Sedimentation Rate of Dunaliella salina in Dark Conditions
Abstract
:1. Introduction
2. Materials and Methods
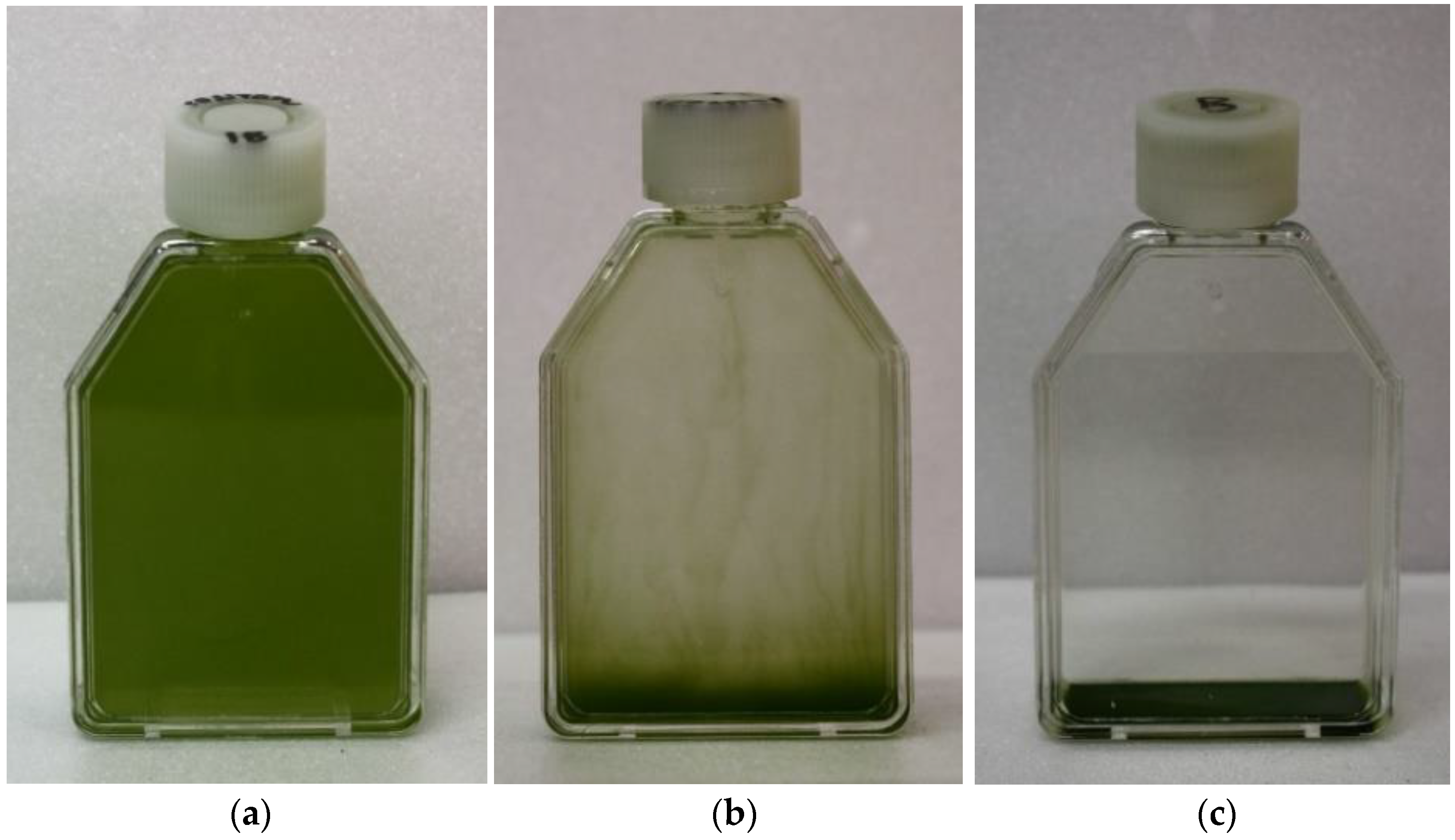
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chisti, Y. Society and microalgae: Understanding the past and present. In Microalgae in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2018; pp. 11–21. [Google Scholar]
- Zhuang, D.; He, N.; Khoo, K.S.; Ng, E.-P.; Chew, K.W.; Ling, T.C. Application progress of bioactive compounds in microalgae on pharmaceutical and cosmetics. Chemosphere 2021, 291, 132932. [Google Scholar] [CrossRef] [PubMed]
- Yarkent, Ç.; Gürlek, C.; Oncel, S.S. Potential of microalgal compounds in trending natural cosmetics: A review. Sustain. Chem. Pharm. 2020, 17, 100304. [Google Scholar] [CrossRef]
- Harun, R.; Danquah, M.K.; Forde, G.M. Microalgal biomass as a fermentation feedstock for bioethanol production. J. Chem. Technol. Biotechnol. 2010, 85, 199–203. [Google Scholar] [CrossRef]
- Nagarajan, D.; Varjani, S.; Lee, D.-J.; Chang, J.-S. Sustainable aquaculture and animal feed from microalgae–Nutritive value and techno-functional components. Renew. Sustain. Energy Rev. 2021, 150, 111549. [Google Scholar] [CrossRef]
- Schenk, P.M.; Thomas-Hall, S.R.; Stephens, E.; Marx, U.C.; Mussgnug, J.H.; Posten, C.; Kruse, O.; Hankamer, B. Second generation biofuels: High-efficiency microalgae for biodiesel production. Bioenergy Res. 2008, 1, 20–43. [Google Scholar] [CrossRef]
- Vanthoor-Koopmans, M.; Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H. Biorefinery of microalgae for food and fuel. Bioresour. Technol. 2013, 135, 142–149. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Pessôa, L.C.; Deamici, K.M.; Pontes, L.A.M.; Druzian, J.I.; de Jesus Assis, D. Technological prospection of microalgae-based biorefinery approach for effluent treatment. Algal Res. 2021, 60, 102504. [Google Scholar] [CrossRef]
- Schlesinger, A.; Eisenstadt, D.; Bar-Gil, A.; Carmely, H.; Einbinder, S.; Gressel, J. Inexpensive non-toxic flocculation of microalgae contradicts theories; overcoming a major hurdle to bulk algal production. Biotechnol. Adv. 2012, 30, 1023–1030. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Muylaert, K. Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef]
- Talukder, M.M.R.; Das, P.; Wu, J.C. Immobilization of microalgae on exogenous fungal mycelium: A promising separation method to harvest both marine and freshwater microalgae. Biochem. Eng. J. 2014, 91, 53–57. [Google Scholar] [CrossRef]
- Estime, B.; Ren, D.; Sureshkumar, R. Cultivation and energy efficient harvesting of microalgae using thermoreversible sol-gel transition. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Poelman, E.; De Pauw, N.; Jeurissen, B. Potential of electrolytic flocculation for recovery of micro-algae. Resour. Conserv. Recycl. 1997, 19, 1–10. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Yeh, K.-L.; Aisyah, R.; Lee, D.-J.; Chang, J.-S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef]
- Najjar, Y.S.H.; .Abu-Shamleh, A. Harvesting of microalgae by centrifugation for biodiesel production: A review. Algal Res. 2020, 51, 102046. [Google Scholar] [CrossRef]
- Li, S.; Hu, T.; Xu, Y.; Wang, J.; Chu, R.; Yin, Z.; Mo, F.; Zhu, L. A review on flocculation as an efficient method to harvest energy microalgae: Mechanisms, performances, influencing factors and perspectives. Renew. Sustain. Energy Rev. 2020, 131, 110005. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Gudin, C.; Thepenier, C. Bioconversion of solar energy into organic chemicals by microalgae. Adv. Biotechnol. Process. (USA) 1986, 6, 73–110. [Google Scholar]
- Molina Grima, E.; Belarbi, E.-H.; Acién Fernández, F.G.; Robles Medina, A.; Chisti, Y. Recovery of microalgal biomass and metabolites: Process options and economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]
- Morais Junior, W.G.; Gorgich, M.; Corrêa, P.S.; Martins, A.A.; Mata, T.M.; Caetano, N.S. Microalgae for biotechnological applications: Cultivation, harvesting and biomass processing. Aquaculture 2020, 528, 735562. [Google Scholar] [CrossRef]
- Singh, G.; Patidar, S. Microalgae harvesting techniques: A review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef]
- Goh, B.H.H.; Ong, H.C.; Cheah, M.Y.; Chen, W.-H.; Yu, K.L.; Mahlia, T.M.I. Sustainability of direct biodiesel synthesis from microalgae biomass: A critical review. Renew. Sustain. Energy Rev. 2019, 107, 59–74. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chen, C.-Y.; Lee, D.-J.; Chang, J.-S. Perspectives on microalgal CO2-emission mitigation systems—A review. Biotechnol. Adv. 2011, 29, 189–198. [Google Scholar] [CrossRef]
- Kim, J.; Yoo, G.; Lee, H.; Lim, J.; Kim, K.; Kim, C.W.; Park, M.S.; Yang, J.-W. Methods of downstream processing for the production of biodiesel from microalgae. Biotechnol. Adv. 2013, 31, 862–876. [Google Scholar] [CrossRef]
- Uduman, N.; Qi, Y.; Danquah, M.K.; Forde, G.M.; Hoadley, A. Dewatering of microalgal cultures: A major bottleneck to algae-based fuels. J. Renew. Sustain. Energy 2010, 2, 012701. [Google Scholar] [CrossRef]
- Besson, A.; Guiraud, P. High-pH-induced flocculation–flotation of the hypersaline microalga Dunaliella salina. Bioresour. Technol. 2013, 147, 464–470. [Google Scholar] [CrossRef]
- Milledge, J.J.; Heaven, S. A review of the harvesting of micro-algae for biofuel production. Rev. Environ. Sci. Bio/Technol. 2013, 12, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Fan, X.; Gao, G.; Beardall, J.; Inaba, K.; Hall-Spencer, J.M.; Xu, D.; Zhang, X.; Han, W.; McMinn, A. Decreased motility of flagellated microalgae long-term acclimated to CO2-induced acidified waters. Nat. Clim. Change 2020, 10, 561–567. [Google Scholar] [CrossRef]
- Guillard, R.R. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Springer: Berlin/Heidelberg, Germany, 1975; pp. 29–60. [Google Scholar]
- Guillard, R.R.; Ryther, J.H. Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Leyendekkers, J. Prediction of the density and viscosity of seawater, its concentrates and other multicomponent solutions using the Tammann-Tait-Gibson (TTG) model. Desalination 1979, 29, 263–274. [Google Scholar] [CrossRef]
- Smayda, T.J. The suspension and sinking of phytoplankton in the sea. Oceanogr. Mar. Biol. Ann. Rev. 1970, 8, 353–414. [Google Scholar]
- Xu, Y.; Milledge, J.J.; Abubakar, A.; Swamy, R.; Bailey, D.; Harvey, P. Effects of centrifugal stress on cell disruption and glycerol leakage from Dunaliella salina. Microalgae Biotechnol. 2015, 1, 20–27. [Google Scholar] [CrossRef]
- Ludwig, W. Zur theorie der flimmerbewegung (dynamik, nutzeffekt, energiebilanz). Z. Für Vgl. Physiol. 1930, 13, 397–504. [Google Scholar] [CrossRef]
- Jahn, T.L.; Bovee, E.C. Motile behavior of protozoa. In Research in Protozoology; Elsevier: Amsterdam, The Netherlands, 1967; pp. 41–200. [Google Scholar]
- Watsuji, T.-o.; Naka, A.; Morita, Y.; Kurahashi, M. Effect of temperature and dissolved oxygen on gravity sedimentation of the unicellular alga Dunaliella salina. Ann. Microbiol. 2021, 71, 1–7. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naka, A.; Kurahashi, M. Sedimentation Rate of Dunaliella salina in Dark Conditions. Appl. Biosci. 2023, 2, 14-20. https://doi.org/10.3390/applbiosci2010002
Naka A, Kurahashi M. Sedimentation Rate of Dunaliella salina in Dark Conditions. Applied Biosciences. 2023; 2(1):14-20. https://doi.org/10.3390/applbiosci2010002
Chicago/Turabian StyleNaka, Angelica, and Midori Kurahashi. 2023. "Sedimentation Rate of Dunaliella salina in Dark Conditions" Applied Biosciences 2, no. 1: 14-20. https://doi.org/10.3390/applbiosci2010002
APA StyleNaka, A., & Kurahashi, M. (2023). Sedimentation Rate of Dunaliella salina in Dark Conditions. Applied Biosciences, 2(1), 14-20. https://doi.org/10.3390/applbiosci2010002





