Alternative Treatments to Exercise for the Attenuation of Disuse-Induced Skeletal Muscle Atrophy in Rats
Abstract
1. Introduction
2. Methods
2.1. Animals
2.2. Cast-Immobilization
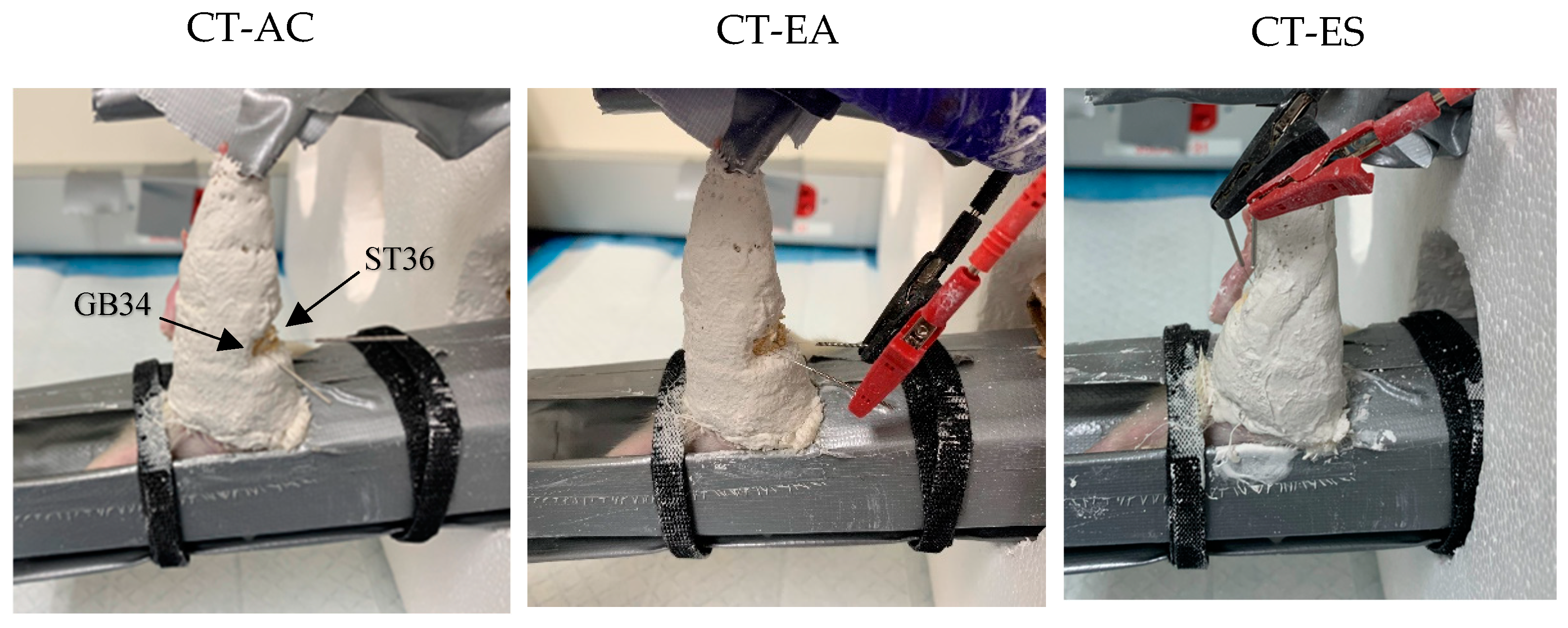
2.3. Treatment
2.4. Contractile Properties, Muscle Characteristics, and Protein Measures
2.5. Statistical Analysis
3. Results
3.1. Animal Characteristics
3.2. Muscle Characteristics
3.3. Contractile Properties of the Gastrocnemius Muscle
3.4. Biochemical Measures
4. Discussion
4.1. Muscle Characteristics
4.2. Contractile Properties of the Gastrocnemius Muscle
4.3. Biochemical Measures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; Campos, N.; Lopez-Pedrosa, J.M.; Rueda, R.; Rodriguez-Mañas, L. Skeletal muscle regulates metabolism via interorgan crosstalk: Roles in health and disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Jackman, R.W.; Kandarian, S.C. The molecular basis of skeletal muscle atrophy. Am. J. Physiol.-Cell Physiol. 2004, 287, C834–C843. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, N.; Lim, J.-Y.; Miljkovic, I.; Frontera, W.R. Aging of skeletal muscle fibers. Ann. Rehabil. Med. 2015, 39, 155. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Kong, J.; Li, Q.; Wang, Y.; Li, J. Role of miRNAs in skeletal muscle aging. Clin. Interv. Aging 2018, 13, 2407–2419. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Evans, W.J. Changes in skeletal muscle with aging: Effects of exercise training. Exerc. Sport Sci. Rev. 1993, 21, 65–102. [Google Scholar] [CrossRef]
- Buford, T.W.; Cooke, M.B.; Manini, T.M.; Leeuwenburgh, C.; Willoughby, D.S. Effects of age and sedentary lifestyle on skeletal muscle NF-κB signaling in men. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2010, 65, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Chon, T.Y.; Lee, M.C. Acupuncture. Mayo Clin Proc. 2013, 88, 1141–1146. [Google Scholar] [CrossRef]
- White, A.; Ernst, E. A brief history of acupuncture. Rheumatology 2004, 43, 662–663. [Google Scholar] [CrossRef]
- Madsen, M.V.; Gøtzsche, P.C.; Hróbjartsson, A. Acupuncture treatment for pain: Systematic review of randomised clinical trials with acupuncture, placebo acupuncture, and no acupuncture groups. BMJ 2009, 338, a3115. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.; Wilson, P.; Kleijnen, J. Acupuncture. Qual. Saf. Health Care 2002, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Onda, A.; Jiao, Q.; Nagano, Y.; Akimoto, T.; Miyamoto, T.; Minamisawa, S.; Fukubayashi, T. Acupuncture ameliorated skeletal muscle atrophy induced by hindlimb suspension in mice. Biochem. Biophys. Res. Commun. 2011, 410, 434–439. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, F.; Liang, X. Acupuncture improves the facial muscular function in a case of facioscapulohumeral muscular dystrophy. J. Acupunct. Meridian Stud. 2019, 12, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Varatharajan, S.; Côté, P.; Collaboration, O. Effectiveness of acupuncture therapies to manage musculoskeletal disorders of the extremities: A systematic review. J. Orthop. Sports Phys. Ther. 2016, 46, 409–429. [Google Scholar] [CrossRef] [PubMed]
- Swathy, S.; Sethu, V.G. Acupuncture and lower back pain. Res. J. Pharm. Technol. 2015, 8, 991–993. [Google Scholar] [CrossRef]
- Symons, T.B.; Park, J.; Kim, J.H.; Kwon, E.H.; Delacruz, J.; Lee, J.; Park, Y.; Chung, E.; Lee, S. Attenuation of skeletal muscle atrophy via acupuncture, electro-acupuncture, and electrical stimulation. Integr. Med. Res. 2023, 12, 100949. [Google Scholar] [CrossRef]
- Heidland, A.; Fazeli, G.; Klassen, A.; Sebekova, K.; Hennemann, H.; Bahner, U.; Di Iorio, B. Neuromuscular electrostimulation techniques: Historical aspects and current possibilities in treatment of pain and muscle waisting. Clin. Nephrol. 2013, 79, S12–S23. [Google Scholar] [CrossRef]
- Moreland, J.; Thomson, M.A. Efficacy of electromyographic biofeedback compared with conventional physical therapy for upper-extremity function in patients following stroke: A research overview and meta-analysis. Phys. Ther. 1994, 74, 534–543. [Google Scholar] [CrossRef]
- Abdellaoui, A.; Préfaut, C.; Gouzi, F.; Couillard, A.; Coisy-Quivy, M.; Hugon, G.; Molinari, N.; Lafontaine, T.; Jonquet, O.; Laoudj-Chenivesse, D. Skeletal muscle effects of electrostimulation after COPD exacerbation: A pilot study. Eur. Respir. J. 2011, 38, 781–788. [Google Scholar] [CrossRef]
- Lake, D.A. Neuromuscular electrical stimulation: An overview and its application in the treatment of sports injuries. Sports Med. 1992, 13, 320–336. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hong, K.-S.; Kim, K. Effect of previous strength training episode and retraining on facilitation of skeletal muscle hypertrophy and contractile properties after long-term detraining in rats. J. Exerc. Rehabil. 2016, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Farrar, R.P. Resistance training induces muscle-specific changes in muscle mass and function in rat. J. Exerc. Physiol. Online 2003, 6, 80–87. [Google Scholar]
- Su, Z.; Hu, L.; Cheng, J.; Klein, J.D.; Hassounah, F.; Cai, H.; Li, M.; Wang, H.; Wang, X.H. Acupuncture plus low-frequency electrical stimulation (Acu-LFES) attenuates denervation-induced muscle atrophy. J. Appl. Physiol. 2016, 120, 426–436. [Google Scholar] [PubMed]
- Suzuki, H.; Yoshikawa, Y.; Tsujimoto, H.; Kitaura, T.; Muraoka, I. Clenbuterol accelerates recovery after immobilization-induced atrophy of rat hindlimb muscle. Acta Histochem. 2020, 122, 151453. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.; Ferian, A.; Alves, P.K.; Silva, W.J.; Bento, M.R.; Gasch, A.; Labeit, S.; Moriscot, A.S. Skeletal muscle anti-atrophic effects of leucine involve myostatin inhibition. DNA Cell Biol. 2020, 39, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, R.; Hung, Y.-L.; Takagi, K.; Nakano, D.; Fujii, T.; Kawanishi, N.; Okamoto, T.; Machida, S. Differential protective effects of Radix astragali, herbal medicine, on immobilization-induced atrophy of slow-twitch and fast-twitch muscles. Biomed. Res. 2020, 41, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Bataa, M.; Kalleny, N.K.; Hamam, G.G. Effect of amino acid L-leucine on the musculo-skeletal changes during cast-immobilization in adult male albino rats. Physiological and Histological study. Life Sci. J. 2011, 8, 1–17. [Google Scholar]
- Machida, S.; Booth, F. Changes in signalling molecule levels in 10-day hindlimb immobilized rat muscles. Acta Physiol. Scand. 2005, 183, 171–179. [Google Scholar] [CrossRef]
- Booth, F.; Kelso, J. Effect of hind-limb immobilization on contractile and histochemical properties of skeletal muscle. Pfluegers Arch. 1973, 342, 231–238. [Google Scholar] [CrossRef]
- Duchateau, J.; Hainaut, K. Effects of immobilization on contractile properties, recruitment and firing rates of human motor units. J. Physiol. 1990, 422, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Belova, S.P.; Kalashnikova, E.P.; Tyganov, S.A.; Kostrominova, T.Y.; Shenkman, B.S.; Nemirovskaya, T.L. Effect of enhanced muscle tone on the expression of atrogenes and cytoskeletal proteins during postural muscle unloading. Arch. Biochem. Biophys. 2022, 725, 109291. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Torii, S.; Machida, S. Differential gene expression of muscle-specific ubiquitin ligase MAFbx/Atrogin-1 and MuRF1 in response to immobilization-induced atrophy of slow-twitch and fast-twitch muscles. J. Physiol. Sci. 2011, 61, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.-M.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.D.; Lecker, S.H.; Jagoe, R.T.; Navon, A.; Goldberg, A.L. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc. Natl. Acad. Sci. USA 2001, 98, 14440–14445. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-B.; Kwon, D.-K.; Jeon, Y.-J.; Song, Y.-J. Mealworm (Tenebrio molitor)-derived protein supplementation attenuates skeletal muscle atrophy in hindlimb casting immobilized rats. Chin. J. Physiol. 2021, 64, 211. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.R.; Haddad, F.; Bodell, P.W.; Tran, P.D.; Baldwin, K.M. Combined isometric, concentric, and eccentric resistance exercise prevents unloading-induced muscle atrophy in rats. J. Appl. Physiol. 2007, 103, 1644–1654. [Google Scholar] [CrossRef] [PubMed]
- Gielen, S.; Sandri, M.; Kozarez, I.; Kratzsch, J.; Teupser, D.; Thiery, J.; Erbs, S.; Mangner, N.; Lenk, K.; Hambrecht, R. Exercise training attenuates MuRF-1 expression in the skeletal muscle of patients with chronic heart failure independent of age: The randomized Leipzig Exercise Intervention in Chronic Heart Failure and Aging catabolism study. Circulation 2012, 125, 2716–2727. [Google Scholar] [CrossRef] [PubMed]
- Crossland, H.; Skirrow, S.; Puthucheary, Z.A.; Constantin-Teodosiu, D.; Greenhaff, P.L. The impact of immobilisation and inflammation on the regulation of muscle mass and insulin resistance: Different routes to similar end-points. J. Physiol. 2019, 597, 1259–1270. [Google Scholar] [CrossRef]
- Barik, A.; Lu, Y.; Sathyamurthy, A.; Bowman, A.; Shen, C.; Li, L.; Xiong, W.C.; Mei, L. LRP4 is critical for neuromuscular junction maintenance. J. Neurosci. 2014, 34, 13892–13905. [Google Scholar] [CrossRef]
- Goddeeris, M.M.; Wu, B.; Venzke, D.; Yoshida-Moriguchi, T.; Saito, F.; Matsumura, K.; Moore, S.A.; Campbell, K.P. LARGE glycans on dystroglycan function as a tunable matrix scaffold to prevent dystrophy. Nature 2013, 503, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Soendenbroe, C.; Heisterberg, M.F.; Schjerling, P.; Kjaer, M.; Andersen, J.L.; Mackey, A.L. Human skeletal muscle acetylcholine receptor gene expression in elderly males performing heavy resistance exercise. American J. Physiol.-Cell Physiol. 2022, 323, C159–C169. [Google Scholar] [CrossRef] [PubMed]
- Tintignac, L.A.; Brenner, H.R.; Rüegg, M.A. Mechanisms regulating neuromuscular junction development and function and causes of muscle wasting. Physiol. Rev. 2015, 95, 809–852. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Group | ||||
|---|---|---|---|---|---|
| CON (n = 8) | CT (n = 8) | CT-AC (n = 8) | CT-EA (n = 8) | CT-ES (n = 8) | |
| Pre Cast Body Weight (g) | 203.1 ± 7.2 | 205.3 ± 16.8 | 207.6 ± 11.9 | 201.1 ± 13.0 | 209.5 ± 9.0 |
| Post Cast Body Weight (g) | 213.1 ± 6.2 * | 203.4 ± 15.1 | 202.3 ± 9.2 | 197.5 ± 11.1 | 208.8 ± 9.2 |
| Characteristics | Group | ||||
|---|---|---|---|---|---|
| CON (n = 8) | CT (n = 8) | CT-AC (n = 8) | CT-EA (n = 8) | CT-ES (n = 8) | |
| Muscle Weight (mg) | 1149.6 ± 121.0 | 666.6 ± 60.2 * | 788.0 ± 103.7 *† | 759.2 ± 108.5 * | 744.9 ± 64.2 * |
| Gastro/body, ×105 | 539.1 ± 49.5 | 329.4 ± 38.1 * | 389.9 ± 52.1 * | 385.1 ± 56.4 * | 357.1 ± 29.6 * |
| Lo (mm) | 37 ± 2.0 | 35 ± 1.3 | 36 ± 2.1 | 34 ± 0.9 | 35 ± 1.1 |
| Gastrocnemius CSA (mm2) | 1.09 | 1.01 | 1.04 | 1.04 | 1.04 |
| Gastrocnemius Fiber CSA (µm2) | 17,748.0 ± 2698.5 | 7473.8 ± 1492.8 * | 9215.2 ± 713.7 *† | 8895.7 ± 807.4 *† | 9188.3 ± 801.7 *† |
| Characteristics | Group | ||||
|---|---|---|---|---|---|
| CON (n = 8) | CT (n = 8) | CT-AC (n = 8) | CT-EA (n = 8) | CT-ES (n = 8) | |
| CT (ms) | 26.14 ± 7.71 | 29.33 ± 6.37 | 34.13 ± 2.17 * | 34.75 ± 3.88 * | 37.00 ± 4.34 * |
| 1/2 RT (ms) | 24.43 ± 8.36 | 19.00 ± 3.02 | 24.63 ± 3.42 | 28.75 ± 7.48 † | 26.63 ± 4.44 |
| Pt (N) | 4.89 ± 1.13 | 2.74 ± 0.33 * | 3.74 ± 0.75 † | 3.70 ± 0.54 † | 3.98 ± 0.58 † |
| SPt (N/cm2) | 4.2 ± 1.0 | 3.8 ± 0.7 | 4.5 ± 0.9 | 4.3 ± 0.6 | 4.8 ± 0.8 * |
| Po (N) | 10.43 ± 3.20 | 7.46 ± 1.32 * | 7.78 ± 1.64 | 8.49 ± 2.23 | 8.89 ± 1.97 |
| SPo (N/cm2) | 8.8 ± 2.3 | 10.1 ± 1.9 | 9.5 ± 2.4 | 10.3 ± 2.2 | 11.1 ± 2.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Symons, T.B.; Kwon, E.H.; Chung, E.; Lee, S. Alternative Treatments to Exercise for the Attenuation of Disuse-Induced Skeletal Muscle Atrophy in Rats. Muscles 2024, 3, 224-234. https://doi.org/10.3390/muscles3030020
Park J, Symons TB, Kwon EH, Chung E, Lee S. Alternative Treatments to Exercise for the Attenuation of Disuse-Induced Skeletal Muscle Atrophy in Rats. Muscles. 2024; 3(3):224-234. https://doi.org/10.3390/muscles3030020
Chicago/Turabian StylePark, Jinho, T. Brock Symons, Eun Hye Kwon, Eunhee Chung, and Sukho Lee. 2024. "Alternative Treatments to Exercise for the Attenuation of Disuse-Induced Skeletal Muscle Atrophy in Rats" Muscles 3, no. 3: 224-234. https://doi.org/10.3390/muscles3030020
APA StylePark, J., Symons, T. B., Kwon, E. H., Chung, E., & Lee, S. (2024). Alternative Treatments to Exercise for the Attenuation of Disuse-Induced Skeletal Muscle Atrophy in Rats. Muscles, 3(3), 224-234. https://doi.org/10.3390/muscles3030020






