Exertional Rhabdomyolysis and Ultra-Trail Races: A Systematic Review Highlighting the Significant Impact of Eccentric Load
Abstract
:1. Introduction
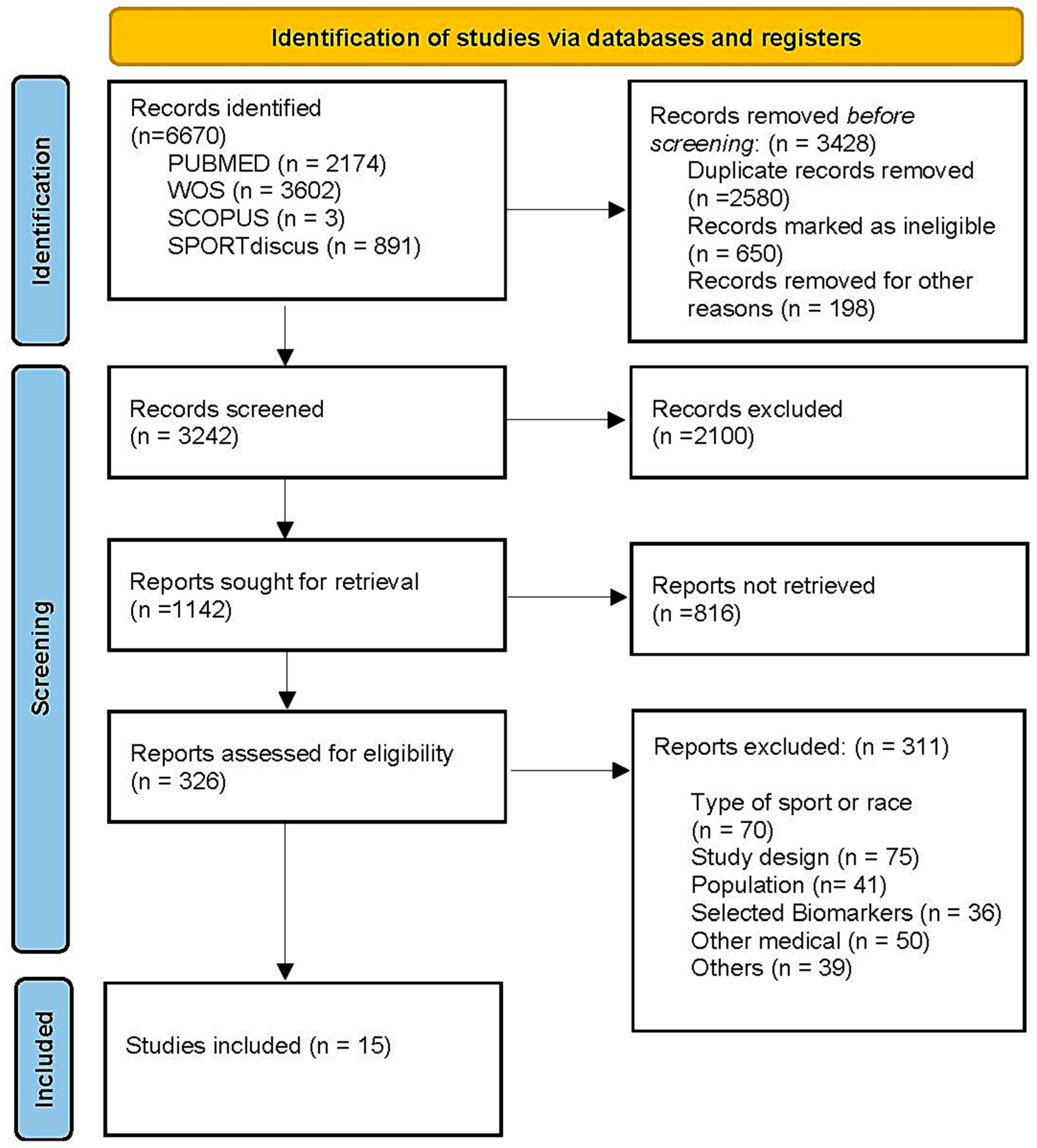
2. Materials and Methods
2.1. Criteria for Study and Selection
2.1.1. Inclusion Criteria
- Adult population of both sexes;
- UT races (>42,195 km) and negative elevation of at least 1000 m;
- Muscle damage biomarkers included CK, LDH, MB, and/or liver damage biomarkers AST and ALT.
2.1.2. Exclusion Criteria
- Population with previous medical conditions related to muscle illness;
- ER was not assessed;
- Combined sports (triathlon or biathlon);
- Another language different to English;
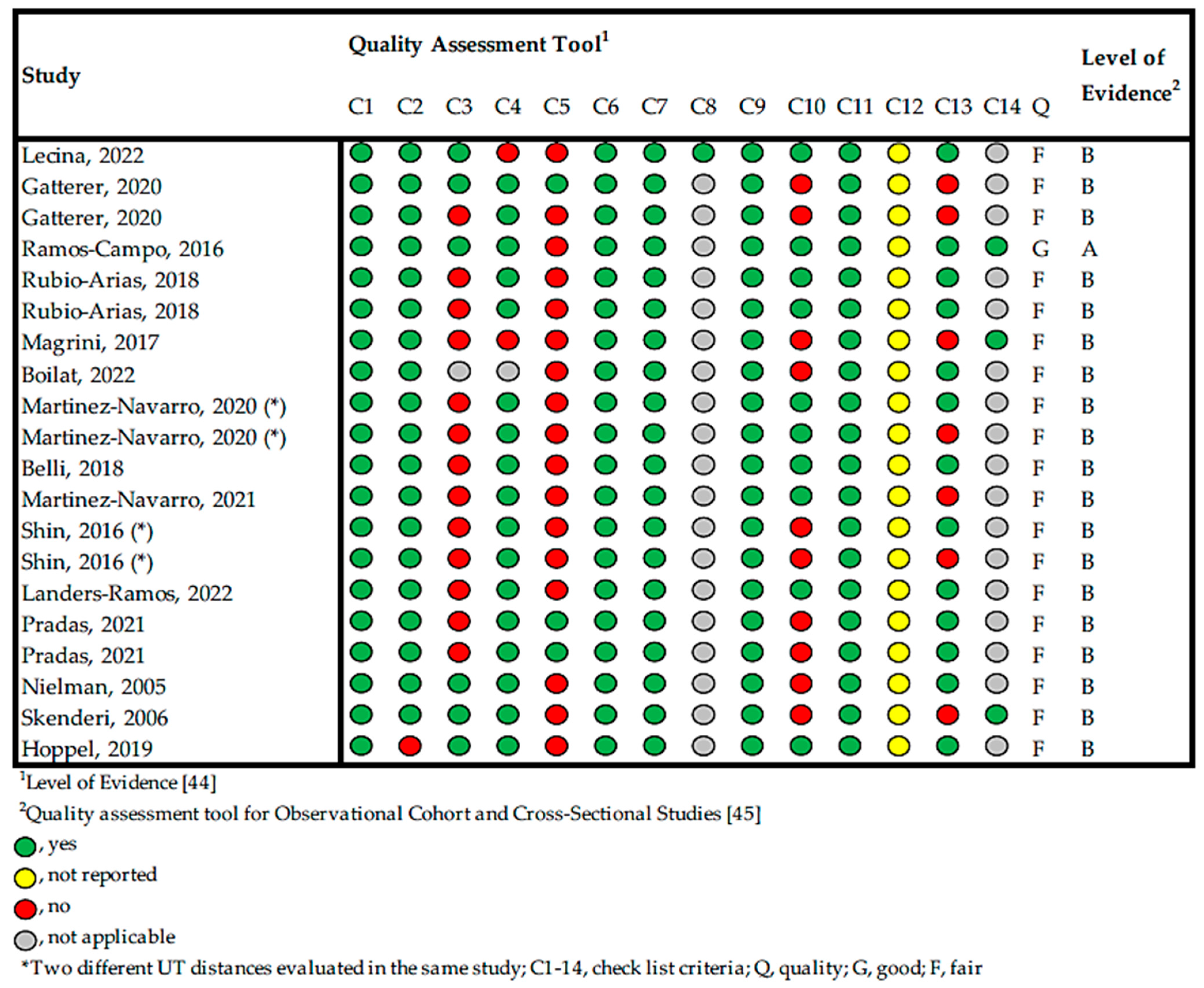
2.2. Overall Quality of the Studies
2.3. Risk of Bias
3. Results
3.1. Types of UT and Participant Data
3.2. ER and Biomarkers
3.3. Liver Damage
4. Discussion
4.1. Exertional Rhabdomyolysis
4.2. Eccentric Load
4.3. Liver Damage
- Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Stöhr, A.; Nikolaidi, P.T.; Villiger, E.; Sousa, C.V.; Scheer, V.; Hill, L.; Knechtle, B. An Analysis of Participation and Performance of 2067 100-Km Ultra-Marathons Worldwide. Int. J. Environ. Res. Public Health 2021, 18, 362. [Google Scholar] [CrossRef]
- Spenceley, K.; Humphrey, R.; Lingam, C.; Introduction, W. Defining ultra-endurance: A survey of athletes and coaches to achieve a consensus definition. Scope 2017, 1, 171–179. [Google Scholar]
- Dawadi, S.; Basyal, B.; Subedi, Y. Morbidity Among Athletes Presenting for Medical Care During 3 Iterations of an Ultratrail Race in the Himalayas. Wilderness Environ. Med. 2020, 31, 437–440. [Google Scholar] [CrossRef]
- Scheer, V. Participation Trends of Ultra Endurance Events. Sports Med. Arthrosc. Rev. 2019, 27, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Waśkiewicz, Z.; Nikolaidis, P.T.; Chalabaev, A.; Rosemann, T.; Knechtle, B. Motivation in Ultra-Marathon Runners. Psychol. Res. Behav. Manag. 2019, 12, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Runsignup. Annual Industry Report. 2020, Volume 9. Available online: https://info.runsignup.com/wp-content/uploads/sites/3/2023/01/2020-RaceTrends-Report.pdf (accessed on 2 May 2024).
- Ehrensperger, L.; Knechtle, B.; Rüst, C.A.; Rosemann, T. Participation and Performance Trends in 6-Hour Ultra-Marathoners—A Retrospective Data Analysis of Worldwide Participation from 1991–2010. J. Hum. Sport Exerc. 2013, 8, 905–924. [Google Scholar] [CrossRef]
- Cejka, N.; Rüst, C.A.; Lepers, R.; Onywera, V.; Rosemann, T.; Knechtle, B. Participation and Performance Trends in 100-Km Ultra-Marathons Worldwide. J. Sports Sci. 2014, 32, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Zaryski, C.; Smith, D.J. Training Principles and Issues for Ultra-Endurance Athletes. Curr. Sports Med. Rep. 2005, 4, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Scheer, V.; Basset, P.; Giovanelli, N.; Vernillo, G.; Millet, G.P.; Costa, R.J.S. Defining Off-Road Running: A Position Statement from the Ultra Sports Science Foundation. Int. J. Sports Med. 2020, 41, 275–284. [Google Scholar] [CrossRef]
- Knechtle, B.; Nikolaidis, P.T. Physiology and Pathophysiology in Ultra-Marathon Running. Front. Physiol. 2018, 9, 634. [Google Scholar] [CrossRef]
- Knechtle, B.; Knechtle, P.; Wirth, A.; Alexander Rüst, C.; Rosemann, T. A Faster Running Speed Is Associated with a Greater Body Weight Loss in 100-Km Ultra-Marathoners. J. Sports Sci. 2012, 30, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Marroyo, J.A.; González-Lázaro, J.; Arribas-Cubero, H.F.; Villa, J.G. Physiological Demands of Mountain Running Races. Kinesiology 2018, 50, 60–66. [Google Scholar]
- Vernillo, G.; Savoldelli, A.; La Torre, A.; Skafidas, S.; Bortolan, L.; Schena, F. Injury and Illness Rates during Ultratrail Running. Int. J. Sports Med. 2016, 37, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, L.; Walter, E.; Venn, R.; Galloway, R.; Pitsiladis, Y.; Sardat, F.; Forni, L. Acute Kidney Injury Associated with Endurance Events—Is It a Cause for Concern? A Systematic Review. BMJ Open Sport. Exerc. Med. 2017, 3, e000093. [Google Scholar] [CrossRef] [PubMed]
- Balducci, P.; Saboul, D.; Trama, R. Monitoring Heart Rates to Evaluate Pacing on a 75-Km MUM. J. Sports Med. Phys. Fit. 2019, 59, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H. The Effect of Long-Distance Running on Bone Strength and Bone Biochemical Markers. J. Exerc. Rehabil. 2019, 15, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Žákovská, A.; Knechtle, B.; Chlíbková, D.; Miličková, M.; Rosemann, T.; Nikolaidis, P.T. The Effect of a 100-Km Ultra-Marathon under Freezing Conditions on Selected Immunological and Hematological Parameters. Front. Physiol. 2017, 8, 638. [Google Scholar] [CrossRef] [PubMed]
- Runacres, A.; Mackintosh, K.A.; McNarry, M.A. Health Consequences of an Elite Sporting Career: Long-Term Detriment or Long-Term Gain? A Meta-Analysis of 165,000 Former Athletes. Sports Med. 2021, 51, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Knapik, J.J.; O’Connor, F.G. Exertional Rhabdomyolysis: Epidemiology, Diagnosis, Treatment, and Prevention. J. Spec. Oper. Med. 2016, 16, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Safari, S.; Yousefifard, M.; Hashemi, B.; Baratloo, A.; Forouzanfar, M.M.; Rahmati, F.; Motamedi, M.; Najafi, I. The Role of Scoring Systems and Urine Dipstick in Prediction of Rhabdomyolysis-Induced Acute Kidney Injury: A Systematic Review. Iran. J. Kidney Dis. 2016, 10, 101–106. [Google Scholar]
- Bergstrom, H.C.; Housh, T.J.; Dinyer, T.K.; Byrd, M.T.; Jenkins, N.D.M.; Cochrane-Snyman, K.C.; Succi, P.J.; Schmidt, R.J.; Johnson, G.O.; Zuniga, J.M. Neuromuscular Responses of the Superficial Quadriceps Femoris Muscles: Muscle Specific Fatigue and Inter-Individual Variability during Severe Intensity Treadmill Running. J. Musculoskelet. Neuronal Interact. 2020, 20, 77–87. [Google Scholar] [PubMed]
- Lemire, M.; Remetter, R.; Hureau, T.J.; Kouassi, B.Y.L.; Lonsdorfer, E.; Geny, B.; Isner-Horobeti, M.E.; Favret, F.; Dufour, S.P. High-Intensity Downhill Running Exacerbates Heart Rate and Muscular Fatigue in Trail Runners. J. Sports Sci. 2021, 39, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.G.F.; Coswig, V.S.; de Oliveira, E.; Farias, D.A. Exercise-Induced Rhabdomyolysis Is Not More Severe or Frequent after Crossfit than after Running or Strength Training Programs. Rev. Andal. Med. Deport. 2019, 12, 206–209. [Google Scholar] [CrossRef]
- Magrini, D.; Khodaee, M.; San-Millán, I.; Hew-Butler, T.; Provance, A.J. Serum Creatine Kinase Elevations in Ultramarathon Runners at High Altitude. Physician Sportsmed. 2017, 45, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Krabak, B.J.; Lipman, G.S.; Waite, B.L.; Rundell, S.D. Exercise-Associated Hyponatremia, Hypernatremia, and Hydration Status in Multistage Ultramarathons. Wilderness Environ. Med. 2017, 28, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Reid, W.A.; McQueen, A. Acute Rhabdomyolysis in a Marathon Runner. Br. J. Sports Med. 1987, 21, 49. [Google Scholar] [CrossRef] [PubMed]
- Cleary, M.A. Creatine Kinase, Exertional Rhabdomyolysis, and Exercise-Associated Hyponatremia in Ultra-Endurance Athletes: A Critically Appraised Paper. Int. J. Athl. Ther. Train. 2016, 21, 13–15. [Google Scholar] [CrossRef]
- Nance, J.R.; Mammen, A.L. Diagnostic Evaluation of Rhabdomyolysis. Muscle Nerve 2015, 51, 793–810. [Google Scholar] [CrossRef] [PubMed]
- Lecina, M.; López, I.; Castellar, C.; Pradas, F. Extreme Ultra-Trail Race Induces Muscular Damage, Risk for Acute Kidney Injury and Hyponatremia: A Case Report. Int. J. Environ. Res. Public Health 2021, 18, 11323. [Google Scholar] [CrossRef]
- Vanhelst, J.; Fardy, P.S.; Chapelot, D.; Czaplicki, G.; Ulmer, Z. Physical Fitness Levels of Adolescents in the Ile de France Region: Comparisons with European Standards and Relevance for Future Cardiovascular Risk. Clin. Physiol. Funct. Imaging 2016, 36, 476–481. [Google Scholar] [CrossRef]
- Torres, P.A.; Helmstetter, J.A.; Kaye, A.M.; Kaye, A.D. Rhabdomyolysis: Pathogenesis, Diagnosis, and Treatment. Ochsner J. 2015, 15, 58–69. [Google Scholar] [PubMed]
- Cacelín-Garza, J.R.; Díaz-Gutiérrez, S. Rabdomiólisis. Comunicación de Dos Casos Relacionados con Esfuerzo y Revisión de la Bibliografía. Med. Interna Mex. 2013, 29, 410–423. [Google Scholar]
- Schiff, H.B.; MacSearraigh, E.T.M.; Kallmeyer, J.C. Myoglobinuria, Rhabdomyolysis and Marathon Running. QJM Int. J. Med. 1978, 47, 463–472. [Google Scholar] [CrossRef]
- Lim, A.K.H. Abnormal Liver Function Tests Associated with Severe Rhabdomyolysis. World J. Gastroenterol. 2020, 26, 1020–1028. [Google Scholar] [CrossRef]
- Skenderi, K.P.; Kavouras, S.A.; Anastasiou, C.A.; Yiannakouris, N.; Matalas, A.L. Exertional Rhabdomyolysis during a 246-Km Continuous Running Race. Med. Sci. Sports Exerc. 2006, 38, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Valverde, D.; Sánchez-Ureña, B.; Crowe, J.; Timón, R.; Olcina, G.J. Exertional Rhabdomyolysis and Acute Kidney Injury in Endurance Sports: A Systematic Review. Eur. J. Sport. Sci. 2020, 21, 261–274. [Google Scholar] [CrossRef]
- Giovanelli, N.; Taboga, P.; Rejc, E.; Lazzer, S. Effects of Strength, Explosive and Plyometric Training on Energy Cost of Running in Ultra-Endurance Athletes. Eur. J. Sport. Sci. 2017, 17, 805–813. [Google Scholar] [CrossRef]
- Royer, N.; Nosaka, K.; Doguet, V.; Jubeau, M. Neuromuscular Responses to Isometric, Concentric and Eccentric Contractions of the Knee Extensors at the Same Torque-Time Integral. Eur. J. Appl. Physiol. 2022, 122, 127–139. [Google Scholar] [CrossRef]
- Gatterer, H.; Gatterer, H.; Rauch, S.; Rauch, S.; Procter, E.; Strapazzon, G.; Schenk, K.; Schenk, K. Performance Determinants in Short (68 Km) and Long (121 Km) Mountain Ultra-Marathon Races. Sportverletz. Sportschaden 2020, 34, 79–83. [Google Scholar] [CrossRef]
- Moseley, A.M.; Herbert, R.D.; Sherrington, C.; Maher, C.G. Evidence for Physiotherapy Practice: A Survey of the Physiotherapy Evidence Database (PEDro). Aust. J. Physiother. 2002, 48, 43–49. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological Quality (Risk of Bias) Assessment Tools for Primary and Secondary Medical Studies: What Are They and Which Is Better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung, and Blood Institute. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 3 May 2024).
- Rosenbrand, K.; Van Croonenborg, J.; Wittenberg, J. Guideline Development. Stud. Health Technol. Inform. 2008, 139, 3–21. [Google Scholar] [PubMed]
- Lecina, M.; Castellar, C.; Pradas, F.; López-Laval, I. 768-Km Multi-Stage Ultra-Trail Case Study-Muscle Damage, Biochemical Alterations and Strength Loss on Lower Limbs. Int. J. Environ. Res. Public Health 2022, 19, 876. [Google Scholar] [CrossRef]
- Rubio-Arias, J.; Ávila-Gandía, V.; López-Román, F.J.; Soto-Méndez, F.; Alcaraz, P.E.; Ramos-Campo, D.J. Muscle Damage and Inflammation Biomarkers after Two Ultra-Endurance Mountain Races of Different Distances: 54 km vs 111 km. Physiol. Behav. 2019, 205, 51–57. [Google Scholar] [CrossRef]
- Jesús Ramos-Campo, D.; Ávila-Gandía, V.; Alacid, F.; Soto-Méndez, F.; Alcaraz, P.E.; Javier López-Román, F.; Ángel Rubio-Arias, J.; Jesús Ramos Campo, D. Muscle Damage, Physiological Changes and Energy Balance in Ultra-Endurance Mountain Event Athletes. Appl. Physiol. Nutr. Metab. 2016, 41, 872–878. [Google Scholar] [CrossRef]
- Boillat, T.; Kourie, A.; Thalange, N.; Du Plessis, S.; Loney, T. Guinness World Record: Personal Experience and Physiological Responses of a Non-Professional Athlete Successfully Covering 620 Km in 7-Days by Foot Across the United Arab Emirates. J. Sports Sci. Med. 2022, 21, 267–276. [Google Scholar] [CrossRef]
- Martínez-Navarro, I.; Collado, E.; Hernando, C.; Hernando, B.; Hernando, C. Inflammation, Muscle Damage and Postrace Physical Activity Following a Mountain Ultramarathon. J. Sports Med. Phys. Fitness 2021, 61, 1668–1674. [Google Scholar] [CrossRef]
- Martínez-Navarro, I.; Sanchez-Gómez, J.M.; Aparicio, I.; Priego-Quesada, J.I.; Pérez-Soriano, P.; Collado, E.; Hernando, B.; Hernando, C. Effect of Mountain Ultramarathon Distance Competition on Biochemical Variables, Respiratory and Lower-Limb Fatigue. PLoS ONE 2020, 15, e0238846. [Google Scholar] [CrossRef]
- Shin, K.A.; Park, K.D.; Ahn, J.; Park, Y.; Kim, Y.J. Comparison of Changes in Biochemical Markers for Skeletal Muscles, Hepatic Metabolism, and Renal Function after Three Types of Long-Distance Running. Medicine 2016, 95, e3657. [Google Scholar] [CrossRef]
- Landers-Ramos, R.Q.; Dondero, K.; Nelson, C.; Ranadive, S.M.; Prior, S.J.; Addison, O. Muscle Thickness and Inflammation during a 50km Ultramarathon in Recreational Runners. PLoS ONE 2022, 17, e0273510. [Google Scholar] [CrossRef]
- Pradas, F.; Falcón, D.; Peñarrubia-Lozano, C.; Toro-Román, V.; Carrasco, L.; Castellar, C. Effects of Ultratrail Running on Neuromuscular Function, Muscle Damage and Hydration Status. Differences According to Training Level. Int. J. Environ. Res. Public Health 2021, 18, 5119. [Google Scholar] [CrossRef]
- Nieman, D.C.; Dumke, C.L.; Henson, D.A.; McAnulty, S.R.; Gross, S.J.; Lind, R.H. Muscle Damage Is Linked to Cytokine Changes Following a 160-Km Race. Brain Behav. Immun. 2005, 19, 398–403. [Google Scholar] [CrossRef]
- Hoppel, F.; Calabria, E.; Pesta, D.; Kantner-Rumplmair, W.; Gnaiger, E.; Burtscher, M. Physiological and Pathophysiological Responses to Ultramarathon Running in Non-Elite Runners. Front. Physiol. 2019, 10, 1300. [Google Scholar] [CrossRef]
- Belli, T.; Macedo, D.V.; De Araújo, G.G.; Masselli dos Reis, I.G.; Menezes Scariot, P.P.; Lazarim, F.L.; Soares Nunes, L.A.; Brenzikofer, R.; Gobatto, C.A. Mountain Ultramarathon Induces Early Increases of Muscle Damage, Inflammation, and Risk for Acute Renal Injury. Front. Physiol. 2018, 9, 1368. [Google Scholar] [CrossRef]
- Bäcker, H.; Richards, J.T.; Kienze, A. Exertional Rhabdomyolysis in Athletes: Systematic Review and Current Perspectives. Clin. J. Sports Med. 2023, 33, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Bäcker, H.C.; Busko, M.; Krause, F.G.; Exadaktylos, A.K.; Klukowska-Roetzler, J.; Deml, M.C. Exertional Rhabdomyolysis and Causes of Elevation of Creatine Kinase. Phys. Sportsmed. 2020, 48, 179–185. [Google Scholar] [CrossRef]
- Scheer, V.; Tiller, N.B.; Doutreleau, S.; Khodaee, M.; Knechtle, B.; Pasternak, A.; Rojas-Valverde, D. Potential Long-Term Health Problems Associated with Ultra-Endurance Running: A Narrative Review. Sports Med. 2021, 52, 725–740. [Google Scholar] [CrossRef]
- Muanjai, P.; Mickevicius, M.; Sniečkus, A.; Sipavičienė, S.; Satkunskiene, D.; Kamandulis, S.; Jones, D.A. Low Frequency Fatigue and Changes in Muscle Fascicle Length Following Eccentric Exercise of the Knee Extensors. Exp. Physiol. 2020, 105, 502–510. [Google Scholar] [CrossRef]
- Hill, O.T.; Wahi, M.M.; Carter, R.; Kay, A.B.; McKinnon, C.J.; Wallace, R.F. Rhabdomyolysis in the US Active Duty Army, 2004–2006. Med. Sci. Sports Exerc. 2012, 44, 442–449. [Google Scholar] [CrossRef]
- Hew-Butler, T.; Rosner, M.H.; Fowkes-Godek, S.; Dugas, J.P.; Hoffman, M.D.; Lewis, D.P.; Maughan, R.J.; Miller, K.C.; Montain, S.J.; Rehrer, N.J.; et al. Statement of the 3rd International Exercise-Associated Hyponatremia Consensus Development Conference, Carlsbad, California, 2015. Br. J. Sports Med. 2015, 49, 1432–1446. [Google Scholar] [CrossRef]
- Lipman, G.S.; Krabak, B.J.; Rundell, S.D.; Shea, K.M.; Badowski, N.; Little, C. Incidence and Prevalence of Acute Kidney Injury during Multistage Ultramarathons. Clin. J. Sport. Med. 2016, 26, 314–319. [Google Scholar] [CrossRef]
- Sawhney, S.; Fraser, S.D. Epidemiology of AKI: Utilizing Large Databases to Determine the Burden of AKI. Adv. Chronic Kidney Dis. 2017, 24, 194–204. [Google Scholar] [CrossRef]
- Rojas-Valverde, D.; Sánchez-Ureña, B.; Pino-Ortega, J.; Gómez-Carmona, C.; Gutiérrez-Vargas, R.; Timón, R.; Olcina, G. External Workload Indicators of Muscle and Kidney Mechanical Injury in Endurance Trail Running. Int. J. Environ. Res. Public Health 2019, 16, 3909. [Google Scholar] [CrossRef]
- Lippi, G.; Schena, F.; Ceriotti, F. Diagnostic Biomarkers of Muscle Injury and Exertional Rhabdomyolysis. Clin. Chem. Lab. Med. 2018, 57, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Scalco, R.S.; Snoeck, M.; Quinlivan, R.; Treves, S.; Laforét, P.; Jungbluth, H.; Voermans, N.C.; Scalco, R. Exertional Rhabdomyolysis: Physiological Response or Manifestation of an Underlying Myopathy? BMJ Open Sport Exerc. Med. 2016, 2, e000151. [Google Scholar] [CrossRef]
- Fernandes, P.M.; Davenport, R.J. How to Do It: Investigate Exertional Rhabdomyolysis (or Not). Pract. Neurol. 2019, 19, 43–48. [Google Scholar] [CrossRef]
- Bruso, J.R.; Hoffman, M.D.; Rogers, I.R.; Lee, L.; Towle, G.; Hew-Butler, T. Rhabdomyolysis and Hyponatremia: A Cluster of Five Cases at the 161-Km 2009 Western States Endurance Run. Wilderness Environ. Med. 2010, 21, 303–308. [Google Scholar] [CrossRef]
- Hody, S.; Rogister, B.; Leprince, P.; Wang, F.; Croisier, J.L. Muscle Fatigue Experienced during Maximal Eccentric Exercise Is Predictive of the Plasma Creatine Kinase (CK) Response. Scand. J. Med. Sci. Sports 2013, 23, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Junglee, N.A.; di Felice, U.; Dolci, A.; Fortes, M.B.; Jibani, M.M.; Lemmey, A.B.; Walsh, N.P.; Macdonald, J.H. Exercising in a Hot Environment with Muscle Damage: Effects on Acute Kidney Injury Biomarkers and Kidney Function. Am. J. Physiol. Renal Physiol. 2013, 305, 813–821. [Google Scholar] [CrossRef]
- Cleary, M.A.; Sadowski, K.A.; Y-c Lee, S.; Miller, G.L.; Nichols, A.W. Exertional Rhabdomyolysis in An Adolescent Athlete During Preseason Conditioning: A Perfect Storm. J. Strength Cond. Res. 2011, 12, 3506–3513. [Google Scholar] [CrossRef] [PubMed]
- Temesi, J.; Rupp, T.; Martin, V.; Arnal, P.J.; Féasson, L.; Verges, S.; Millet, G.Y. Central Fatigue Assessed by Transcranial Magnetic Stimulation in Ultratrail Running. Med Sci Sports Exerc 2014, 46, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Espeit, L.; Brownstein, C.G.; Royer, N.; Besson, T.; Martin, V.; Millet, G.Y.; Lapole, T. Central Fatigue Aetiology in Prolonged Trail Running Races. Exp. Physiol. 2021, 106, 663–672. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Keech, A.; Lonergan, A.; Abernethy, P. Differential Expression of Muscle Damage in Humans Following Acute Fast and Slow Velocity Eccentric Exercise. J. Sci. Med. Sport 2005, 8, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Theodorou, A.A.; Chatzinikolaou, P.N.; Kyparos, A.; Nikolaidis, M.G.; Paschalis, V. Eccentric Exercise per Se Does Not Affect Muscle Damage Biomarkers: Early and Late Phase Adaptations. Eur. J. Appl. Physiol. 2021, 121, 549–559. [Google Scholar] [CrossRef]
- Coratella, G.; Varesco, G.; Rozand, V.; Cuinet, B.; Sansoni, V.; Lombardi, G.; Vernillo, G.; Mourot, L. Downhill Running Increases Markers of Muscle Damage and Impairs the Maximal Voluntary Force Production as well as the Late Phase of the Rate of Voluntary Force Development. Eur. J. Appl. Physiol. 2024, 124, 1875–1883. [Google Scholar] [CrossRef]
- Cleary, M.; Ruiz, D.; Eberman, L.; Mitchell, I.; Binkley, H. Dehydration, Cramping, and Exertional Rhabdomyolysis: A Case Report. With Suggestions for Recovery. J. Sport Rehabil. 2007, 16, 244–259. [Google Scholar] [CrossRef]
- De Castro, R.R.T.; Filho, R.B.C.; Da Nóbrega, A.C.L. Fulminant Liver Failure in a Street Runner: Effects of Heat Stroke. Rev. Assoc. Med. Bras. 2018, 64, 208–211. [Google Scholar] [CrossRef]
- Huang, X.-J.; Choi, Y.-K.; Im, H.-S.; Yarimaga, O.; Yoon, E.; Kim, H.-S. Aspartate Aminotransferase (AST/GOT) and Alanine Aminotransferase (ALT/GPT) Detection Techniques. Sensors 2006, 6, 756–782. [Google Scholar] [CrossRef]
- Tirabassi, J.N.; Olewinski, L.; Khodaee, M. Variation of Traditional Biomarkers of Liver Injury After an Ultramarathon at Altitude. Sports Health 2018, 10, 361–365. [Google Scholar] [CrossRef]
- Pal, S.; Chaki, B.; Chattopadhyay, S.; Bandyopadhyay, A. High-intensity exercise-induced oxidative stress and skeletal muscle damage in postpubertal boys and girls: A comparative study. J. Strength Cond. Res. 2018, 32, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, C.E.A.; Vargas-Flores, S.M. Rabdomiólisis Secundaria a Hepatitis Medicamentosa: A Propósito de Un Caso Rhabdomyolysis Secondary to Drug-Induced Hepatitis: A Case Report. Salud ConCiencia 2022, 1, e5. [Google Scholar] [CrossRef]
| Author; Year | Population; Number; Sex; Age | Race Length; Stages; Elevation +/− | CK Pre, Post & Rec | LDH Pre, Post, and Rec | AST Pre, Post, and Rec | ALT Pre, Post, and Rec | ER Cases (%) |
|---|---|---|---|---|---|---|---|
| Lecina, 2022 [46] | Highly trained; 4; 0 F, 4 M; 38.0 ± 4.11 yrs | 786 km; 11; 46,865+ m; 46,845− m; | ↑CK Pre 98.51 ± 24.53 Post 974 ± 402.66 R2 474.85 ± 185.70 ↓CK R9 88.00 ± 16.27 | ↑LDH Pre 172.75 ± 14.71 Post 470.30 ± 104.80 R2 316 ± 70.88 R9 208 ± 31.55 | ↑AST Pre 23.75 ± 3.20 Post 63.50 ± 9.68 R2 44.50 ± 8.74 R9 48.25 ± 27.45 | ↑ALT Pre 17.25 ± 3.59 Post 44.75 ± 12.44 R2 37.25 ± 8.80 R9 56.25 ± 34.25 | 1 (25%) |
| Gatterer, 2020 (*) [40] | Amateur; 11; 0 F, 11 M; 42.0 ± 9.0 yrs | 68 km; 1; 4260+ m; 4260− m | ↑CK Post 1562 ± 1250 | - | - | - | 0 (0%) |
| Gatterer, 2020 (*) [40] | Amateur; 7; 0 F, 7 M; 41.0 ± 10.0 yrs | 121 km; 1; 7554+ m; 7554− m | ↑CK Post 4933 ± 3760 | - | - | - | 0 (0%) |
| Ramos-Campo, 2016 [48] | Amateur; 11; 0 F, 11 M; 29.7 ± 10.2 yrs | 54 km; 1; 2726+ m; 2665− m | ↑CK Pre 820 ± 2087.3 Post 2421.1 ± 2336.2 | ↑LDH Pre 383 ± 178.6 Post 795 ± 260.7 | - | - | 0 (0%) |
| Rubio-Arias, 2018 (*) [47] | Amateur; 10; 0 F, 10 M; 27.0 ± 5.7 yrs | 54 km; 1; 2726+ m; 2665− m | ↑CK Pre 886.6 ± 2187.9 Post 2213.8 ± 2354.5 R1 1014 ± 732.6 ↓CK R2 495.8 ± 358 R3 309.4 ± 191.6 | ↑LDH Pre 406.9 ± 135 Post 731.3 ± 161.1 R1 449.8 ± 66.9 R2 430.9 ± 72.1 R3 409.9 ± 59.8 | - | - | 0 (0%) |
| Rubio-Arias, 2018 (*) [47] | Amateur; 6; 0 F, 6 M; 30.5 ± 8 yrs | 111 km; 1; 4474+ m; 4420− m | ↑CK Pre 174 ± 197.4 Post 8976 ± 4327.1 R1 2132.5 ± 1399.6 R2 1277.7 ± 1368.2 R3 604.2 ± 878.3 | ↑LDH Pre 335.1 ± 35.2 Post 751 ± 57.2 R1 588.7 ± 90.7 R2 551.1 ± 72.7 R3 509.1 ± 56.7 | ↑LDH Pre 335.1 ± 35.2 Post 751 ± 57.2 R1 588.7 ± 90.7 R2 551.1 ± 72.7 R3 509.1 ± 56.7 | - | 0 (0%) |
| Magrini, 2017 [25] | Amateur; 36; 8 F, 28 M; 43 yrs (26–68) | 160 km; 1; 4800+ m; 4800− m | ↑CK Pre 126 ± 64 Post 14,569 ± 14,729 | - | - | - | 0 (0%) |
| Boillat, 2022 [49] | Highly trained; 1; 0 F, 1 M; 34 yrs | 619 km; 7; 600+ m; 1100− m | ↑CK Inc post vs. pre (102%) | - | - | - | 0 (0%) |
| Martínez-Navarro, 2020 (*) [51] | Amateur; 17; 4 F, 13 M; 41 ± 7 yrs | 65 km; 1; 4200+ m; 4200− m | ↑CK Pre-Post Pre-R1 | ↑LDH Pre-Post Pre-R1 | - | - | 0 (0%) |
| Martínez-Navarro, 2020 (*) [51] | Amateur; 32; 13 F, 19 M; 41 ± 6 yrs | 107.4 km; 1; 5604+ m; 4356− m | ↑CK Pre-Post Pre-R1 | ↑LDH Pre-Post Pre-R1 | - | - | 0 (0%) |
| Belli, 2018 [57] | Highly trained; 6; 0 F, 6 M; 47 ± 5 yrs | 217 km; 1; 12,200+ m; 12,200− m | ↑CK Pre 132 ± 18 84 k 3988 ± 1004 177 k 18,667 ± 10,664 Post 19,157 ± 12,369 | ↑LDH Pre 371 ± 66 84k 677 ± 80 177k 2026 ± 671 Post 1986 ± 687 | ↑AST Pre 28 ± 3 84k 109 ± 13 177k 677 ± 406 Post 668 ± 407 | - | 0 (0%) |
| Martínez-Navarro, 2021 [50] | Amateur; 34; 5 F, 29 M; 39 ± 7 yrs | 118 km; 1; 5439+ m; 4227− m | ↑CK Pre-Post Pre-R3 | ↑LDH Pre-Post Pre-R3 Pre-R7 | - | - | 0 (0%) |
| Shin, 2016 (*) [52] | Amateur; 17; 0 F, 17 M; 48.35 ± 3.14 yrs | 100 km; 1; 1000+ m; 1000− m | ↑CK Pre 132.76 ± 55.17 Post 2983.1 ± 715.56 | ↑LDH Pre 338.82 ± 97.02 Post 693.58 ± 323.56 | ↑AST Pre 26.35 ± 14.02 Post 126.58 ± 35.58 | ↑ALT Pre 23.88 ± 18.05 Post 43.64 ± 32.98 | 0 (0%) |
| Shin, 2016 (*) [52] | Amateur; 16; 0 F, 16 M; 51.43 ± 2.89 yrs | 308 km; 1; 1000+ m; 1000− m | ↑CK Pre 131.75 ± 39.34 Post 4970.31 ± 2222.48 | ↑LDH Pre 385.62 ± 57.55 Post 1002.31 ± 224.60 | ↑AST Pre 25.87 ± 7.33 Post 203.50 ± 99.70 | ↑ALT Pre 21.75 ± 6.01 Post 78.06 ± 30.76 | 0 (0%) |
| Landers-Ramos, 2022 [53] | Amateur; 11; 3 F, 8 M; 40 ± 7 yrs | 50 km; 1; 762+ m 762− m | ↑CK Pre-10km Pre-Post Pre-R1 | - | - | - | 0 (0%) |
| Pradas, 2021 (*) [54] | Amateur; 10; 0 F, 10 M; 43.3 ± 4.52 yrs | 108 km; 1; 5800+ m 5800− m | ↑CK Pre 164.3 ± 69.39 Post 3251.6 ± 1011.89 | - | - | ↑ALT Pre 18.6 ± 2.5 Post 31.7 ± 9.67 | 0 (0%) |
| Pradas, 2021 (*) [54] | Highly trained; 10; 0 F, 10 M; 41.4 ± 6.18 yrs | 108 km; 1; 5800+ m 5800− m | ↑CK Pre 193.6 ± 42.11 Post 4261.5 ± 1469.6 | - | - | ↑ALT Pre 23.5 ± 3.92 Post 37.1 ± 7.46 | 0 (0%) |
| Nieman, 2005 [55] | Amateur; 60; 15 F, 45 M; 45.3 ± 1.1 yrs | 160 km; 1; 5500+ m 6700− m | ↑CK Pre 159 ± 21 Post 17,833 ± 2883 | - | - | 0 (0%) | |
| Skenderi, 2006 [36] | Amateur; 39; N/A; 41 ± 1 yrs | 246 km; 1; 1200+ m 1200− m | - | - | - | - | 0 (0%) |
| Hoppel, 2019 [56] | Amateur; 8; 0 F, 8 M; 41.5 yrs | 67 km; 1; 2500+ m; 2500− m | - | - | - | - | 0 (0%) |
| UT Race Classification | Distance (km) (Range) | Elevation Gain (m) (Range) | Elevation Loss (m) (Range) | Negative Relative Elevation Loss (Range) |
|---|---|---|---|---|
| Medium | (50–60) | (762–4260+) | (762–4260−) | (15.24–64.62) |
| Extra | (100–308) | (1200–12,200+) | (1200–12,200−) | (1.02–62.43) |
| Multi-stage | (619–786) | (600–46,865+) | (1100–46,865−) | (1.78–59.62) |
| Author; Year; Reference | Rank | Negative Relative Elevation Loss |
|---|---|---|
| Martínez-Navarro, 2020 (*) [50] | 1 | 64.62% |
| Gatterer, 2020 (*) [40] | 2 | 62.65% |
| Gatterer, 2020 (*) [40] | 3 | 62.43% |
| Belli, 2018 [51] | 4 | 56.22% |
| Pradas, 2021 (*) [55] | 5 | 53.70% |
| Pradas, 2021 (*) [55] | 6 | 53.70% |
| Rubio-Arias, 2019 (*) [48] | 7 | 49.35% |
| Ramos Campo, 2016 [47] | 8 | 49.35% |
| Nieman, 2005 [56] | 9 | 41.88% |
| Martínez-Navarro, 2020 (*) [50] | 10 | 40.71% |
| Rubio Arias, 2019 (*) [48] | 11 | 39.82% |
| Martínez-Navarro, 2021 [52] | 12 | 35.82% |
| Magrini, 2017 [25] | 13 | 30.00% |
| Hoppel, 2019 [57] | 14 | 29.85% |
| Shin, 2016 (*) [53] | 15 | 21.00% |
| Landers-Ramos, 2022 [54] | 16 | 15.24% |
| Lecina 2022 [46] | 17 | 5.42% |
| Skenderi, 2006 [36] | 18 | 4.88% |
| Boillat, 2022 [49] | 19 | 1.78% |
| Shin, 2016 (*) [53] | 20 | 1.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lecina, M.; Castellar-Otín, C.; García-Giménez, A.; Pradas, F. Exertional Rhabdomyolysis and Ultra-Trail Races: A Systematic Review Highlighting the Significant Impact of Eccentric Load. Muscles 2024, 3, 242-258. https://doi.org/10.3390/muscles3030022
Lecina M, Castellar-Otín C, García-Giménez A, Pradas F. Exertional Rhabdomyolysis and Ultra-Trail Races: A Systematic Review Highlighting the Significant Impact of Eccentric Load. Muscles. 2024; 3(3):242-258. https://doi.org/10.3390/muscles3030022
Chicago/Turabian StyleLecina, Miguel, Carlos Castellar-Otín, Alejandro García-Giménez, and Francisco Pradas. 2024. "Exertional Rhabdomyolysis and Ultra-Trail Races: A Systematic Review Highlighting the Significant Impact of Eccentric Load" Muscles 3, no. 3: 242-258. https://doi.org/10.3390/muscles3030022
APA StyleLecina, M., Castellar-Otín, C., García-Giménez, A., & Pradas, F. (2024). Exertional Rhabdomyolysis and Ultra-Trail Races: A Systematic Review Highlighting the Significant Impact of Eccentric Load. Muscles, 3(3), 242-258. https://doi.org/10.3390/muscles3030022









