Abstract
Cancer cachexia is characterized by irreversible muscle loss which is a critical factor in the prognosis of cancer patients. Myoblasts are myogenic precursor cells that are required to maintain skeletal muscle tissue. Previous studies reported that cancer-released factors deteriorate myoblast differentiation, which is one of the causes of cachexia-associated muscle wasting. We recently identified the myogenetic oligodeoxynucleotide, iSN04, which serves as an anti-nucleolin aptamer and promotes myogenesis. The present study investigated the effects of iSN04 on human myoblasts exposed to a conditioned medium (CM) of cancer cells. CM of colon cancer cell lines LoVo and HCT-116 significantly impaired myogenic differentiation and the myotube formation of human myoblasts by inducing the expression of inflammatory cytokines such as interleukin (IL)-1β and tumor necrosis factor-α (TNF-α); however, the CM of the colon fibroblast cell line CCD-18Co did not. Intriguingly, iSN04 completely reversed the deterioration of myoblast differentiation by LoVo-CM by upregulating MyoD and myogenin, and downregulating myostatin, IL-1β, and TNF-α. TNF-α, of which a high level was produced in LoVo, alone inhibited myogenic differentiation and induced IL-1β, IL-6, and IL-8 transcriptions of myoblasts; however, pre-treatment with iSN04 reversed TNF-α-induced cachectic phenotypic features. The results indicate that iSN04 protects myoblasts against the effects of cancer-released factors and maintains their myogenic activity. This study provides a novel therapeutic strategy to prevent muscle loss associated with cancer cachexia.
1. Introduction
Cancer cachexia is a wasting syndrome characterized by an irreversible loss of adipose tissue and skeletal muscle mass along with anorexia, asthenia, and anemia [1]. Approximately 30–80% of cancer patients present with weight loss associated with chronic inflammation, and cachexia accounts for around 20% of cancer deaths [2]. As skeletal muscle is the primary organ for thermogenesis [3], energy storage [4], and insulin-responsive glucose uptake [5], a decrease in muscle mass disturbs systemic homeostasis and eventually increases the mortality risk of cancer patients. For instance, muscle wasting is a survival predictor for patients with metastatic colorectal cancer underlying chemotherapy [6]. Therefore, the prevention of muscle loss is a major challenge in cancer therapy. However, no specific treatments or interventions have been made available for cachectic muscle wasting with chronic inflammation [7].
Myoblasts are the principal myogenic precursors involved in muscle formation. Myoblasts initially differentiate into myocytes, a process that is driven by myogenic transcription factors such as MyoD and myogenin [8]. Then, the myocytes fuse to form multinuclear myotubes by muscle-specific membrane proteins, myomaker and myomixer [9,10]. The factors released by the cancer cells have been reported to impair the myogenic program. The conditioned medium (CM) of colon cancer cells, including myostatin, promotes protein degradation in the C2C12 murine myoblast cell line [11]. Prostate cancer-CM inhibits the myogenic differentiation of murine myoblasts by upregulating CCAAT/enhancer-binding protein β and interleukin (IL)-1β [12]. Hsp70 and Hsp90, associated with extracellular vesicles from lung and colon carcinoma cells, deteriorate murine myotube formation by disrupting catabolism [13]. Exosome microRNAs, miR-125b and miR-195a, in colon cancer-CM induce atrophy and apoptosis in murine myoblasts [14]. These cancerous factors have been confirmed to recapitulate muscle wasting in vivo [12,13,14]. Thus, the exposure of myoblasts to the factors secreted by cancer cells is considered one of the causes of muscle loss, and the myogenic ability of the myoblasts needs to be recovered to prevent cancer cachexia.
We recently reported that single-strand short DNAs named myogenetic oligodeoxynucleotides (myoDNs) promote the differentiation of myoblasts and rhabdomyosarcoma [15,16,17,18]. One of the myoDNs, iSN04, serves as an anti-nucleolin aptamer to increase p53 translation [15] and recovers myoblast differentiation attenuated by diabetes mellitus [16], demonstrating that iSN04 is a potential nucleic acid drug for disease-associated muscle wasting. The present study investigated whether iSN04 affects myogenesis and inflammation in human myoblasts cultured in colon cancer-CM mimicking cancer cachexia.
2. Results
2.1. iSN04 Recovers Differentiation of Human Myoblasts Aggravated by LoVo-CM
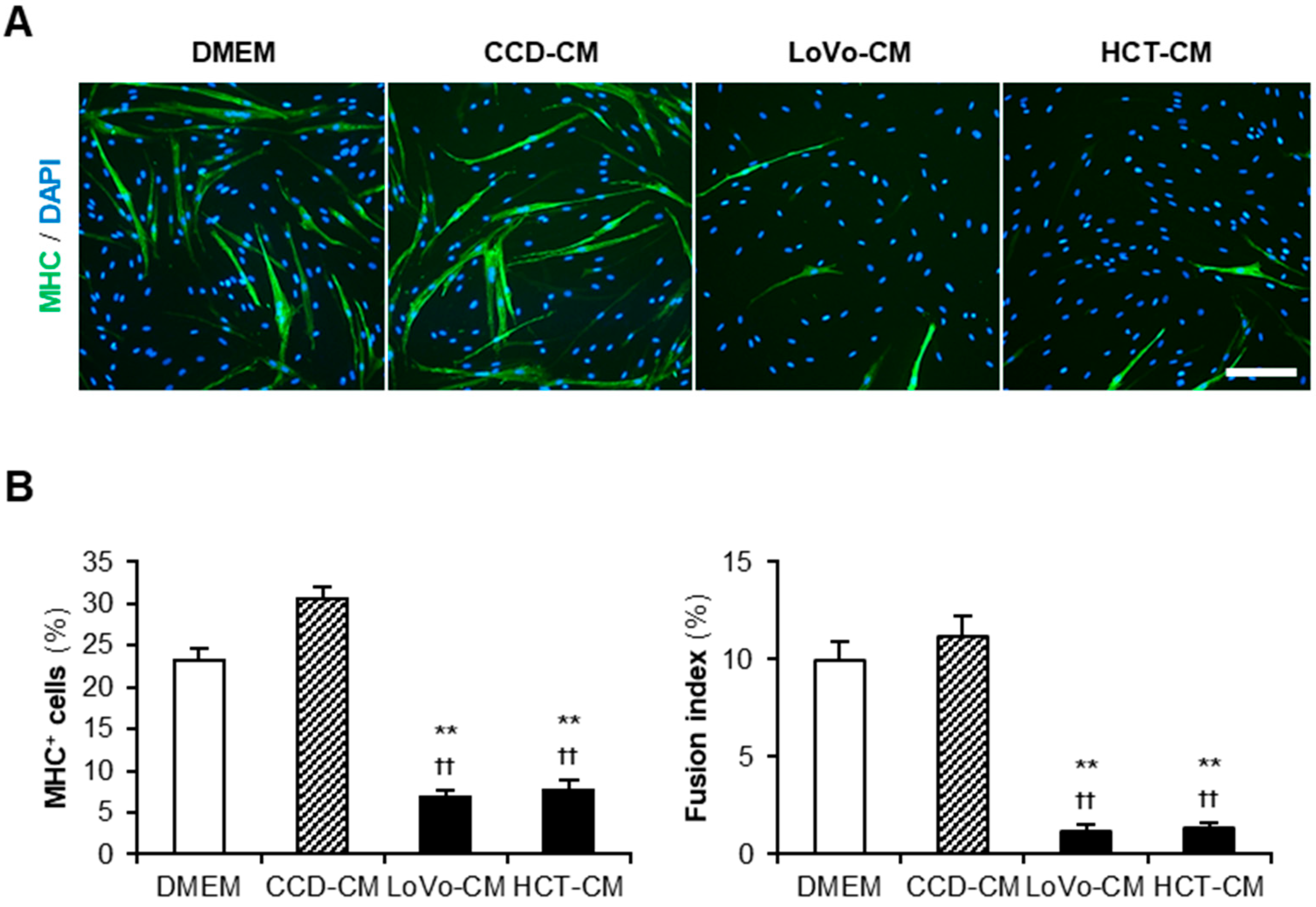
Cancer-CM has been reported to deteriorate myogenesis in murine myoblasts [12,13,14]. We first investigated the impact of colon cancer-CM on human myoblasts. Colon cancer cell lines LoVo and HCT-116 (HCT) were used to harvest cancer-CM. The colon fibroblast cell line CCD-18Co (CCD) was used for control-CM. Non-supplemented Dulbecco’s modified Eagle medium (DMEM) served as the negative control. Human myoblasts were subjected to a differentiation medium with 50% CM for 48 h and immunostained for the myosin heavy chain (MHC) (Figure 1A). Both LoVo-CM and HCT-CM significantly decreased the ratio of MHC+ cells and multinuclear myotubes compared to that of non-supplemented DMEM and CCD-CM (Figure 1B). This demonstrated that the colon cancer-released factors aggravate the myogenesis of human myoblasts. In the following experiments, LoVo showing undifferentiated signatures such as primary tumors [19] was used as a representative colon cancer cell line.
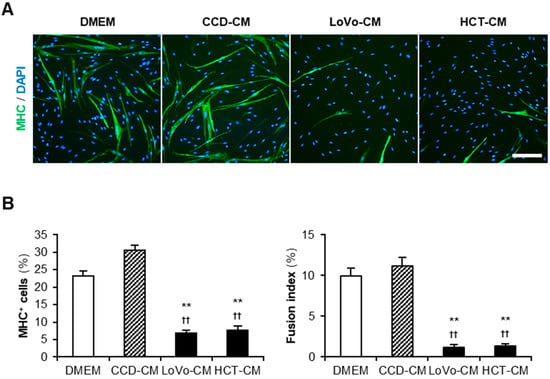
Figure 1.
Colon cancer-CM deteriorate human myoblast differentiation. (A) Representative immunofluorescent images of the human myoblasts differentiated in differentiation medium with 50% CM for 48 h. Scale bar, 200 μm. (B) Quantification of the ratio of MHC+ cells and multinuclear myotubes. ** p < 0.01 vs. DMEM; †† p < 0.01 vs. CCD-CM (Tukey–Kramer post hoc test with one-way ANOVA). n = 6. Abbreviations in the figure are: CCD, CCD-18Co; CM, conditioned medium; DMEM, Dulbecco’s modified Eagle medium; HCT, HCT-116; MHC, myosin heavy chain.
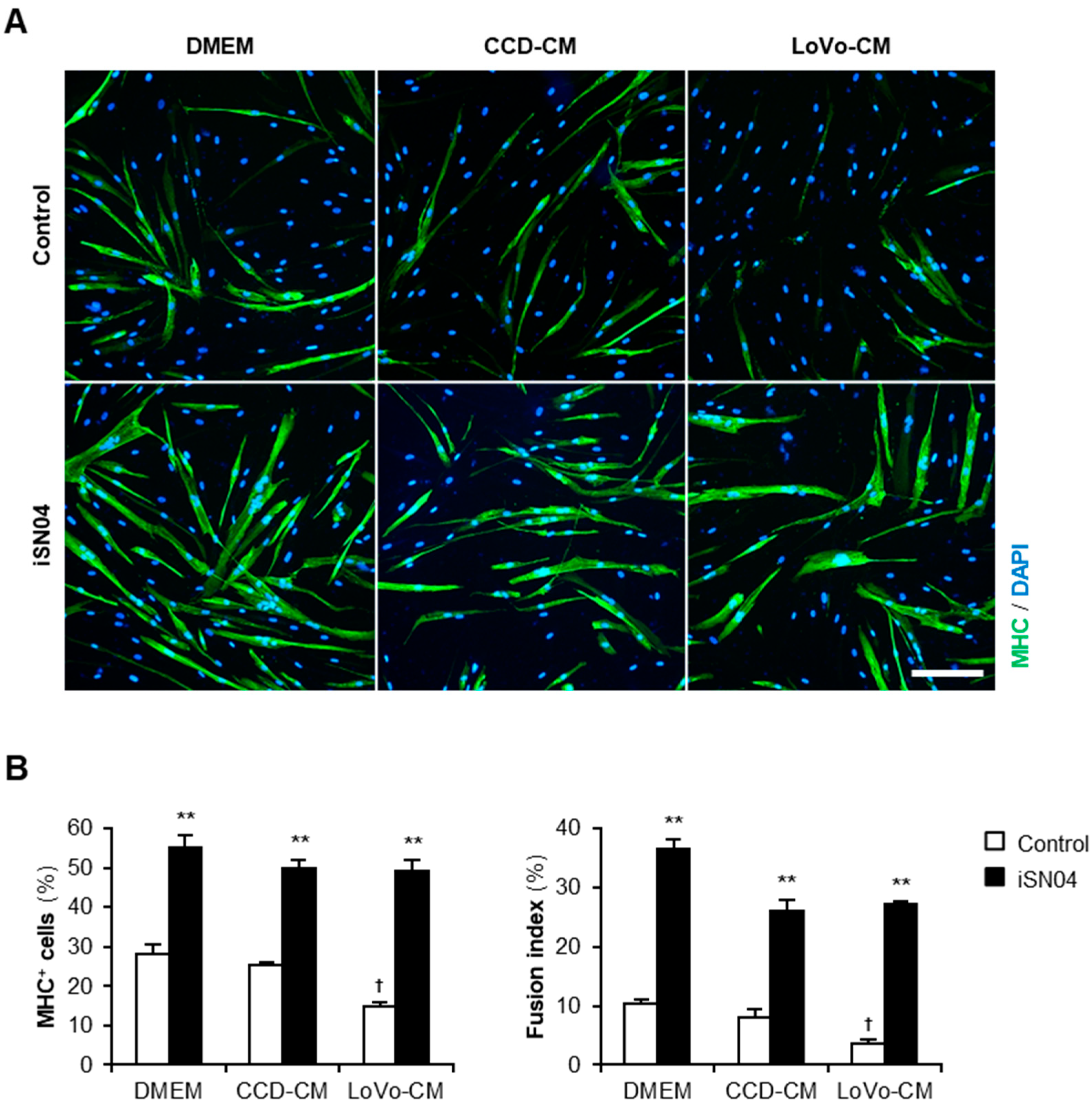
Next, we investigated whether iSN04 recovers the myogenic differentiation deteriorated by LoVo-CM. Human myoblasts were subjected to a differentiation medium with 30% CM and 30 μM iSN04 for 48 h (Figure 2A). Consistent with the previous results, in the absence of iSN04, LoVo-CM significantly inhibited differentiation into MHC+ myocytes and myotubes compared to that of non-supplemented DMEM and CCD-CM. Interestingly, iSN04 markedly promoted myogenesis even in the presence of LoVo-CM (Figure 2B). iSN04 completely restored the differentiation of the myoblasts exposed to LoVo-CM to the same extent as non-supplemented DMEM and CCD-CM. These results indicate that iSN04 facilitates myoblast differentiation even in the presence of cancer-released factors.
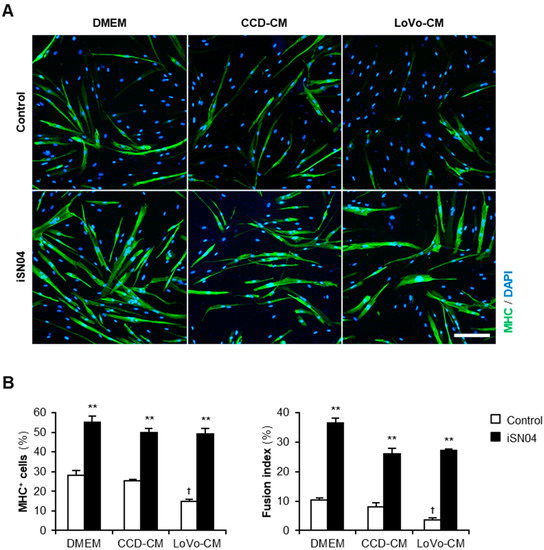
Figure 2.
iSN04 recovers the human myoblast differentiation deteriorated by LoVo-CM. (A) Representative immunofluorescent images of the human myoblasts differentiated in differentiation medium with 30% CM and 30 μM iSN04 for 48 h. Scale bar, 200 μm. (B) Quantification of the ratio of MHC+ cells and multinuclear myotubes. ** p < 0.01 vs. control in each medium; † p < 0.05 vs. DMEM-control (Tukey–Kramer post hoc test with one-way ANOVA). n = 4. Abbreviations in the figure are: CCD, CCD-18Co; CM, conditioned medium; DMEM, Dulbecco’s modified Eagle medium; MHC, myosin heavy chain.
2.2. iSN04 Reverses Gene Expression in the Human Myoblasts Exposed to LoVo-CM
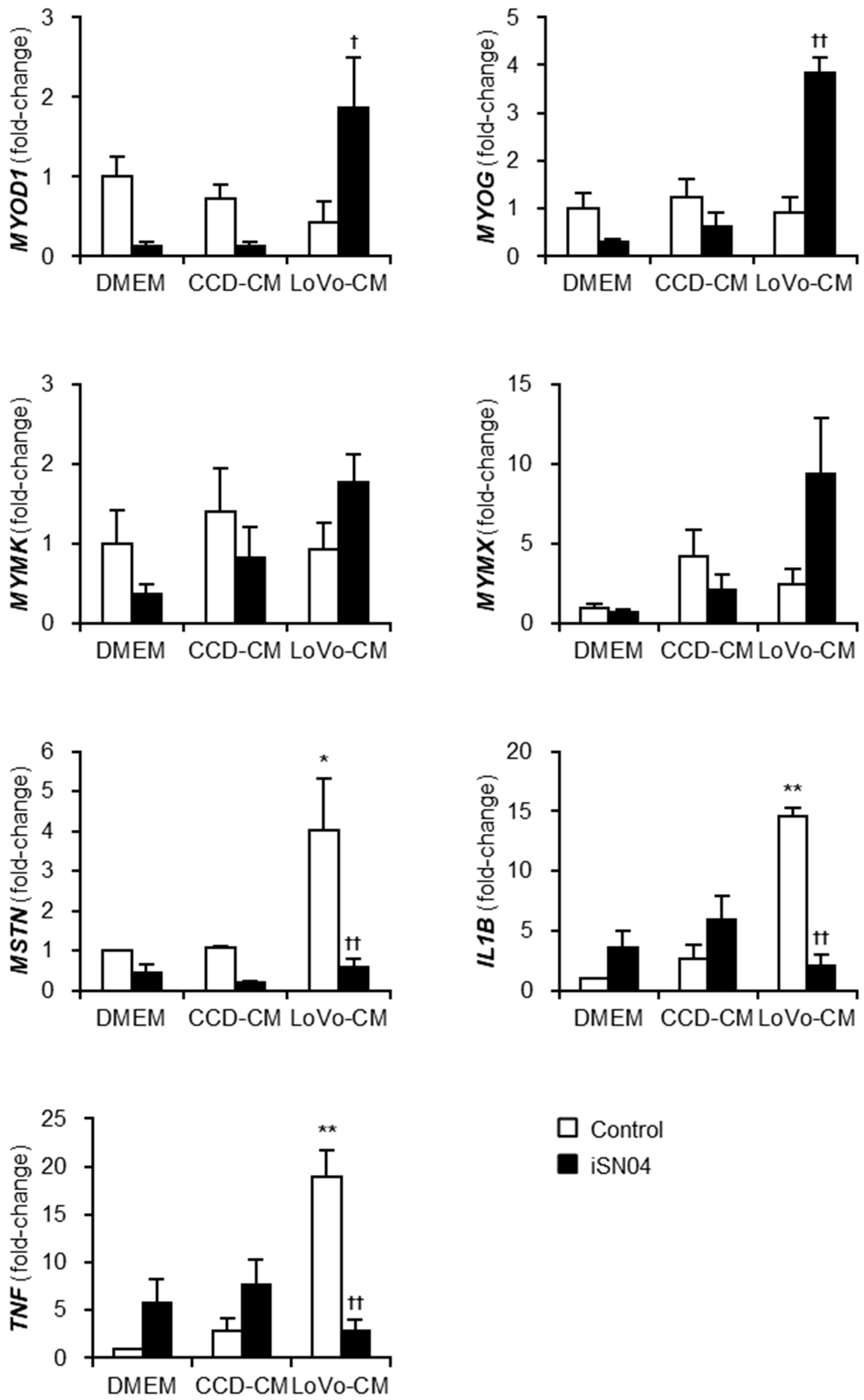
To investigate the mechanism by which LoVo-CM deteriorates and iSN04 recovers myogenesis, the gene expression patterns in the myoblasts treated with LoVo-CM and iSN04 were quantified using quantitative real-time RT-PCR (qPCR) (Figure 3). The mRNA levels of myogenic transcription factors, MyoD (MYOD1) and myogenin (MYOG), were significantly upregulated by iSN04 in LoVo-CM-treated myoblasts. Similarly, myomixer (MYMX) expression tended to increase under the same condition. iSN04-induced muscle gene expression is considered to cause the recovery of myogenesis in myoblasts cultured with LoVo-CM. However, it is still unclear how LoVo-CM impairs myoblast differentiation because LoVo-CM did not alter myogenic gene expression. qPCR revealed that LoVo-CM significantly elevated the mRNA levels of myostatin (MSTN), IL-1β (IL1B), and tumor necrosis factor-α (TNF-α) (TNF) in myoblasts, which are the cytokines inhibiting myogenesis [20,21,22]. iSN04 completely reversed LoVo-CM-induced transcription of the cytokines. These data demonstrate that iSN04 recovers myoblast differentiation by inducing myogenic genes and suppressing anti-myogenic cytokines.
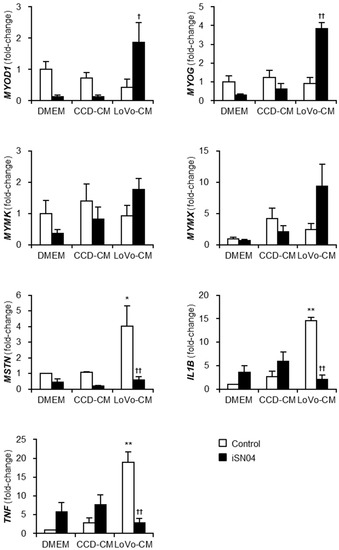
Figure 3.
iSN04 reverses the gene expression patterns altered by LoVo-CM. qPCR results of gene expression in the human myoblasts differentiated in differentiation medium with 30% CM and 30 μM iSN04 for 24 h. Mean value of DMEM-control was set to 1.0 for each gene. * p < 0.05, ** p < 0.01 vs. DMEM-control; † p < 0.05, †† p < 0.01 vs. LoVo-CM-control (Tukey–Kramer post hoc test with one-way ANOVA). n = 3. Abbreviations in the figure are: CCD, CCD-18Co; CM, conditioned medium; DMEM, Dulbecco’s modified Eagle medium; IL1B, interleukin-1β; MSTN, myostatin; MYMK, myomaker; MYMX, myomixer; MYOD1, MyoD; MYOG, myogenin; TNF, tumor necrosis factor-α.
2.3. iSN04 Reverses Inflammation and Differentiation of TNF-α-Treated Human Myoblasts
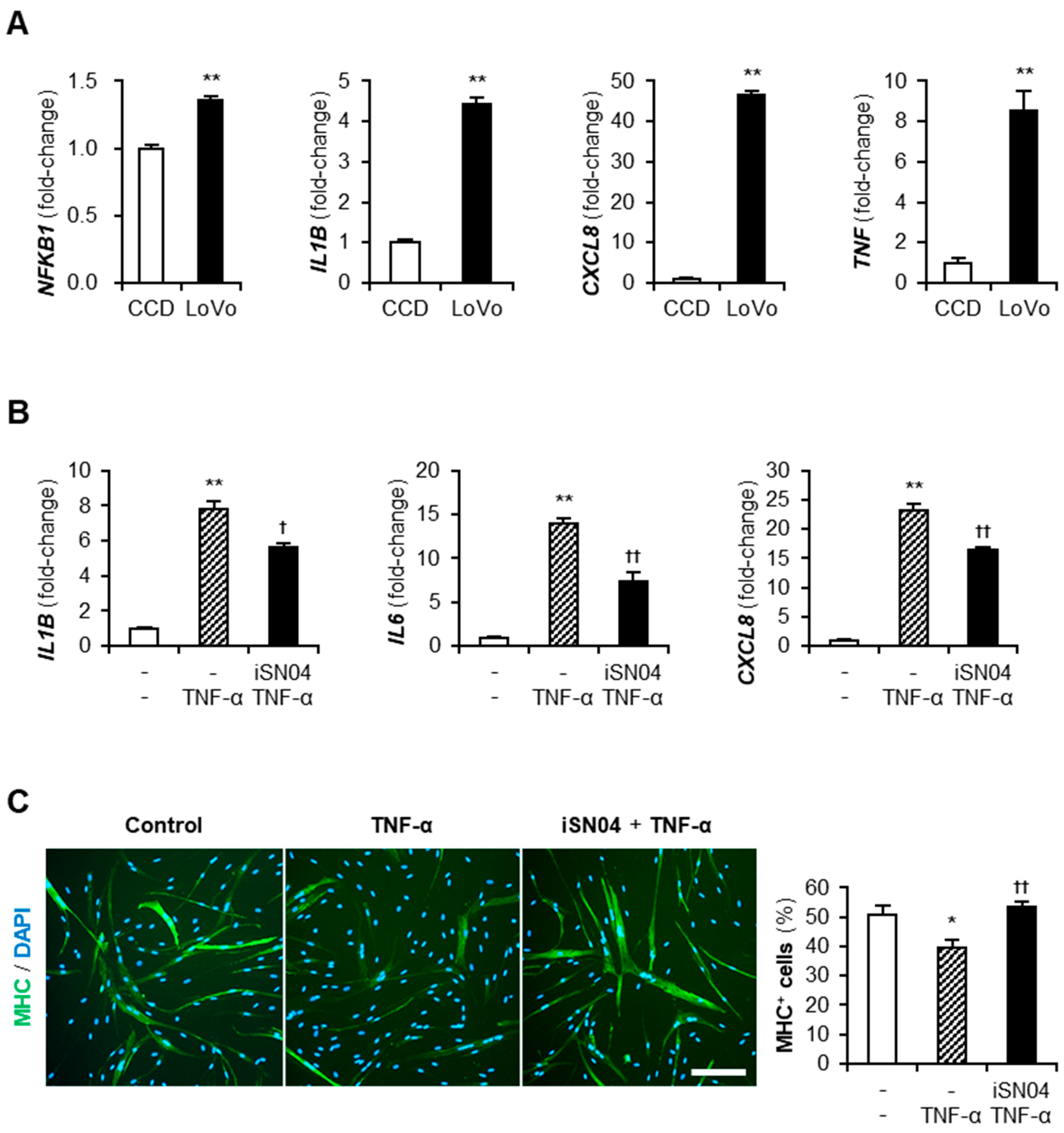
Cancer-CM contains a variety of cancer-released factors, which include multiple inflammatory cytokines [23]. qPCR indicated that LoVo expressed an inflammatory transcription factor, nuclear factor-κB (NF-κB) p50 subunit (NFKB1), and its downstream cytokines, IL-1β, IL-8 (CXCL8), and TNF-α, at significantly high levels compared to CCD (Figure 4A). These cytokines present in LoVo-CM were speculated to initiate inflammatory responses and attenuate the differentiation of myoblasts. TNF-α is a typical cytokine contained in cancer-CM [23] and was reported to induce inflammation and impair the myogenesis of myoblasts [20,24]. To investigate the effect of iSN04 on TNF-α-induced inflammation, human myoblasts were pre-treated with iSN04 for 3 h and then treated with 10 ng/mL TNF-α. The mRNA levels of IL-1β, IL-6, and IL-8 in the myoblasts were markedly increased by TNF-α, but significantly suppressed by pre-treatment with iSN04 (Figure 4B). Correspondingly, pre-treatment with iSN04 completely reversed the attenuation of myoblast differentiation by TNF-α (Figure 4C). These results demonstrate that iSN04 reverses the inflammation and differentiation of myoblasts deteriorated by TNF-α.
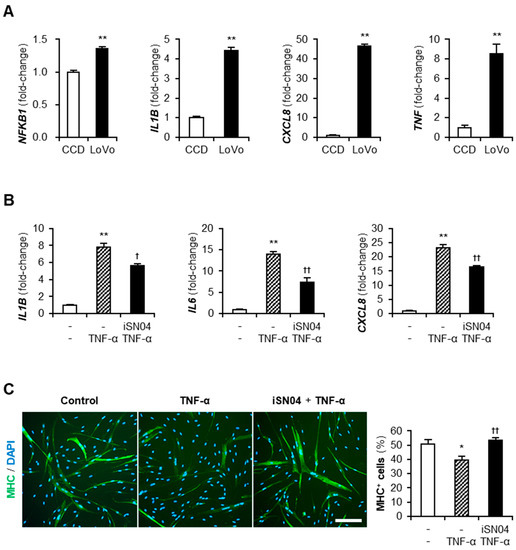
Figure 4.
iSN04 reverses inflammation and restores differentiation of TNF-α-treated human myoblasts. (A) qPCR results of gene expression in CCD and LoVo. ** p < 0.01 vs. CCD (Student’s t-test). n = 3. (B) qPCR results of gene expression in human myoblasts pre-treated with 30 μM iSN04 for 3 h and then treated with 10 ng/mL TNF-α for 1 h. ** p < 0.01 vs. control; † p < 0.05, †† p < 0.01 vs. TNF-α (Tukey–Kramer post hoc test with one-way ANOVA). n = 3. (C) Representative immunofluorescent images of human myoblasts pre-treated with 30 μM iSN04 for 3 h and then treated with 10 ng/mL TNF-α in differentiation medium for 48 h. Scale bar, 200 μm. The ratio of MHC+ cells was quantified. * p < 0.05 vs. control; †† p < 0.01 vs. TNF-α (Tukey–Kramer post hoc test with one-way ANOVA). n = 5. Abbreviations in the figure are: CCD, CCD-18Co; CXCL8, interleukin-8; IL1B, interleukin-1β; IL6, interleukin-6; MHC, myosin heavy chain; NFKB1, nuclear factor-κB p50 subunit; TNF-α and TNF, tumor necrosis factor-α.
3. Discussion
The present study indicated that iSN04 rescued myogenic differentiation and reversed inflammatory responses in human myoblasts exposed to colon cancer-CM. Cancer-released factors, including cytokines and miRNAs, have been reported to impair myogenesis, one of the mechanisms of cachexia-associated muscle loss [12,13,14]. In this study, the colon cancer cell line LoVo showed a high-level expression of IL-1β, IL-8, and TNF-α. As previously reported, TNF-α disturbed myoblast differentiation and evoked inflammatory responses; however, iSN04 treatment successfully reversed TNF-α-induced cachectic phenotypic features in myoblasts. The protective effect of iSN04 against cancer secretions is expected to prevent deteriorated differentiation and inflammation in myoblasts of cancer patients. The myogenic and anti-inflammatory effects of iSN04 were also reported in diabetic human myoblasts [16]. These studies support that iSN04 may be a potential nucleic acid drug that can be used to prevent disease-associated muscle wasting along with systemic chronic inflammation such as cancer cachexia. Although this study examined the effect of iSN04 on myoblasts during myogenic differentiation, it needs to be confirmed on differentiated myotubes and matured myofibers. As myofibers represent the majority of muscle tissue and significantly contribute to systemic inflammation [25], the pharmacological actions of iSN04 on myotubes and myofibers should be examined in further studies for clinical application.
It is well known that inflammatory cytokines, particularly TNF-α, activate NF-κB, which not only enhances inflamed gene expression but also impairs the myogenic differentiation of myoblasts [20,24]. Myostatin is downstream of NF-κB [26] and a critical inhibitor of myogenesis [21]. As iSN04 reverses cancer-CM-induced upregulation of NF-κB-dependent genes (myostatin, IL-1β, and TNF-α), iSN04 is predicted to interfere with the NF-κB signaling pathway. iSN04 serves as an anti-nucleolin aptamer [15], and nucleolin mediates the NF-κB-dependent expression of IL-1β and TNF-α in monocytes [27]. AS1411, another anti-nucleolin aptamer that promotes myogenesis as well as iSN04 [15], forms a complex with nucleolin and NF-κB essential modulator (NEMO) to block the NF-κB signaling, and eventually suppresses TNF-α-induced inflammation in cancer cells [28]. Although the anti-inflammatory mechanism of iSN04 is speculated to be identical to that of AS1411, this needs to be further investigated using myoblasts.
In myoblasts, NF-κB downregulates the myogenic transcription factors MyoD and myogenin [20,24,29]. The present study indicated that iSN04 significantly induces MyoD and myogenin in myoblasts cultured with cancer-CM. In our previous study, we demonstrated that iSN04 upregulates MyoD and myogenin expression by improving the p53 protein level in myoblasts [15]. Nucleolin binds to the 5′ untranslated region of p53 mRNA and interferes with its translation [30,31]; however, antagonizing nucleolin by iSN04 recovers p53 translation [15]. Numerous studies reported that p53 promotes myogenesis [32,33]; consequently, iSN04 accelerates myoblast differentiation by activating the p53 signaling pathway [15]. Intriguingly, NF-κB and p53 antagonize each other’s activity by competing with p300 [34]. This might explain why iSN04 upregulated myogenic gene expression even in the presence of cancer-CM.
The present study revealed the dual role of iSN04, specifically its myogenic activity and anti-inflammatory effect, that restores the myoblasts exposed to cancer-CM. iSN04 is therefore anticipated to maintain myoblast activity in cancer patients by promoting myogenesis and suppressing inflammation, and eventually preventing cachectic muscle loss. Clinically, cachexia is complex muscle atrophy that includes dysregulated protein metabolism, apoptosis, and the impaired regeneration of muscle cells [1]. These pathological changes accompany those of the various types of cells that make up the microenvironment in muscle tissue. For example, immune cells producing numerous cytokines for inflammation and muscle repair, fibroblast that remodel the extracellular matrix and fibrosis, and endothelial cells associated with vascularization during muscle regeneration [35]. They are closely and intricately related to each other in the progression of cachectic muscle wasting. Thus, the beneficial effects of iSN04 on cancer cachexia need to be examined in vivo using animal models to establish a concrete therapeutic strategy.
4. Materials and Methods
4.1. Chemicals
Phosphorothioated iSN04 (5′-AGA TTA GGG TGA GGG TGA-3′) was synthesized and purified using HPLC (GeneDesign, Osaka, Japan) and dissolved in endotoxin-free water [15,16,17,18,36]. Recombinant mouse TNF-α (Fujifilm Wako Chemicals, Osaka, Japan) was rehydrated in phosphate-buffered saline. Equal volumes of the solvent were used as the negative control.
4.2. CM Preparation
The human colon fibroblast cell line CCD-18Co (CRL-1459; ATCC, Manassas, VA, USA) was used as a non-cancer cell line. The human colon adenocarcinoma cell line LoVo (IFO50067; JCRB Cell Bank, Osaka, Japan) and the human colon carcinoma cell line HCT-116 (EC91091005-F0; ECACC, Salisbury, UK) were used as colon cancer cells. The cells were cultured in DMEM (Nacalai, Osaka, Japan) supplemented with 10% fetal bovine serum (GE Healthcare, Salt Lake City, UT, USA) and a mixture of 100 units/mL penicillin and 100 μg/mL of streptomycin (P/S) (Nacalai) at 37 °C under 5% CO2. The culture medium was replaced with non-supplemented DMEM when the cells became confluent. After 48 h, the DMEM was harvested as CM, filtered through 0.22-μm filters, and stored at −80 °C. The remaining cells after CM preparation were subjected to qPCR.
4.3. Human Myoblast Culture
The commercially available human myoblast stock isolated from a healthy subject (CC-2580; Lonza, Walkersville, MD, USA) was used [15,16,17,37]. The myoblasts were cultured on dishes coated with collagen type I-C (Cellmatrix; Nitta Gelatin, Osaka, Japan) at 37 °C under 5% CO2. Undifferentiated myoblasts were maintained in Skeletal Muscle Growth Media-2 (CC-3245; Lonza). Subcultured myoblasts grown to 70% confluency were dissociated using 0.25% trypsin with 1 mM EDTA; then, 7.5 × 104 myoblasts were seeded on 30 mm dishes for immunocytochemistry and 1.5 × 105 myoblasts were seeded on 60 mm dishes for qPCR. The next day, confluent myoblasts were used to induce myogenic differentiation by replacing the medium with a differentiation medium consisting of DMEM supplemented with 2% horse serum (GE Healthcare) and P/S. Myoblasts in the differentiation medium were immediately treated as follows. For CM experiments, the myoblasts were simultaneously treated with 30 μM iSN04 and 30–50% of CM for 24 h (qPCR) or 48 h (immunocytochemistry). Non-supplemented DMEM filtered through 0.22-μm filters was used as a negative control for CM. For TNF-α experiments, the myoblasts were pre-treated with 30 μM iSN04 for 3 h and then subsequently treated with 10 ng/mL TNF-α for 1 h (qPCR) or 48 h (immunocytochemistry) without medium replacement.
4.4. Immunocytochemistry
The myoblasts were fixed with 2% paraformaldehyde, permeabilized with 0.2% Triton X-100 (Nacalai), and immunostained with 0.5 μg/mL mouse monoclonal anti-MHC antibody (MF20; R&D Systems, Minneapolis, MN, USA) overnight at 4 °C and then with 0.1 μg/mL of Alexa Fluor 488-conjugated donkey polyclonal anti-mouse IgG antibody (Jackson ImmunoResearch, West Grove, PA, USA) for 1 h at room temperature. Cell nuclei were stained with DAPI (Nacalai). Fluorescent images were captured using an EVOS FL Auto microscope (AMAFD1000; Thermo Fisher Scientific, Waltham, MA, USA). The ratio of MHC+ cells was defined as the number of nuclei in all MHC+ cells divided by the total number of nuclei, and the fusion index was defined as the number of nuclei in multinuclear MHC+ myotubes divided by the total number of nuclei, which was calculated using the ImageJ software version 1.52a (National Institutes of Health, Bethesda, MD, USA).
4.5. qPCR
Total RNA was isolated using NucleoSpin RNA Plus (Macherey-Nagel, Düren, Germany) and reverse transcribed using the Revera Trace qPCR RT Master Mix (TOYOBO, Osaka, Japan). qPCR was performed using GoTaq qPCR Master Mix (Promega, Madison, WI, USA) with the StepOne Real-Time PCR System (Thermo Fisher Scientific). The amount of each transcript was calculated using the 2−ΔΔCt method and normalized to that of the 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta gene (YWHAZ) [15]. The results are presented as fold-changes. Primer sequences (5′-3′) were as follows: myomaker (MYMK), CCC TGA TGC TAC GCT TCT TC and TCC AGC CTT CTT GTT GAC CT; myomixer (MYMX), ATC CAG CCA GAG ACT GAT TC and AGG ACA GCA GCA ATC GAA G. Primer sequences of IL-1β (IL1B), IL-6 (IL6), IL-8 (CXCL8), MyoD (MYOD1), myogenin (MYOG), myostatin (MSTN), NF-κB p50 subunit (NFKB1), and TNF-α (TNF) were described previously [15,16].
4.6. Statistical Analysis
The results are presented as the mean ± standard error. Normality of the data was tested by performing a chi-squared test for goodness of fit. Statistical comparison between two groups was performed using unpaired two-tailed Student’s t-test and among multiple groups using the Tukey–Kramer post hoc test following a one-way analysis of variance (ANOVA). Statistical significance was set at p < 0.05.
5. Conclusions
This study revealed that an anti-nucleolin aptamer, iSN04, restores the differentiation and inflammation of myoblasts exposed to a cancer-conditioned medium. iSN04 reversed the TNF-α-dependent expression of inflammatory cytokines in myoblasts, resulting in a protective effect for myogenesis. It demonstrated that iSN04 could be beneficial for muscle wasting along with chronic inflammation such as cancer cachexia. Further studies of the anti-inflammatory effect of iSN04 could provide a novel therapeutic strategy for cachexia using nucleic acid drugs.
6. Patents
Shinshu University was accredited with the invention of myoDNs by T.T., Koji Umezawa, and T.S. Japan Patent Application 2018-568609 was filed on 15 February 2018.
Author Contributions
Y.N. and T.T. designed the study. T.T. wrote the manuscript. Y.N. and M.Y. performed experiments and data analyses. T.S. designed and provided iSN04. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Japan Society for the Promotion of Science, grant numbers 19K05948 and 22K05554.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Acknowledgments
The preprint is posted on bioRxiv [38].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Argiles, J.M.; Busquets, S.; Stemmler, B.; Lopez-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Bossola, M.; Aversa, Z.; Bellantone, R.; Faneli, F.R. Prevention and treatment of cancer cachexia: New insights into an old problem. Eur. J. Cancer 2006, 42, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Rowland, L.A.; Bal, N.C.; Periasamy, M. The role of skeletal-muscle-based thermogenic mechanisms in vertebrate endothermy. Biol. Rev. Camb. Philos. Soc. 2015, 90, 1279–1297. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Rustad, P.I.; Kolnes, A.J.; Lai, Y.C. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front. Physiol. 2011, 2, 112. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, M.; Herrera, J.L.; Reis, F.C.G. Skeletal muscle thermogenesis and its role in whole body energy metabolism. Diabetes Metab. J. 2017, 41, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Blauwhoff-Buskermolen, S.; Versteeg, K.S.; de van der Schueren, M.A.; den Braver, N.R.; Berkhof, J.; Langius, J.A.; Verheul, H.M. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J. Clin. Oncol. 2016, 34, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.M.; Kempen, L.J.A.P.; Hardy, R.S.; Langen, R.C.J. Inflammation and skeletal muscle wasting during cachexia. Front. Physiol. 2020, 11, 597675. [Google Scholar] [CrossRef]
- Dumont, N.A.; Bentzinger, C.F.; Sincennes, M.C.; Rudnicki, M.A. Satellite cells and skeletal muscle regeneration. Compr. Physiol. 2015, 5, 1027–1059. [Google Scholar]
- Millay, D.P.; O’Rourke, J.R.; Sutherland, L.B.; Bezprozvannaya, S.; Shelton, J.M.; Bassel-Duby, R.; Olson, E.N. Myomaker is a membrane activator of myoblast fusion and muscle formation. Nature 2013, 499, 301–305. [Google Scholar] [CrossRef]
- Bi, P.; Ramirez-Matinez, A.; Li, H.; Cannavino, J.; McAnally, J.R.; Shelton, J.M.; Sanchez-Otiz, E.; Bassel-Duby, R.; Olson, E.N. Control of muscle formation by the fusogenic micropeptide myomixer. Science 2017, 356, 323–327. [Google Scholar] [CrossRef]
- Lokireddy, S.; Wijesoma, I.W.; Bonala, S.; Wei, M.; Sze, S.K.; McFarlane, C.; Kambadur, R.; Sharma, M. Myostatin is a novel tumoral factor that induces cancer cachexia. Biochem. J. 2012, 446, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Marchildon, F.; Lamarche, E.; Lala-Tabbert, N.; St-Louis, C.; Wiper-Bergeron, N. Expression of CCAAT/enhancer binding protein beta in muscle satellite cells inhibits myogenesis in cancer cachexia. PLoS ONE 2015, 10, e0145583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, Z.; Ding, H.; Zhou, Y.; Doan, H.A.; Sin, K.W.T.; Zhu, Z.J.; Flores, R.; Wen, Y.; Gong, X.; et al. Tumor induces muscle wasting in mice through releasing extracellular Hsp70 and Hsp90. Nat. Commun. 2017, 8, 589. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Zhang, W.; Feng, L.; Gu, X.; Shen, Q.; Lu, S.; Fan, M.; Li, Y.; Guo, X.; Ma, Y.; et al. Cancer-derived exosome miRNAs induce skeletal muscle wasting by Bcl-2-mediated apoptosis in colon cancer cachexia. Mol. Ther. Nucleic Acids 2021, 24, 923–938. [Google Scholar] [CrossRef] [PubMed]
- Shinji, S.; Umezawa, K.; Nihashi, Y.; Nakamura, S.; Shimosato, T.; Takaya, T. Identification of the myogenetic oligodeoxynucleotides (myoDNs) that promote differentiation of skeletal muscle myoblasts by targeting nucleolin. Front. Cell Dev. Biol. 2021, 8, 606706. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Yonekura, S.; Shimosato, T.; Takaya, T. Myogenetic oligodeoxynucleotide (myoDN) recovers the differentiation of skeletal muscle myoblasts deteriorated by diabetes mellitus. Front. Physiol. 2021, 12, 679152. [Google Scholar] [CrossRef] [PubMed]
- Nihashi, Y.; Shinji, S.; Umezawa, K.; Shimosato, T.; Ono, T.; Kagami, H.; Takaya, T. Myogenetic oligodeoxynucleotide complexed with berberine promotes differentiation of chicken myoblasts. Anim. Sci. J. 2021, 92, e13597. [Google Scholar] [CrossRef]
- Nohira, N.; Shinji, S.; Nakamura, S.; Nihashi, Y.; Shimosato, T.; Takaya, T. Myogenetic oligodeoxynucleotides as anti-nucleolin aptamers inhibit the growth of embryonal rhabdomyosarcoma cells. bioRxiv 2021, 464889. [Google Scholar]
- Berg, K.C.G.; Eide, P.W.; Eilertsen, I.A.; Johannessen, B.; Bruun, J.; Danielsen, S.A.; Bjornslett, M.; Meza-Zepeda, L.A.; Eknaes, M.; Lind, G.E.; et al. Multi-omics of 34 colorectal cancer cell lines—A resource for biomedical studies. Mol. Cancer 2017, 16, 116. [Google Scholar] [CrossRef]
- Langen, R.C.; Schols, A.M.; Kelders, M.C.; Wouters, E.F.; Janssen-Heininger, Y.M. Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-κB. FASEB J. 2001, 15, 1169–1180. [Google Scholar] [CrossRef]
- Langley, B.; Thomas, M.; Bishop, A.; Sharma, M.; Gilmour, S.; Kambadur, R. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J. Biol. Chem. 2002, 277, 49831–49840. [Google Scholar] [CrossRef] [PubMed]
- Broussard, S.R.; McCusker, R.H.; Novakofski, J.E.; Strle, K.; Shen, W.H.; Johnson, R.W.; Dantzer, R.; Kelley, K.W. IL-1β impairs insulin-like growth factor I-induced differentiation and downstream activation signals of the insulin-like growth factor I receptor in myoblasts. J. Immunol. 2004, 172, 7713–7720. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Kumar, A.; Laskar, S.; Pandey, B.N. Cytokine profile of conditioned medium from human tumor cell lines after acute and fractionated doses of gamma radiation and its effect on survival of bystander tumor cells. Cytokine 2013, 61, 54–62. [Google Scholar] [CrossRef]
- Ladner, K.J.; Caligiuri, M.A.; Guttridge, D.C. Tumor necrosis factor-regulated biphasic activation of NF-κB is required for cytokine-induced loss of skeletal muscle gene products. J. Biol. Chem. 2003, 278, 2294–2303. [Google Scholar] [CrossRef] [PubMed]
- Bivona Ill, J.J.; Mank, M.M.; Stapleton, R.D.; Files, D.C.; Toth, M.J.; Poynter, M.E. Skeletal muscle myofibers directly contribute to LPS-induced systemic inflammatory tone. Front. Pharmacol. 2022, 13, 917917. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Thapaliya, S.; Runkana, A.; Yang, Y.; Tsien, C.; Mohan, M.L.; Narayanan, A.; Eghtesad, B.; Mozdziak, P.E.; McDonald, C.; et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 18162–18167. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Wang, K.K.; Huang, Q.; Cheng, F.; Huang, F.; Liu, W.W. Nucleolin mediates LPS-induced expression of inflammatory mediators and activation of signaling pathways. Curr. Med. Sci. 2020, 40, 646–653. [Google Scholar] [CrossRef]
- Girvan, A.C.; Teng, Y.; Casson, L.K.; Thomas, S.D.; Juliger, S.; Ball, M.W.; Klein, J.B.; Pierce, W.M., Jr.; Barve, S.S.; Bates, P.J. AGRO100 inhibits activation of nuclear factor-κB (NF-κB) by forming a complex with NF-κB essential modulator (NEMO) and nucleolin. Mol. Cancer Ther. 2006, 5, 1790–1799. [Google Scholar] [CrossRef]
- Guttridge, D.C.; Mayo, M.W.; Madrid, L.V.; Wang, C.Y.; Baldwin, A.S., Jr. NF-κB-induced loss of MyoD messenger RNA: Possible role in muscle decay and cachexia. Science 2000, 289, 2363–2366. [Google Scholar] [CrossRef]
- Takagi, M.; Absalon, M.J.; McLure, K.G.; Kastan, M.B. Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell 2005, 123, 49–63. [Google Scholar] [CrossRef]
- Chen, J.; Guo, K.; Kastan, M.B. Interactions of nucleolin and ribosomal protein L26 (RPL26) in translational control of human p53 mRNA. J. Biol. Chem. 2012, 287, 16467–16476. [Google Scholar] [CrossRef] [PubMed]
- Cerone, M.A.; Marchetti, A.; Bossi, G.; Blandino, G.; Sacchi, A.; Soddu, S. p53 is involved in the differentiation but not in the differentiation-associated apoptosis of myoblasts. Cell Death Differ. 2000, 7, 506–508. [Google Scholar] [CrossRef]
- Porrello, A.; Cerone, M.A.; Coen, S.; Gurtner, A.; Fontemaggi, G.; Cimino, L.; Piaggio, G.; Sacchi, A.; Soddu, S. p53 regulates myogenesis by triggering the differentiation activity of pRb. J. Cell Biol. 2000, 151, 1295–1304. [Google Scholar] [CrossRef]
- Pal, S.; Bhattacharjee, A.; Ali, A.; Mandal, N.C.; Mandal, S.C.; Pal, M. Chronic inflammation and cancer: Potential chemoprevention through nuclear factor kappa B and p53 mutual antagonism. J. Inflamm. 2014, 11, 23. [Google Scholar] [CrossRef]
- VanderVeen, B.N.; Murphy, E.A.; Carson, J.A. The impact of immune cells on the skeletal muscle microenvironment during cancer cachexia. Front. Physiol. 2020, 11, 1037. [Google Scholar] [CrossRef] [PubMed]
- Nihashi, Y.; Miyoshi, M.; Umezawa, K.; Shimosato, T.; Takaya, T. Identification of a novel osteogenetic oligodeoxynucleotide (osteoDN) that promotes osteoblast differentiation in a TLR9-independent manner. Nanomaterials 2022, 12, 1680. [Google Scholar] [CrossRef] [PubMed]
- Nihashi, Y.; Umezawa, K.; Shinji, S.; Hamaguchi, Y.; Kobayashi, H.; Kono, T.; Ono, T.; Kagami, H.; Takaya, T. Distinct cell proliferation, myogenic differentiation, and gene expression in skeletal muscle myoblasts of layer and broiler chickens. Sci. Rep. 2019, 9, 16527. [Google Scholar] [CrossRef]
- Nihashi, Y.; Yamamoto, M.; Shimosato, T.; Takaya, T. Myogenetic oligodeoxynucleotide restores differentiation and reverses inflammation of myoblasts aggravated by cancer-conditioned medium. bioRxiv 2021, 469038. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).