Resting Systemic Irisin Concentrations Are Lower in Older versus Younger Males after 12 Weeks of Resistance-Exercise Training While Apelin and IL-15 Concentrations Were Increased in the Whole Cohort
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Participants
4.3. Training Protocol
4.4. Blood Analysis
4.5. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kunz, H.E.; Lanza, I.R. Age-Associated Inflammation and Implications for Skeletal Muscle Responses to Exercise. Exp. Gerontol. 2023, 177, 112177. [Google Scholar] [CrossRef]
- Abou Sawan, S.; Nunes, E.A.; Lim, C.; McKendry, J.; Phillips, S.M. The Health Benefits of Resistance Exercise: Beyond Hypertrophy and Big Weights. Exerc. Sport Mov. 2023, 1, e00001. [Google Scholar] [CrossRef]
- Cornish, S.M.; Chilibeck, P.D.; Candow, D.G. Potential Importance of Immune System Response to Exercise on Aging Muscle and Bone. Curr. Osteoporos. Rep. 2020, 18, 350–356. [Google Scholar] [CrossRef]
- Zunner, B.E.M.; Wachsmuth, N.B.; Eckstein, M.L.; Scherl, L.; Schierbauer, J.R.; Haupt, S.; Stumpf, C.; Reusch, L.; Moser, O. Myokines and Resistance Training: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 3501. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The Anti-Inflammatory Effects of Exercise: Mechanisms and Implications for the Prevention and Treatment of Disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Pedersen, B.K. The Anti-Inflammatory Effect of Exercise: Its Role in Diabetes and Cardiovascular Disease Control. Essays Biochem. 2006, 42, 105–117. [Google Scholar] [CrossRef]
- de Melo Madureira, Á.N.; de Oliveira, J.R.S.; de Menezes Lima, V.L. The Role of IL-6 Released during Exercise to Insulin Sensitivity and Muscle Hypertrophy. Mini Rev. Med. Chem. 2022, 22, 2419–2428. [Google Scholar] [CrossRef]
- Slate-Romano, J.J.; Yano, N.; Zhao, T.C. Irisin Reduces Inflammatory Signaling Pathways in Inflammation-Mediated Metabolic Syndrome. Mol. Cell Endocrinol. 2022, 552, 111676. [Google Scholar] [CrossRef]
- Khalafi, M.; Akbari, A.; Symonds, M.E.; Pourvaghar, M.J.; Rosenkranz, S.K.; Tabari, E. Influence of Different Modes of Exercise Training on Inflammatory Markers in Older Adults with and without Chronic Diseases: A Systematic Review and Meta-Analysis. Cytokine 2023, 169, 156303. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Steensberg, A.; Fischer, C.; Keller, C.; Keller, P.; Plomgaard, P.; Wolsk-Petersen, E.; Febbraio, M. The Metabolic Role of IL-6 Produced during Exercise: Is IL-6 an Exercise Factor? Proc. Nutr. Soc. 2004, 63, 263–267. [Google Scholar] [CrossRef]
- Rogeri, P.S.; Gasparini, S.O.; Martins, G.L.; Costa, L.K.F.; Araujo, C.C.; Lugaresi, R.; Kopfler, M.; Lancha, A.H. Crosstalk Between Skeletal Muscle and Immune System: Which Roles Do IL-6 and Glutamine Play? Front. Physiol. 2020, 11, 582258. [Google Scholar] [CrossRef]
- Cornish, S.M.; Bugera, E.M.; Duhamel, T.A.; Peeler, J.D.; Anderson, J.E. A Focused Review of Myokines as a Potential Contributor to Muscle Hypertrophy from Resistance-Based Exercise. Eur. J. Appl. Physiol. 2020, 120, 941–959. [Google Scholar] [CrossRef]
- Pan, L.; Xie, W.; Fu, X.; Lu, W.; Jin, H.; Lai, J.; Zhang, A.; Yu, Y.; Li, Y.; Xiao, W. Inflammation and Sarcopenia: A Focus on Circulating Inflammatory Cytokines. Exp. Gerontol. 2021, 154, 111544. [Google Scholar] [CrossRef]
- Forcina, L.; Franceschi, C.; Musarò, A. The Hormetic and Hermetic Role of IL-6. Ageing Res. Rev. 2022, 80, 101697. [Google Scholar] [CrossRef]
- Prokopchuk, O.; Liu, Y.; Wang, L.; Wirth, K.; Schmidtbleicher, D.; Steinacker, J.M. Skeletal Muscle IL-4, IL-4Ralpha, IL-13 and IL-13Ralpha1 Expression and Response to Strength Training. Exerc. Immunol. Rev. 2007, 13, 67–75. [Google Scholar]
- Chen, D.; Tang, T.-X.; Deng, H.; Yang, X.-P.; Tang, Z.-H. Interleukin-7 Biology and Its Effects on Immune Cells: Mediator of Generation, Differentiation, Survival, and Homeostasis. Front. Immunol. 2021, 12, 747324. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, Exercise and Obesity: Skeletal Muscle as a Secretory Organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in Health, Resilience and Disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Vinel, C.; Lukjanenko, L.; Batut, A.; Deleruyelle, S.; Pradère, J.-P.; Le Gonidec, S.; Dortignac, A.; Geoffre, N.; Pereira, O.; Karaz, S.; et al. The Exerkine Apelin Reverses Age-Associated Sarcopenia. Nat. Med. 2018, 24, 1360–1371. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Li, P.; Zheng, Y.; Yang, Y.; Ji, S. Apelin/APJ System in Inflammation. Int. Immunopharmacol. 2022, 109, 108822. [Google Scholar] [CrossRef]
- Broholm, C.; Pedersen, B.K. Leukaemia Inhibitory Factor--an Exercise-Induced Myokine. Exerc. Immunol. Rev. 2010, 16, 77–85. [Google Scholar]
- Hunt, L.C.; White, J. The Role of Leukemia Inhibitory Factor Receptor Signaling in Skeletal Muscle Growth, Injury and Disease. Adv. Exp. Med. Biol. 2016, 900, 45–59. [Google Scholar] [CrossRef]
- Chodorowska, G.; Głowacka, A.; Tomczyk, M. Leukemia Inhibitory Factor (LIF) and Its Biological Activity. Ann. Univ. Mariae Curie Sklodowska Med. 2004, 59, 189–193. [Google Scholar]
- Kim, C.-S.; Joe, Y.; Choi, H.-S.; Back, S.H.; Park, J.W.; Chung, H.T.; Roh, E.; Kim, M.-S.; Ha, T.Y.; Yu, R. Deficiency of Fibroblast Growth Factor 21 Aggravates Obesity-Induced Atrophic Responses in Skeletal Muscle. J. Inflamm. 2019, 16, 17. [Google Scholar] [CrossRef]
- Cordingley, D.M.; Anderson, J.E.; Cornish, S.M. Myokine Response to Blood-Flow Restricted Resistance Exercise in Younger and Older Males in an Untrained and Resistance-Trained State: A Pilot Study. J. Sci. Sport Exerc. 2022, 5, 203–217. [Google Scholar] [CrossRef]
- Latroche, C.; Weiss-Gayet, M.; Muller, L.; Gitiaux, C.; Leblanc, P.; Liot, S.; Ben-Larbi, S.; Abou-Khalil, R.; Verger, N.; Bardot, P.; et al. Coupling between Myogenesis and Angiogenesis during Skeletal Muscle Regeneration Is Stimulated by Restorative Macrophages. Stem Cell Rep. 2017, 9, 2018–2033. [Google Scholar] [CrossRef]
- Kilpiö, T.; Skarp, S.; Perjés, Á.; Swan, J.; Kaikkonen, L.; Saarimäki, S.; Szokodi, I.; Penninger, J.M.; Szabó, Z.; Magga, J.; et al. Apelin Regulates Skeletal Muscle Adaptation to Exercise in a High-Intensity Interval Training Model. Am. J. Physiol.-Cell Physiol. 2024, 326, C1437–C1450. [Google Scholar] [CrossRef]
- O’Leary, M.F.; Wallace, G.R.; Bennett, A.J.; Tsintzas, K.; Jones, S.W. IL-15 Promotes Human Myogenesis and Mitigates the Detrimental Effects of TNFα on Myotube Development. Sci. Rep. 2017, 7, 12997. [Google Scholar] [CrossRef]
- Furmanczyk, P.S.; Quinn, L.S. Interleukin-15 Increases Myosin Accretion in Human Skeletal Myogenic Cultures. Cell Biol. Int. 2003, 27, 845–851. [Google Scholar] [CrossRef]
- Quinn, L.S.; Anderson, B.G.; Drivdahl, R.H.; Alvarez, B.; Argilés, J.M. Overexpression of Interleukin-15 Induces Skeletal Muscle Hypertrophy in Vitro: Implications for Treatment of Muscle Wasting Disorders. Exp. Cell Res. 2002, 280, 55–63. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Mohammad Rahimi, G.R.; Hejazi, K.; Hofmeister, M. The Effect of Exercise Interventions on Irisin Level: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. EXCLI J. 2022, 21, 524, ISSN 1611-2156. [Google Scholar] [CrossRef]
- Löffler, D.; Müller, U.; Scheuermann, K.; Friebe, D.; Gesing, J.; Bielitz, J.; Erbs, S.; Landgraf, K.; Wagner, I.V.; Kiess, W.; et al. Serum Irisin Levels Are Regulated by Acute Strenuous Exercise. J. Clin. Endocrinol. Metab. 2015, 100, 1289–1299. [Google Scholar] [CrossRef]
- Miyamoto-Mikami, E.; Sato, K.; Kurihara, T.; Hasegawa, N.; Fujie, S.; Fujita, S.; Sanada, K.; Hamaoka, T.; Tabata, I.; Iemitsu, M. Endurance Training-Induced Increase in Circulating Irisin Levels Is Associated with Reduction of Abdominal Visceral Fat in Middle-Aged and Older Adults. PLoS ONE 2015, 10, e0120354. [Google Scholar] [CrossRef]
- Huh, J.Y.; Dincer, F.; Mesfum, E.; Mantzoros, C.S. Irisin Stimulates Muscle Growth-Related Genes and Regulates Adipocyte Differentiation and Metabolism in Humans. Int. J. Obes. 2014, 38, 1538–1544. [Google Scholar] [CrossRef]
- Endo, Y.; Nourmahnad, A.; Sinha, I. Optimizing Skeletal Muscle Anabolic Response to Resistance Training in Aging. Front. Physiol. 2020, 11, 874. [Google Scholar] [CrossRef]
- Pérez-López, A.; McKendry, J.; Martin-Rincon, M.; Morales-Alamo, D.; Pérez-Köhler, B.; Valadés, D.; Buján, J.; Calbet, J.A.L.; Breen, L. Skeletal Muscle IL-15/IL-15Rα and Myofibrillar Protein Synthesis after Resistance Exercise. Scand. J. Med. Sci. Sports 2018, 28, 116–125. [Google Scholar] [CrossRef]
- Quinn, L.S.; Anderson, B.G.; Strait-Bodey, L.; Wolden-Hanson, T. Serum and Muscle Interleukin-15 Levels Decrease in Aging Mice: Correlation with Declines in Soluble Interleukin-15 Receptor Alpha Expression. Exp. Gerontol. 2010, 45, 106–112. [Google Scholar] [CrossRef]
- Hennigar, S.R.; McClung, J.P.; Pasiakos, S.M. Nutritional Interventions and the IL-6 Response to Exercise. FASEB J. 2017, 31, 3719–3728. [Google Scholar] [CrossRef]
- Sabaratnam, R.; Wojtaszewski, J.F.P.; Højlund, K. Factors Mediating Exercise-induced Organ Crosstalk. Acta Physiol. 2022, 234, e13766. [Google Scholar] [CrossRef]
- Senesi, P.; Luzi, L.; Terruzzi, I. Adipokines, Myokines, and Cardiokines: The Role of Nutritional Interventions. Int. J. Mol. Sci. 2020, 21, 8372. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.D.; Wewege, M.A.; Hackett, D.A.; Keogh, J.W.L.; Hagstrom, A.D. Sex Differences in Adaptations in Muscle Strength and Size Following Resistance Training in Older Adults: A Systematic Review and Meta-Analysis. Sports Med. 2021, 51, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.M.; Nuckols, G.; Krieger, J.W. Sex Differences in Resistance Training: A Systematic Review and Meta-Analysis. J. Strength Cond. Res. 2020, 34, 1448–1460. [Google Scholar] [CrossRef]
- Cornish, S.M.; Myrie, S.B.; Bugera, E.M.; Chase, J.E.; Turczyn, D.; Pinder, M. Omega-3 Supplementation with Resistance Training Does Not Improve Body Composition or Lower Biomarkers of Inflammation More so than Resistance Training Alone in Older Men. Nutr. Res. 2018, 60, 87–95. [Google Scholar] [CrossRef]
| Variable | Younger (n = 8) | Older (n = 7) | p-Values | ||||
|---|---|---|---|---|---|---|---|
| Pre-Training | Post-Training | Pre-Training | Post-Training | Training Status | Age Group | Age Group × Training Status | |
| Apelin (pg/mL) | 476.0 ± 155.8 | 521.8 ± 141.9 | 363.5 ± 110.6 | 529.0 ± 135.9 | 0.003 | 0.432 | 0.066 |
| Fibroblast Growth Factor—21 (pg/mL) | 266.5 ± 148.6 | 262.5 ± 151.6 | 157.5 ± 61.6 | 183.6 ± 42.7 | 0.711 | 0.100 | 0.616 |
| Interleukin-4 (pg/mL) | 19.7 ± 17.1 | 20.1 ± 15.6 | 139.9 ± 190.4 | 118.2 ± 164.7 | 0.675 | 0.153 | 0.516 |
| Interleukin-6 (pg/mL) | 1.2 ± 1.6 | 1.7 ± 2.7 | 15.0 ± 21.9 | 13.6 ± 20.3 | 0.758 | 0.197 | 0.041 |
| Interleukin-7 (pg/mL) | 3.0 ± 1.6 | 3.4 ± 2.2 | 3.3 ± 1.4 | 3.4 ± 1.3 | 0.418 | 0.873 | 0.593 |
| Interleukin-15 (pg/mL) | 15.0 ± 4.9 | 17.6 ± 5.2 | 11.1 ± 2.6 | 17.5 ± 2.2 | <0.001 | 0.299 | 0.066 |
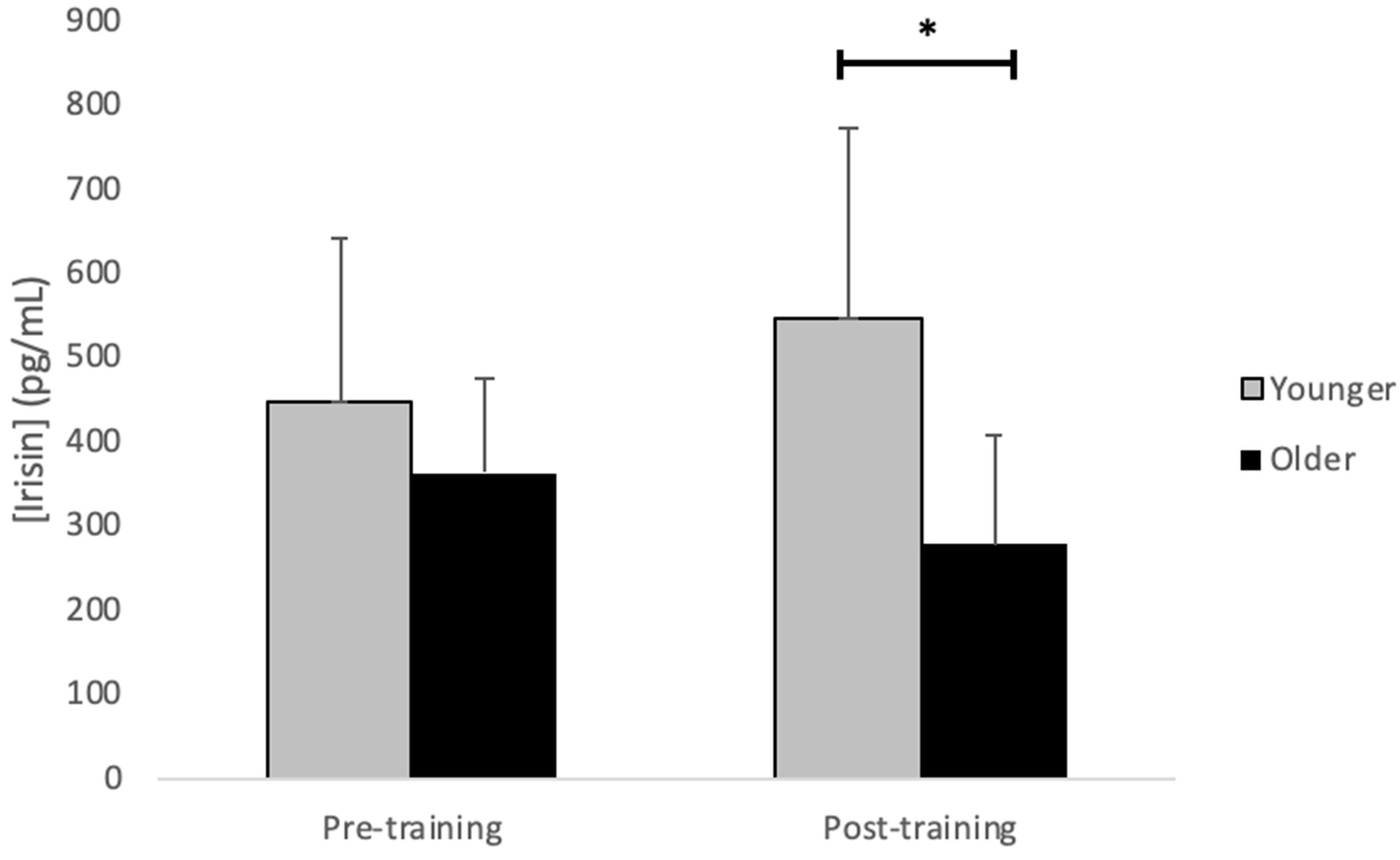
| Irisin (pg/mL) | 447.7 ± 191.7 | 548.0 ± 222.4 | 363.2 ± 112.3 | 278.3 ± 127.1 * | 0.817 | 0.053 | 0.014 |
| Leukemia inhibitory factor (pg/mL) | 23.4 ± 20.4 | 26.0 ± 9.4 | 17.5 ± 6.5 | 15.2 ± 4.5 | 0.395 | 0.221 | 0.076 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordingley, D.M.; Anderson, J.E.; Cornish, S.M. Resting Systemic Irisin Concentrations Are Lower in Older versus Younger Males after 12 Weeks of Resistance-Exercise Training While Apelin and IL-15 Concentrations Were Increased in the Whole Cohort. Muscles 2024, 3, 202-211. https://doi.org/10.3390/muscles3030018
Cordingley DM, Anderson JE, Cornish SM. Resting Systemic Irisin Concentrations Are Lower in Older versus Younger Males after 12 Weeks of Resistance-Exercise Training While Apelin and IL-15 Concentrations Were Increased in the Whole Cohort. Muscles. 2024; 3(3):202-211. https://doi.org/10.3390/muscles3030018
Chicago/Turabian StyleCordingley, Dean M., Judy E. Anderson, and Stephen M. Cornish. 2024. "Resting Systemic Irisin Concentrations Are Lower in Older versus Younger Males after 12 Weeks of Resistance-Exercise Training While Apelin and IL-15 Concentrations Were Increased in the Whole Cohort" Muscles 3, no. 3: 202-211. https://doi.org/10.3390/muscles3030018
APA StyleCordingley, D. M., Anderson, J. E., & Cornish, S. M. (2024). Resting Systemic Irisin Concentrations Are Lower in Older versus Younger Males after 12 Weeks of Resistance-Exercise Training While Apelin and IL-15 Concentrations Were Increased in the Whole Cohort. Muscles, 3(3), 202-211. https://doi.org/10.3390/muscles3030018






