The Muscle Cells in Pelvic Floor Dysfunctions: Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
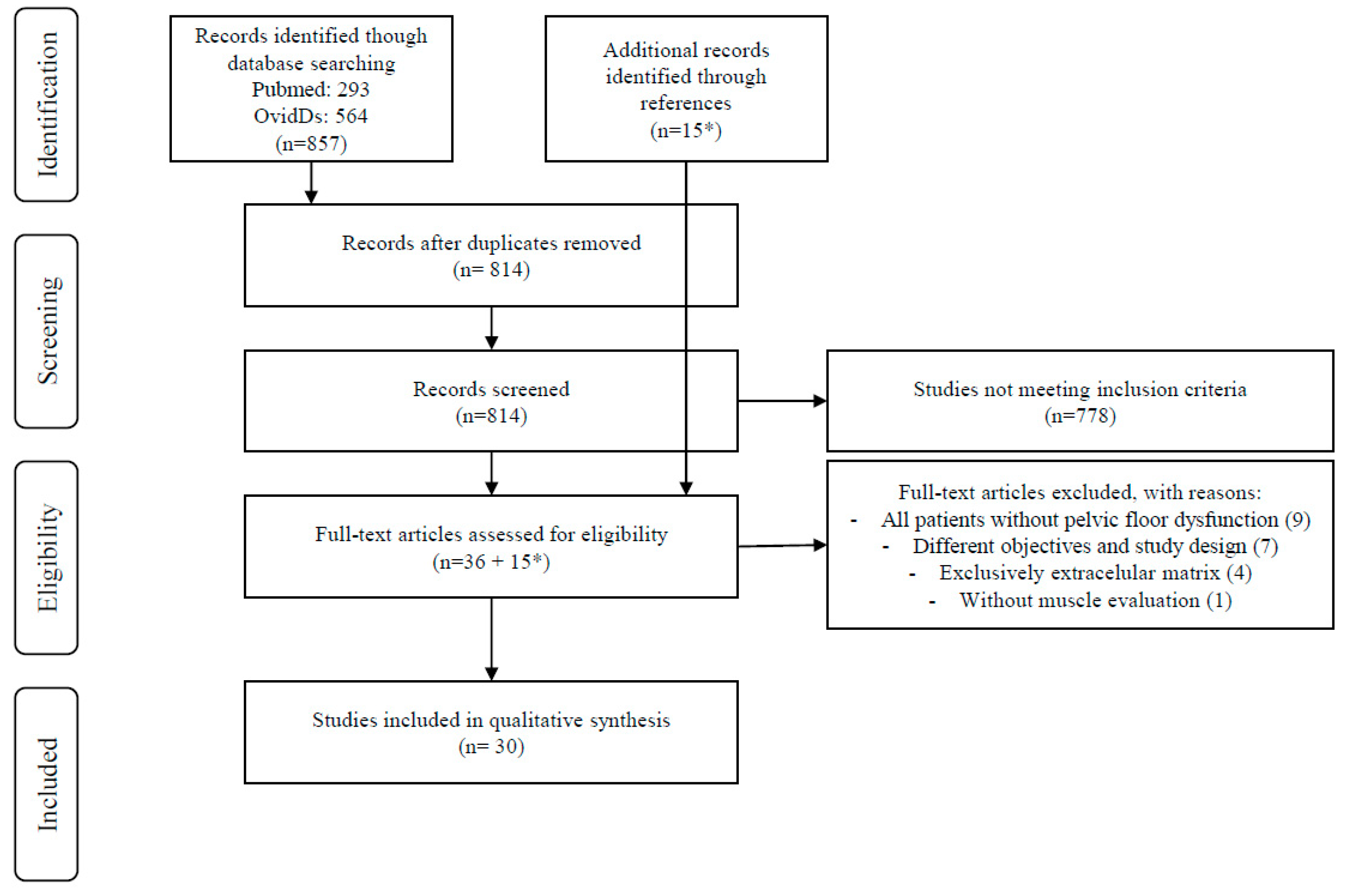
Database Search and Study Selection
2.2. Eligibility Criteria
2.3. Search Strategies
2.4. Study Selection and Data Extraction
2.5. Risk-of-Bias Assessment and Data Synthesis
3. Results
3.1. Study Characteristics
3.2. Study Quality Appraisal and Bias Assessment
3.2.1. Cellular Pelvic Floor Muscle Alterations
3.2.2. Structural Pelvic Floor Muscle Changes
3.2.3. Muscle Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PFD | Pelvic floor dysfunction |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| NOS | Newcastle–Ottawa Scale |
| POP | Pelvic organ prolapse |
| ArfGAP3 | Adenosine diphosphate ribosylation factor GTPase activating protein 3 |
| PGP | Protein gene product |
| SMCs | Smooth muscle cells |
| PDGF | Platelet-derived Growth Factor |
| α-SMA | α- Smooth muscle actin |
| LAM | Levator ani muscle |
| OASI | Obstetric anal sphincter injury |
| MRI | Magnetic Resonance Imaging |
| RR | Relative risk |
| SUI | Stress urinary incontinence |
| FI | Fecal incontinence |
| LAD | Levator ani defect |
| AP | Anteroposterior diameter |
| LR | Coronal diameter |
| HA | Hiatal area |
| LAW | Levator attachment width |
| PVM | Pubovisceral muscle |
| PFM | Pelvic floor muscle |
References
- Nygaard, I. Prevalence of Symptomatic Pelvic Floor Disorders in US Women. JAMA 2008, 300, 1311. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.M.; Oldroyd, J.; Rana, J.; Romero, L.; Karim, M.N. Prevalence of symptomatic pelvic floor disorders in community-dwelling women in low and middle-income countries: A systematic review and meta-analysis. Int. Urogynecol. J. 2019, 30, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Vaughan, C.P.; Goode, P.S.; Redden, D.T.; Burgio, K.L.; Richter, H.E.; Markland, A.D. Prevalence and trends of symptomatic pelvic floor disorders in U.S. women. Obstet. Gynecol. 2014, 123, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Frawley, H.; Shelly, B.; Morin, M.; Bernard, S.; Kari, B.; Digesu, G.A.; Dickinson, T.; Goonewardene, S.; McClurg, D.; Rahnama’i, M.S.; et al. An International Continence Society (ICS) report on the terminology for pelvic floor muscle assessment. Neurourol. Urodyn. 2021, 40, 1217–1260. [Google Scholar] [CrossRef]
- Salvador, J.C.; Coutinho, M.P.; Venâncio, J.M.; Viamonte, B. Dynamic magnetic resonance imaging of the female pelvic floor—A pictorial review. Insights Imaging 2019, 10, 4. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Prisma-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. Available online: http://www.systematicreviewsjournal.com/content/4/1/1 (accessed on 3 November 2022). [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2011; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 5 February 2013).
- Sun, Y.; Li, B.; Lu, D.; Liu, C.; Hong, S.; Hong, L. Expression of ArfGAP3 in Vaginal Anterior Wall of Patients with Pelvic Floor Organ Prolapse in Pelvic Organ Prolapse and Non–Pelvic Organ Prolapse Patients. Female Pelvic Med. Reconstr. Surg. 2021, 27, e64–e69. [Google Scholar] [CrossRef]
- Busacchi, P.; Perri, T.; Paradisi, R.; Oliverio, C.; Santini, D.; Guerrini, S.; Barbara, G.; Stanghellini, V.; Corinaldesi, R.; De Giorgio, R. Abnormalities of somatic peptide-containing nerves supplying the pelvic floor of women with genitourinary prolapse and stress urinary incontinence. Urology 2004, 63, 591–595. [Google Scholar] [CrossRef]
- North, C.E.; Creighton, S.M.; Smith, A.R.B. A comparison of genital sensory and motor innervation in women with pelvic organ prolapse and normal controls including a pilot study on the effect of vaginal prolapse surgery on genital sensation: A prospective study. BJOG Int. J. Obstet. Gynaecol. 2013, 120, 193–199. [Google Scholar] [CrossRef]
- Altman, D.; Zhang, A.; Falconer, C. Innervation of the rectovaginal wall in patients with rectocele compared to healthy controls. Neurourol. Urodyn. 2006, 25, 776–781. [Google Scholar] [CrossRef]
- Vetuschi, A.; D’Alfonso, A.; Sferra, R.; Zanelli, D.; Pompili, S.; Patacchiola, F.; Gaudio, E.; Carta, G. Changes in muscularis propria of anterior vaginal wall in women with pelvic organ prolapse. Eur. J. Histochem. 2016, 60, 32–38. [Google Scholar] [CrossRef]
- Heilbrun, M.E.; Nygaard, I.E.; Lockhart, M.E.; Richter, H.E.; Brown, M.B.; Kenton, K.S.; Rahn, D.D.; Thomas, J.V.; Weidner, A.C.; Nager, C.W.; et al. Correlation between levator ani muscle injuries on magnetic resonance imaging and fecal incontinence, pelvic organ prolapse, and urinary incontinence in primiparous women. Am. J. Obstet. Gynecol 2010, 202, 488.e1–488.e6. [Google Scholar] [CrossRef] [PubMed]
- Van Delft, K.; Sultan, A.H.; Thakar, R.; Schwertner-Tiepelmann, N.; Kluivers, K. The relationship between postpartum levator ani muscle avulsion and signs and symptoms of pelvic floor dysfunction. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 1164–1172. [Google Scholar] [CrossRef]
- Dietz, H.P. Quantification of major morphological abnormalities of the levator ani. Ultrasound Obstet. Gynecol. 2007, 29, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Volløyhaug, I.; Taithongchai, A.; Van Gruting, I.; Sultan, A.; Thakar, R. Levator ani muscle morphology and function in women with obstetric anal sphincter injury. Ultrasound Obstet. Gynecol. 2019, 53, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Lammers, K.; Fütterer, J.J.; Inthout, J.; Prokop, M.; Vierhout, M.E.; Kluivers, K.B. Correlating signs and symptoms with pubovisceral muscle avulsions on magnetic resonance imaging. Am. J. Obstet. Gynecol. 2013, 208, 148.e1–148.e7. [Google Scholar] [CrossRef]
- Dietz, H.; Simpson, J. Levator trauma is associated with pelvic organ prolapse. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 979–984. [Google Scholar] [CrossRef]
- DeLancey, J.O.L.; Kearney, R.; Chou, Q.; Speights, S.; Binno, S. The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet. Gynecol. 2003, 101, 46–53. [Google Scholar]
- Oberwalder, M.; Dinnewitzer, A.; Baig, M.K.; Thaler, K.; Cotman, K.; Nogueras, J.J.; Weiss, E.G.; Efron, J.; Vernava, A.M., III; Wexner, S.D. The Association between Late-Onset Fecal Incontinence and Obstetric Anal Sphincter Defects. Arch. Surg. 2004, 139, 429–432. [Google Scholar] [CrossRef]
- Dietz, H.P.; Kirby, A.; Shek, K.L.; Bedwell, P.J. Does avulsion of the puborectalis muscle affect bladder function? Int. Urogynecol. J. 2009, 20, 967–972. [Google Scholar] [CrossRef]
- Handa, V.L.; Blomquist, J.L.; Roem, J.; Muñoz, A.; Dietz, H.P. Pelvic Floor Disorders After Obstetric Avulsion of the Levator Ani Muscle. Female Pelvic Med. Reconstr. Surg. 2019, 25, 3–7. [Google Scholar] [CrossRef]
- Tunn, R.; Goldammer, K.; Neymeyer, J.; Gauruder-Burmester, A.; Hamm, B.; Beyersdorff, D. MRI morphology of the levator ani muscle, endopelvic fascia, and urethra in women with stress urinary incontinence. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 126, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yang, L.; Lin, N.; Fan, Z. Comparison of translabial three-dimensional ultrasonography and magnetic resonance imaging for the grading of levator ani defects. Medicine 2021, 100, e25997. [Google Scholar] [CrossRef] [PubMed]
- Murad-Regadas, S.M.; Fernandes, G.O.D.S.; Regadas, F.S.P.; Rodrigues, L.V.; Pereira, J.D.J.R.; Dealcanfreitas, I.D.; Regadas Filho, F.S.P. Assessment of pubovisceral muscle defects and levator hiatal dimensions in women with faecal incontinence after vaginal delivery: Is there a correlation with severity of symptoms? Color. Dis. 2014, 16, 1010–1018. [Google Scholar] [CrossRef]
- Hsu, Y.; Chen, L.; Huebner, M.; Ashton-Miller, J.A.; DeLancey, J.O.L. Quantification of levator ani cross-sectional area differences between women with and those without prolapse. Obstet. Gynecol. 2006, 108, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Del Vescovo, R.; Piccolo, C.L.; Vecchia NDella Giurazza, F.; Cazzato, R.L.; Grasso, R.F.; Zobel, B.B. MRI role in morphological and functional assessment of the levator ani muscle: Use in patients affected by stress urinary incontinence (SUI) before and after pelvic floor rehabilitation. Eur. J. Radiol. 2014, 83, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Castro-Pardiñas, M.A.; Torres-Lacomba, M.; Navarro-Brazález, B. Muscle function of the pelvic floor in healthy and puerperal women and with pelvic floor dysfunction. Actas Urol. Españ. 2017, 41, 249–257. [Google Scholar] [CrossRef]
- Morin, M.; Bourbonnais, D.; Gravel, D.; Dumoulin, C.; Lemieux, M.C. Pelvic floor muscle function in continent and stress urinary incontinent women using dynamometric measurements. Neurourol. Urodyn. 2004, 23, 668–674. [Google Scholar] [CrossRef]
- DeLancey, J.O.L.; Morgan, D.M.; Fenner, D.E.; Kearney, R.; Guire, K.; Miller, J.M.; Hussain, H.; Umek, W.; Hsu, Y.; Ashton-Miller, J.A. Comparison of Levator Ani Muscle Defects and Function in Women With and Without Pelvic Organ Prolapse. Obstet. Gynecol. 2007, 109 Pt 1, 295–302. [Google Scholar] [CrossRef]
- Tosun, G.; Peker, N.; Tosun, Ö.Ç.; Yeniel, Ö.A.; Ergenoğlu, A.M.; Elvan, A.; Yıldırım, M. Pelvic floor muscle function and symptoms of dysfunctions in midwifes and nurses of reproductive age with and without pelvic floor dysfunction. Taiwan J. Obstet. Gynecol. 2019, 58, 505–513. [Google Scholar] [CrossRef]
- Borello-france, D.F.; Handa, V.L.; Brown, M.B.; Goode, P.; Kreder, K.; Scheufele, L.L.; Weber, A.M.; Pelvic Floor Disorders Network. Pelvic-Floor Muscle Function in Women with Pelvic Organ Prolapse. Phys. Ther. 2007, 87, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Handa, V.L.; Roem, J.; Blomquist, J.L.; Dietz, H.P.; Muñoz, A. Pelvic organ prolapse as a function of levator ani avulsion, hiatus size, and strength. Am. J. Obstet. Gynecol. 2019, 221, 41.e1–41.e7. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.J.; Nielsen, P.M.F.; Taberner, A.J.; Kruger, J.A. Change in levator ani muscle stiffness and active force during pregnancy and post-partum. Int. Urogynecol. J. 2020, 31, 2345–2351. [Google Scholar] [CrossRef]
- Hilde, G.; Stær-Jensen, J.; Siafarikas, F.; Engh, M.E.; Brækken, I.H.; Bo, K. Impact of childbirth and mode of delivery on vaginal resting pressure and on pelvic floor muscle strength and endurance. Am. J. Obstet. Gynecol. 2013, 208, 50.e1–50.e7. [Google Scholar] [CrossRef]
- Chantarasorn, V.; Shek, K.L.; Dietz, H.P. Sonographic detection of puborectalis muscle avulsion is not associated with anal incontinence. Aust. N. Z. J. Obstet. Gynaecol. 2011, 51, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Lewicky-Gaupp, C.; Brincat, C.; Yousuf, A.; Patel, D.A.; Delancey, J.O.L.; Fenner, D.E. Fecal incontinence in older women: Are levator ani defects a factor? Am. J. Obstet. Gynecol. 2010, 202, 491.e1–491.e6. [Google Scholar] [CrossRef]
- Muro, S.; Moue, S.; Akita, K. Twisted orientation of the muscle bundles in the levator ani functional parts in women: Implications for pelvic floor support mechanism. J. Anat. 2024, 244, 486–496. [Google Scholar] [CrossRef]
- Kato, M.K.; Muro, S.; Kato, T.; Miyasaka, N.; Akita, K. Spatial distribution of smooth muscle tissue in the female pelvic floor and surrounding the urethra and vagina. Anat. Sci. Int. 2020, 95, 516–522. [Google Scholar] [CrossRef]
- Nyangoh Timoh, K.; Moszkowicz, D.; Zaitouna, M.; Lebacle, C.; Martinovic, J.; Diallo, D.; Creze, M.; Lavoue, V.; Darai, E.; Benoit, G.; et al. Detailed muscular structure and neural control anatomy of the levator ani muscle: A study based on female human fetuses. Am. J. Obstet. Gynecol. 2018, 218, 121.e1–121.e12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, A.M.; Faleiro, M.L.; Mascarenhas-Saraiva, M.; Pais, S. The Muscle Cells in Pelvic Floor Dysfunctions: Systematic Review. Muscles 2025, 4, 9. https://doi.org/10.3390/muscles4010009
Vieira AM, Faleiro ML, Mascarenhas-Saraiva M, Pais S. The Muscle Cells in Pelvic Floor Dysfunctions: Systematic Review. Muscles. 2025; 4(1):9. https://doi.org/10.3390/muscles4010009
Chicago/Turabian StyleVieira, Ana Margarida, Maria Leonor Faleiro, Miguel Mascarenhas-Saraiva, and Sandra Pais. 2025. "The Muscle Cells in Pelvic Floor Dysfunctions: Systematic Review" Muscles 4, no. 1: 9. https://doi.org/10.3390/muscles4010009
APA StyleVieira, A. M., Faleiro, M. L., Mascarenhas-Saraiva, M., & Pais, S. (2025). The Muscle Cells in Pelvic Floor Dysfunctions: Systematic Review. Muscles, 4(1), 9. https://doi.org/10.3390/muscles4010009







