Treadmill Stepping after Epidural Stimulation Cessation in Decerebrated Cats
Abstract
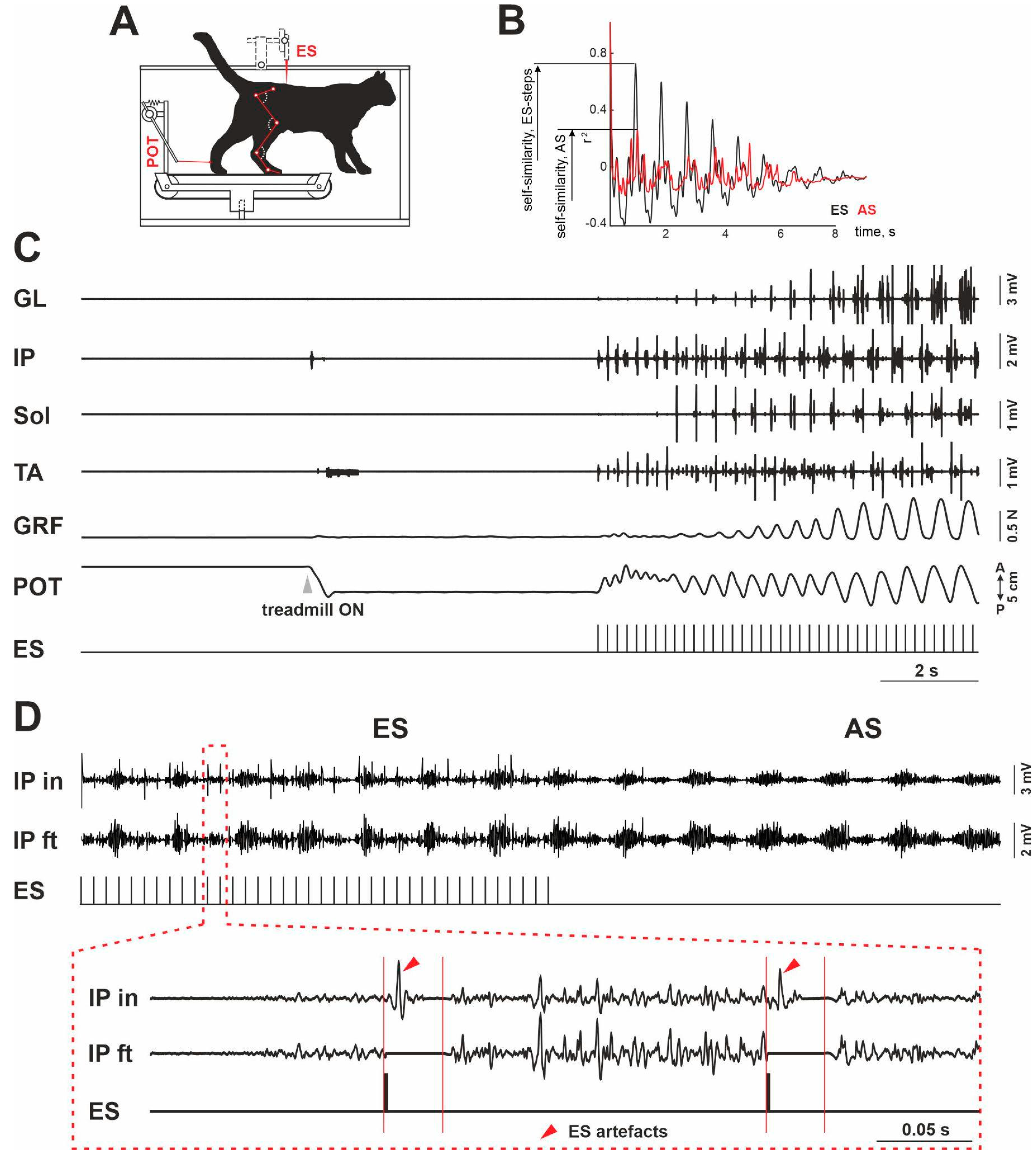
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kiehn, O. Decoding the organization of spinal circuits that control locomotion. Nat. Rev. Neurosci. 2016, 17, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Grillner, S.; El Manira, A. Current principles of motor control, with special reference to vertebrate locomotion. Physiol. Rev. 2020, 100, 271–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlovsky, G.N.; Deliagina, T.G.; Grillner, S. Neuronal Control of Locomotion; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Rossignol, S. Neuronal control of stereotypic limb movements. In Handbook of Physiology; Rowell, L.B., Sheperd, J.T., Eds.; Oxford UP: New York, NY, USA, 1996; pp. 173–216. [Google Scholar]
- Gerasimenko, Y.; Roy, R.R.; Edgerton, V.R. Epidural stimulation: Comparison of the spinal circuits that generate and control locomotion in rats, cats and humans. Exp. Neurol. 2008, 209, 417–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgerton, V.R.; Harkema, S. Epidural stimulation of the spinal cord in spinal cord injury: Current status and future challenges. Expert Rev. Neurother. 2011, 11, 1351–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shik, M.L.; Severin, F.V.; Orlovskiĭ, G.N. Control of walking and running by means of electric stimulation of the midbrain. Biofizika 1966, 11, 659–666. [Google Scholar] [PubMed]
- Iwahara, T.; Atsuta, Y.; Garcia-Rill, E.; Skinner, R.D. Spinal cord stimulation-induced locomotion in the adult cat. Brain Res. Bull. 1992, 28, 99–105. [Google Scholar] [CrossRef]
- Musienko, P.E.; Bogacheva, I.N.; Gerasimenko, Y.P. Significance of peripheral feedback in the generation of stepping movements during epidural stimulation of the spinal cord. Neurosci. Behav. Physiol. 2007, 37, 181–190. [Google Scholar] [CrossRef]
- Gerasimenko, Y.; Musienko, P.; Bogacheva, I.; Moshonkina, T.; Savochin, A.; Lavrov, I.; Roy, R.R.; Edgerton, V.R. Propriospinal bypass of the serotonergic system that can facilitate stepping. J. Neurosci. 2009, 29, 5681–5689. [Google Scholar] [CrossRef] [Green Version]
- Musienko, P.E.; Zelenin, P.V.; Lyalka, V.F.; Gerasimenko, Y.P.; Orlovsky, G.N.; Deliagina, T.G. Spinal and supraspinal control of the direction of stepping during locomotion. J. Neurosci. 2012, 32, 17442–17453. [Google Scholar] [CrossRef] [Green Version]
- Merkulyeva, N.; Veshchitskii, A.; Gorsky, O.; Pavlova, N.; Zelenin, P.V.; Gerasimenko, Y.; Deliagina, T.G.; Musienko, P. Distribution of spinal neuronal networks controlling forward and backward locomotion. J. Neurosci. 2018, 38, 4695–4707. [Google Scholar] [CrossRef]
- Merkulyeva, N.; Lyakhovetskii, V.; Veshchitskii, A.; Gorskii, O.; Musienko, P. Rostrocaudal distribution of the C-Fos-immunopositive spinal network defined by muscle activity during locomotion. Brain Sci. 2021, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Bogacheva, I.N.; Kucher, V.I.; Shcherbakova, N.A.; Musienko, P.E.; Gerasimenko, I.P. Mathematical modeling of the mechanisms of locomotory pattern formation under epidural spinal cord stimulation with consideration of peripheral feedback. Biofizika 2005, 50, 1125–1130. (In Russian) [Google Scholar] [PubMed]
- Kim, S.A.; Heinze, K.G.; Schwille, P. Fluorescence correlation spectroscopy in living cells. Nat. Methods 2007, 4, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Nichols, T.R. The organization of heterogenic reflexes among muscles crossing the ankle joint in the decerebrate cat. J. Physiol. 1989, 410, 463–477. [Google Scholar] [CrossRef]
- Engberg, I.; Lundberg, A. An electromyographic analysis of muscular activity in the hindlimb of the cat during unrestrained locomotion. Acta Physiol. Scand. 1969, 75, 614–630. [Google Scholar] [CrossRef]
- Burke, R.E.; Levine, D.N.; Salcman, M.; Tsairis, P. Motor units in cat soleus muscle: Physiological, histochemical and morphological characteristics. J. Physiol. 1974, 238, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Côté, M.-P.; Murray, L.M.; Knikou, M. Spinal control of locomotion: Individual neurons, their circuits and functions. Front. Physiol. 2018, 9, 784. [Google Scholar] [CrossRef] [Green Version]
- Walmsley, B.; Hodgson, J.A.; Burke, R.E. Forces produced by medial gastrocnemius and soleus muscles during locomotion in freely moving cats. J. Neurophysiol. 1978, 41, 1203–1216. [Google Scholar] [CrossRef]
- Gregor, R.J.; Smith, D.W.; Prilutsky, B.I. Mechanics of slope walking in the cat: Quantification of muscle load, length change, and ankle extensor EMG patterns. J. Neurophysiol. 2006, 95, 1397–1409. [Google Scholar] [CrossRef] [Green Version]
- Alaimo, M.A.; Smith, J.L.; Roy, R.R.; Edgerton, V.R. EMG activity of slow and fast ankle extensors following spinal cord transection. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 56, 1608–1613. [Google Scholar] [CrossRef]
- Hensbergen, E.; Kernell, D. Daily durations of spontaneous activity in cat’s ankle muscles. Exp. Brain Res. 1997, 115, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Mildren, R.L.; Peters, R.M.; Carpenter, M.G.; Blouin, J.-S.; Inglis, J.T. Soleus single motor units show stronger coherence with Achilles tendon vibration across a broad bandwidth relative to medial gastrocnemius units while standing. J. Neurophysiol. 2019, 122, 2119–2129. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Hagio, S.; Kibushi, B.; Moritani, T.; Kouzaki, M. Comparison of muscle synergies for running between different foot strike patterns. PLoS ONE 2017, 12, e0171535. [Google Scholar] [CrossRef] [PubMed]
- Klishko, A.N.; Akyildiz, A.; Mehta-Desai, R.; Prilutsky, B.I. Common and distinct muscle synergies during level and slope locomotion in the cat. J. Neurophysiol. 2021, 126, 493–515. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.A. The relationship between soleus and gastrocnemius muscle activity in conscious cats—A model for motor unit recruitment? J. Physiol. 1983, 337, 553–562. [Google Scholar] [CrossRef] [Green Version]
- Kaya, M.; Leonard, T.R.; Herzog, W. Control of ground reaction forces by hindlimb muscles during cat locomotion. J. Biomech. 2006, 39, 2752–2766. [Google Scholar] [CrossRef]
- Smith, J.L.; Carlson-Kuhta, P.; Trank, T.V. Forms of forward quadrupedal locomotion. III. A comparison of posture, hindlimb kinematics, and motor patterns for downslope and level walking. J. Neurophysiol. 1998, 79, 1702–1716. [Google Scholar] [CrossRef]
- Shik, M.L.; Orlovsky, G.N. Neurophysiology of locomotor automatism. Physiol. Rev. 1976, 56, 465–501. [Google Scholar] [CrossRef]
- Frigon, A.; Thibaudier, Y.; Hurteau, M.-F. Modulation of forelimb and hindlimb muscle activity during quadrupedal tied-belt and split-belt locomotion in intact cats. Neuroscience 2015, 290, 266–278. [Google Scholar] [CrossRef]
- Hník, P.; Vejsada, R.; Goldspink, D.F.; Kasicki, S.; Krekule, I. Quantitative evaluation of electromyogram activity in rat extensor and flexor muscles immobilized at different lengths. Exp. Neurol. 1985, 88, 515–528. [Google Scholar] [CrossRef]
- Schomburg, E.D. Spinal sensorimotor systems and their supraspinal control. Neurosci. Res. 1990, 7, 265–340. [Google Scholar] [CrossRef]
- Takakusaki, K.; Chiba, R.; Nozu, T.; Okumura, T. Brainstem control of locomotion and muscle tone with special reference to the role of the mesopontine tegmentum and medullary reticulospinal systems. J. Neural Transm. 2016, 123, 695–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimamura, M.; Kogure, I.; Wada, S. Reticular neuron activities associated with locomotion in thalamic cats. Brain Res. 1982, 231, 51–62. [Google Scholar] [CrossRef]
- Drew, T.; Dubuc, R.; Rossignol, S. Discharge patterns of reticulospinal and other reticular neurons in chronic, unrestrained cats walking on a treadmill. J. Neurophysiol. 1986, 55, 375–401. [Google Scholar] [CrossRef]
- Rybak, I.A.; Stecina, K.; Shevtsova, N.A.; McCrea, D.A. Modelling spinal circuitry involved in locomotor pattern generation: Insights from the effects of afferent stimulation. J. Physiol. 2006, 577, 641–658. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merkulyeva, N.; Lyakhovetskii, V.; Gorskii, O.; Musienko, P. Treadmill Stepping after Epidural Stimulation Cessation in Decerebrated Cats. Muscles 2022, 1, 102-110. https://doi.org/10.3390/muscles1020011
Merkulyeva N, Lyakhovetskii V, Gorskii O, Musienko P. Treadmill Stepping after Epidural Stimulation Cessation in Decerebrated Cats. Muscles. 2022; 1(2):102-110. https://doi.org/10.3390/muscles1020011
Chicago/Turabian StyleMerkulyeva, Natalia, Vsevolod Lyakhovetskii, Oleg Gorskii, and Pavel Musienko. 2022. "Treadmill Stepping after Epidural Stimulation Cessation in Decerebrated Cats" Muscles 1, no. 2: 102-110. https://doi.org/10.3390/muscles1020011
APA StyleMerkulyeva, N., Lyakhovetskii, V., Gorskii, O., & Musienko, P. (2022). Treadmill Stepping after Epidural Stimulation Cessation in Decerebrated Cats. Muscles, 1(2), 102-110. https://doi.org/10.3390/muscles1020011






