accelerate metal corrosion via oxidation of the metal surfaces to generate energy [32] and accumulate acidic metabolites [33]. These results suggest that Sphingomonas bacteria initiate biofilm formation when their growth is not inhibited by environmental stresses or nutrient limitations. The resulting growths of Massilia and Pseudomonas bacteria inside the biofilm encourage corrosion of the metal scrap surfaces.
In this study, bacterial community analysis was performed on metal pieces without biofilm or corrosion using a synthetic medium with carbonate as the single carbon source as a preliminary study for developing guidelines to prevent biofilm formation. The bacterial community on the metal pieces could not be analyzed without an enrichment culture approach. More than 80 bacterial genera were detected by applying an enrichment culture approach. In addition, many kinds of bacteria in the community were different from the commonly known MIC-causing bacteria, suggesting that the bacteria that act on biofilm formation are different from the MIC-causing bacteria. These results demonstrated that our idea of using low concentrations of carbonate for the enrichment culture media is useful for bacterial community analysis for bacteria below the detection limits. Moreover, the results of this study will serve as a basis for the development of guidelines for the prevention of biofilm formation on metal surfaces of infrastructure in the marine environment. To facilitate the development of the guidelines, we are planning to confirm the correlation between culture conditions and the type of sample that affects the bacterial community, which may be useful in improving the accuracy of the guidelines. These results will be presented in a future report.
4. Conclusions
The bacterial community before biofilm formation on metal surfaces has not been reported, but the analysis is necessary to develop guidelines for the prevention of biofilm formation. In this study, we demonstrated the effectiveness of bacterial community analysis using a low-nutrient media for an enrichment culture approach to identify the bacteria below detection limits. To develop accurate guidelines, it is necessary to clarify how enrichment culture conditions affect the bacterial community.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/2813-0464/2/1/4/s1, Table S1: Bacterial species with less than 1% of relative abundance observed in the culture sample.
Author Contributions
Conceptualization, H.A.; methodology, H.A. and Y.S.; validation, H.A. and Y.S.; formal analysis, H.A. and Y.S.; investigation, H.A. and Y.S.; resources, H.A.; data curation, H.A. and Y.S.; writing—original draft preparation, H.A.; writing—review and editing, H.A., Y.S. and Z.-i.K.; visualization, H.A.; supervision, H.A. and Z.-i.K.; project administration, H.A.; funding acquisition, H.A. and Z.-i.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from The Salt Science Research Foundation (No. 2101).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The 16S rRNA gene sequence of A. hongkongensis HKU9T, A. illinoisensis MVAB Hex1T, M. agri K-3-1T, M. timonae UR/MT95T, M. bullatum F3.2T, M. hispanicum DSM 16372T, M. mesophilicum JCM 2829T, M. organophilum JCM 2833T, M. paludis TPT56T, M. terrae CCM 8645T, P. aeruginosa DSM 50071T, P. koreensis Ps 9-14T, S. hankookensis ODN7T, S. kyungheensis THG-B283T, S. paucimobilis DSM 1098T, S. spiritivorum JCM 1277T, and Sulfurisphaera tokodaii 7T are available in the GenBank/EMBL/DDBJ databases under accession numbers NR_114676, NR_025254, KX672812, U54470, NR_116548, NR_112613, NR_115550, AJ400920, AM490402, NR_158094, NR_026078, NR_025228, NR_116570, NR_118263, X72722, D14026, and NR_028609, respectively.
Acknowledgments
We are grateful to all members of the Bio-conversion Research Group at our Institute [Research Institute for Sustainable Chemistry, National Institute of Advanced Industrial Science and Technology (AIST)] for their technical assistance and valuable discussion.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the readability of figure (Figure 1). This change does not affect the scientific content of the article.
References
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm matrixome: Extracellular components in structured microbial communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Little, B.J.; Lee, J.S. Microbiologically Influenced Corrosion; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 1–279. [Google Scholar]
- Dai, X.; Wang, H.; Ju, L.K.; Cheng, G.; Cong, H.; Newby, B.Z. Corrosion of aluminum alloy 2024 caused by Aspergillus niger. Int. Biodeterior. Biodegrad. 2016, 115, 1–10. [Google Scholar] [CrossRef]
- Xu, D.; Wen, J.; Fu, W.; Gu, T.; Raad, I.I. D-Amino acids for the enhancement of a binary biocide cocktail consisting of THPS and EDDS against an SRB biofilm. World J. Microbiol. Biotechnol. 2012, 28, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xu, D.; Li, Y.; Yang, K.; Gu, T. Electron mediators accelerate the microbiologically influenced corrosion of 304 stainless steel by the Desulfovibrio vulgaris biofilm. Bioelectrochemistry 2015, 101, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Yang, C.; Xu, D.; Sun, D.; Nan, L.; Sun, Z.; Li, Q.; Gu, T.; Yang, K. Laboratory investigation of the microbiologically influenced corrosion (MIC) resistance of a novel Cu-bearing 2205 duplex stainless steel in the presence of an aerobic marine Pseudomonas aeruginosa biofilm. Biofouling 2015, 31, 481–492. [Google Scholar] [CrossRef]
- Li, H.; Zhou, E.; Zhang, D.; Xu, D.; Xia, J.; Yang, C.; Feng, H.; Jiang, Z.; Li, X.; Gu, T.; et al. Microbiologically influenced corrosion of 2707 hyper-duplex stainless steel by marine Pseudomonas aeruginosa biofilm. Sci. Rep. 2016, 6, 20190. [Google Scholar] [CrossRef]
- Li, H.; Yang, C.; Zhou, E.; Yang, C.; Feng, H.; Jiang, Z.; Xu, D.; Gu, T.; Yang, K. Microbiologically influenced corrosion behavior of S32654 super austenitic stainless steel in the presence of marine Pseudomonas aeruginosa biofilm. J. Mater. Sci. Technol. 2017, 33, 1596–1603. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Zhang, R.; Guan, F.; Hou, B.; Duan, J. Microbiologically influenced corrosion of marine steels within the interaction between steel and biofilms: A brief view. Appl. Microbiol. Biotechnol. 2020, 104, 515–525. [Google Scholar] [CrossRef]
- Näslund, J.; Johnsson, J.I. Environmental enrichment for fish in captive environments: Effects of physical structures and substrates. Fish Fish. 2016, 17, 1–30. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Cabrera-Álvarez, M.J.; Maia, C.M.; Saraiva, J.L. Environmental enrichment in fish aquaculture: A review of fundamental and practical aspects. Rev. Aquacult. 2022, 14, 704–728. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, L.; Zhang, X. Environmental enrichment increases aquatic animal welfare: A systematic review and meta-analysis. Rev. Aquacult. 2022, 14, 1120–1135. [Google Scholar] [CrossRef]
- Guangfeng, X.; Xiaodong, Z.; Shuai, W.; Jie, Y.; Jie, S.; Zhongyi, A.; Yan, L.; Xinlei, Q. Synergistic effect between sulfate-reducing bacteria and Pseudomonas aeruginosa on corrosion behavior of Q235 steel. Int. J. Electrochem. Sci. 2020, 15, 361–370. [Google Scholar]
- Tran, T.T.T.; Kannoorpatti, K.; Padovan, A.; Thennadil, S. A study of bacteria adhesion and microbial corrosion on different stainless steels in environment containing Desulfovibrio vulgaris. R. Soc. Open Sci. 2021, 8, 201577. [Google Scholar] [CrossRef]
- Prasanna, J.; Rosalie, C.; Sun, S.; Martin, S.; Florentin, C.; Sudesh, W.L.; Dominique, T.; Daniel, B.J.; Diane, M.; Scott, R.A.; et al. Onset of microbial influenced corrosion (MIC) in stainless steel exposed to mixed species biofilms from equatorial seawater. J. Electrochem. Soc. 2017, 164, C532–C538. [Google Scholar]
- Bloomfield, S.F.; Stewart, G.S.A.B.; Dodd, C.E.R.; Booth, I.R.; Power, E.G.M. The viable but non-culturable phenomenon explained? Microbiology 1998, 144, 1–3. [Google Scholar] [CrossRef]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gel electrophoresis analysis of polymerase chain reaction amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef]
- Herlemann, D.P.; Labrenz, M.; Jürgens, K.; Bertilsson, S.; Waniek, J.J.; Andersson, A.F. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 2011, 5, 1571–1679. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 15 December 2021).
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Caporaso, J.G. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Akita, H.; Shinto, Y.; Kimura, Z.-I. Bacterial Community Analysis of Biofilm Formed on Metal Joint. Appl. Biosci. 2022, 1, 221–228. [Google Scholar] [CrossRef]
- Bukin, Y.S.; Galachyants, Y.P.; Morozov, I.V.; Bukin, S.V.; Zakharenko, A.S.; Zemskaya, T.I. The effect of 16S rRNA region choice on bacterial community metabarcoding results. Sci. Data 2019, 6, 190007. [Google Scholar] [CrossRef]
- Fadeev, E.; Cardozo-Mino, M.G.; Rapp, J.Z.; Bienhold, C.; Salter, I.; Salman-Carvalho, V.; Molari, M.; Tegetmeyer, H.E.; Buttigieg, P.L.; Boetius, A. Comparison of two 16S rRNA primers (V3–V4 and V4–V5) for studies of arctic microbial communities. Front. Microbiol. 2021, 12, 637526. [Google Scholar] [CrossRef]
- White, D.C.; Sutton, S.D.; Ringelberg, D.B. The genus Sphingomonas: Physiology and ecology. Curr. Opin. Biotechnol. 1996, 7, 301–306. [Google Scholar] [CrossRef]
- Waigi, M.G.; Sun, K.; Gao, Y. Sphingomonads in microbe-assisted phytoremediation: Tackling soil pollution. Trends Biotechnol. 2017, 35, 883–899. [Google Scholar] [CrossRef]
- Venugopalan, V.P.; Kuehn, M.; Hausner, M.; Springael, D.; Wilderer, P.A.; Wuertz, S. Architecture of a nascent Sphingomonas sp. biofilm under varied hydrodynamic conditions. Appl. Environ. Microbiol. 2005, 71, 2677–2686. [Google Scholar] [CrossRef]
- Beale, D.J.; Morrison, P.D.; Key, C.; Palombo, E.A. Metabolic profiling of biofilm bacteria known to cause microbial influenced corrosion. Water Sci. Technol. 2014, 69, 1–8. [Google Scholar] [CrossRef]
- Meiying, L.; Min, D. A review: Microbiologically influenced corrosion and the effect of cathodic polarization on typical bacteria. Rev. Environ. Sci. Biotechnol. 2018, 17, 431–446. [Google Scholar]
- Kaewyaia, J.; Noophana, P.; Wantawina, C.; Munakata-Marrb, J. Microbiologically influenced corrosion and current mitigation strategies: A state of the art review. Int. Biodeterior. Biodegrad. 2019, 137, 42–58. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).