Anti-Tumour Activities from Secondary Metabolites and Their Derivatives in Bryophytes: A Brief Review
Abstract
:1. Bryophytes and Their Antiproliferative Activity
2. The Hallmarks of Cancer
2.1. Multidrug Resistance in Cancer Cells
2.2. Apoptotic Blockade
2.3. Angiogenesis
| Compounds | Chemical Class | Species | Cell Lines/Animal | Activity | References |
|---|---|---|---|---|---|
| Marchantin A | (Bis)bibenzyls | Marchantia ssp. | Mammary cancer cells MCF7 | Cell growth inhibition; Apoptosis inducer; Cell cycle arrest; | [39] |
| Human melanoma A375 cells | Cytotoxic; | [40] | |||
| Breast cancer A256 cells Breast cancer T47D cells | Reduction in cell viability | [41] | |||
| MCF-7 | Increase in cleaved caspase-3, cleaved caspase-9, and cleaved PARP | ||||
| Marchantin C | (Bis)bibenzyls | Marchantia ssp. | T98G | P-gp inhibition; microtubules depolymerization; angiogenesis inhibition; cell migration inhibition | [42,43,44,45,46] |
| 12-bromomarchantin C | Halogenated (bis)bibenzyl | Marchantia C synthetic derivative | PC-3 | Tubulin depolymerization induction | [47] |
| Marchantin M | (Bis)bibenzyls | Marchantia ssp. | Human prostatic carcinoma PC-3 cells | Induction of apoptosis through ER stress | [48] |
| Mice model with APC gene mutation | Apoptotic induction through caspase-3 and topoisomerase II inhibition; P-gp expression decrease; angiogenesis inhibition | [49] | |||
| Riccardin D | (Bis)bibenzyls | Dumortiera hirsuta | Human colon cancer HT-29 cells | Apoptosis inducer by suppressing the NF-kB signaling pathway | [50] |
| Riccardin D-26 | (Bis)bibenzyls | Riccardin D synthetic derivative | Apoptosis induction; xenograft tumor growth inhibition; cell cycle regulator | [51] | |
| 3, 12, 30-Tri(2-(dimethylamino)ethoxy)-riccardin D | Tri-O-alkylated (bis)bibenzyls | Riccardin D synthetic derivative | A549 | Lysosomal membrane permeabilization; apoptosis induction | [52] |
| 13, 40-Bis((pyrrolidin-1-yl)methyl)-riccardin D | Amino methylated (bis)bibenzyls | Riccardin D synthetic derivative | A549 | Lysosomal membrane permeabilization; apoptosis induction | [52] |
| 10-bromoriccardin D | Halogenated (bis)bibenzyls | Riccardin D synthetic derivative | PC-3 | Cell cycle arrest; tubulin depolymerization | [47] |
| Riccardin F | (Bis)bibenzyls | Plagiochasma intermedium | Myelogenous leukaemia K562 cells; multidrug resistant K562/A02 | MDR reversal through P-gp inhibition | [53] |
| Plagiochin E | (Bis)bibenzyls | Marchantia polymorpha | K562/A02 | MDR reversal through P-gp inhibition | [54] |
| 12-bromoplagiochin E | Halogenated (bis)bibenzyls | Plagiochin E synthetic derivative | PC-3 | Tubulin depolymerization | [47] |
| Isoplagiochin A | (Bis)bibenzyls | Plagiochila fruticosa | In vitro tubulin polymerization assay | Tubulin polymerization inhibition | [55] |
| Isoplagiochin B | (Bis)bibenzyls | Plagiochila fruticosa | In vitro tubulin polymerization assay | Tubulin polymerization inhibition | [55] |
| Brittonin A Brittonin B | Methoxylated bibenzyls | Frullania inouei | KB; KB/VCR; K562; K562/A02 | Proliferation inhibition; MDR reversal | [56] |
| (±)-Radulapin D | Prenylated bibenzyl | Radula apiculata | PC-3 | Apoptosis via ROS accumulation; Bax increase; caspase-9 increase | [57] |
| Ent-11α-hydroxy-16-kauren-15-one | Ent-kaurane diterpenoids | Jungermannia ssp. | H60 cells | With TNF-α inducing DNA fragmentation | [58] |
| Jungermannenone A-B | Ent-kaurane diterpenoids | Jungermannia fauriana | PC-3 | Apoptosis via ROS accumulation; Induction of cell cycle arrest | [59] |
| Isomanool | Diterpenoids | Heteroscyphus tener | PC-3; DU145 | Cell cycle arrest; caspase-3 increase; ROS accumulation | [60] |
| Lepidozin G | Triterpenoids | Lepidozia reptans | PC-3 | Apoptosis through ROS accumulation; mitochondrial membrane potential disruption | [61] |
| Compounds | Chemical Class | Species | Cell Lines/Animal | Activity | References |
|---|---|---|---|---|---|
| Muscicolone | Diterpenoids | Frullania muscicola | KB, PG, HT-29, BEL-7402 | IC50 20–60 mg/mL | [62] |
| 5α, 8α, 9α-trihydroxy-13E-labden-12-one; 5α, 8α-dihydroxy-13E-labden-12-one | Labdane diterpenoids | Scapania undulate | 1) A549 2) K562 3) A2780 | 1) IC50 37.6 μmol/L 2) IC50 36.8 μmol/L 3) IC50 19.5 μmol/L | |
| Scapaundulin C | Labdane diterpenoids | Scapania undulate | 1) A549 2) K562 3) A2780 | 1) IC50 39.2 μmol/L 2) IC50 37.1 μmol/L 3) IC50 16.8 μmol/L | [63] |
| **nn | Atisane diterpenoids | Lepidolaena clavigera | P388 | IC50 16 μg/mL | [64] |
| Notolutesin A | Diterpenoids | Notoscyphus lutescens | PC-3 | IC50 6.2 μM | [65] |
| Perrotettianal A | Diterpenoids | Porella viridissima | A2780 ovarian cancer cells | IC50 1.6 μg/mL | [66] |
| Chiloscyphenols A | Sesquiterpenoids | Bazzania albifolia | MCF-7 | IC50 5.6 μM | [67] |
| Chandolide | Zierane sesquiterpene-γ-lactone | Chandonantus hirtellus | HL-60 | IC50 5.3 μg/mL | |
| Anadensin | Zierane sesquiterpene-γ-lactone | Chandonantus hirtellus | HL-60 | IC50 17.0 μg/mL | [68] |
| 3,18,20-epi-iso-chandonanth- one | Diterpenoids | Chandonantus hirtellus | HL-60 | IC50 17.0 μg/mL | |
| 13,18,20-epi-iso-chandonanthone | Diterpenoids | Chandonantus hirtellus | HL-60 | IC50 17.0 μg/mL | |
| (+)-3α-[4′Methoxybenzyl]-5,7-dimethoxyphthalide | Bibenzyls | Frullania ssp.; Porella perrottetiana | 1) HL-60 2) KB | 1) IC50 0.92 μM 2) IC50 0.96 μM | |
| (−)-3α-[3′-Methoxy-4′,5′-methylenedioxybenzyl]-5,7dimethoxyphthalide | Bibenzyls | Frullania ssp.; Porella perrottetiana | 1) HL-60 2) KB | 1) IC50 6.30 μM 2) IC50 5.47 μM | |
| Tulipinolide | Sesquiterpene lactone | Frullania ssp.; Porella perrottetiana | 1) HL-60 2) KB | 1) IC50 4.59 μM 2) *nd | [69] |
| 7-oxopinguisenol-12-methyl ester | Sesquiterpenoid | Frullania ssp.; Porella perrottetiana | 1) HL-60 2) KB | 1) IC50 8.53 μM 2) IC50 52.64 μM | |
| 4α-5β-Epoxy-8-epiinunolide | Sesquiterpene lactone | Frullania ssp.; Porella perrottetiana | 1) HL-60 2) KB | 1) IC50 2.68 μM 2) IC50 46.27 μM | |
| (-)-herbertenediol | Sesquiterpenoids | Mastigophora diclados | 1) HL cells 2)KB cells | 1) IC50 < 5 μg/mL 2) IC50 > 10 μg/mL | |
| (-)-a-Herbertenol | Sesquiterpenoids | Mastigophora diclados | 1) HL cells 2)KB cells | 1) IC50 > 10 μg/mL 2) IC50 > 10 μg/mL | [70] |
| (-)-Diplohyllolide A | Sesquiterpenoids | Mastigophora diclados | 1) HL cells 2)KB cells | 1) IC50 < 5 μg/mL 2) IC50 < 5 μg/mL | |
| (3S,5S,7R,10S)-3-hydroperoxy-7-hydroxy-eudesma-4(15)-ene | Sesquiterpenoids | Chiloscyphus polyanthus var. rivularis | A549 | Cytotoxic IC50 > 10 μM | [71] |
| Diplophyllolide | Sesquiterpene lactone | Clasmatocolea vermicularis | P388 | IC50 0.4 ug/ml | [72] |
| Scapairiin G Scapairrin H Scapairrin I Scapairrin J | Labdane diterpenoids | Scapania irrigua | MDA-MB231; A2780; HeLa; HT-29 | IC50 < 10 μM (see reference for details) | [73] |
| Lycophlegmarinol B | Triterpenoids | Lycopodium phlegmaria | 1) HuCCA-1 2) A549 3) HepG2 4) MOLT-3 | 1) Inactive 2) Inactive 3) Inactive 4) IC50 14.7 μM | |
| Lycophlegmarinol D | Serratene triterpenoids | Lycopodium phlegmaria | 1) HuCCA-1 2) A549 3) HepG2 4) MOLT-3 | 1) IC50 26.72 μM 2) IC50 47.5 μM 3)Inactive 4) IC50 3.0 μM | [74] |
| 21b-Hydroxy-serrat-14-en-3a-ol | Serratene triterpenoids | Lycopodium phlegmaria | 1) HuCCA-1 2) A549 3) HepG2 4) MOLT-3 | 1) IC50 2.62 μM 2) Inactive 3) IC50 2.42 μM 4) IC50 2.94 μM | |
| Lepidozin A | Cycloartane triterpenoids | Lepidozia reptans | 1) PC-3 2) A549 3) H3255 4) H446 | 1) IC50 6.5 μM 2) IC50 7.0 μM 3) IC50 9.4 μM 4) IC50 7.5 μM | |
| Lepidozin F | Cycloartane triterpenoids | Lepidozia reptans | 1) PC-3 2) A549 3) H3255 4) H446 | 1) IC50 8.6 μM 2) IC50 8.8 μM 3) Inactive 4) IC50 9.6 μM | [61] |
| Naviculyn caffeate | Caffeate esters | Bazzania novaezelandiae | PP38 murine leukaemia cells | IC50 1.1 μg/mL | [75] |
| (±)-Radulapin A (±)-Radulapin C (±)-Radulapin D (±)-Radulapin E (±)-Radulapin F (±)-Radulapin G (±)-Radulapin H | Prenylated Bibenzyl | Radula apiculata | PC-3 A549 MCF-7 NCI-H121199 | IC50 < 10 μM (see reference for details) | [57] |
| 2-carbomethoxy- 3,5dihydroxystilbene | Bibenzyls | Radula amoena | 1) HepG-2 2) SMMC-7721 3) A549 | 1) 22.5 μg/mL 2) 27.63 μg/mL 3) 18 μg/mL | [76] |
| 3,5-dimethoxybibenzyl | Bibenzyls | Radula amoena | 1) HepG-2 2) SMMC-7721 3) A549 | 1) 19.1 μg/mL 2) 18.49 μg/mL 3) 16.55 μg/mL | [76] |
| Perrotettin E | (Bis)bibenzyls | Pellia endivifolia | 1) Promyelocytic HL-60 cells 3) Pro-monocytic Human myeloid leukaemia U-937 cells 4) Myelogenous leukaemia cell line K562 5) NT2/D1 6) A-172 7) U-251 | 1) IC50 14.2 μM 2) IC50 50.5 μM 3) IC50 37.2 μM 4) IC50 11.2 μM 5) IC50 8.8 μM 6) IC50 15 μM | [77] |
| 10′-hydroxyperrottetin E | (Bis)bibenzyls | Pellia endivifolia | 1) HL-60 2) U-937 3) K562 4) NT2/D1 5) A-172 6) U-251 | 1) IC50 > 100 μM 2) IC50 38.5 μM 3) IC50 > 100 μM 4) IC50 15.5 μM 5) IC50 26.2 μM 6) IC50 47.2 μM | [77] |
| 10,10′-dihydroxyperrottetin E | (Bis)bibenzyls | Pellia endivifolia | 1) HL-60 2) U-937 3) K562 4) NT2/D1 5) A-172 6) U-251 | 1) IC50 > 100 μM 2) IC50 > 100 μM 3) IC50 > 100 μM 4) IC50 6.8 μM 5) IC50 53.8 μM 6) IC50 54.8 μM | [77] |
3. Methodology
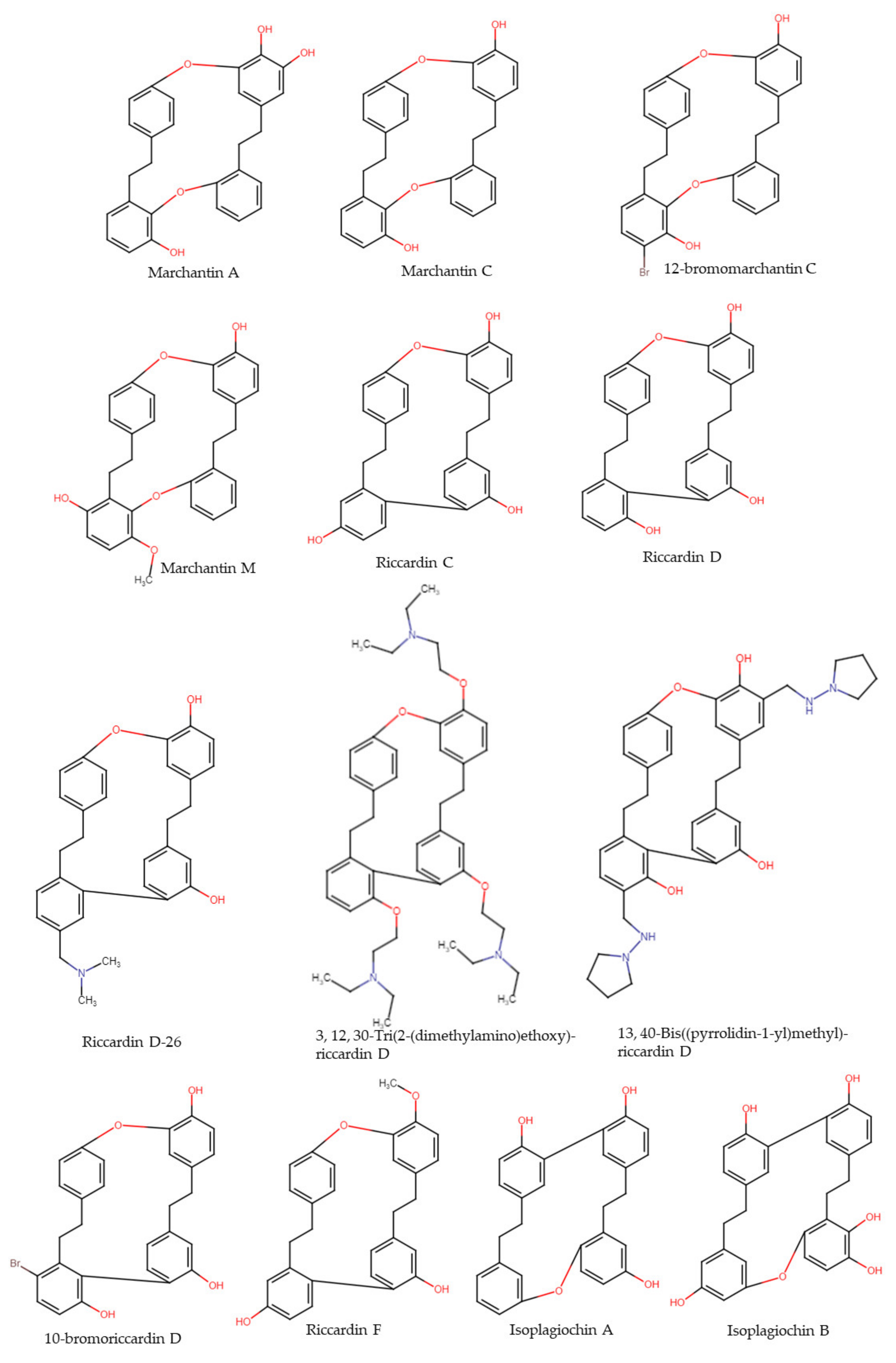
4. Macrocyclic and Acyclic (Bis)bibenzyls
4.1. Marchantin A
4.2. Marchantin C and Derivatives
4.3. Marchantin M
4.4. Riccardin C
4.5. Riccardin D and Derivatives
4.6. Riccardin F
4.7. Isoplagiochin A and B
4.8. Plagiochin E and Derivatives
4.9. Pakyonol
4.10. Dihydroptychantol A and Derivatives
5. Bibenzyls
5.1. Brittonin A, B, and Chrysotobibenzyl
5.2. (±)-Radulapin D
6. Terpenoids
6.1. Jungermannenone A and B
6.2. Ent-11α-hydroxy-16-kauren-15-one
6.3. Isomanool
6.4. Lepidozin G
7. Discussion and Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Plants as a Source of Anti-Cancer Agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of Natural Products on Developing New Anti-Cancer Agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef] [PubMed]
- Taneja, S.C.; Qazi, G.N. Bioactive Molecules in Medicinal Plants: A Perspective on Their Therapeutic Action. In Drug Discovery and Development; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; Volume 2, pp. 1–50. ISBN 978-0-471-39847-9. [Google Scholar]
- Desai, A.G.; Qazi, G.N.; Ganju, R.K.; El-Tamer, M.; Singh, J.; Saxena, A.K.; Bedi, Y.S.; Taneja, S.C.; Bhat, H.K. Medicinal Plants and Cancer Chemoprevention. Curr. Drug Metab. 2008, 9, 581–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asakawa, Y.; Ludwiczuk, A. Chemical Constituents of Bryophytes: Structures and Biological Activity. J. Nat. Prod. 2018, 81, 641–660. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Chandra, D.; Barh, A.; Pankaj; Pandey, R.K.; Sharma, I.P. Bryophytes: Hoard of Remedies, an Ethno-Medicinal Review. J. Tradit. Complement. Med. 2016, 7, 94–98. [Google Scholar] [CrossRef] [Green Version]
- Sabovljević, M.S.; Sabovljević, A.D.; Ikram, N.K.K.; Peramuna, A.; Bae, H.; Simonsen, H.T. Bryophytes—An Emerging Source for Herbal Remedies and Chemical Production. Plant Genet. Resour. 2016, 14, 314–327. [Google Scholar] [CrossRef]
- Asakawa, Y.; Ludwiczuk, A.; Novakovic, M.; Bukvicki, D.; Anchang, K.Y. Bis-Bibenzyls, Bibenzyls, and Terpenoids in 33 Genera of the Marchantiophyta (Liverworts): Structures, Synthesis, and Bioactivity. J. Nat. Prod. 2021, 85, 729–762. [Google Scholar] [CrossRef]
- Cianciullo, P.; Maresca, V.; Sorbo, S.; Basile, A. Antioxidant and Antibacterial Properties of Extracts and Bioactive Compounds in Bryophytes. Appl. Sci. 2022, 12, 160. [Google Scholar] [CrossRef]
- Castaldo-Cobianchi, R.; Giordano, S.; Basile, A.; Violante, U. Occurrence of Antibiotic Activity in Conocephalum Conicum, Mnium Undulatum and Leptodictyum Riparium (Bryophytes). G. Bot. Ital. 1988, 122, 303–311. [Google Scholar] [CrossRef]
- Belkin, M.; Fitzgerald, D.B.; Felix, M.D. Tumor-Damaging Capacity of Plant Materials. II. Plants Used as Diuretics. J. Natl. Cancer Inst. 1952, 13, 741–744. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Screening Data Summary; National Cancer Institute: Bethesda, MD, USA, 1977. [Google Scholar]
- National Cancer Institute. Screening Data Summary; National Cancer Institute: Bethesda, MD, USA, 1980. [Google Scholar]
- Spjut, R.W.; Suffness, M.; Cragg, G.M.; Norris, D.H. Mosses, Liverworts, and Hornworts Screened for Antitumor Agents. Econ. Bot. 1986, 40, 310. [Google Scholar] [CrossRef]
- Abay, G.; Altun, M.; Koldaş, S.; Tüfekçi, A.R.; Demirtas, I. Determination of Antiproliferative Activities of Volatile Contents and HPLC Profiles of Dicranum Scoparium (Dicranaceae, Bryophyta). Comb. Chem. High Throughput Screen. 2022, 18, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Klavina, L.; Springe, G.; Nikolajeva, V.; Martsinkevich, I.; Nakurte, I.; Dzabijeva, D.; Steinberga, I. Chemical Composition Analysis, Antimicrobial Activity and Cytotoxicity Screening of Moss Extracts (Moss Phytochemistry). Molecules 2015, 20, 17221–17243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, H.; Toyang, N.; Bryant, J. In Vitro and In Vivo Anti-Cancer Effects of Tillandsia Recurvata (Ball Moss) from Jamaica. West Indian Med. J. 2017, 62, 177–180. [Google Scholar] [CrossRef] [Green Version]
- Oztopcu-Vatan, P.; Savaroglu, F.; Iscen, C.F.; Kabadere, S.; Ozturk, N.; Ilhan, S. Screening of Antimicrobial, Cytotoxic Effects and Phenolic Compounds of the Moss Aulacomnium Androgynum (Hedw.) Schwagr (Bryophyta). J. Anim. Plant Sci. 2017, 27, 1909–1917. [Google Scholar]
- Oztopcu-Vatan, P.; Savaroglu, F.; Filik-Iscen, C.; Kabadere, S.; Ilhan, S.; Uyar, R. Antimicrobial and Antiproliferative Activities of Homalothecium Sericeum (Hedw.) Schimp. Extracts. Fresenius Environ. Bull. 2011, 20, 461–466. [Google Scholar]
- Vollár, M.; Gyovai, A.; Szucs, P.; Zupkó, I.; Marschall, M.; Csupor-Lffler, B.; Bérdi, P.; Vecsernyés, A.; Csorba, A.; Liktor-Busa, E.; et al. Antiproliferative and Antimicrobial Activities of Selected Bryophytes. Molecules 2018, 23, 1520. [Google Scholar] [CrossRef] [Green Version]
- Wolski, G.J.; Sadowska, B.; Fol, M.; Podsędek, A.; Kajszczak, D.; Kobylińska, A. Cytotoxicity, Antimicrobial and Antioxidant Activities of Mosses Obtained from Open Habitats. PLoS ONE 2021, 16, e0257479. [Google Scholar] [CrossRef]
- Yuan, W.; Cheng, X.; Wang, P.; Jia, Y.; Liu, Q.; Tang, W.; Wang, X. Polytrichum Commune L.Ex Hedw Ethyl Acetate Extract-Triggered Perturbations in Intracellular Ca2+ Homeostasis Regulates Mitochondrial-Dependent Apoptosis. J. Ethnopharmacol. 2015, 172, 410–420. [Google Scholar] [CrossRef]
- Hawrył, A.; Bogucka-Kocka, A.; Świeboda, R.; Hawrył, M.; Stebel, A.; Waksmundzka-Hajnos, M. Thin-Layer Chromatography Fingerprint and Chemometric Analysis of Selected Bryophyta Species with Their Cytotoxic Activity. JPC—J. Planar Chromatogr.–Mod. TLC 2018, 31, 28–35. [Google Scholar] [CrossRef]
- Yayintas, O. Determination of Antioxidant, Antimicrobial and Antitumor Activity of Bryophytes from Mount Ida (Canakkale, Turkey); Council of Scientific and Industrial Research–National Institute of Science Communication and Policy Research (CSIR–NIScPR): New Delhi, India, 2019. [Google Scholar]
- Savaroğlu, F.; İŞçen, C.F.; Vatan, A.P.Ö.; Kabadere, S.; İLhan, S.; Uyar, R. Determination of Antimicrobial and Antiproliferative Activities of the Aquatic Moss Fontinalis Antipyretica Hedw. Turk. J. Biol. 2011, 35, 361–369. [Google Scholar]
- Guo, L.; Wu, J.; Han, T.; Cao, T.; Rahman, K.; Qin, L. Chemical Composition, Antifungal and Antitumor Properties of Ether Extracts of Scapania Verrucosa Heeg. and Its Endophytic Fungus Chaetomium Fusiforme. Molecules 2008, 13, 2114–2125. [Google Scholar] [CrossRef] [Green Version]
- Önder, A.; Özenoğlu, H. Evaluation of Cytotoxic Effects on Ethereal Extracts of Some Selected Liverworts. Fabad J. Pharm. Sci. 2019, 44, 119–125. [Google Scholar]
- Zhou, F.; Aipire, A.; Xia, L.; Halike, X.; Yuan, P.; Sulayman, M.; Wang, W.; Li, J. Marchantia Polymorpha L. Ethanol Extract Induces Apoptosis in Hepatocellular Carcinoma Cells via Intrinsic- and Endoplasmic Reticulum Stress-Associated Pathways. Chin. Med. 2021, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Krishna, R.; Mayer, L.D. Multidrug Resistance (MDR) in Cancer: Mechanisms, Reversal Using Modulators of MDR and the Role of MDR Modulators in Influencing the Pharmacokinetics of Anticancer Drugs. Eur. J. Pharm. Sci. 2000, 11, 265–283. [Google Scholar] [CrossRef]
- Cole, S.P.C.; Deeley, R.G. Multidrug Resistance Mediated by the ATP-Binding Cassette Transporter Protein MRP. BioEssays 1998, 20, 931–940. [Google Scholar] [CrossRef]
- Sharom, F.J. The P-Glycoprotein Multidrug Transporter. Essays Biochem. 2011, 50, 161–178. [Google Scholar] [CrossRef] [Green Version]
- Thiebaut, F.; Tsuruo, T.; Hamada, H.; Gottesman, M.M.; Pastan, I.; Willingham, M.C. Cellular Localization of the Multidrug-Resistance Gene Product P-Glycoprotein in Normal Human Tissues. Proc. Natl. Acad. Sci. USA 1987, 84, 7735–7738. [Google Scholar] [CrossRef] [Green Version]
- Fernald, K.; Kurokawa, M. Evading Apoptosis in Cancer. Trends Cell Biol. 2013, 23, 620–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- HemaIswarya, S.; Doble, M. Potential Synergism of Natural Products in the Treatment of Cancer. Phytother. Res. 2006, 20, 239–249. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Jayson, G.C.; Kerbel, R.; Ellis, L.M.; Harris, A.L. Antiangiogenic Therapy in Oncology: Current Status and Future Directions. Lancet 2016, 388, 518–529. [Google Scholar] [CrossRef]
- Huang, W.-J.; Wu, C.-L.; Lin, C.-W.; Chi, L.-L.; Chen, P.-Y.; Chiu, C.-J.; Huang, C.-Y.; Chen, C.-N. Marchantin A, a Cyclic Bis(Bibenzyl Ether), Isolated from the Liverwort Marchantia Emarginata Subsp. Tosana Induces Apoptosis in Human MCF-7 Breast Cancer Cells. Cancer Lett. 2010, 291, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Gaweł-Bęben, K.; Osika, P.; Asakawa, Y.; Antosiewicz, B.; Głowniak, K.; Ludwiczuk, A. Evaluation of Anti-Melanoma and Tyrosinase Inhibitory Properties of Marchantin A, a Natural Macrocyclic Bisbibenzyl Isolated from Marchantia Species. Phytochem. Lett. 2019, 31, 192–195. [Google Scholar] [CrossRef]
- Jensen, J.S.R.E.; Omarsdottir, S.; Thorsteinsdottir, J.B.; Ogmundsdottir, H.M.; Olafsdottir, E.S. Synergistic Cytotoxic Effect of the Microtubule Inhibitor Marchantin A from Marchantia Polymorpha and the Aurora Kinase Inhibitor MLN8237 on Breast Cancer Cells In Vitro. Planta Med. 2012, 78, 448–454. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.-Q.; Liao, Y.-X.; Qu, X.-J.; Yuan, H.-Q.; Li, S.; Qu, J.-B.; Lou, H.-X. Marchantin C, a Macrocyclic Bisbibenzyl, Induces Apoptosis of Human Glioma A172 Cells. Cancer Lett. 2008, 262, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhu, C.; Yuan, H.; Li, B.; Gao, J.; Qu, X.; Sun, B.; Cheng, Y.; Li, S.; Li, X.; et al. Marchantin C, a Novel Microtubule Inhibitor from Liverwort with Anti-Tumor Activity Both in Vivo and in Vitro. Cancer Lett. 2009, 276, 160–170. [Google Scholar] [CrossRef]
- Shen, J.; Li, G.; Liu, Q.; He, Q.; Gu, J.; Shi, Y.; Lou, H. Marchantin C: A Potential Anti-Invasion Agent in Glioma Cells. Cancer Biol. Ther. 2010, 9, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Xi, G.; Sun, B.; Jiang, H.; Kong, F.; Yuan, H.; Lou, H. Bisbibenzyl Derivatives Sensitize Vincristine-Resistant KB/VCR Cells to Chemotherapeutic Agents by Retarding P-Gp Activity. Bioorganic Med. Chem. 2010, 18, 6725–6733. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Song, Q.; Shao, Q.; Gao, W.; Mao, H.; Lou, H.; Qu, X.; Li, X. Comparison of the Effects of Marchantin C and Fucoidan on SFlt-1 and Angiogenesis in Glioma Microenvironment. J. Pharm. Pharmacol. 2012, 64, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Sun, B.; Wang, Y.; Cui, M.; Zhang, L.; Cui, C.; Wang, Y.; Liu, X.; Lou, H. Synthesis of Macrocyclic Bisbibenzyl Derivatives and Their Anticancer Effects as Anti-Tubulin Agents. Bioorganic Med. Chem. 2012, 20, 2382–2391. [Google Scholar] [CrossRef]
- Zhang, T.-W.; Xing, L.; Tang, J.-L.; Lu, J.-X.; Liu, C.-X. Marchantin M Induces Apoptosis of Prostate Cancer Cells Through Endoplasmic Reticulum Stress. Med. Sci. Monit. 2015, 21, 3570–3576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.-P.; Gao, Z.-H.; Cui, S.-X.; Sun, D.-F.; Wang, Y.; Zhao, C.-R.; Lou, H.-X.; Qu, X.-J. Inhibition of Intestinal Adenoma Formation in APCMin/+ Mice by Riccardin D, a Natural Product Derived from Liverwort Plant Dumortiera Hirsuta. PLoS ONE 2012, 7, e33243. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, G.; Zhang, B.; Sun, D.; Wu, J.; Chen, F.; Kong, F.; Luan, Y.; Jiang, W.; Wang, R.; et al. Suppression of the NF-κB Signaling Pathway in Colon Cancer Cells by the Natural Compound Riccardin D from Dumortierahirsute. Mol. Med. Rep. 2018, 17, 5837–5843. [Google Scholar] [CrossRef] [Green Version]
- Yue, B.; Zhao, C.-R.; Xu, H.-M.; Li, Y.-Y.; Cheng, Y.-N.; Ke, H.-N.; Yuan, Y.; Wang, R.-Q.; Shi, Y.-Q.; Lou, H.-X.; et al. Riccardin D-26, a Synthesized Macrocyclic Bisbibenzyl Compound, Inhibits Human Oral Squamous Carcinoma Cells KB and KB/VCR: In Vitro and in Vivo Studies. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2013, 1830, 2194–2203. [Google Scholar] [CrossRef]
- Sun, B.; Liu, J.; Gao, Y.; Zheng, H.; Li, L.; Hu, Q.; Yuan, H.; Lou, H. Design, Synthesis and Biological Evaluation of Nitrogen-Containing Macrocyclic Bisbibenzyl Derivatives as Potent Anticancer Agents by Targeting the Lysosome. Eur. J. Med. Chem. 2017, 136, 603–618. [Google Scholar] [CrossRef]
- Ji, M.; Shi, Y.; Lou, H. Overcoming of P-Glycoprotein-Mediated Multidrug Resistance in K562/A02 Cells Using Riccardin F and Pakyonol, Bisbibenzyl Derivatives from Liverworts. BioScience Trends 2011, 5, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.-Q.; Qu, X.-J.; Liao, Y.-X.; Xie, C.-F.; Cheng, Y.-N.; Li, S.; Lou, H.-X. Reversal Effect of a Macrocyclic Bisbibenzyl Plagiochin E on Multidrug Resistance in Adriamycin-Resistant K562/A02 Cells. Eur. J. Pharmacol. 2008, 584, 66–71. [Google Scholar] [CrossRef]
- Morita, H.; Tomizawa, Y.; Tsuchiya, T.; Hirasawa, Y.; Hashimoto, T.; Asakawa, Y. Antimitotic Activity of Two Macrocyclic Bis(Bibenzyls), Isoplagiochins A and B from the Liverwort Plagiochila Fruticosa. Bioorganic Med. Chem. Lett. 2009, 19, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.-X.; Xiang, F.; Wang, X.-N.; Yuan, H.-Q.; Xi, G.-M.; Wang, Y.-Y.; Yu, W.-T.; Lou, H.-X. Labdane Diterpenoids and Highly Methoxylated Bibenzyls from the Liverwort Frullania Inouei. Phytochemistry 2010, 71, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Y.; Gao, Y.; Zhou, J.-C.; Xu, Z.-J.; Qiao, Y.-N.; Zhang, J.-Z.; Lou, H.-X. Diverse Prenylated Bibenzyl Enantiomers from the Chinese Liverwort Radula Apiculata and Their Cytotoxic Activities. J. Nat. Prod. 2021, 84, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, I.; Kondoh, M.; Harada, M.; Koizumi, N.; Fujii, M.; Nagashima, F.; Asakawa, Y.; Watanabe, Y. An ent-Kaurene Diterpene Enhances Apoptosis Induced by Tumor Necrosis Factor in Human Leukemia Cells. Planta Med. 2004, 70, 723–727. [Google Scholar] [CrossRef]
- Guo, Y.; Lin, Z.; Wang, M.; Dong, Y.; Niu, H.; Young, C.Y.; Lou, H.; Yuan, H. Jungermannenone A and B Induce ROS- and Cell Cycle-Dependent Apoptosis in Prostate Cancer Cells in Vitro. Acta Pharmacol. Sin. 2016, 37, 814–824. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.-M.; Guo, Y.-X.; Wang, S.-Q.; Wang, X.-N.; Chang, W.-Q.; Zhou, J.-C.; Yuan, H.; Lou, H. Diterpenoids from the Chinese Liverwort Heteroscyphus Tener and Their Antiproliferative Effects. J. Nat. Prod. 2014, 77, 1336–1344. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Chu, Z.-J.; Zhou, J.-C.; Liu, S.-G.; Zhang, J.-Z.; Qian, L.; Lou, H.-X. Cytotoxic Activities of 9,10-Seco-Cycloartane-Type Triterpenoids from the Chinese Liverwort Lepidozia Reptans. J. Nat. Prod. 2021, 84, 3020–3028. [Google Scholar] [CrossRef]
- Lou, H.-X.; Li, G.-Y.; Wang, F.-Q. A Cytotoxic Diterpenoid and Antifungal Phenolic Compounds from Frullania Muscicola Steph. J. Asian Nat. Prod. Res. 2002, 4, 87–94. [Google Scholar] [CrossRef]
- Kang, Y.-Q.; Zhou, J.-C.; Fan, P.-H.; Wang, S.-Q.; Lou, H.-X. Scapaundulin C, a Novel Labdane Diterpenoid Isolated from Chinese Liverwort Scapania Undulate, Inhibits Acetylcholinesterase Activity. Chin. J. Nat. Med. 2015, 13, 933–936. [Google Scholar] [CrossRef]
- Perry, N.B.; Burgess, E.J.; Baek, S.-H.; Weavers, R.T. The First Atisane Diterpenoids from a Liverwort: Polyols from Lepidolaena Clavigera. Org. Lett. 2001, 3, 4243–4245. [Google Scholar] [CrossRef]
- Wang, S.; Li, R.-J.; Zhu, R.-X.; Hu, X.-Y.; Guo, Y.-X.; Zhou, J.-C.; Lin, Z.-M.; Zhang, J.-Z.; Wu, J.-Y.; Kang, Y.-Q.; et al. Notolutesins A–J, Dolabrane-Type Diterpenoids from the Chinese Liverwort Notoscyphus Lutescens. J. Nat. Prod. 2014, 77, 2081–2087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Métoyer, B.; Lebouvier, N.; Hnawia, E.; Thouvenot, L.; Wang, F.; Harinantenaina Rakotondraibe, L.; Raharivelomanana, P.; Asakawa, Y.; Nour, M. Chemotaxonomy and Cytotoxicity of the Liverwort Porella Viridissima. Nat. Prod. Res. 2021, 35, 2099–2102. [Google Scholar] [CrossRef]
- Liu, N.; Guo, D.-X.; Wang, S.-Q.; Wang, Y.-Y.; Zhang, L.; Li, G.; Lou, H.-X. Bioactive Sesquiterpenoids and Diterpenoids from the Liverwort Bazzania Albifolia. Chem. Biodivers. 2012, 9, 2254–2261. [Google Scholar] [CrossRef] [PubMed]
- Komala, I.; Ito, T.; Nagashima, F.; Yagi, Y.; Kawahata, M.; Yamaguchi, K.; Asakawa, Y. Zierane Sesquiterpene Lactone, Cembrane and Fusicoccane Diterpenoids, from the Tahitian Liverwort Chandonanthus Hirtellus. Phytochemistry 2010, 71, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Komala, I.; Ito, T.; Nagashima, F.; Yagi, Y.; Asakawa, Y. Cytotoxic Bibenzyls, and Germacrane- and Pinguisane-Type Sesquiterpenoids from Indonesian, Tahitian and Japanese Liverworts. Nat. Prod. Commun. 2011, 6, 1934578X1100600301. [Google Scholar] [CrossRef] [Green Version]
- Komala, I.; Ito, T.; Nagashima, F.; Yagi, Y.; Asakawa, Y. Cytotoxic, Radical Scavenging and Antimicrobial Activities of Sesquiterpenoids from the Tahitian Liverwort Mastigophora Diclados (Brid.) Nees (Mastigophoraceae). J. Nat. Med. 2010, 64, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Z.; Qiao, Y.-N.; Li, L.; Wang, Y.-J.; Li, Y.; Fei, X.; Zhou, J.-C.; Wang, X.; Fan, P.-H.; Lou, H.-X. Ent-Eudesmane-Type Sesquiterpenoids from the Chinese Liverwort Chiloscyphus Polyanthus Var. Rivularis. Planta Med. 2016, 82, 1128–1133. [Google Scholar] [CrossRef] [Green Version]
- Lorimer, S.D.; Burgess, E.J.; Perry, N.B. Diplophyllolide: A Cytotoxic Sesquiterpene Lactone from the Liverworts Clasmatocolea Vermicularis and Chiloscyphus Subporosa. Phytomedicine 1997, 4, 261–263. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Zhu, R.; Li, L.; Wang, Y.; Zhou, J.; Qiao, Y.; Zhang, Z.; Lou, H. Scapairrins A–Q, Labdane-Type Diterpenoids from the Chinese Liverwort Scapania Irrigua and Their Cytotoxic Activity. J. Nat. Prod. 2015, 78, 2087–2094. [Google Scholar] [CrossRef]
- Wittayalai, S.; Sathalalai, S.; Thorroad, S.; Worawittayanon, P.; Ruchirawat, S.; Thasana, N. Lycophlegmariols A–D: Cytotoxic Serratene Triterpenoids from the Club Moss Lycopodium Phlegmaria L. Phytochemistry 2012, 76, 117–123. [Google Scholar] [CrossRef]
- Burgess, E.J.; Larsen, L.; Perry, N.B. A Cytotoxic Sesquiterpene Caffeate from the Liverwort Bazzania Novae-Zelandiae. J. Nat. Prod. 2000, 63, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zhu, R.; Zhou, J.; Li, Y.; Qiao, Y.; Zhang, C.; Zhang, J.; Gao, Y.; Chen, W.; Lou, H. Prenyl Bibenzyls Isolated from Chinese Liverwort Radula Amoena and Their Cytotoxic Activities. Phytochem. Lett. 2019, 31, 53–57. [Google Scholar] [CrossRef]
- Ivković, I.; Novaković, M.; Veljić, M.; Mojsin, M.; Stevanović, M.; Marin, P.D.; Bukvički, D. Bis-Bibenzyls from the Liverwort Pellia Endiviifolia and Their Biological Activity. Plants 2021, 10, 1063. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Sun, Y.; Zhu, R.-L. The Oil Bodies of Liverworts: Unique and Important Organelles in Land Plants. Crit. Rev. Plant Sci. 2013, 32, 293–302. [Google Scholar] [CrossRef]
- Tori, M.; Toyota, M.; Harrison, L.J.; Takikawa, K.; Asakawa, Y. Total Assignment of 1H and 13C NMR Spectra of Marchantins Isolated from Liverworts and Its Application to Structure Determination of Two New Macrocyclic Bis(Bibenzyls) from Plagiochasma Intermedium and Riccardia Multifida. Tetrahedron Lett. 1985, 26, 4735–4738. [Google Scholar] [CrossRef]
- Friederich, S.; Rueffer, M.; Asakawa, Y.; Zenk, M.H. Cytochromes P-450 Catalyze the Formation of Marchantins A and C in Marchantia Polymorpha. Phytochemistry 1999, 52, 1195–1202. [Google Scholar] [CrossRef]
- Kámory, E.; Keserü, G.M.; Papp, B. Isolation and Antibacterial Activity of Marchantin A, a Cyclic Bis(Bibenzyl) Constituent of Hungarian Marchantia Polymorpha. Planta Med. 1995, 61, 387–388. [Google Scholar] [CrossRef]
- Toyota, M.; Yoshida, T.; Matsunami, J.; Asakawa, Y. Sesquiterpene and Other Constituents of the Liverwort Dumortiera Hirsuta. Phytochemistry 1997, 44, 293–298. [Google Scholar] [CrossRef]
- Asakawa, Y.; Lin, X.; Kondo, K.; Fukuyama, Y. Terpenoids and Aromatic Compounds from Selected East Malaysian Liverworts. Phytochemistry 1991, 30, 4019–4024. [Google Scholar] [CrossRef]
- Adam, K.-P.; Becker, H. Bisbibenzyl Formation in Aseptic Cultures of Marchantia Polymorpha L. Z. Für Nat. C 1993, 48, 839–842. [Google Scholar] [CrossRef] [Green Version]
- Asakawa, Y.; Toyota, M.; Matsuda, R.; Takikawa, K.; Takemoto, T. Distribution of Novel Cyclic Bisbibenzyls in Marchantia and Riccardia Species. Phytochemistry 1983, 22, 1413–1415. [Google Scholar] [CrossRef]
- Bardón, A.; Kamiya, N.; Toyota, M.; Asakawa, Y. A 7-Nordumortenone and Other Dumortane Derivatives from the Argentine Liverwort Dumortiera Hirsuta. Phytochemistry 1999, 51, 281–287. [Google Scholar] [CrossRef]
- Wei, H.-C.; Ma, S.-J.; Wu, C.-L. Sesquiterpenoids and Cyclic Bisbibenzyls from the Liverwort Reboulia Hemisphaerica. Phytochemistry 1995, 39, 91–97. [Google Scholar] [CrossRef]
- Kroemer, G. The Proto-Oncogene Bcl-2 and Its Role in Regulating Apoptosis. Nat. Med. 1997, 3, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.C. Double Identity for Proteins of the Bcl-2 Family. Nature 1997, 387, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Hu, Z.; Qu, J.; Liu, S.; Syed, A.K.A.; Yuan, H.; Lou, H. Cyclic Bisbibenzyls Induce Growth Arrest and Apoptosis of Human Prostate Cancer PC3 Cells. Acta Pharmacol. Sin. 2010, 31, 609–615. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Sun, J.; Xu, Q.; Liu, Y.; Wei, J.; Young, C.Y.F.; Yuan, H.; Lou, H. Marchantin M: A Novel Inhibitor of Proteasome Induces Autophagic Cell Death in Prostate Cancer Cells. Cell Death Dis. 2013, 4, e761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asakawa, Y.; Matsuda, R. Riccardin C, a Novel Cyclic Bibenzyl Derivative from Reboulia Hemisphaerica. Phytochemistry 1982, 21, 2143–2144. [Google Scholar] [CrossRef]
- Asakawa, Y.; Okada, K.; Perold, G.W. Distribution of Cyclic Bis(Bibenzyls) in the South African Liverwort Marchantia Polymorpha. Phytochemistry 1988, 27, 161–163. [Google Scholar] [CrossRef]
- Asakawa, Y.; Tori, M.; Takikawa, K.; Krishnamurty, H.G.; Kar, S.K. Cyclic Bis(Bibenzyls) and Related Compounds from the Liverworts Marchantia Polymorpha and Marchantia Palmata. Phytochemistry 1987, 26, 1811–1816. [Google Scholar] [CrossRef]
- Harinantenaina, L.; Asakawa, Y. Chemical Constituents of Malagasy Liverworts, Part II: Mastigophoric Acid Methyl Ester of Biogenetic Interest from Mastigophora Diclados (Lepicoleaceae Subf. Mastigophoroideae). Chem. Pharm. Bull. 2004, 52, 1382–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardón, A.; Kamiya, N.; Toyota, M.; Takaoka, S.; Asakawa, Y. Sesquiterpenoids, Hopanoids and Bis(Bibenzyls) from the Argentine Liverwort Plagiochasma Rupestre. Phytochemistry 1999, 52, 1323–1329. [Google Scholar] [CrossRef]
- Kunz, S.; Becker, H. Bibenzyl Glycosides from the Liverwort Ricciocarpos Natans. Phytochemistry 1992, 31, 3981–3983. [Google Scholar] [CrossRef]
- Asakawa, Y. Chemical Constituents of the Bryophytes. In Progress in the Chemistry of Organic Natural Products; Asakawa, Y., Ed.; Fortschritte der Chemie organischer Naturstoffe; Springer: Vienna, Austria, 1995; pp. 1–562. ISBN 978-3-7091-6896-7. [Google Scholar]
- Hu, Z.; Zhang, D.; Hao, J.; Tian, K.; Wang, W.; Lou, H.; Yuan, H. Induction of DNA Damage and P21-Dependent Senescence by Riccardin D Is a Novel Mechanism Contributing to Its Growth Suppression in Prostate Cancer Cells in Vitro and in Vivo. Cancer Chemother Pharm. 2014, 73, 397–407. [Google Scholar] [CrossRef]
- Valcic, S.; Zapp, J.; Becker, H. Plagiochilines and Other Sesquiterpenoids from Plagiochila (Hepaticae). Phytochemistry 1997, 44, 89–99. [Google Scholar] [CrossRef]
- Xue, X.; Sun, D.-F.; Sun, C.-C.; Liu, H.-P.; Yue, B.; Zhao, C.-R.; Lou, H.-X.; Qu, X.-J. Inhibitory Effect of Riccardin D on Growth of Human Non-Small Cell Lung Cancer: In Vitro and in Vivo Studies. Lung Cancer 2012, 76, 300–308. [Google Scholar] [CrossRef]
- Xue, X.; Qu, X.-J.; Gao, Z.-H.; Sun, C.-C.; Liu, H.-P.; Zhao, C.-R.; Cheng, Y.-N.; Lou, H.-X. Riccardin D, a Novel Macrocyclic Bisbibenzyl, Induces Apoptosis of Human Leukemia Cells by Targeting DNA Topoisomerase II. Investig. New Drugs 2012, 30, 212–222. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, Y.; Xue, X.; Cheng, Y.; Liu, H.; Zhao, C.; Lou, H.; Qu, X. Inhibition of Angiogenesis Involves in Anticancer Activity of Riccardin D, a Macrocyclic Bisbibenzyl, in Human Lung Carcinoma. Eur. J. Pharmacol. 2011, 667, 136–143. [Google Scholar] [CrossRef]
- Xie, C.-F.; Qu, J.-B.; Wu, X.-Z.; Liu, N.; Ji, M.; Lou, H.-X. Antifungal Macrocyclic Bis(Bibenzyls) from the Chinese Liverwort Ptagiochasm Intermedlum L. Nat. Prod. Res. 2010, 24, 515–520. [Google Scholar] [CrossRef]
- Hashimoto, T.; Kanayama, S.; Fukuyama, Y.; Takaoka, S.; Tori, M.; Asakawa, Y. Two Novel Macrocyclic Bis(Bibenzyls), Isoplagiochins A and B from the Liverwort Plagiochila Fruticosa. Tetrahedron Lett. 1994, 35, 911–912. [Google Scholar] [CrossRef]
- Wu, X.; Cheng, A.; Sun, L.; Lou, H. Effect of Plagiochin E, an Antifungal Macrocyclic Bis(Bibenzyl), on Cell Wall Chitin Synthesis in Candida Albicans. Acta Pharmacol. Sin. 2008, 29, 1478–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Sun, B.; Zhu, C.-J.; Yuan, H.-Q.; Shi, Y.-Q.; Gao, J.; Li, S.-J.; Lou, H.-X. Reversal of P-Glycoprotein-Mediated Multidrug Resistance by Macrocyclic Bisbibenzyl Derivatives in Adriamycin-Resistant Human Myelogenous Leukemia (K562/A02) Cells. Toxicol. Vitr. 2009, 23, 29–36. [Google Scholar] [CrossRef]
- Pang, Y.; Si, M.; Sun, B.; Niu, L.; Xu, X.; Lu, T.; Yuan, H.; Lou, H. DHA2, a Synthesized Derivative of Bisbibenzyl, Exerts Antitumor Activity against Ovarian Cancer through Inhibition of XIAP and Akt/MTOR Pathway. Food Chem. Toxicol. 2014, 69, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lu, J.-J.; Huang, M.-Q.; Bao, J.-L.; Chen, X.-P.; Wang, Y.-T. Terpenoids: Natural Products for Cancer Therapy. Expert Opin Investig. Drugs 2012, 21, 1801–1818. [Google Scholar] [CrossRef] [PubMed]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.-M.; Chan, T.-F.; Hui, J.H.L. Terpenes and Terpenoids in Plants: Interactions with Environment and Insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef]
- Kamran, S.; Sinniah, A.; Abdulghani, M.A.M.; Alshawsh, M.A. Therapeutic Potential of Certain Terpenoids as Anticancer Agents: A Scoping Review. Cancers 2022, 14, 1100. [Google Scholar] [CrossRef]
- Lin, Z.; Guo, Y.; Gao, Y.; Wang, S.; Wang, X.; Xie, Z.; Niu, H.; Chang, W.; Liu, L.; Yuan, H.; et al. Ent-Kaurane Diterpenoids from Chinese Liverworts and Their Antitumor Activities through Michael Addition As Detected in Situ by a Fluorescence Probe. J. Med. Chem. 2015, 58, 3944–3956. [Google Scholar] [CrossRef]
- Nagashima, F.; Kondoh, M.; Kawase, M.; Simizu, S.; Osada, H.; Fujii, M.; Watanabe, Y.; Sato, M.; Asakawa, Y. Apoptosis-Inducing Properties of ent-Kaurene-Type Diterpenoids from the Liverwort Jungermannia truncata. Planta Med. 2003, 69, 377–379. [Google Scholar] [CrossRef]
- Nagashima, F.; Kondoh, M.; Uematsu, T.; Nishiyama, A.; Saito, S.; Sato, M.; Asakawa, Y. Cytotoxic and Apoptosis-Inducing Ent-Kaurane-Type Diterpenoids from the Japanese Liverwort Jungermannia Truncata NEES. Chem. Pharm. Bull. 2002, 50, 808–813. [Google Scholar] [CrossRef] [Green Version]
- Bloom, J.; Cross, F.R. Multiple Levels of Cyclin Specificity in Cell-Cycle Control. Nat. Rev. Mol. Cell Biol. 2007, 8, 149–160. [Google Scholar] [CrossRef]
- Miceli, M.; Bontempo, P.; Nebbioso, A.; Altucci, L. Natural Compounds in Epigenetics: A Current View. Food Chem. Toxicol. 2014, 73, 71–83. [Google Scholar] [CrossRef]
- Scafuri, B.; Bontempo, P.; Altucci, L.; De Masi, L.; Facchiano, A. Molecular docking simulations on histone deacetylases (Hdac)-1 and-2 to investigate the flavone binding. Biomedicines 2020, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ludwiczuk, A.; Wei, G.; Chen, X.; Crandall-Stotler, B.; Bowman, J.L. Terpenoid Secondary Metabolites in Bryophytes: Chemical Diversity, Biosynthesis and Biological Functions. Crit. Rev. Plant Sci. 2018, 37, 210–231. [Google Scholar] [CrossRef]
- Gupta, S.; Kesarla, R.; Omri, A. Formulation Strategies to Improve the Bioavailability of Poorly Absorbed Drugs with Special Emphasis on Self-Emulsifying Systems. ISRN 2013, 2013, 848043. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cianciullo, P.; Cimmino, F.; Maresca, V.; Sorbo, S.; Bontempo, P.; Basile, A. Anti-Tumour Activities from Secondary Metabolites and Their Derivatives in Bryophytes: A Brief Review. Appl. Biosci. 2022, 1, 73-94. https://doi.org/10.3390/applbiosci1010005
Cianciullo P, Cimmino F, Maresca V, Sorbo S, Bontempo P, Basile A. Anti-Tumour Activities from Secondary Metabolites and Their Derivatives in Bryophytes: A Brief Review. Applied Biosciences. 2022; 1(1):73-94. https://doi.org/10.3390/applbiosci1010005
Chicago/Turabian StyleCianciullo, Piergiorgio, Francesca Cimmino, Viviana Maresca, Sergio Sorbo, Paola Bontempo, and Adriana Basile. 2022. "Anti-Tumour Activities from Secondary Metabolites and Their Derivatives in Bryophytes: A Brief Review" Applied Biosciences 1, no. 1: 73-94. https://doi.org/10.3390/applbiosci1010005
APA StyleCianciullo, P., Cimmino, F., Maresca, V., Sorbo, S., Bontempo, P., & Basile, A. (2022). Anti-Tumour Activities from Secondary Metabolites and Their Derivatives in Bryophytes: A Brief Review. Applied Biosciences, 1(1), 73-94. https://doi.org/10.3390/applbiosci1010005









