Efficacy of Electromyographic Biofeedback in the Recovery of the Vastus Lateralis after Knee Injury: A Single-Group Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Measurements and Instruments
2.4. Procedure
2.5. Data Analysis
3. Results
3.1. Variance Component Analysis
3.2. Generalizability Analysis
3.3. Trials with BF vs. Trials without BF and Trials before BF vs. Trials after BF
3.4. Session One vs. Session Ten
4. Discussion
4.1. Practical Applications
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abril-Rodríguez, S.; Herrero, R. Biofeedback electromiográfico y electroglotográfico aplicado a la terapia vocal: Una revisión sistemática. Rev. Investig. Logopedia 2022, 12, e75581. [Google Scholar] [CrossRef]
- Duarte-Moreira, R.J.; Castro, K.V.F.; Luz-Santos, C.; Martins, J.V.P.; Sá, K.N.; Baptista, A.F. Electromyographic biofeedback in motor function recovery after peripheral nerve injury: An integrative review of the literature. Appl. Psychophys. Biof. 2018, 43, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Giggins, O.M.; Persson, U.; Caulfield, B. Biofeedback in rehabilitation. J. Neuroeng. Rehabil. 2013, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, P.; Kaur, K.; Sharma, A.; Shah, K.; Huseby, R.; Bhavsar, J.; Sgobba, P.; Zhang, Y. Heart rate variability biofeedback improves emotional and physical health and performance: A systematic review and meta analysis. Appl. Psychophys. Biof. 2020, 45, 109–129. [Google Scholar] [CrossRef]
- Chowdhury, R.H.; Reaz, M.B.I.; Ali, M.A.M. Surface Electromyography Signal Processing and Classification Techniques. Sensors 2013, 13, 12431–12466. [Google Scholar] [CrossRef]
- De Luca, C.J. Electromyography. In Encyclopaedia of Medical Devices and Instrumentation; Webster, J.G., Ed.; Wiley: New York, NY, USA, 1988; pp. 1111–1120. [Google Scholar]
- Phinyomark, A.; Campbell, E.; Scheme, E. Surface Electromyography (EMG) Signal Processing, Classification, and Practical Considerations. In Biomedical Signal Processing: Advances in Theory, Algorithms and Applications; Naik, G., Ed.; Springer: Singapore, 2020; pp. 3–29. [Google Scholar] [CrossRef]
- Jaramillo-Yánez, A.; Benalcázar, M.E.; Mena-Maldonado, E. Real-Time Hand Gesture Recognition Using Surface Electromyography and Machine Learning: A Systematic Literature Review. Sensors 2020, 20, 2467. [Google Scholar] [CrossRef]
- McGill, K. Surface electromyogram signal modelling. Med. Biol. Eng. Comput. 2004, 42, 446–454. [Google Scholar] [CrossRef]
- Sklempe-Kokic, I.; Vuksanic, M.; Kokic, T.; Peric, I.; Duvnjak, I. Effects of Electromyographic Biofeedback on Functional Recovery of Patients Two Months after Total Knee Arthroplasty: A Randomized Controlled Trial. J. Clin. Med. 2022, 11, 3182. [Google Scholar] [CrossRef]
- Jing, G. Clinical effect evaluation and experience of motomed virtual scene training combined with electromyographic biofeedback therapy in the treatment of spastic cerebral palsy. Ann. Phys. Rehab. Med. 2018, 61, e322. [Google Scholar] [CrossRef]
- Kamonseki, D.H.; Calixtre, L.B.; Barreto, R.P.G.; Camargo, P.R. Effects of electromyographic biofeedback interventions for shoulder pain and function: Systematic review and meta-analysis. Clin. Rehab. 2021, 35, 952–963. [Google Scholar] [CrossRef]
- Raeissadat, S.A.; Rayegani, S.M.; Sedighipour, L.; Bossaghzade, Z.; Abdollahzadeh, M.H.; Nikray, R.; Mollayi, F. The efficacy of electromyographic biofeedback on pain, function, and maximal thickness of vastus medialis oblique muscle in patients with knee osteoarthritis: A randomized clinical trial. J. Pain Res. 2018, 11, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Liang, M.; Shi, S.; Liu, Y.; Xiong, R. Rehabilitation programme including EMG-biofeedback-assisted pelvic floor muscle training for rectus diastasis after childbirth: A randomised controlled trial. Physiotherapy 2022, 117, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Florjanski, W.; Malysa, A.; Orzeszek, S.; Smardz, J.; Olchowy, A.; Paradowska-Stolarz, A.; Wieckiewicz, M. Evaluation of biofeedback usefulness in masticatory muscle activity management—A systematic review. J. Clin. Med. 2019, 8, 766. [Google Scholar] [CrossRef] [PubMed]
- Neblett, R. Surface Electromyographic (SEMG) Biofeedback for Chronic Low Back Pain. Healthcare 2016, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Mendo, A. Biofeedback electromiográfico en la rehabilitación de lesiones de rodilla. Estudio de dos casos en futbolistas profesionales. Cuad. Psicol. Deporte 2011, 11, 71–80. [Google Scholar]
- Hernández-Mendo, A.; Morales-Sánchez, V. Efectividad del biofeedback electromiográfico en la rehabilitación de lesiones deportivas. Rev. Psicol. Deporte 2014, 23, 489–500. [Google Scholar]
- Draper, V.; Ballard, L. Electrical stimulation versus electromyographic biofeedback in the recovery of quadriceps femoris muscle function following anterior cruciate ligament surgery. Phys. Ther. 1991, 71, 455–461. [Google Scholar] [CrossRef]
- Karaborklu-Argut, S.; Celik, D.; Yasacı, Z. Effectiveness of therapeutic electromyographic biofeedback after orthopedic knee surgeries: A systematic review. Disabil. Rehabil. 2022, 44, 3364–3372. [Google Scholar] [CrossRef]
- Christanell, F.; Hoser, C.; Huber, R.; Fink, C.; Luomajoki, H. The influence of electromyographic biofeedback therapy on knee extension following anterior cruciate ligament reconstruction: A randomized controlled trial. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2012, 4, 41. [Google Scholar] [CrossRef]
- Xie, Y.J.; Wang, S.; Gong, Q.J.; Wang, J.X.; Sun, F.H.; Miyamoto, A.; Ou, X.; Wang, S.Q.; Zhang, C. Effects of electromyography biofeedback for patients after knee surgery: A systematic review and meta-analysis. J. Biomech. 2021, 120, 110386. [Google Scholar] [CrossRef]
- López-Valenciano, A.; Ruiz-Pérez, I.; Garcia-Gómez, A.; Vera-Garcia, F.J.; Croix, M.D.S.; Myer, G.D.; Ayala, F. Epidemiology of injuries in professional football: A systematic review and meta-analysis. Brit. J. Sport Med. 2020, 54, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gómez, J.; Adsuar, J.C.; Alcaraz, P.E.; Carlos-Vivas, J. Physical exercises for preventing injuries among adult male footballsoccer players: A systematic review. J. Sport Health Sci. 2020, 20, 30152–30156. [Google Scholar] [CrossRef]
- Morgan, J.P.M.; Hamm, M.; Schmitz, C.; Brem, M.H. Return to play after treating acute muscle injuries in elite football players with radial extracorporeal shock wave therapy. J. Orthop. Surg. Res. 2021, 16, 708. [Google Scholar] [CrossRef] [PubMed]
- Larruskain, J.; Lekue, J.A.; Martin-Garetxana, I.; Barrio, I.; McCall, A.; Gil, S.M. Injuries Are Negatively Associated with Player Progression in an Elite Football Academy. Sci. Med. Footb. 2022, 6, 405–414. [Google Scholar] [CrossRef]
- Jones, A.; Jones, G.; Greig, N.; Bower, P.; Brown, J.; Hind, K.; Francis, P. Epidemiology of injury in English Professional Football players: A cohort study. Phys. Ther. Sport 2019, 35, 18–22. [Google Scholar] [CrossRef]
- Read, P.J.; Oliver, J.L.; De Ste Croix, M.; Myer, G.D.; Lloyd, R.S. Neuromuscular risk factors for knee and ankle ligament injuries in male youth soccer players. Sports Med. 2016, 46, 1059–1066. [Google Scholar] [CrossRef]
- Bollen, S. Epidemiology of knee injuries: Diagnosis and triage. Br. J. Sports Med. 2000, 34, 227–228. [Google Scholar] [CrossRef]
- Arundale, A.J.; Silvers-Granelli, H.J.; Marmon, A.; Zarzycki, R.; Dix, C.; Snyder-Mackler, L. Changes in biomechanical knee injury risk factors across two collegiate soccer seasons using the 11+ prevention program. Scand. J. Med. Sci. Sport 2018, 28, 2592–2603. [Google Scholar] [CrossRef]
- Nishida, Y.; Nishino, T.; Tanaka, K.; Onishi, S.; Kanamori, A.; Yamazaki, M. An Objective Measure of Patellar Tendon Thickness Based on Ultrasonography and MRI in University Athletes. J. Clin. Med. 2021, 10, 4092. [Google Scholar] [CrossRef]
- Adams, B.G.; Houston, M.N.; Cameron, K.L. The epidemiology of meniscus injury. Sports Med. Arthrosc. 2021, 29, e24–e33. [Google Scholar] [CrossRef]
- Warden, S.J.; Kiss, Z.S.; Malara, F.A.; Ooi, A.B.T.; Cook, J.L.; Crossley, K. Comparative Accuracy of Magnetic Resonance Imaging and Ultrasonography in Confirming Clinically Diagnosed Patellar Tendinopathy. Am. J. Sports Med. 2007, 35, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Steinbacher, G.; Alentorn-Geli, E.; Alvarado-Calderón, M.; Barastegui, D.; Álvarez-Díaz, P.; Cugat, R. Meniscal fixation is a successful treatment for hypermobile lateral meniscus in soccer players. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Drobnič, M.; Ercin, E.; Gamelas, J.; Papacostas, E.T.; Slynarski, K.; Zdanowicz, U.; Spalding, T.; Verdonk, P. Treatment options for the symptomatic post-meniscectomy knee. Knee. Surg. Sports Traumatol. Arthrosc. 2019, 27, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Diaz, P.; Alentorn-Geli, E.; Llobet, F.; Granados, N.; Steinbacher, G.; Cugat, R. Return to play after all-inside meniscal repair in competitive football players: A minimum 5-year follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1997–2001. [Google Scholar] [CrossRef]
- Vaquero, J.; Forriol, F. Meniscus tear surgery and meniscus replacement. Muscles Ligaments Tendons J. 2016, 19, 71–89. [Google Scholar] [CrossRef]
- Jacob, G.; Shimomura, K.; Krych, A.J.; Nakamura, N. The Meniscus Tear: A Review of Stem Cell Therapies. Cells 2020, 9, 92. [Google Scholar] [CrossRef]
- Marcacci, M.; Marcheggiani-Muccioli, G.M.; Grassi, A.; Ricci, M.; Tsapralis, K.; Nanni, G.; Bonanzinga, T.; Zaffagnini, S. Arthroscopic meniscus allograft transplantation in male professional soccer players: A 36-month follow-up study. Am. J. Sport Med. 2014, 42, 382–388. [Google Scholar] [CrossRef]
- Jeong, H.J.; Lee, S.H.; Ko, C.S. Meniscectomy. Knee Surg. Rel. Res. 2012, 24, 129–136. [Google Scholar] [CrossRef]
- Lee, D.H.; D’Lima, D.D.; Lee, S.H. Clinical and radiographic results of partial versus total meniscectomy in patients with symptomatic discoid lateral meniscus: A systematic review and meta-analysis. Orthop. Traumatol. Sur. 2019, 105, 669–675. [Google Scholar] [CrossRef]
- Niering, M.; Muehlbauer, T. Effects of Physical Training on Physical and Psychological Parameters in Individuals with Patella Tendinopathy: A Systematic Review and Meta-Analysis. Sports 2021, 9, 12. [Google Scholar] [CrossRef]
- Lavoie-Gagne, O.Z.; Korrapati, A.; Retzky, J.; Bernstein, D.N.; Diaz, C.C.; Berlinberg, E.J.; Forlenza, E.M.; Fury, M.S.; Mehta, N.; O’Donnell, E.A.; et al. Return to Play and Player Performance After Meniscal Tear Among Elite-Level European Soccer Players: A Matched Cohort Analysis of Injuries From 2006 to 2016. Orthop. J. Sport Med. 2022, 10, 23259671211059541. [Google Scholar] [CrossRef]
- Núñez-Sánchez, F.J.; Cabrera, F.I.M.; Abad, F.H.; Suárez-Arrones, L. Progressive Rehabilitation of a Professional Soccer Player After an Anterior Cruciate Ligament Reconstruction in Phase 1: Clinical Perspective with Video Demonstration. J. Athl. Train. 2021, 56, 1132–1136. [Google Scholar] [CrossRef]
- Chambless, D.L.; Hollon, S.D. Defining empirically supported therapies. J. Consult. Clin. Psych. 1998, 66, 7–18. [Google Scholar] [CrossRef]
- Jarvela, T.; Kannus, P.; Latvala, K.; Jarvinen, M. Simple measurements in assessing muscle performance after an ACL reconstruction. Int. J. Sports Med. 2002, 23, 196–201. [Google Scholar] [CrossRef]
- Criado, L.; de La Fuente, A.; Heredia, M.; Montero, J.; Albaladejo, A.; Criado, J.-M. Electromyographic biofeedback training for reducing muscle pain and tension on masseter and temporal muscles: A pilot study. J. Clin. Exp. Dent. 2016, 8, e571–e576. [Google Scholar] [CrossRef]
- Simón, M.A.; Bueno, A.M. Psychophysiological Profile in Dyssynergic Defecation Patients: An Individual and Situational Response Specificity Analysis. Appl. Psychophysiol. Biofeedback 2009, 34, 93–97. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Med. Assoc. 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Solé, V.; Moliner, L. Metodología para el estudio de la fatiga y la contracción muscular. Rehabilitación 1988, 22, 37–50. [Google Scholar]
- Byrne, B. Structural Equation Modeling with AMOS. Basic Concepts, Applications, and Programming, 3rd ed.; Routledge: Oxfordshire, UK, 2016. [Google Scholar]
- Hojat, M.; Xu, G. A visitor’s guide to effect sizes: Statistical significance versus practical (clinical) importance of research findings. Adv. Health Sci. Educ. Theory Pract. 2004, 9, 241–249. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute. User’s Guide, 8th ed.; SAS/STAT SAS Institute: Cary, NC, USA, 1999. [Google Scholar]
- Schlotzhauer, S.D.; Littell, R. SAS System for Elementary Statistical Analysis; SAS Institute Inc.: Cary, NC, USA, 1997. [Google Scholar]
- Hernández-Mendo, A.; Blanco-Villaseñor, A.; Pastrana, J.L.; Morales-Sánchez, V.; Ramos-Pérez, F.J. SAGT: New software for generalizability analysis. Rev. Iberoam. Psicol. Ejerc. Deporte 2016, 11, 77–89. [Google Scholar]
- Hemmerle, W.; Hartley, H. Computing maximum likelihood estimates for the mixed AOV Model using the w-transformation. Technometrics 1973, 15, 819–831. [Google Scholar] [CrossRef]
- Searle, S.; Casella, G.; McCulloch, C. Variance Components; John Wiley & Sons: New York, NY, USA, 1992. [Google Scholar] [CrossRef]
- Blanco-Villaseñor, Á.; Castellano, J.; Hernández Mendo, A.; Sánchez-López, C.R.; Usabiaga, O. Aplicación de la TG en el deporte para el estudio de la fiabilidad, validez y estimación de la muestra. Rev. Psicol. Deporte 2014, 2, 131–137. [Google Scholar] [CrossRef]
- Morales-Sánchez, V.; Falcó, C.; Hernández-Mendo, A.; Reigal, R.E. Efficacy of Electromyographic Biofeedback in Muscle Recovery after Meniscectomy in Soccer Players. Sensors 2022, 22, 4024. [Google Scholar] [CrossRef] [PubMed]
| Sources of Variation | Sum of Squares | Degree of Freedom | Mean Square | % |
|---|---|---|---|---|
| [p] | 8,394,421.45 | 3 | 2,798,140.48 | 69.53 |
| [s] | 955,988.74 | 9 | 106,220.97 | 3.82 |
| [p][s] | 210,894.61 | 27 | 7810.91 | 0 |
| [tt] | 1,191,352.64 | 1 | 1,191,352.64 | 14.15 |
| [p][tt] | 117,122.04 | 3 | 39,040.68 | 1.53 |
| [s][tt] | 330,312.53 | 9 | 36,701.39 | 3.53 |
| [p][s][tt] | 233,506.63 | 27 | 8648.39 | 4.35 |
| [tn] | 64,863.69 | 2 | 32,431.85 | 0.43 |
| [p][tn] | 1048.08 | 6 | 174.68 | 0 |
| [s][tn] | 192,699.31 | 18 | 10,705.52 | 1.82 |
| [p][s][tn] | 58,809.88 | 54 | 1089.07 | 0.82 |
| [tt][tn] | 0 | 2 | 0 | 0 |
| [p][tt][tn] | 0 | 6 | 0 | 0 |
| [s][tt][tn] | 0 | 18 | 0 | 0 |
| [p][s][tt][tn] | 0 | 54 | 0 | 0 |
| Face | Levels | Size Universe | Description | % Variance | Model Generalizability | G Relative | G Absolute |
|---|---|---|---|---|---|---|---|
| [p] | 4 | INF | participants | 69.53 | [s] [tt] [tn]/[p] | 0.934 | 0.555 |
| [s] | 10 | INF | session | 3.82 | [p] [tt] [tn]/[s] | 0.988 | 0.984 |
| [tt] | 2 | INF | type of test | 14.15 | [p] [s] [tn]/[tt] | 0.942 | 0.866 |
| [tn] | 3 | INF | test number | 0.42 | [p] [s] [tt]/[tn] | 0.991 | 0.990 |
| Electromyographic Activity (µV) | ||||||
|---|---|---|---|---|---|---|
| Values | M | SD | S | K | S-W | |
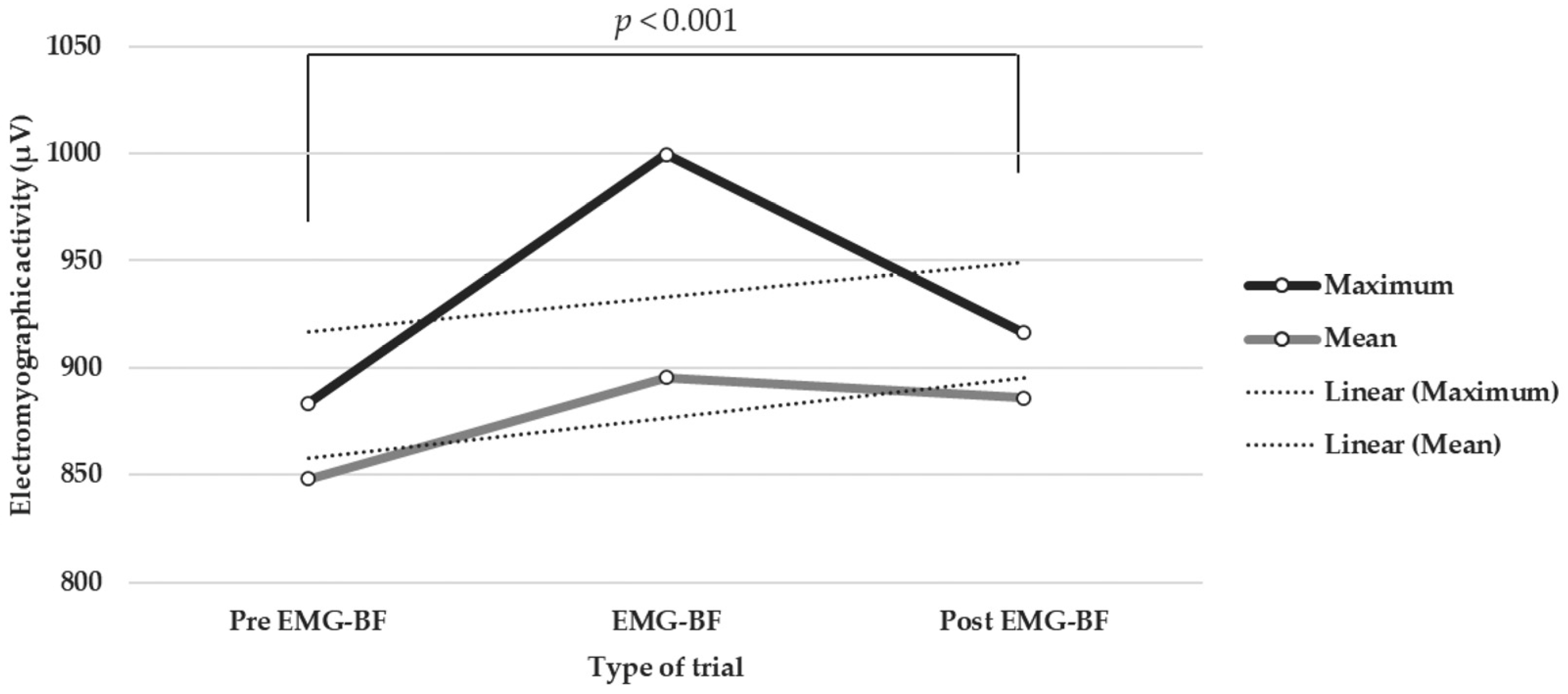
| Pre-EMG-BF trials | Maximum | 883.54 | 135.57 | −1.03 | 0.03 | 0.29 *** |
| Mean | 848.34 | 135.22 | −0.92 | −0.18 | 0.25 *** | |
| Post-EMG-BF trials | Maximum | 916.41 | 123.06 | −1.23 | −0.25 | 0.14 *** |
| Mean | 885.83 | 120.01 | −1.21 | −0.01 | 0.26 *** | |
| EMG-BF trials | Maximum | 999.61 | 176.97 | −0.13 | −0.10 | 0.36 *** |
| Mean | 895.39 | 110.10 | −1.18 | 0.174 | 0.30 *** | |
| Without EMG-BF trials (pre and post) | Maximum | 899.98 | 130.23 | −1.12 | −0.04 | 0.33 *** |
| Mean | 867.08 | 128.95 | −1.06 | −0.07 | 0.28 *** | |
| Electromyographic Activity (µV) | |||||||
|---|---|---|---|---|---|---|---|
| Session | Values | M | SD | S | K | S-W | |
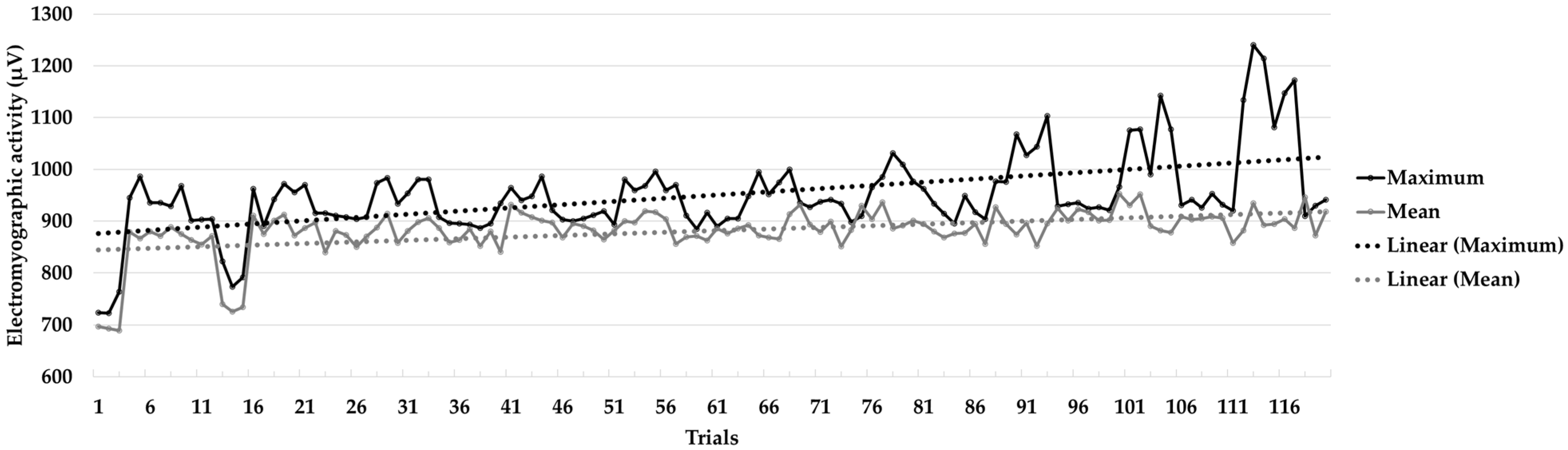
| Without EMG-BF trials | 1 | Maximum | 819.90 | 91.94 | −0.10 | −3.08 | 0.76 * |
| Mean | 778.34 | 93.38 | 00.01 | −3.29 | 0.73 * | ||
| 10 | Maximum | 930.82 | 15.28 | 0.11 | −0.29 | 0.99 | |
| Mean | 901.60 | 32.04 | −0.12 | −0.64 | 0.96 | ||
| EMG-BF trials | 1 | Maximum | 950.08 | 22.58 | 1.02 | −0.48 | 0.86 |
| Mean | 876.66 | 7.71 | 0.26 | 0.17 | 0.99 | ||
| 10 | Maximum | 1165.05 | 57.54 | −0.10 | −0.59 | 0.98 | |
| Mean | 898.91 | 18.79 | 1.68 | 3.10 | 0.84 | ||
| All trials (mean) | 1 | Maximum | 884.99 | 93.25 | −1.07 | −0.37 | 0.80 * |
| Mean | 827.50 | 81.40 | −1.29 | −0.36 | 0.65 *** | ||
| 10 | Maximum | 1047.94 | 128.74 | 0.27 | −1.87 | 0.83 * | |
| Mean | 900.26 | 25.08 | 0.26 | −0.09 | 0.98 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Sánchez, V.; Reigal, R.E.; Antunes, R.; Matos, R.; Hernández-Mendo, A.; Monteiro, D. Efficacy of Electromyographic Biofeedback in the Recovery of the Vastus Lateralis after Knee Injury: A Single-Group Case Study. Muscles 2023, 2, 361-373. https://doi.org/10.3390/muscles2040028
Morales-Sánchez V, Reigal RE, Antunes R, Matos R, Hernández-Mendo A, Monteiro D. Efficacy of Electromyographic Biofeedback in the Recovery of the Vastus Lateralis after Knee Injury: A Single-Group Case Study. Muscles. 2023; 2(4):361-373. https://doi.org/10.3390/muscles2040028
Chicago/Turabian StyleMorales-Sánchez, Verónica, Rafael E. Reigal, Raul Antunes, Rui Matos, Antonio Hernández-Mendo, and Diogo Monteiro. 2023. "Efficacy of Electromyographic Biofeedback in the Recovery of the Vastus Lateralis after Knee Injury: A Single-Group Case Study" Muscles 2, no. 4: 361-373. https://doi.org/10.3390/muscles2040028
APA StyleMorales-Sánchez, V., Reigal, R. E., Antunes, R., Matos, R., Hernández-Mendo, A., & Monteiro, D. (2023). Efficacy of Electromyographic Biofeedback in the Recovery of the Vastus Lateralis after Knee Injury: A Single-Group Case Study. Muscles, 2(4), 361-373. https://doi.org/10.3390/muscles2040028







