Sarcopenia and Pleural Effusions: Exploring a Potential Link
Abstract
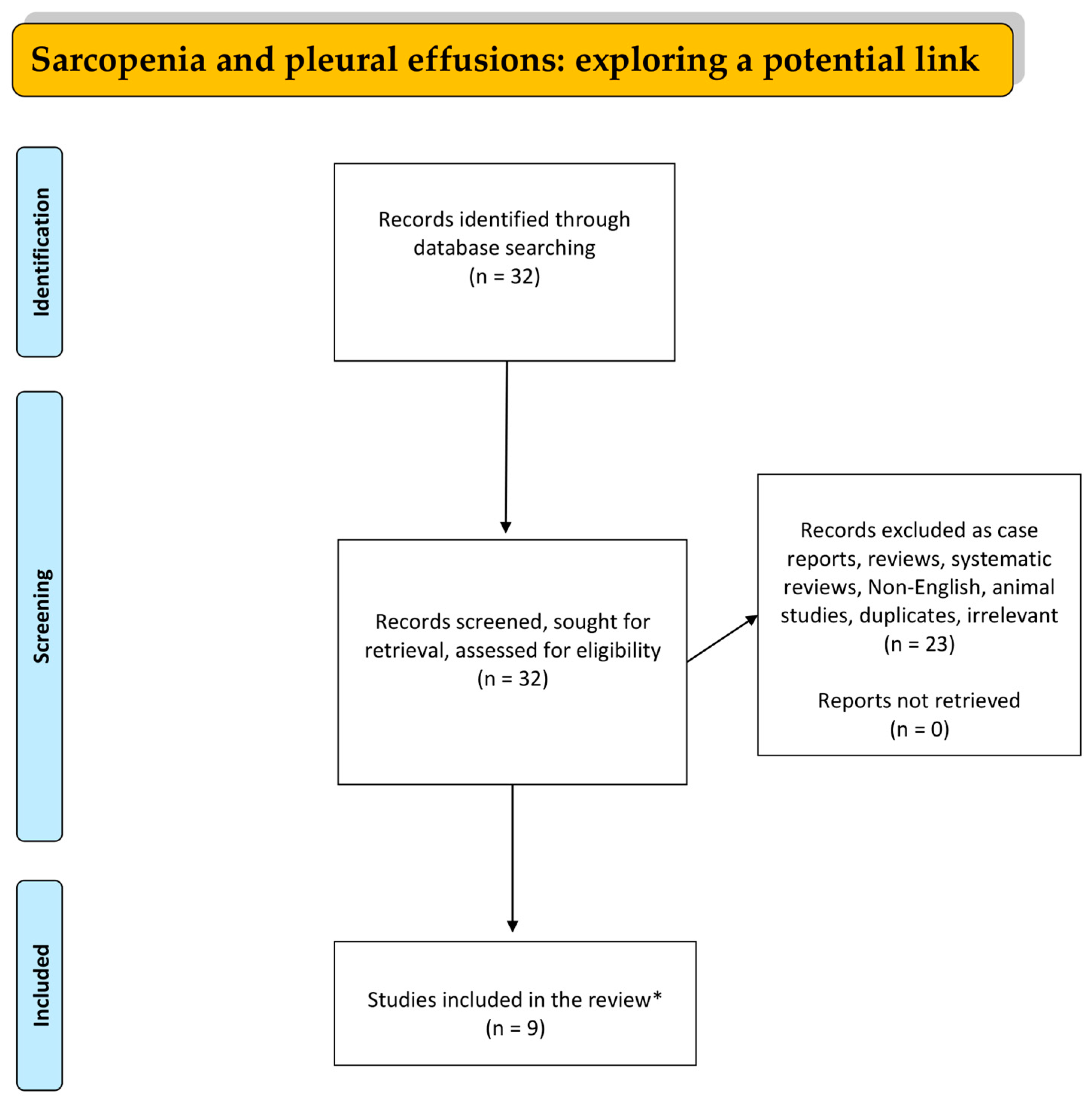
:1. Introduction
2. Materials and Methods
3. Discussion
3.1. Sarcopenia, Pleural Effusion, and Liver Transplantation
3.2. Malignant Pleural Effusions and Sarcopenia
3.3. Sarcopenia, Pes, and Cancer
3.4. Sarcopenia, Frailty, Pleural Effusions, and Mitral Valve Surgery
3.5. Pleural Effusions, Sarcopenia, and Esophagectomy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Woo, J. Sarcopenia. Clin. Geriatr. Med. 2017, 33, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Urano, T.; Inoue, S. Recent Genetic Discoveries in Osteoporosis, Sarcopenia and Obesity [Review]. Endocr. J. 2015, 62, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Sayer, A.A.; Syddall, H.E.; Gilbody, H.J.; Dennison, E.M.; Cooper, C. Does Sarcopenia Originate in Early Life? Findings From the Hertfordshire Cohort Study. J. Gerontol. Ser. A 2004, 59, M930–M934. [Google Scholar] [CrossRef] [PubMed]
- Wochenschr, W.K.; Morley, J.E. Frailty and Sarcopenia in Elderly. Wien. Klin. Wochenschr. 2016, 128, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Dodds, R.; Sayer, A.A. Sarcopenia and Frailty: New Challenges for Clinical Practice. Clin. Med. 2016, 16, 455. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Morley, J.E.; Schols, A.M.W.J.; Ferrucci, L.; Cruz-Jentoft, A.J.; Dent, E.; Baracos, V.E.; Crawford, J.A.; Doehner, W.; Heymsfield, S.B.; et al. Sarcopenia: A Time for Action. An SCWD Position Paper. J. Cachexia Sarcopenia Muscle 2019, 10, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, R.J.S.; Hasni, S. Pathogenesis and Management of Sarcopenia. Clin. Geriatr. Med. 2017, 33, 17–26. [Google Scholar] [CrossRef]
- Papadopoulou, S.K. Sarcopenia: A Contemporary Health Problem among Older Adult Populations. Nutrients 2020, 12, 1293. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global Prevalence of Sarcopenia and Severe Sarcopenia: A Systematic Review and Meta-Analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef]
- Thompson, M.Q.; Jadczak, A.D.; Yu, S.; Tucker, G.R.; Visvanathan, R. Sarcopenia Risk in Nursing Home Residents Using SARC-F: FIRST Study Findings. Geriatr. Gerontol. Int. 2022, 22, 206–212. [Google Scholar] [CrossRef]
- Do, J.Y.; Seo, J.H.; Kang, S.H. Validation of the SARC-F for Assessing Sarcopenia in Patients on Peritoneal Dialysis. J. Ren. Nutr. 2022, 32, 341–346. [Google Scholar] [CrossRef]
- Duarte, M.P.; Ribeiro, H.S.; Almeida, L.S.; Baião, V.M.; Inda-Filho, A.; Avesani, C.M.; Ferreira, A.P.; Lima, R.M. SARC-F and SARC-CalF Are Associated with Sarcopenia Traits in Hemodialysis Patients. Nutr. Clin. Pract. 2022, 37, 1356–1365. [Google Scholar] [CrossRef]
- Ida, S.; Kaneko, R.; Murata, K. SARC-F for Screening of Sarcopenia Among Older Adults: A Meta-Analysis of Screening Test Accuracy. J. Am. Med. Dir. Assoc. 2018, 19, 685–689. [Google Scholar] [CrossRef]
- Bahat, G.; Erdoǧan, T.; Ilhan, B. SARC-F and Other Screening Tests for Sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 37–42. [Google Scholar] [CrossRef]
- Malmstrom, T.K.; Miller, D.K.; Simonsick, E.M.; Ferrucci, L.; Morley, J.E. SARC-F: A Symptom Score to Predict Persons with Sarcopenia at Risk for Poor Functional Outcomes: SARC-F. J. Cachexia Sarcopenia Muscle 2016, 7, 28–36. [Google Scholar] [CrossRef]
- Nishikawa, H.; Asai, A.; Fukunishi, S.; Takeuchi, T.; Goto, M.; Ogura, T.; Nakamura, S.; Kakimoto, K.; Miyazaki, T.; Nishiguchi, S.; et al. Screening Tools for Sarcopenia. Vivo 2021, 35, 3001–3009. [Google Scholar] [CrossRef]
- Karakousis, N.D.; Gourgoulianis, K.I.; Kotsiou, O.S. Sarcopenia and Tuberculosis: Is There Any Connection? J. Pers. Med. 2023, 13, 1102. [Google Scholar] [CrossRef]
- Ryu, J.Y.; Kim, M.; Kim, K.S.; Kim, S.; Won, C.W. Chair Stand Test as a Proxy for Physical Performance and Muscle Strength in Sarcopenia Diagnosis: The Korean Frailty and Aging Cohort Study. Aging Clin. Exp. Res. 2022, 34, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Zwart, A.T.; Becker, J.N.; Lamers, M.J.; Dierckx, R.A.J.O.; de Bock, G.H.; Halmos, G.B.; van der Hoorn, A. Skeletal Muscle Mass and Sarcopenia Can Be Determined with 1.5-T and 3-T Neck MRI Scans, in the Event That No Neck CT Scan Is Performed. Eur. Radiol. 2021, 31, 4053–4062. [Google Scholar] [CrossRef]
- Beer, L.; Bastati, N.; Ba-Ssalamah, A.; Pötter-Lang, S.; Lampichler, K.; Bican, Y.; Lauber, D.; Hodge, J.; Binter, T.; Pomej, K.; et al. MRI-Defined Sarcopenia Predicts Mortality in Patients with Chronic Liver Disease. Liver Int. 2020, 40, 2797–2807. [Google Scholar] [CrossRef]
- Cheng, K.Y.K.; Chow, S.K.H.; Hung, V.W.Y.; Wong, C.H.W.; Wong, R.M.Y.; Tsang, C.S.L.; Kwok, T.; Cheung, W.H. Diagnosis of Sarcopenia by Evaluating Skeletal Muscle Mass by Adjusted Bioimpedance Analysis Validated with Dual-Energy X-Ray Absorptiometry. J. Cachexia Sarcopenia Muscle 2021, 12, 2163–2173. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, G.; Ponti, F.; Agostini, M.; Amadori, M.; Battista, G.; Bazzocchi, A. The Role of DXA in Sarcopenia. Aging Clin. Exp. Res. 2016, 28, 1047–1060. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Meschi, T.; Narici, M.V.; Lauretani, F.; Maggio, M. Muscle Ultrasound and Sarcopenia in Older Individuals: A Clinical Perspective. J. Am. Med. Dir. Assoc. 2017, 18, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Tagliafico, A.S.; Bignotti, B.; Torri, L.; Rossi, F. Sarcopenia: How to Measure, When and Why. Radiol. Med. 2022, 127, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, Y.; Kusakabe, T.; Arai, H.; Yamamoto, Y.; Nakao, K.; Ikeue, K.; Ishihara, Y.; Tagami, T.; Yasoda, A.; Ishii, K.; et al. Phase Angle from Bioelectrical Impedance Analysis Is a Useful Indicator of Muscle Quality. J. Cachexia Sarcopenia Muscle 2022, 13, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Bise, T.; Yoshimura, Y.; Wakabayashi, H.; Nagano, F.; Kido, Y.; Shimazu, S.; Shiraishi, A.; Matsumoto, A. Association between BIA-Derived Phase Angle and Sarcopenia and Improvement in Activities of Daily Living and Dysphagia in Patients Undergoing Post-Stroke Rehabilitation. J. Nutr. Health Aging 2022, 26, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.; et al. Prevalence of and Interventions for Sarcopenia in Ageing Adults: A Systematic Review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Beckwée, D.; Delaere, A.; Aelbrecht, S.; Baert, V.; Beaudart, C.; Bruyere, O.; de Saint-Hubert, M.; Bautmans, I. Exercise Interventions for the Prevention and Treatment of Sarcopenia. A Systematic Umbrella Review. J. Nutr. Health Aging 2019, 23, 494–502. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Wakabayashi, H.; Yamada, M.; Kim, H.; Harada, A.; Arai, H. Interventions for Treating Sarcopenia: A Systematic Review and Meta-Analysis of Randomized Controlled Studies. J. Am. Med. Dir. Assoc. 2017, 18, 553.e1–553.e16. [Google Scholar] [CrossRef]
- Giallauria, F.; Cittadini, A.; Smart, N.A.; Vigorito, C. Resistance Training and Sarcopenia. Monaldi Arch. Chest Dis. 2016, 84, 738. [Google Scholar] [CrossRef] [PubMed]
- Hurst, C.; Robinson, S.M.; Witham, M.D.; Dodds, R.M.; Granic, A.; Buckland, C.; De Biase, S.; Finnegan, S.; Rochester, L.; Skelton, D.A.; et al. Resistance Exercise as a Treatment for Sarcopenia: Prescription and Delivery. Age Ageing 2022, 51, afac003. [Google Scholar] [CrossRef] [PubMed]
- Sayer, A.A.; Cruz-Jentoft, A. Sarcopenia Definition, Diagnosis and Treatment: Consensus Is Growing. Age Ageing 2022, 51, afac22. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.C.; O’Halloran, A.M. Tools for Assessing Frailty in Older People: General Concepts. Adv. Exp. Med. Biol. 2020, 1216, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Bibby, A.C.; Dorn, P.; Psallidas, I.; Porcel, J.M.; Janssen, J.; Froudarakis, M.; Subotic, D.; Astoul, P.; Licht, P.; Schmid, R.; et al. ERS/EACTS Statement on the Management of Malignant Pleural Effusions. Eur. Respir. J. 2018, 52, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Bedawi, E.O.; Hassan, M.; Rahman, N.M. Recent Developments in the Management of Pleural Infection: A Comprehensive Review. Clin. Respir. J. 2018, 12, 2309–2320. [Google Scholar] [CrossRef] [PubMed]
- Jany, B.; Welte, T. Pleural Effusion in Adults—Etiology, Diagnosis, and Treatment. Dtsch. Arztebl. Int. 2019, 116, 377. [Google Scholar] [CrossRef] [PubMed]
- Porcel, J.M.; Light, R.W. Diagnostic Approach to Pleural Effusion in Adults. Am. Fam. Physician 2006, 73, 1211–1220. [Google Scholar]
- Karkhanis, V.S.; Joshi, J.M. Pleural Effusion: Diagnosis, Treatment, and Management. Open Access Emerg. Med. 2012, 4, 31. [Google Scholar] [CrossRef]
- He, T.; Oh, S. Diagnostic Approach to Pleural Effusions. AME Med. J. 2018, 3, 116. [Google Scholar] [CrossRef]
- Rahman, N.M.; Chapman, S.J.; Davies, R.J.O. Pleural Effusion: A Structured Approach to Care. Br. Med. Bull. 2004, 72, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Gayen, S. Malignant Pleural Effusion: Presentation, Diagnosis, and Management. Am. J. Med. 2022, 135, 1188–1192. [Google Scholar] [CrossRef] [PubMed]
- Sundaralingam, A.; Bedawi, E.O.; Rahman, N.M. Diagnostics in Pleural Disease. Diagnostics 2020, 10, 1046. [Google Scholar] [CrossRef] [PubMed]
- Zocchi, L. Physiology and Pathophysiology of Pleural Fluid Turnover. Eur. Respir. J. 2002, 20, 1545–1558. [Google Scholar] [CrossRef] [PubMed]
- Light, R.W. Pleural Effusions. Med. Clin. N. Am. 2011, 95, 1055–1070. [Google Scholar] [CrossRef] [PubMed]
- Meriggi, F. Malignant Pleural Effusion: Still a Long Way to Go. Rev. Recent Clin. Trials 2019, 14, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Du Rand, I.; Maskel, N. Introduction and Methods: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010, 65, ii1–ii3. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.E.; Rahman, N.M.; Maskell, N.A.; Bibby, A.C.; Blyth, K.G.; Corcoran, J.P.; Edey, A.; Evison, M.; De Fonseka, D.; Hallifax, R.; et al. British Thoracic Society Guideline for Pleural Disease. Thorax 2023, 78, 1143–1156. [Google Scholar] [CrossRef]
- Roberts, M.E.; Rahman, N.M.; Maskell, N.A.; Bibby, A.C.; Blyth, K.G.; Corcoran, J.P.; Edey, A.; Evison, M.; De Fonseka, D.; Hallifax, R.; et al. British Thoracic Society Guideline for Pleural Disease. Thorax 2023, 78, s1–s42. [Google Scholar] [CrossRef]
- Markatis, E.; Perlepe, G.; Afthinos, A.; Pagkratis, K.; Varsamas, C.; Chaini, E.; Papanikolaou, I.C.; Gourgoulianis, K.I. Mortality Among Hospitalized Patients with Pleural Effusions. A Multicenter, Observational, Prospective Study. Front. Med. 2022, 9, 828783. [Google Scholar] [CrossRef]
- Clouse, J.W.; Mangus, R.S.; Vega, C.A.; Cabrales, A.E.; Bush, W.J.; Clouse, I.T.; Ekser, B.; Mihaylov, P.; Kubal, C.A. Pleural Effusion and Malnutrition Are Associated with Worse Early Outcomes after Liver Transplant. Am. Surg. 2023, 89, 5881–5890. [Google Scholar] [CrossRef]
- Jain, A.; Haussner, D.; Hranjec, T.; Butt, F.; Stine, J.G.; Ankola, A.; Al Yousif, H.; Dicristina, R.; Krok, K.L.; Arenas, J. Review of Sarcopenia and Testosterone Deficiency with Chronic Liver Disease and Postoperative Liver Transplant Utility of Short-Term Testosterone Replacement Therapy. Exp. Clin. Transpl. 2022, 20, 1000–1008. [Google Scholar] [CrossRef]
- Wu, M.Y.; Lim, W.X.; Cheng, Y.F.; Chang, C.D.; Hsu, H.W.; Lin, C.C.; Chen, C.L.; Chang, W.C.; Yu, C.Y.; Tsang, L.L.C.; et al. Sarcopenia Adversely Impacts Postoperative Complications in Living-Donor Liver Transplantation Recipients. Sci. Rep. 2021, 11, 19247. [Google Scholar] [CrossRef]
- Rodríguez-Torres, J.; López-López, L.; Cabrera-Martos, I.; Valenza-Demet, G.; Cahalin, L.P.; Valenza, M.C. Sarcopenia in Patients with Malignant Pleural Effusion: Impact on Symptoms, Health Status, and Response to Hospitalization. Support. Care Cancer 2019, 27, 4655–4663. [Google Scholar] [CrossRef]
- Meggyesy, A.M.; Wilshire, C.L.; Chang, S.C.; Gorden, J.A.; Gilbert, C.R. Muscle Mass Cross-Sectional Area Is Associated with Survival Outcomes in Malignant Pleural Disease Related to Lung Cancer. Respir. Med. 2023, 217, 107371. [Google Scholar] [CrossRef]
- Aro, R.; Mäkäräinen-Uhlbäck, E.; Ämmälä, N.; Rautio, T.; Ohtonen, P.; Saarnio, J.; Meriläinen, S. The Impact of Sarcopenia and Myosteatosis on Postoperative Outcomes and 5-Year Survival in Curatively Operated Colorectal Cancer Patients—A Retrospective Register Study. Eur. J. Surg. Oncol. 2020, 46, 1656–1662. [Google Scholar] [CrossRef]
- Lee, J.; Moon, S.W.; Choi, J.S.; Hyun, K.; Moon, Y.K.; Moon, M.H. Impact of Sarcopenia on Early Postoperative Complications in Early-Stage Non-Small-Cell Lung Cancer. Korean J. Thorac. Cardiovasc. Surg. 2020, 53, 93–103. [Google Scholar] [CrossRef]
- Ostovar, R.; Schröter, F.; Kühnel, R.U.; Hartrumpf, M.; Albes, J.M. What Exactly Makes Age a Risk Factor for an Unfavorable Outcome after Mitral Valve Surgery? J. Clin. Med. 2022, 11, 6907. [Google Scholar] [CrossRef]
- Kemper, M.; Molwitz, I.; Krause, L.; Reeh, M.; Burdelski, C.; Kluge, S.; Yamamura, J.; Izbicki, J.R.; de Heer, G. Are Muscle Parameters Obtained by Computed Tomography Associated with Outcome after Esophagectomy for Cancer? Clin. Nutr. 2021, 40, 3729–3740. [Google Scholar] [CrossRef]
| Author/Ref. | Study Design | Study Population | Main Findings |
|---|---|---|---|
| Clouse et al. [51] | Retrospective study | A total of 512 LTs were performed. | A total of 21% of LT patients developed PE while PE was related to poorer outcomes across all clinical parameters. PE patients had a longer hospital stay (17 vs. 9 days, p < 0.001) and were more likely to be sent to a care facility (48% vs. 21%, p < 0.001). A total of 69% of effusion patients required a 90-day readmission, compared to 44% (p < 0.001). Patients with any effusion had a one-year survival rate of 86% (vs. 94%, p < 0.01). |
| Jain et al. [52] | Retrospective study | A total of 16 liver transplant recipients receiving posttransplant testosterone replacement therapy with functional sarcopenia were included. | Pleural effusions, as part of the overall body composition assessment, can influence the evaluation of sarcopenia in patients undergoing liver transplantation. |
| Wu et al. [53] | Retrospective study | A total of 271 LDLT recipients were included. | Postoperative massive PE requiring pigtail drainage performed more frequently in the sarcopenia group than in the non-sarcopenia group (p = 0.003). The 1-, 3-, 5- and 10-year overall survival rates in females were importantly poorer in the sarcopenia group (n = 14) compared with the non-sarcopenia group (n = 108), at 92.9% versus 97.2%, 85.7% versus 95.4%, 85.7% versus 92.5%, and 70.1 versus 82.0%, respectively (p = 0.041), and rates were 94.6%, 89.9%, 85.9%, and 78.5% in male patients. Sarcopenia is related to a significantly increased hazard of major postoperative complications in females. |
| Rodriquez–Torres et al. [54] | Observational prospective cohort | A total of 74 pts with MPE underwent measurements of symptoms, health-related quality of life, and functional status upon admission, discharge, and 3 months after hospital discharge. | Health-related quality of life and functional status were worse in subjects with MPE and sarcopenia, subjects with MPE and sarcopenia were symptomatic during hospitalization and at discharge. The distribution of pleural fluid location was comparable between both groups (93.75% vs. 80.95%). The duration of hospitalization was longer in the group with sarcopenia (14.69 vs. 10.84 days). Therefore, sarcopenia is a clinical feature with substantial negative effects in patients with MPE. |
| Meggyesy et al. [55] | Cross-sectional study | A total of 309 patients with MPE were available for analysis. | The presence of decreased muscle mass within a lung cancer population that has malignant pleural effusions was related to decreased survival. Multivariable analysis stratified by gender (female: HR = 0.81, 95% C.I. (0.57–1.16), p = 0.249; male: HR = 0.67, 95% C.I. (0.42–1.08), p = 0.101) suggests that higher muscle area, particularly in males, may have a protective effect on overall survival. Nevertheless, the presence of decreased muscle mass within a heterogenous population of malignant pleural disease was not related to decreased overall survival time. |
| Aro et al. [56] | Retrospective study | A total of 348 colorectal cancer patients were included. | A total of 208 patients had sarcopenia while 108 had myosteatosis. Sarcopenia was related to increased hazard of postoperative pneumonia (6.7% vs. 1.4%, p = 0.021). Sarcopenic colon cancer subjects had an increased rate of cardiorespiratory complications compared to non-sarcopenic (6.3% vs. 0.0%, p = 0.023), and sarcopenic rectum cancer subjects developed pneumonia more often than non-sarcopenic patients (8.5% vs. 0.0%, p = 0.041). Sarcopenia increases the pneumonia and cardiorespiratory complication rates including PE. |
| Lee et al. [57] | Retrospective study | A total of 236 patients with pathologic stage I/II NSCLC who underwent curative pulmonary resection were eligible and included. | Sarcopenia, as represented by a low PVI in this study, was demonstrated as a negative prognostic factor for overall early postoperative complications. Respiratory complications included prolonged air leak (16.9% in the low-PVI group vs. 9.6% in the normal-to-high-PVI group (p = 0.125)) and recurrent PE (11.9% in the low-PVI group vs. 6.8% in the normal-to-high-PVI group (p = 0.267)). Results confirmed an important correlation between sarcopenia and impaired pulmonary function recurrent pleural effusion was also more frequently observed in the low-PVI group. |
| Ostovar et al. [58] | Cohort study | The study enrolled 1627 patients who underwent mitral valve surgery. Patients younger than 40 years who had been diagnosed with endocarditis were excluded. | It appears that elderly subjects with advanced renal failure have a significantly increased risk of mortality, postoperative renal failure, need for dialysis, and possibly the development of pleural and pericardial effusions in mitral valve surgery. Furthermore, the proportion of pleural effusions and pericardial effusions was importantly higher with aging (p < 0.001 and p = 0.016, respectively). |
| Kemper et al. [59] | Observational study | A total of 98 patients undergoing esophagectomy. | No relevant association to lengths of stay in intensive care or hospital was recorded. If the SMI increased, the odds for pleural effusion and pleural empyema decreased, but the odds of a pulmonary embolism increased. Univariate, unadjusted long-term survival analysis demonstrated that decreased MRA and lower SMI were associated with shorter survival (p = 0.03). However, if the analysis was adjusted for confounders, e.g., Charlson Comorbidity Index, no relevant association regarding long-term survival was detected. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barkas, G.I.; Karakousis, N.D.; Daniil, Z.; Gourgoulianis, K.I.; Kotsiou, O.S. Sarcopenia and Pleural Effusions: Exploring a Potential Link. Muscles 2024, 3, 189-201. https://doi.org/10.3390/muscles3030017
Barkas GI, Karakousis ND, Daniil Z, Gourgoulianis KI, Kotsiou OS. Sarcopenia and Pleural Effusions: Exploring a Potential Link. Muscles. 2024; 3(3):189-201. https://doi.org/10.3390/muscles3030017
Chicago/Turabian StyleBarkas, Georgios I., Nikolaos D. Karakousis, Zoe Daniil, Konstantinos I. Gourgoulianis, and Ourania S. Kotsiou. 2024. "Sarcopenia and Pleural Effusions: Exploring a Potential Link" Muscles 3, no. 3: 189-201. https://doi.org/10.3390/muscles3030017
APA StyleBarkas, G. I., Karakousis, N. D., Daniil, Z., Gourgoulianis, K. I., & Kotsiou, O. S. (2024). Sarcopenia and Pleural Effusions: Exploring a Potential Link. Muscles, 3(3), 189-201. https://doi.org/10.3390/muscles3030017










