Abstract
Creatine monohydrate supplementation in females is largely under-represented in the literature, and their potentially differential hemodynamic responses are unknown. Methods: Twenty-eight resistance-trained women (25.5 ± 6.1 years, 59.7 ± 6.3 kg, 163 ± 5 cm) were randomly assigned to the supplement creatine monohydrate (CRE; 5 g creatine monohydrate + 5 g dextrose) or placebo (PLA; 10 g dextrose) four times per day for 7 days in a double-blind fashion. Each subject subsequently completed resistance training sessions (3 × week) for four weeks with four sets to muscular failure of both half-squat and leg press exercises. The change in body mass (BM), exercise repetition number (REP), rated perceived exertion (RPE), and cardiovascular variables were assessed (sessions 1, 6, and 12). Statistical analyses were performed at a significance level of p ≤ 0.05. Results: Analyses revealed a significant CRE-specific BM increase (p = 0.013), as well as significantly greater half-squat (p = 0.006) and leg press (p = 0.017) REP per set versus PLA. Additionally, CRE demonstrated significantly lower relative RPE values at session 12 compared with previous sessions. Any significant main or interaction effects were observed for the studied cardiovascular variable. Conclusions: The present data substantiate the creatine’s efficacy to improve muscular performance in females while demonstrating the safety of combined creatine monohydrate supplementation and resistance training on cardiovascular parameters.
1. Introduction
Creatine monohydrate supplementation has been exhaustively explored for its wide-ranging impacts, largely mediated through augmented phosphocreatine content [1,2]. Specifically, the latter both spatially and temporally buffers intracellular adenosine triphosphate (ATP) concentrations from the addition of a high-energy phosphate to adenosine diphosphate (ADP) via the creatine kinase enzyme [3]. Notwithstanding the multiplicity of meta-analyses and position stands touting its ergogenic capabilities in athletic populations and especially those subjected to anaerobic or repeated bouts of high-intensity exercise, creatine supplementation has been further demonstrated to pleiotropically enhance additional parameters [1,2,4]. Amazingly, varying doses of creatine monohydrate amidst equally diverse populations have displayed multiple improved cognitive indices, protected against birth-induced fetal hypoxia and oxidative stress, as well as generally ameliorated metabolic impairments such as oxidative stress, dyslipidemia, and inflammation [1,5,6,7,8,9].
The impacts of creatine supplementation on human cardiovascular outcomes are particularly sparse [10]. Similar to other cell types, creatine enters into cardiomyocytes via the membrane-bound sodium-dependent transporter (SLC6A8) and is ostensibly vital to their function due to the presence of expressed alanine-glycine amino transferase (AGAT; synthesizes guanidinoacetate as a creatine precursor) and that attenuated creatine concentrations in these tissues are inexorably linked to complications such as congestive heart failure [10]. It was previously—and erroneously—believed that creatine’s osmolytic properties potentially had a deleterious effect on blood pressure via total body water elevation-mediated increases in plasma volume; however, more recent data otherwise contend that supplementation does not alter the intracellular-to-extracellular water ratio nor detrimentally affect cardiac function [10,11,12]. Moreover, there is increasing evidence that supplementation of this widely beneficial compound may maintain sustain vascular tone, as well as attenuate exercise-induced increases in systolic blood pressure, heart rate, left ventricular afterload, and lactate production [10]. While promising in several animal models and males recovering from heart failure, these creatine supplementation-driven cardiovascular benefits remain hitherto uninvestigated in many healthy human demographics [10,13].
Although creatine supplementation is extensively reported to improve relative body mass (largely through skeletal muscle accretion) and performance parameters similarly in both males and females, the overwhelming majority has focused on the former [4]. Furthermore, prior authors have also posited that females may glean reduced strength gains and/or muscle mass during combined resistance training and supplementation [14,15,16]. The reasons for this largely remain unclear due to conflicting results concerning any sex-specific impacts, although many of these discrepancies may correspond with a general paucity in female-focused interventions [4]. Females have nevertheless been previously characterized as less fatigable, ostensibly due to an increased slow-twitch/type I fiber predisposition, higher capillarization, and several other physiologically aerobic-oriented attributes [17,18,19,20]. Incidentally, type I muscle fibers display relatively lower total creatine content compared with their fast-twitch counterparts, suggesting a type-II-fiber-dominant male advantage with regards to muscle creatine content. Despite these supposed sex differences, the impacts of creatine supplementation on females during their reproductive lives are not only heavily under-investigated, but wholly non-existent with respect towards its effect on the clearly aerobic-related cardiovascular parameters [21,22]. Therefore, the purpose of this investigation was to test the hypothesis that young, healthy females would display increased body mass and enhanced performance, commensurate to favorable cardiovascular parameters, relative to placebo-matched controls across a four-week training timeline.
2. Results
No significant differences were found between groups in initial subject characteristics (see Table 1).

Table 1.
Subject baseline characteristics.
As the body mass variables violated homoscedasticity and sphericity, we performed nonparametric ANCOVA (Quade’s test; baseline body mass as the covariate) to verify the difference between moments and treatments. Analyses demonstrated no significant differences between groups (F = 0.768; p = 0.389) or treatments (F = 0.538; p = 0.470). However, body mass variation (delta = post − pre) showed greater differences between groups (p = 0.01; Cohen’s d = −1.01) (see Figure 1).
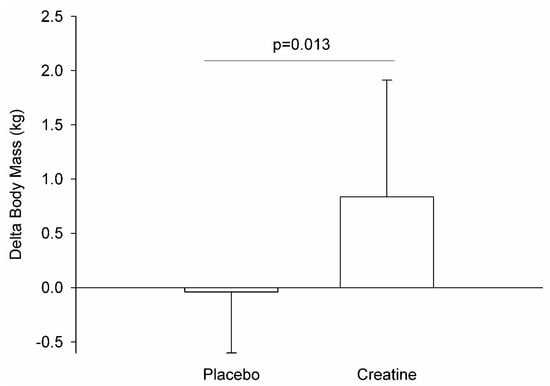
Figure 1.
Delta (post−pre) from body mass variation during experiment.
Analyses revealed significant medium-strong main session effects for both half-squat (F = 63.675; p < 0.001; η2 = 0.391) and leg press (F = 47.329; p < 0.001; η2 = 0.366) repetitions. In either exercise modality, pairwise comparisons specifically demonstrated both that the number of repetitions in session 1 was significantly (p > 0.001 in all cases) lower than in session 6 or 12, and that the number of repetitions was also lower in session 6 relative to session 12 (p > 0.01 in all cases). There was a weak significant supplement main effect (F = 8.760; p = 0.006; η2 = 0.085) for half-squat repetitions (see Figure 2A,B), whereby creatine supplementation significantly increased the volume per set. A similar weak finding was observed in the leg press (F = 6.540; p = 0.017; η2 = 0.074), whereby creatine-supplemented participants performed significantly greater repetitions per set versus PLA (see Figure 2C,D). Although analyses failed to reveal any significant main effects for sets, there were significant weak session x group interactions for both half-squat (F = 6.191; p = 0.004; η2 = 0.038) and leg press (F = 3.922; p = 0.026; η2 = 0.03) repetition number. Pairwise comparisons demonstrated that the CRE group performed significantly more repetitions within-group for both the half-squat and leg press exercises from session 1 to 6 (half-squat, p < 0.001; leg press, p = 0.001) and from session 6 to 12 (both p < 0.001), whereas PLA-specific repetition volume was only significantly increased from session 6 to 12 (half-squat, p < 0.001; leg press, p = 0.02). Conversely, the repetition number per set was not enhanced when comparing supplement groups within the same set (p > 0.05 in all cases). All other interaction effects failed to reach statistical significance.
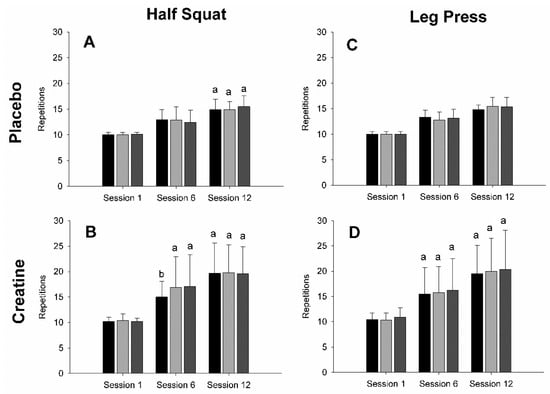
Figure 2.
Number of repetitions per set for the half squat placebo (A) and creatine (B) groups, as well as for the leg press placebo (C) and creatine (D) groups. Black, light gray, and dark gray bar colors indicate the first, second, and third sets, respectively. (a) Indicates a significant difference in set repetition number in session 1 (p < 0.001); (b) indicates a significant difference in set repetition number amidst the same session (p < 0.05).
All RPE data are displayed below in Figure 3. There were no significant supplement effects for the half-squat (F = 1.98; p = 0.17; η2 = 0.038) nor leg press (F = 2.480; p = 0.13; η2 = 0.043) with regards to RPE (see Figure 3). Conversely, analyses revealed significant weak session effects for both half-squat (F = 6.09; p = 0.004; η2 = 0.067) and leg press exercises (F = 7.730; p = 0.001; η2 = 0.095), whereby session 12 demonstrated a lower subjective exertion relative to session 6 (both p < 0.001). Main effects for set were similarly significant for the half-squat (F = 16.25; p < 0.001; η2 = 0.01) and leg press (F = 4.711; p = 0.013; η2 = 0.004) exercises. While Bonferroni adjustments rendered pairwise comparisons for the latter non-significant (p > 0.05), the half-squat-specific RPE was significantly greater in sets 2 (p = 0.018) and 3 (p < 0.001) relative to the first set, along with set 3 displaying greater subjective exertion than set 2 (p = 0.017). Additionally, analyses revealed weak significant session x group interaction effects for both the half-squat (F = 3.866; p < 0.027; η2 = 0.043) and leg press exercises (F = 3.336; p < 0.043; η2 = 0.041), whereby CRE displayed a lower subjective exertion in session 12 relative to PLA in session 6 (half-squat, p = 0.027; leg press, p = 0.01; see Figure 3B) and 12 (half-squat, p < 0.001; leg press, p = 0.002; see Figure 3D). There were also significant weak set x group (F = 4.333; p = 0.018; η2 = 0.005) and session x set interaction effects (F = 3.637; p = 0.008; η2 = 0.005) for half-squat RPE. Specifically, the third set was significantly greater than the first (p < 0.001) and second (p = 0.039) sets amongst PLA. Within the first session, the set 3 RPE was significantly greater than set 1 (p = 0.004). Furthermore, the first set in session 6 was more subjectively difficult than the second set in session 12 (p = 0.045), and the second set in session 6 demonstrated a significantly higher RPE than every set in session 12 (p > 0.02 in all cases). Analyses failed to reveal any additional significant interactions for leg press RPE.
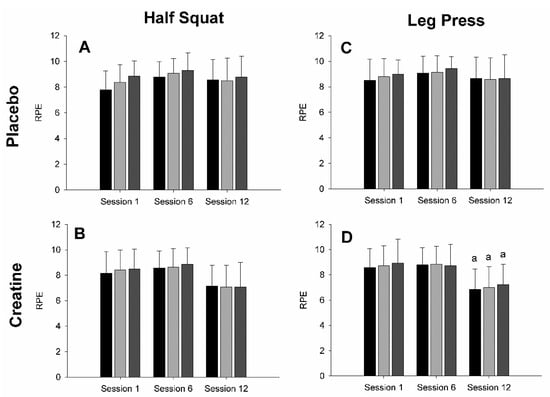
Figure 3.
Rate of perceived exertion (RPE) measured with 1–10 scale during sessions 1, 6, and 16 evaluated on number of repetitions per set for the half squat placebo (A) and creatine (B) groups, as well as for the leg press placebo (C) and creatine (D) groups. Black, light gray, and dark gray bar colors indicate the first, second, and third sets, respectively. (a) Indicates a significant difference in set repetition number in session 1 (p < 0.001).
Analyses failed to reveal any significant between-group differences in HR (F = 0.431; p = 0.52; η2 = 0.003), SBP (F = 0.226; p = 0.64; η2 = 0.002), and DBP (F = 0.0443; p = 0.84; η2 = 0.001) for the half-squat (see Table 2). Similarly, the supplement x session x set interaction effect was not significant for HR (F = 0.376; p = 0.89; η2 = 0.001), SBP (F = 0.584; p = 0.74; η2 = 0.006), or DBP in the half-squat (F = 0.719; p = 0.64; η2 = 0.006) (see Table 2).

Table 2.
Variations in heart rate (HR) and systolic (SBP) and diastolic (DBP) blood pressure during the half-squat exercise.
There were no significant between-supplement HR (F = 0.0854; p = 0.77; η2 = 0.001), SBP (F = 0.0077; p = 0.93; η2 = 0.000), or DBP (F = 0.120; p = 0.73; η2 = 0.002) differences in the leg press (see Table 3). Likewise, the supplement x session x set interaction was not significant for HR (F = 1.0691; p = 0.38; η2 = 0.002), SBP (F = 0.818; p = 0.56; η2 = 0.003), or DBP in the leg press (F = 2.206; p = 0.07; η2 = 0.014) (see Table 3).

Table 3.
Variations in heart rate (HR) and systolic (SBP) and diastolic (DBP) blood pressure during the leg press exercise.
3. Discussion
The present investigation is the first to jointly assess the efficacy of creatine monohydrate supplementation on both resistance training performance and cardiovascular parameters within a female population. Incidentally, our data demonstrate a creatine-specific augmentation in body mass that supports both our hypothesis and the prior literature in female participants [14,23]. This enhancement was commensurate with an overall greater creatine-specific increase in half-squat and leg press repetition number, ostensibly via realizing a greater volume-load earlier amidst the 12-week training timeframe. While it is reasonable to assume that the significantly—albeit minimally—increased repetitions achieved by CRE may explain the aforementioned greater relative body mass largely through skeletal muscle mass accretion, these results should be interpreted cautiously; it remains possible that the aforementioned body mass augmentations were due to increases in creatine-mediated total body water content [11,24]. Therefore, the use of more sensitive techniques such as bioelectrical impedance analysis, commensurate with detailed skeletal muscle mass assessment via dual X-ray absorptiometry or—more ideally—ultrasonography may provide further insight into the mechanisms underlying the observed weight changes [25,26,27]. Substantial variation may also have been introduced into both our total body-water-associated body weight and exercise capacity data due to the present supplementation protocol. Although our participants appropriately loaded an empirically supported creatine monohydrate dose, the current investigation did not provide a sustained “maintenance” dose, possibly reversing the effects of supplementation over the four-week training period [28]. Conversely, Rawson et al. [29] demonstrated that some individuals may require more than 30 days before muscle creatine and any supplementation-mediated body mass alterations return to baseline levels [30]. We therefore posit that while suboptimal, the present administration protocol still meaningfully demonstrates the impacts of creatine monohydrate on the targeted performance and cardiovascular indices.
Contrary to our original hypothesis, all cardiovascular parameters did not differ between groups following supplementation and the training intervention. It has been previously reported that cardiomyocytes may require larger and longer supplemental dosing and administration timeframes, respectively [10]. Therefore, the predominantly high relative creatine levels in this tissue type may have rendered the present protocol’s methodology insufficient to appropriately saturate cardiac muscle and yield any ostensible cardiovascular enhancements [10]. The present findings do nevertheless corroborate previous the authors’ support of efficacy, tolerability, and safety for human cardiovascular outcomes [8,10].
Although the present investigation reinforced the preponderance of ergogenically focused creatine literature whilst innovatively and simultaneously corroborating its efficacy in females and cardiovascular safety profile, there are many inherent limitations within our research design [4,14,23]. Specifically, the strength of the current data is potentially tempered by menstrual cycle phase-mediated variability; prior data describe impaired recovery amidst the early follicular phase relative to ovulatory or luteal phases [17]. Notwithstanding our inability to control for this factor, the relevance of a menstrual phase-specific recovery difference is inconsistent within the present literature and thus requires further investigation before dismissing the present findings [17,31,32]. Furthermore, our research design singularly recruited young, healthy females given the male-oriented precedent amongst prior literature. It is therefore unknown how both females in a broader age and health predisposition range might vary with respect to strength- and/or hypertrophy-associated outcomes, as well as how these demographics and males along the full demographic spectrum may differentially display cardiovascular outcomes [23]. Nevertheless, it was largely impractical within the present investigation to recruit and sustain such a broad range of participants across a supplemental protocol and subsequent training intervention. Although we also instructed all participants to refrain from dietary changes throughout the study protocol, we likewise were unable to feasibly enforce stringent dietary constraints nor collect comprehensive nutritional records. This weakens our ability to infer that the previously displayed greater CRE-specific body mass increases were not mediated via dietary modulations in energy intake. It is thereby necessary for future investigations to elucidate the combined influence creatine supplementation and resistance training confer upon the vast population with appropriately comprehensive dietary records to limit confounding variables.
Although our findings are limited by a relatively short supplementation period and inadequate supporting biological samples (i.e., serum and/or muscle creatine parameters) to substantiate a wholly creatine-supplementation-mediated enhancement, these data overall further substantiate creatine monohydrate’s already substantial body of evidence [1,4,14]. Specifically, the present CRE group demonstrated a statistically augmented resistance training capacity alongside increased body mass in females, additionally supporting a hitherto undescribed safety profile with respect to multiple cardiovascular parameters [10,23]. Despite the ostensible novelty of our findings, the female-specific and generalized hemodynamically focused creatine literature remains sparse and warrants further investigation; more rigorous methods should be employed that cumulatively facilitate a longer supplementation period amidst more extensive skeletal muscle and cardiovascular assessments. Nevertheless, these data demonstrate that the future of this supplement remains persistently optimistic; future research will likely only continue to elucidate creatine’s expanding pleiotropic roles.
4. Materials and Methods
4.1. Experimental Approach to the Problem
The current longitudinal, randomized, double-blinded, and placebo-controlled study was conducted over six weeks; during the first week, anthropometric measurements (e.g., height, body mass) were collected and 10 RM (half-squat and leg press) tests were repeated 72 h apart to verify reliable loads. In the second week, in a double-blinded and counterbalanced fashion, subjects were supplemented with either creatine monohydrate (CRE) or placebo (PLA; both at 20 g per day for 7 days). During the subsequent four weeks, subjects performed twelve resistance exercise sessions (three sessions per week), consisting of four sets of half-squat and leg press. Heart rate, blood pressure, total repetitions of movements, and rating of perceived exertion were recorded.
The present investigation was largely aimed at determining performance- rather than health-associated health outcomes. Our trial was not intended to alter cardiovascular outcomes, but more so to observe how cardiovascular parameters were impacted as the participants were supplemented creatine and improved upon their resistance training adaptations. In this way, our manuscript is not characterized as a randomized “clinical” trial, and in our understanding, there is no need for registration.
4.2. Subjects
To determine the efficacy of creatine supplementation on female-specific resistance training and cardiovascular-associated parameters, twenty-eight women (age = 25.5 ± 6.1 years, body mass = 59.7 ± 6.3 kg, height = 163 ± 5 cm) volunteered to participate in the present investigation. All subjects were characterized by the following training history: consistent participation in a resistance training program in the past two years with a minimum training frequency of three 1 h sessions per week; three to five sets per exercise; six to fifteen repetitions per set; bench press and smith machine squat experience; experience employing resistance training sets to muscular failure; and one- to two-minute rest intervals between sets. The exclusion criteria were: (a) using drugs or nutritional supplements in the past six months that could affect exercise performance (including all forms of creatine compounds); (b) indication of bone, joint, or muscular problems that could limit the effective execution of leg press exercise. All subjects read and signed the informed consent forms, which thoroughly explained the testing procedures. The experimental procedures were approved by the University of Iguaçu—Campus V/Itaperuna—UNIG Institutional Review Board (5.413.810) and conformed to the ethical considerations of the Helsinki Code.
4.3. Procedures
The first week was the preparatory period, during which 10 RM loads were established for the half-squat and leg press according to previously published procedures by Kraemer and Fry (33). The 10 RM was assessed twice with 72 h between tests (ICC half-squat = 0.8638; ICC leg press = 0.7522); reported/perceived half-squat 10 RM = 44.7 ± 12.8 Kg, leg press 10 RM = 170.2 ± 38.1) [33]. Before the 10 RM tests, each subject completed 5 min of low-intensity aerobic activity (i.e., jogging/walking). Two warm-up sets preceded testing of each exercise at 50% of the perceived 10 RM load for 10 repetitions each. After the warm-up sets were completed, the load was increased to the perceived 10 RM, and one set was performed to voluntary exhaustion (i.e., muscle failure). The same spotters closely supervised each 10 RM attempt, and subjects were instructed to give a verbal signal when voluntary exhaustion was reached. If less than or more than 10 repetitions were accomplished during a given 10 RM attempt, the load was adjusted by approximately 5% during the next testing session. All resistance training strength assessments were supervised by a laboratory technician to ensure appropriate technique and/or range of motion.
During the second week, subjects were randomized using a computer random number generator (Graphpad software) into CRE (n = 14) or PLA (n = 14) groups. Subjects’ characteristics are displayed in Table 1. Subjects in the CRE group ingested 20 g of creatine monohydrate powder (Midway, Santos-SP, Brazil) daily (in four equal 5 g doses) as supported by prior loading regimen literature, combined with 5 g of dextrose (New Millen Ltd., Cajamar-SP, Brazil) four times per day for 7 days [28]. Subjects in the PLA group ingested 10 g of dextrose four times per day. Dextrose was supplied to the CRE group in an attempt to more comprehensively blind the participants to any apparent taste or appearance differences between experimental conditions. All supplements were visually identical and participant compliance was enforced via communication with laboratory staff. Analyses from three independent laboratories confirmed that the creatine supplements were 99.9% pure, with no microbiological contaminants.
Each subject completed three exercise sessions per week for four weeks, the initiation of which coincided with the aforementioned supplementation protocol. Each exercise session was conducted on the same day and time each week. Similar to strength testing procedures, a laboratory technician familiarized with standardized resistance training movements supervised each exercise session to ensure proper technique and provided both spotting and verbal encouragement. The loads for the half-squat and leg press were not changed during the experimental period. Furthermore, all repetitions were performed at a goal 2/0/2/0 (eccentric–transition–concentric–transition) cadence as determined by a metronome.
Each training session began with 5 min of low-intensity aerobic activity (i.e., jogging/walking), whereby each participant was asked to maintain a pace in which a “conversation could not be maintained”. Subsequently, two warm-up sets were performed at 50% of the predetermined 10 RM for 15 repetitions. Three minutes after the warm-up sets, four consecutive sets were performed to voluntary exhaustion (i.e., repetition maximum) for the half-squat and leg press. Subjects rested two minutes between sets and five minutes between exercises [34].
Throughout training sessions 1 (pre-training), 6 (mid-point), and 12 (post-training), the following variables were collected prior to (i.e., at rest) and immediately following the completion of each set: pre-training heart rate (HR) and systolic and diastolic blood pressure (SBP and DBP); intra-session HR, SBP, DBP, repetitions, and rating of perceived exertion (RPE) were evaluated immediately after each set. Cardiovascular parameters were assessed by a trained laboratory technician using standardized techniques. HR was specifically monitored with a chest strap monitor (Polar, Kempele, Finland). Furthermore, systolic and diastolic blood pressures were manually measured using a calibrated aneroid sphygmomanometer and stethoscope (BD®, Heidelberg, Germany). SBP and DBP were recorded as the first and last apparent Korotkoff sound, respectively. Rating of perceived exertion (RPE) was assessed using the 10-point Borg scale [35]. Body mass was evaluated in the first week and after the twelfth exercise session.
All subjects were instructed not to perform any ancillary exercise throughout the testing and collection period. All received a list of supplements, drugs (e.g., NSAIDs), and procedures (e.g., massage, cooling, heat, etc.) that were to be avoided during the experimental period.
4.4. Statistical Analysis
All variables were tested for normality and homogeneity of variance using the Shapiro–Wilk test and Levene’s test of homogeneity of variance. Furthermore, the reliability of the 10 RM loads for half-squat and leg press exercise was assessed with the intraclass correlation (ICC) [36] and the reliability was described as ‘excellent’ for ICC values in the range of 0.8–1.0. Statistical analysis was completed using Jamovi Version 1.8.1 (Jamovi Project, Sydney, Australia). All baseline characteristics (anthropometrics (age, body mass, height), training experience, hemodynamics (HR, SBP, DBP, and strength parameters [half-squat and leg press 10 RM]) and the change in body mass (session 12–session 1 weights) between supplement groups were assessed via individual independent t-tests. In the event of significantly different baseline values, an analysis of covariance (ANCOVA) or nonparametric equivalent (Quade’s Test; used if data violate normality assumptions) was employed with baseline values as the covariate. Subsequently, all experimental dependent variables (half-squat and leg press repetition number, RPE, as well as all hemodynamic parameters (HR, SBP, DBP)) were assessed with multiple three-way (supplement [CRE, PLA] × session [1,6,12] × set [1st, 2nd, 3rd]) mixed-model ANOVA with repeated measures. Multiple comparisons were performed according to Bonferroni’s method; if Mauchly’s test of sphericity was violated, the Greenhouse–Geisser correction was adopted. Cohen’s d was employed to infer the between-group magnitude differences for body mass changes, whereby effect size values were classified according to Cohen [37] as trivial, <0.2; small, 0.2–0.49; moderate, 0.5–0.79; and large, >0.8. Furthermore, Eta squared (η2) was used to estimate the proportion of variance in the dependent variables explained by the independent variable within all factorial mixed-model analyses. Eta squared effect sizes are determined to be: weak = 0.17, medium = 0.24, strong = 0.51, very strong = 0.70 [38,39]. All data are presented as mean ± standard deviation and significance was set at p ≤ 0.05.
Author Contributions
Conceptualization, K.S.A., W.J.S.A. and M.M.; methodology, K.S.A., W.J.S.A., R.P. and M.M.; formal analysis, K.S.A., S.B.M., W.J.S.A., R.P. and M.M.; investigation, K.S.A., S.B.M., W.J.S.A., J.M.W., R.P. and M.M.; resources, K.S.A., W.J.S.A. and M.M.; data curation, K.S.A., W.J.S.A. and M.M.; writing—original draft preparation, K.S.A., S.B.M., A.E.L., W.J.S.A., J.M.W. and M.M.; writing—review and editing, K.S.A., S.B.M., A.E.L., W.J.S.A., J.M.W., R.P. and M.M.; visualization, K.S.A., S.B.M., A.E.L., W.J.S.A., J.M.W., R.P. and M.M.; supervision, S.B.M. and M.M.; project administration, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Universidade Iguaçu—Campus V (protocol code 5.413.810—17 May 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank Mojtaba Kaviani and Citlaly Jaregui for their suggestions and improvements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Machek, S.B.; Bagley, J.R. Creatine Monohydrate Supplementation. Strength Cond. J. 2018, 40, 82–93. Available online: http://journals.lww.com/00126548-201804000-00007 (accessed on 4 November 2020). [CrossRef]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Wallimann, T.; Tokarska-Schlattner, M.; Schlattner, U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids 2011, 40, 1271–1296. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21448658 (accessed on 14 May 2022). [CrossRef] [PubMed]
- Branch, J.D. Effect of creatine supplementation on body composition and performance: A meta-analysis. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 198–226. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12945830 (accessed on 14 May 2022). [CrossRef] [PubMed]
- Dickinson, H.; Bain, E.; Wilkinson, D.; Middleton, P.; Crowther, C.A.; Walker, D.W. Creatine for women in pregnancy for neuroprotection of the fetus. Cochrane Database Syst. Rev. 2014, 19, CD010846. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25523279 (accessed on 14 May 2022). [CrossRef]
- Dickinson, J.M.; D’Lugos, A.C.; Naymik, M.A.; Siniard, A.L.; Wolfe, A.J.; Curtis, D.R. Transcriptome response of human skeletal muscle to divergent exercise stimuli. J. Appl. Physiol. 2018, 124, 1529–1540. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29543133 (accessed on 14 May 2022). [CrossRef]
- Borchio, L.; Machek, S.B.; Machado, M. Supplemental creatine monohydrate loading improves cognitive function in experienced mountain bikers. J. Sports Med. Phys. Fitness 2020, 60, 1168–1170. Available online: https://www.minervamedica.it/index2.php?show=R40Y2020N08A1168 (accessed on 14 October 2020). [CrossRef]
- Clarke, H.; Hickner, R.C.; Ormsbee, M.J. The Potential Role of Creatine in Vascular Health. Nutrients 2021, 13, 857. Available online: http://www.ncbi.nlm.nih.gov/pubmed/33807747 (accessed on 14 May 2022). [CrossRef]
- Dickinson, H.; Ellery, S.; Ireland, Z.; LaRosa, D.; Snow, R.; Walker, D.W. Creatine supplementation during pregnancy: Summary of experimental studies suggesting a treatment to improve fetal and neonatal morbidity and reduce mortality in high-risk human pregnancy. BMC Pregnancy Childbirth 2014, 14, 150. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24766646 (accessed on 14 May 2022). [CrossRef]
- Balestrino, M. Role of Creatine in the Heart: Health and Disease. Nutrients 2021, 13, 1215. Available online: http://www.ncbi.nlm.nih.gov/pubmed/33917009 (accessed on 14 May 2022). [CrossRef]
- Blaustein, M.P.; Zhang, J.; Chen, L.; Hamilton, B.P. How does salt retention raise blood pressure? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R514–R523. Available online: http://www.ncbi.nlm.nih.gov/pubmed/16467498 (accessed on 14 May 2022). [CrossRef]
- Ribeiro, A.S.; Avelar, A.; Kassiano, W.; Nunes, J.P.; Schoenfeld, B.J.; Aguiar, A.F.; Trindade, M.C.; Silva, A.M.; Sardinha, L.B.; Cyrino, E.S. Creatine Supplementation Does Not Influence the Ratio Between Intracellular Water and Skeletal Muscle Mass in Resistance-Trained Men. Int. J. Sport Nutr. Exerc. Metab. 2020, 30, 405–411. Available online: http://www.ncbi.nlm.nih.gov/pubmed/32916658 (accessed on 14 May 2022). [CrossRef]
- Carvalho, A.P.P.F.; Rassi, S.; Fontana, K.E.; Correa, K.d.S.; Feitosa, R.H.F. Influence of creatine supplementation on the functional capacity of patients with heart failure. Arq. Bras. Cardiol. 2012, 99, 623–629. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22735863 (accessed on 14 May 2022). [CrossRef]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN exercise & sports nutrition review update: Research & recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30068354 (accessed on 20 October 2021).
- Tarnopolsky, M.A.; MacLennan, D.P. Creatine Monohydrate Supplementation Enhances High-Intensity Exercise Performance in Males and Females. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 452–463. Available online: https://journals.humankinetics.com/view/journals/ijsnem/10/4/article-p452.xml (accessed on 8 October 2020). [CrossRef]
- Almeida, D.; Colombini, A.; Machado, M. Creatine supplementation improves performance, but is it safe? Double-blind placebo-controlled study. J. Sports Med. Phys. Fit. 2020, 60, 1034–1039. [Google Scholar]
- Amdi, C.H.; Cleather, D.J.; Tallent, J. Impact of Training Protocols on Lifting Velocity Recovery in Resistance Trained Males and Females. Sports 2021, 9, 157. Available online: http://www.ncbi.nlm.nih.gov/pubmed/34822356 (accessed on 14 May 2022). [CrossRef]
- Machek, S.B.; Hwang, P.S.; Cardaci, T.D.; Wilburn, D.T.; Bagley, J.R.; Blake, D.T.; Galpin, A.J.; Willoughby, D.S. Myosin Heavy Chain Composition, Creatine Analogues, and the Relationship of Muscle Creatine Content and Fast-Twitch Proportion to Wilks Coefficient in Powerlifters. J. Strength Cond. Res. 2020, 34, 3022–3030. Available online: http://www.ncbi.nlm.nih.gov/pubmed/33105350 (accessed on 14 May 2022). [CrossRef]
- Haizlip, K.M.; Harrison, B.C.; Leinwand, L.A. Sex-based differences in skeletal muscle kinetics and fiber-type composition. Physiology 2015, 30, 30–39. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25559153 (accessed on 14 May 2022). [CrossRef]
- Staron, R.S.; Hagerman, F.C.; Hikida, R.S.; Murray, T.F.; Hostler, D.P.; Crill, M.T.; Ragg, K.E.; Toma, K. Fiber type composition of the vastus lateralis muscle of young men and women. J. Histochem. Cytochem. 2000, 48, 623–629. Available online: http://www.ncbi.nlm.nih.gov/pubmed/10769046 (accessed on 14 May 2022). [CrossRef]
- Ellery, S.J.; Walker, D.W.; Dickinson, H. Creatine for women: A review of the relationship between creatine and the reproductive cycle and female-specific benefits of creatine therapy. Amino Acids 2016, 48, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Mihic, S.; MacDonald, J.R.; McKenzie, S.; Tarnopolsky, M.A. Acute creatine loading increases fat-free mass, but does not affect blood pressure, plasma creatinine, or CK activity in men and women. Med. Sci. Sport Exerc. 2000, 32, 291. Available online: http://journals.lww.com/00005768-200002000-00007 (accessed on 8 October 2020). [CrossRef] [PubMed]
- Smith-Ryan, A.E.; Cabre, H.E.; Eckerson, J.M.; Candow, D.G. Creatine Supplementation in Women’s Health: A Lifespan Perspective. Nutrients 2021, 13, 877. Available online: http://www.ncbi.nlm.nih.gov/pubmed/33800439 (accessed on 5 November 2021). [CrossRef] [PubMed]
- Powers, M.E.; Arnold, B.L.; Weltman, A.L.; Perrin, D.H.; Mistry, D.; Kahler, D.M.; Kraemer, W.; Volek, J. Creatine Supplementation Increases Total Body Water Without Altering Fluid Distribution. J. Athl. Train. 2003, 38, 44–50. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12937471 (accessed on 14 May 2022).
- Sartorio, A.; Malavolti, M.; Agosti, F.; Marinone, P.G.; Caiti, O.; Battistini, N. Body water distribution in severe obesity and its assessment from eight-polar bioelectrical impedance analysis. Eur. J. Clin. Nutr. 2005, 59, 155–160. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15340370 (accessed on 14 May 2022). [CrossRef]
- Miller, R.M.; Chambers, T.L.; Burns, S.P.; Godard, M.P. Validating InBody® 570 Multi-frequency Bioelectrical Impedance Analyzer versus DXA for Body Fat Percentage Analysis. J. Exerc. Physiol. 2016, 19, 71–78. Available online: https://www.asep.org/asep/asep/JEPonlineOCTOBER2016_Miller.pdf (accessed on 14 May 2022). [CrossRef]
- Haun, C.T.; Vann, C.G.; Roberts, B.M.; Vigotsky, A.D.; Schoenfeld, B.J.; Roberts, M.D. A Critical Evaluation of the Biological Construct Skeletal Muscle Hypertrophy: Size Matters but So Does the Measurement. Front. Physiol. 2019, 10, 247. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30930796 (accessed on 14 May 2022). [CrossRef]
- Hultman, E.; Söderlund, K.; Timmons, J.A.; Cederblad, G.; Greenhaff, P.L. Muscle creatine loading in men. J. Appl. Physiol. 1996, 81, 232–237. Available online: http://www.ncbi.nlm.nih.gov/pubmed/8828669 (accessed on 14 May 2022). [CrossRef]
- Rawson, E.S.; Persky, A.M.; Price, T.B.; Clarkson, P.M. Effects of repeated creatine supplementation on muscle, plasma, and urine creatine levels. J. Strength Cond. Res. 2004, 18, 162–167. Available online: http://www.ncbi.nlm.nih.gov/pubmed/14971966 (accessed on 14 May 2022).
- Vandenberghe, K.; Goris, M.; Van Hecke, P.; Van Leemputte, M.; Vangerven, L.; Hespel, P. Long-term creatine intake is beneficial to muscle performance during resistance training. J. Appl. Physiol. 1997, 83, 2055–2063. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9390981 (accessed on 14 May 2022). [CrossRef]
- Mackay, K.; González, C.; Zbinden-Foncea, H.; Peñailillo, L. Effects of oral contraceptive use on female sexual salivary hormones and indirect markers of muscle damage following eccentric cycling in women. Eur. J. Appl. Physiol. 2019, 119, 2733–2744. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31686212 (accessed on 14 May 2022). [CrossRef]
- Anderson, L.J.; Baker, L.L.; Schroeder, E.T. Blunted Myoglobin and Quadriceps Soreness After Electrical Stimulation During the Luteal Phase or Oral Contraception. Res. Q. Exerc. Sport 2017, 88, 193–202. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28388333 (accessed on 14 May 2022). [CrossRef]
- Kraemer, W.J.; Fry, A.C. Strength testing: Development and evaluation of methodology. In Physiological Assessment of Human Fitness; Maud, P., Foster, C., Eds.; Human Kinetics: Champaign, IL, USA, 1995; pp. 115–138. [Google Scholar]
- Kraemer, W.J.; Adams, K.; Cafarelli, E.; A Dudley, G.; Dooly, C.; Feigenbaum, M.S.; Fleck, S.J.; Franklin, B.; Fry, A.C.; Hoffman, J.; et al. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar]
- Arney, B.E.; Glover, R.; Fusco, A.; Cortis, C.; de Koning, J.J.; van Erp, T.; Jaime, S.; Mikat, R.P.; Porcari, J.P.; Foster, C. Comparison of RPE (Rating of Perceived Exertion) Scales for Session RPE. Int. J. Sports Physiol. Perform. 2019, 14, 994–996. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30569764 (accessed on 14 May 2022). [CrossRef]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. Available online: http://doi.apa.org/getdoi.cfm?doi=10.1037/0033-2909.86.2.420 (accessed on 8 October 2020). [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge, Lawrence Erlbaum: Hillsdale, NJ, USA, 1998; 567p. [Google Scholar]
- O’Connor, K.; Stip, E.; Pélissier, M.-C.; Aardema, F.; Guay, S.; Gaudette, G.; van Haaster, I.; Robillard, S.; Grenier, S.; Careau, Y.; et al. Treating delusional disorder: A comparison of cognitive-behavioural therapy and attention placebo control. Can. J. Psychiatry 2007, 52, 182–190. Available online: http://www.ncbi.nlm.nih.gov/pubmed/17479527 (accessed on 14 May 2022). [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. Available online: http://journal.frontiersin.org/article/10.3389/fpsyg.2013.00863/abstract (accessed on 14 May 2022). [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).