Impact on the Rheological Properties and Amino Acid Compositions of the Industrial Evaporation of Waste Vinasse in the Production of Nutritional Supplements for Livestock
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Samples and Analysis of Physicochemical Properties
2.2. Rheological Analysis of the Quintuple-Effect Evaporator System
2.3. Analysis of Proteins, Amino Acids, and Nucleic Acids
2.4. Statistical Techniques
3. Results and Discussion
3.1. Physicochemical Characterization of Vinasse
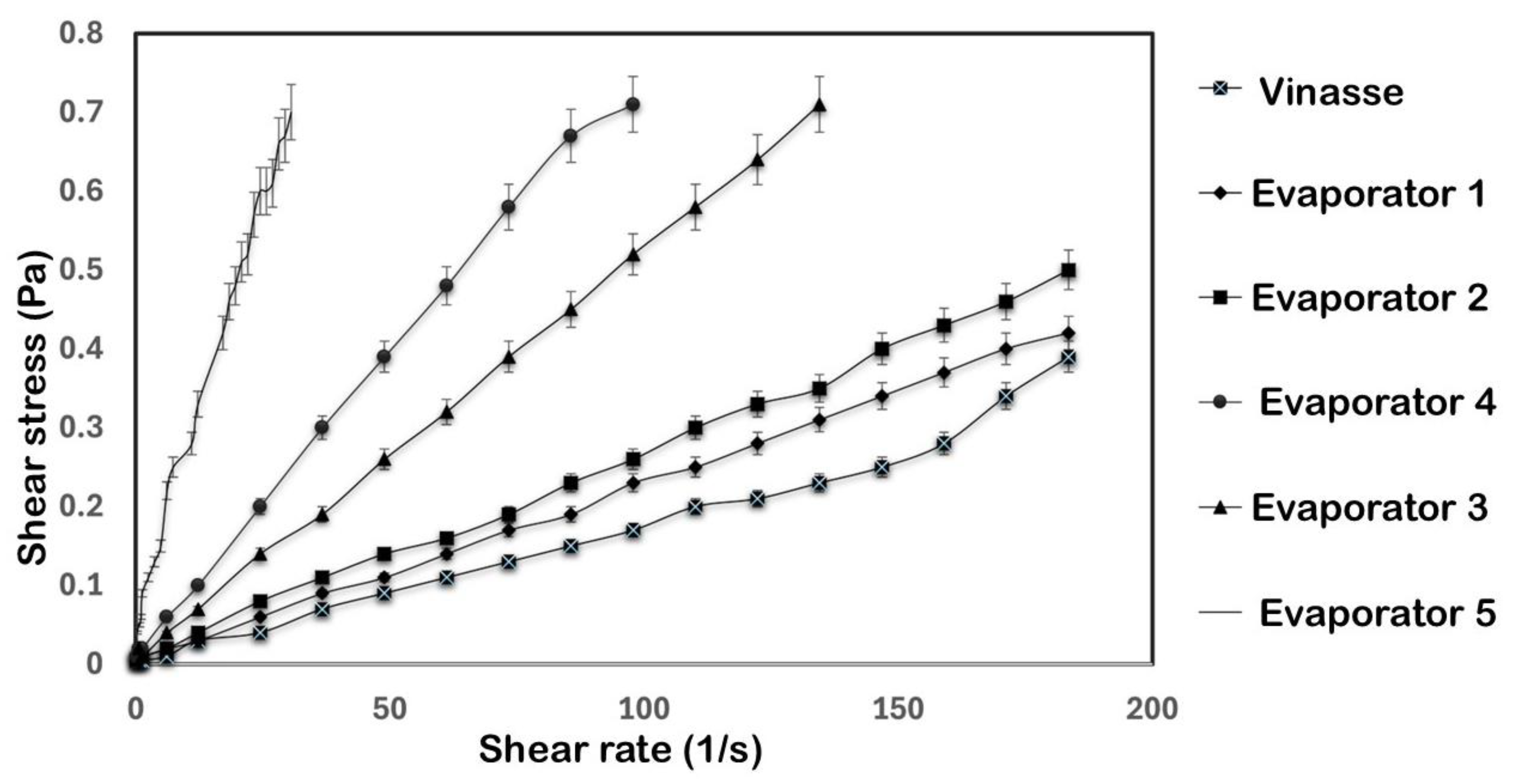
3.2. Rheological Characterization of the Evaporation Stages
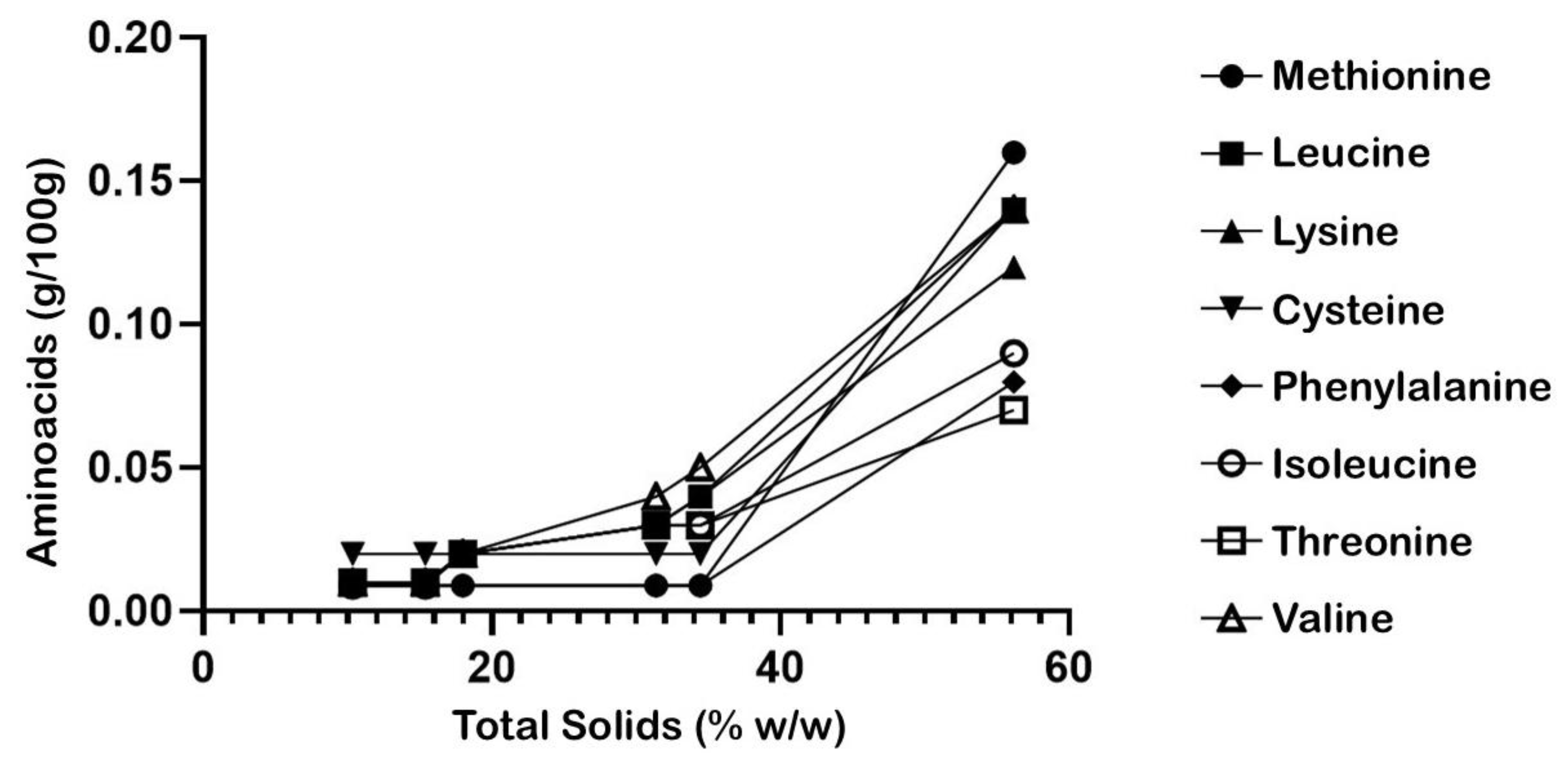
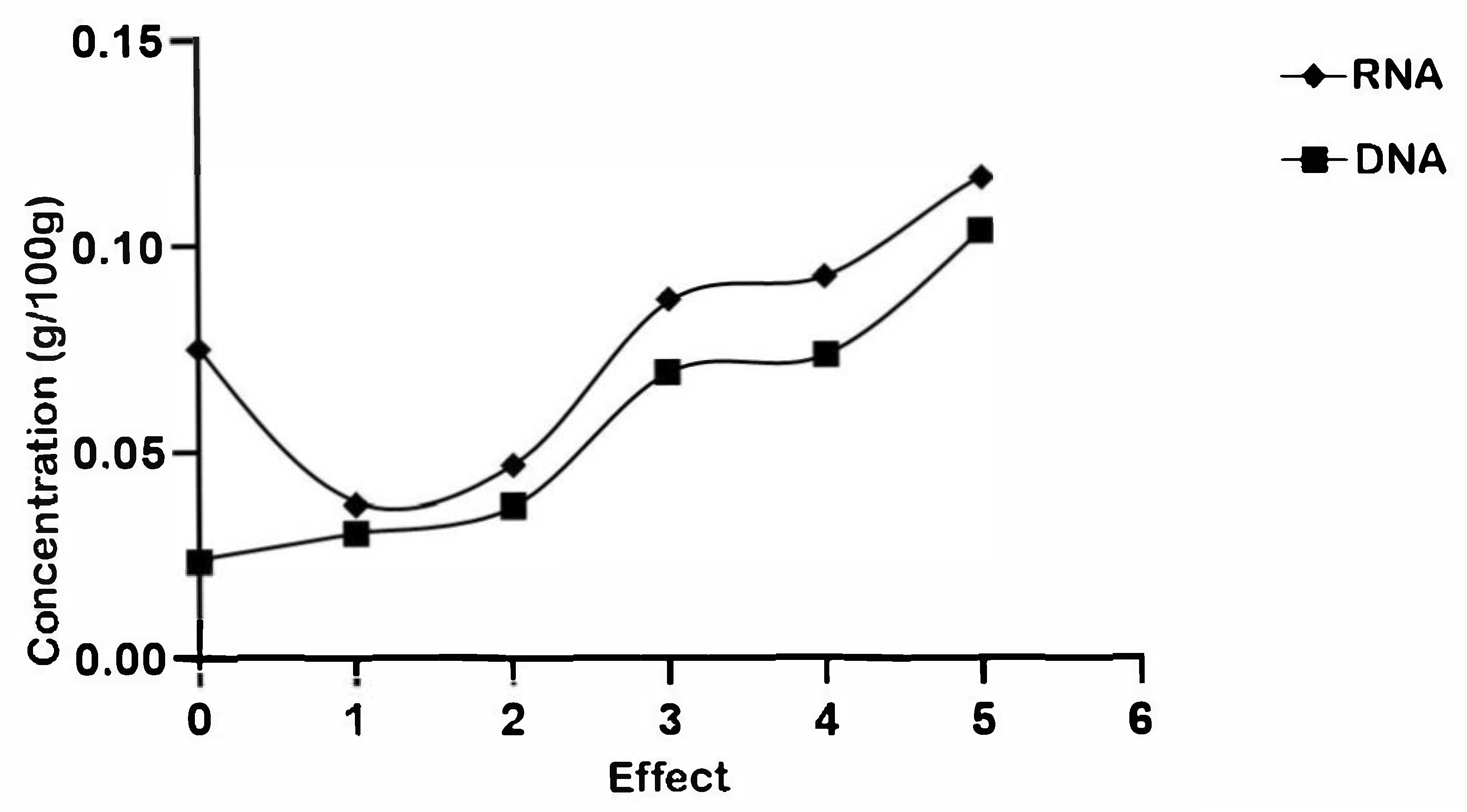
3.3. Physicochemical and Chemical Structure Changes in the Vinasse Evaporation Process
| Amino Acid | Evaporated Vinasse | Baker et al. (1994) [53] | Wu et al. (2014) [54] |
|---|---|---|---|
| Ala | 0.22 | 0.9 | |
| Gly | 0.1 | 0.6 | 1 |
| Val | 0.14 | 0.69 | 0.78 |
| Leu | 0.14 | 0.98 | 1.52 |
| Ile | 0.09 | 0.6 | 0.7 |
| Thr | 0.07 | 0.6 | 0.61 |
| Ser | 0.09 | 0.8 | |
| Pro | 0.07 | 0.4 | 1.31 |
| Asp | 1.29 | 1.03 | |
| Met | 0.16 | 0.325 | 0.38 |
| Glu | 1.24 | 12 | 1.45 |
| Phe | 0.08 | 0.5 | 0.53 |
| Lys | 0.12 | 0.9 | 0.82 |
| His | 0.08 | 0.32 | 0.41 |
| Tyr | 0.05 | 0.45 | 0.41 |
| Cys | 0.14 | 0.325 | 0.29 |
| Total amino acids | 4.08 | 19.8 |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christofoletti, C.A.; Escher, J.P.; Correia, J.E.; Urbano-Marinho, J.F.; Fontanetti, C.S. Sugarcane vinasse: Environmental implications of its use. Waste Manag. 2013, 33, 2752–2761. [Google Scholar] [CrossRef]
- España-Gamboa, E.; Mijangos-Cortes, J.; Barahona-Pérez, L.; Domínguez-Maldonado, J.; Hernández-Zárate, G.; Alzate-Gaviria, L. Vinasses: Characterization and treatments. Waste Manag. Res. 2011, 29, 1235–1250. [Google Scholar] [CrossRef]
- Parnaudeau, V.; Condom, N.; Oliver, R.; Cazevieille, P.; Recous, S. Vinasse organic matter quality and mineralization potential, as influenced by raw material, fermentation and concentration proceses. Bioresour. Technol. 2008, 99, 1553–1562. [Google Scholar] [CrossRef]
- Contreras-Contreras, J.A.; Bernal-González, M.; Solís-Fuentes, J.A.; Durán-Domínguez-de-Bazúa, M.C. Polyphenols from Sugarcane Vinasses, Quantification, and Removal Using Activated Carbon After Biochemical Treatment in Laboratory Scale Thermophilic Upflow Anaerobic Sludge Blanket Reactors. Water Air Soil Pollut. 2020, 231, 401. [Google Scholar] [CrossRef]
- Fuess, L.T.; Rodrigues, I.J.; Garcia, M.L. Fertirrigation with sugarcane vinasse: Foreseeing potential impacts on soil and water resources through vinasse characterization. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2017, 52, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Mijangos-Cortes, J.O.; González-Muñoz, M.E.; España-Gamboa, E.I.; Domínguez-Maldonado, J.A.; Alzate-Gaviria, L. Fertigation of sweet sorghum (Sorghum bicolor L. Moench.) in laboratory and nursery assays with treated vinasses of hidrated ethanol of UASB reactor. Rev. Mex. Ing. Quim. 2014, 13, 713–722. [Google Scholar]
- Ferraresi de Araujo, G.J.; Borges de Oliveira, S.V.W. Analysis of financial and economic feasibility of the use of vinasse for electricity generation in Brazil. Cad. EBAPE Br. 2020, 18, 936–955. [Google Scholar] [CrossRef]
- Vázquez, H.J.; Dacosta, O. Alcoholic fermentation: An option for renewable energy production from agricultural residues. Ing. Investig. Tecnol. 2007, 8, 249–259. [Google Scholar]
- Santos-Gomes, M.T.M.; Eça, K.S.; Viotto, L.A. Concentração da vinhaça por microfiltração seguida de nanofiltração com membranas. Pesq. Agropec. Bras. 2011, 46, 633–638. [Google Scholar] [CrossRef]
- Carrilho, E.N.V.M.; Labuto, G.; Kamogawa, M.Y. Chapter 2—Destination of Vinasse, a Residue From Alcohol Industry. Resource Recovery and Prevention of Pollution. In Environmental Materials and Waste, 1st ed.; Prasad, M.N.V., Shih, K., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 21–43. [Google Scholar]
- Cortés-Rodríguez, E.F.; Fukushima, N.A.; Palacios-Bereche, R.; Ensinas, A.V.; Nebra, S.A. Vinasse concentration and juice evaporation system integrated to the conventional ethanol production process from sugarcane—Heat integration and impacts in cogeneration system. Renew. Energy 2018, 115, 474–488. [Google Scholar] [CrossRef]
- Fukushima, N.A.; Palacios-Bereche, M.C.; Palacios-Bereche, R.; Nebra, S.A. Energy analysis of the ethanol industry considering vinasse concentration and incineration. Renew. Energy 2019, 142, 96–109. [Google Scholar] [CrossRef]
- Da Silva, A.; Rossetto, R.; Bombecini, J.; Piemonte, M.; Muraoka, T. Nitrogen Mineralization from Sugarcane Vinasse. J. Plant Nutr. 2014, 37, 1227–1236. [Google Scholar] [CrossRef]
- Atenodoro-Alonso, J.; Ruíz-Espinoza, J.E.; Alvarado-Lassman, A.; Martínez-Sibaja, A.; Martínez-Delgadillo, S.A.; Méndez-Contreras, J.M. The enhanced anaerobic degradability and kinetic parameters of pathogenic inactivation of wastewater sludge using pre- and post- thermal treatments part 2. Rev. Mex. Ing. Chim. 2015, 14, 311–319. [Google Scholar]
- Chacua, L.M.; Ayala, G.; Rojas, H.; Agudelo, A.C. Mathematical models for prediction of rheological parameters in vinasses derived from sugar cane. Int. Agrophys. 2016, 30, 135–141. [Google Scholar] [CrossRef]
- Torres- Maza, A.; Yupanqui-Bacilio, C.; Castro, V.; Aguirre, E.; Villanueva, E.; Rodríguez, G. Comparison of the hydrocolloids Nostoc commune and Nostoc sphaericum: Drying, spectroscopy, rheology and application in nectar. Sci. Agropecu. 2020, 11, 583–589. [Google Scholar] [CrossRef]
- Zaim, S.; Cherkaoui, O.; Rchid, H.; Nmila, R.; El Moznine, R. Rheological investigations of water-soluble polysaccharides extracted from Moroccan seaweed Cystoseira myriophylloides algae. Polym. Renew. Resour. 2020, 11, 49–63. [Google Scholar] [CrossRef]
- Nordqvist, D.; Vilgis, T.A. Rheological Study of the Gelation Process of Agarose-Based Solutions. Food Biophys. 2011, 6, 450–460. [Google Scholar] [CrossRef]
- Ahmed, J.; Ptaszek, P.; Basu, S. Chapter 1—Food Rheology: Scientific Development and Importance to Food Industry. In Advances in Food Rheology and Its Applications, 1st ed.; Ahmed, J., Ed.; Woodhead Publishing: Cambridge, MA, USA, 2023; pp. 1–22. [Google Scholar]
- Rodríguez-Sandoval, E.; Fernández-Quintero, A.; Ayala-Aponte, A. Reología y textura de masas: Aplicaciones en trigo y maíz. Ing. Investig. 2005, 25, 72–78. [Google Scholar]
- NMX-AA-003-1980; Aguas Residuales—Muestreo. Diario Oficial de la Federación: Mexico City, Mexico, 1980.
- NMX-AA-008-SCFI-2016; Análisis de Aguas—Medición del pH en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2016.
- NMX-AA-093-SCFI-2018; Análisis de Agua—Medición de la Conductividad Eléctrica en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2018.
- NMX-AA-030-SCFI-2001; Análisis de Agua—Determinación de la Demanda Química de Oxígeno en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2001.
- NMX-AA-012-SCFI-2001; Análisis de Agua—Determinación de Oxígeno Disuelto en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2001.
- NOM-CH-50-1984; Métodos de Medición—Determinación de la Densidad de Líquidos. Principio de Arquímedes. Diario Oficial de la Federación: Mexico City, Mexico, 1984.
- NMX-AA-038-SCFI-2001; Análisis de Agua—Determinación de Turbiedad en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2001.
- NMX-F-312-NORMEX-2016; Alimentos—Determinación de Azúcares Reductores en Alimentos y Bebidas No Alcohólicas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2016.
- NMX-AA-135-SCFI-2007; Potabilización del Agua Para Uso y Consumo Humano—Poliaminas—Especificaciones y Métodos de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2007.
- NMX-AA-007-SCFI-2013; Análisis de Agua—Medición de la Temperatura en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2013.
- NMX-AA-034-SCFI-2015; Análisis de Agua—Medición de Sólidos y Sales Disueltas en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2015.
- Ibarra-Camacho, R.; León-Duharte, L.; Osoria-Leyva, A. Caracterización físico-química de vinaza de destilerías. Rev. Cub. Quím. 2019, 31, 246–257. [Google Scholar]
- Zúñiga-Cerón, V.; Gandini-Ayerbe, M.A. Caracterización ambiental de las vinazas de residuos de caña de azúcar resultantes de la producción de etanol. DYNA 2013, 80, 24–131. [Google Scholar]
- Parsaee, M.; Kiani, M.K.D.; Karimi, K. A review of biogas production from sugarcane vinasse. Biomass Bioenergy 2019, 122, 117–125. [Google Scholar] [CrossRef]
- Aristizábal-Alzate, C.E. Caracterización físico-química de una vinaza resultante de la producción de alcohol de una industria licorera, a partir del aprovechamiento de la caña de azúcar. Ing. USBMed. 2015, 6, 36–41. [Google Scholar] [CrossRef]
- González, J.A.; Buedo, S.E.; Prado, F.E.; Álvarez, S. Efecto de la vinaza sobre el crecimiento y productividad de la Soja (Glycine max) en condiciones semicontroladas. Boletín Soc. Argent. Botánica 2018, 53, 597. [Google Scholar] [CrossRef]
- Tapie, W.A.; Prato-García, D.; Sánchez-Guerrero, H. Biodegradación de vinazas de caña de azúcar mediante el hongo de pudrición blanca Pleurotus ostreatus en un reactor de lecho empacado. Trop. Subtrop. Agroecosystems 2016, 19, 145–150. [Google Scholar] [CrossRef]
- Brossard-Perez, L.E.; Bezzon, G.; Olivares-Gómez, E.; Cortez, L.A.B. Use of a rotational bench viscometer to study the influence of temperature and agitation speed on vinasse viscosity. Braz. J. Chem. Eng. 2000, 17, 133–141. [Google Scholar] [CrossRef]
- Dávila-Rincón, J.; Marriaga-Cabrales, N.; Machuca-Martínez, F. Remoción de sólidos totales de vinazas por electrocoagulación—Electroflotación. Dyna 2009, 72, 41–47. [Google Scholar]
- Gouvêa de Godoi, L.A.; Rosseto-Camiloti, P.; Bernardes, A.N.; Sanches, B.L.S.; Rodrigues-Torres, A.P.; Gomes, A.d.C.; Silva-Botta, L. Seasonal variation of the organic and inorganic composition of sugarcane vinasse: Main implications for its environmental uses. Environ. Sci. Pollut. Res. 2019, 26, 29267–29282. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena, 2nd ed.; Limusa Wiley: St. Milwaukee, WI, USA, 2006; p. 281. [Google Scholar]
- Monsalve, A.G. Reología, la ciencia que estudia el movimiento de fluidos. Remetallica 2010, 30, 21–27. [Google Scholar]
- Rapp, B.E. Microfluidics, Modeling, Mechanics and Mathematics, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 243–263. [Google Scholar]
- Wu, G. Amino Acids Biochemistry and Nutrition, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Ayala-Limaylla, F.; Farfán-Farfán, F.; Díaz-Coahila, D. Efecto de la suplementación de aminoácidos esenciales y no esenciales en la nutrición de caninos. Rev. Investig. Vet. Perú. 2023, 34, e21831. [Google Scholar] [CrossRef]
- Kumar, V.; Kaladharan, P. Amino acids in the seaweeds as an alternate source of protein for animal feed. J. Mar. Biol. Assoc. India 2007, 49, 35–40. [Google Scholar]
- Gao, J.; Liu, Z.; Wang, C.; Ma, L.; Chen, Y.; Li, T. Effects of Dietary Protein Level on the Microbial Composition and Metabolomic Profile in Postweaning Piglets. Oxid. Med. Cell. Longev. 2022, 2022, 3355687. [Google Scholar] [CrossRef]
- Wu, Z.; Hou, Y.; Hu, S.; Bazer, F.W.; Meininger, C.J.; McNeal, C.J.; Wu, G. Catabolism and safety of supplemental L-arginine in animals. Amino Acids 2016, 48, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Rahman, M.A.U.; Yang, H.; Shao, T.; Qiu, Q.; Su, H.; Cao, B. Effect of increased dietary crude protein levels on production performance, nitrogen utilisation, blood metabolites and ruminal fermentation of Holstein bulls. Asian-Australas. J. Anim. Sci. 2018, 31, 1643–1653. [Google Scholar] [CrossRef] [PubMed]
- Karau, A.; Grayson, I. Amino acids in human and animal nutrition. Adv. Biochem. Eng. Biotechnol. 2014, 143, 189–228. [Google Scholar] [CrossRef]
- Sanches-Muros, M.J.; Barroso, F.G.; Manzano-Aguliaro, F. Insect meal as renewable spurce of food for animal feeding: A review. J. Clean. Prod. 2014, 65, 16–27. [Google Scholar] [CrossRef]
- Cavagnero, S.; Debe, D.A.; Zhou, Z.H.; Adams, M.W.W.; Chan, S.I. Kinetic Role of Electrostatic Interactions in the Unfolding of Hyperthermophilic and Mesophilic Rubredoxins. Biochemistry 1998, 37, 3369–3376. [Google Scholar] [CrossRef]
- Sun, X.S. 9—Thermal and Mechanical Properties of Soy Proteins. In Bio-Based Polymers and Composites, 1st ed.; Wool, R.P., Sun, X.S., Eds.; Academic Press: San Diego, CA, USA, 2005; pp. 292–326. [Google Scholar]
- Bhagavan, N.V. Chapter 4—Three-Dimensional Structure of Proteins. In Medical Biochemistry, 4th ed.; Bhagavan, N.V., Ed.; Academic Press: San Diego, CA, USA, 2002; pp. 51–65. [Google Scholar]
- Matsuura, Y.; Takehira, M.; Joti, Y.; Ogasahara, K.; Tanaka, T.; Ono, N.; Kunishima, N.; Yutani, K. Thermodynamics of protein denaturation at temperatures over 100 ºC: CutA1 mutant proteins substituted with hydrophobic and changed residues. Sci. Rep. 2015, 5, 15545. [Google Scholar] [CrossRef]
- Pelegrine, D.H.G.; Gasparetto, C.A. Whey proteins solubility as function of temperature and pH. LWT Food Sci. Technol. 2005, 38, 77–80. [Google Scholar] [CrossRef]
- Gonçalves, A.D.; Alexander, C.; Roberts, C.J.; Spain, S.G.; Uddin, S.; Allen, S. The effect of protein concentration on the viscosity of a recombinant albumin solution formulation. RSC Adv. 2016, 18, 15143–15154. [Google Scholar] [CrossRef]
- Cantú-Lozano, D.; Velázquez-Macario, M.V.; Vallejo-Cantú, N.A.; Mauro, M.; Del Bianchi, V.L.; Telis-Romero, J. Rheological behaviour of vinasses from a mexican bioethanol factory. In Proceedings of the International Society of Sugar Cane Technologists, Veracruz, Mexico, 7–11 March 2010; p. 27. [Google Scholar]
- Lewin, B. Genes, 2nd ed.; Editorial Reverté: Barcelona, España, 1996. [Google Scholar]
- Camacho, J.E.; Gómez, M.I.; Villamizar, L.F. Efecto de la temperatura y de dos procesos de secado sobre la actividad insecticida de un nucleopoliedrovirus de Spodoptera frugiperda. Rev. Mex. Ing. Chim. 2013, 12, 437–450. [Google Scholar]
- Casas-Alencáster, N.B.; Pardo-García, D.G. Análisis de perfil de textura y propiedades de relajación de geles de mezclas almidón de maíz ceroso entrecruzado-gelana. Rev. Mex. Ing. Chim. 2005, 4, 107–121. [Google Scholar]
- Carvajal-Carvajal, C. El ácido úrico: De la gota y otros males. Med. Leg. Costa Rica 2016, 33, 182–189. [Google Scholar]
- Waititu, S.M.; Heo, J.M.; Patterson, R.; Nyachoti, C.M. Dietary yeast-based nucleotides as an alternative to in-feed antibiotics in promoting growth performance and nutrient utilization in weaned pigs. Can. J. Anim. Sci. 2016, 96, 289–293. [Google Scholar] [CrossRef]
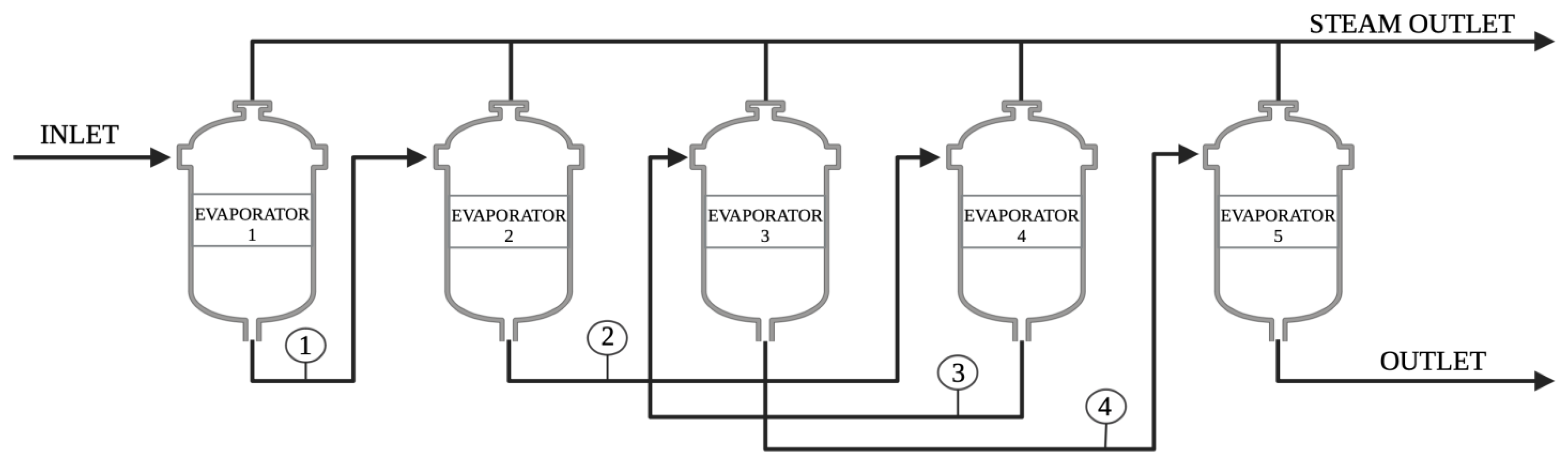

| Parameter | Regulation | Equipment |
|---|---|---|
| pH | NMX-AA-008-SCFI-2016 [22] | Hanna® potentiometer HI2002-01 Model, Mexico City, Mexico |
| Conductivity | NMX-AA-093-SCFI-2018 [23] | Conductronic® potentiometer PC45 Model, Mexico City, Mexico |
| Total COD | NMX-AA-030-SCFI-2012 [24] | ThermoScientific® spectrophotometer UV-VIS Genesys 10S Model, Mexico City, Mexico |
| Dissolved oxygen and saturation | NMX-AA-012-SCFI-2001 [25] | Hatch® multiparameter HQ40D model, Mexico City, Mexico |
| Density | NOM-CH-50-1984 [26] | Ohaus® analytical balance Scout model, Mexico City, Mexico |
| Turbidity | NMX-AA-038-SCFI-2001 [27] | Hatch® turbidimeter 2100Q model, Mexico City, Mexico |
| Reducing sugars | NMX-F-312-NORMEX-2016 [28] | Thermofisher Multiskan EX® microplate reader, Mexico City, Mexico |
| Viscosity | NMX-AA-135-SCFI-2007 [29] | Brookfield® viscometer DV2T model, Mexico City, Mexico |
| Temperature | NMX-AA-007-SCFI-2013 [30] | Brannan® thermometer, Mexico City, Mexico |
| TS and TVS | NMX-AA-034-SCFI-2015 [31] | Felisa® stove 4840 model, Mexico City, Mexico |
| Evaporator 1 | Evaporator 2 | Evaporator 3 | Evaporator 4 | Evaporator 5 |
|---|---|---|---|---|
| 80 °C | 80 °C | 78 °C | 34 °C | 67.7 °C |
| Analysis | Value | Units |
|---|---|---|
| pH | 4.73 | NA |
| Conductivity | 3.8 | µS |
| COD | 181.6 | g O2 |
| Dissolved O2 | 0.0619 | g/L |
| Density | 1.0385 | g/mL |
| Turbidity | 469 | NTU |
| Reductor sugars | 104.605 | g/L |
| Viscosity | 2.01 | cP |
| Total Solids | 10.35 | % w/w |
| Evaporator | Temperature (°C) | Bingham | Ostwald–de Waele | Herschel–Bulkley | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 80 | 0.0223 | 0.0019 | 0.9713 | 0.0106 | 0.5798 | 0.8134 | 0.1258 | 0.0001 | 1.5138 | 0.999 |
| 2 | 80 | 0.0265 | 0.002 | 0.9634 | 0.0119 | 0.5701 | 0.7856 | 0.0161 | 0.0001 | 1.6055 | 0.99 |
| 3 | 78 | 0.0091 | 0.0028 | 0.9974 | 0.014 | 0.6576 | 0.9334 | 0.0091 | 0.0028 | 1.0001 | 0.9974 |
| 4 | 34 | 0.006 | 0.0038 | 0.9993 | 0.0158 | 0.6682 | 0.9407 | 0.0089 | 0.0033 | 1.0269 | 0.9994 |
| 5 | 67.7 | 1.088 | 0.0267 | 0.9081 | 0.0933 | 0.6452 | 0.9802 | 0.0372 | 0.1375 | 0.5196 | 0.9712 |
| AMINOGRAM ANALYSIS | ||||||||
|---|---|---|---|---|---|---|---|---|
| AMINO ACID | VINASSE | E1 | E2 | E3 | E4 | E5 | UNITS | METHOD |
| Ala | 0.3 | 0.02 | 0.04 | 0.10 | 0.1 | 0.22 | g/100 g | GC-MS |
| Gly | 0.02 | 0.03 | 0.03 | 0.05 | 0.05 | 0.1 | ||
| Val | 0.01 | 0.01 | 0.02 | 0.05 | 0.04 | 0.14 | ||
| Leu | 0.01 | 0.01 | 0.02 | 0.04 | 0.03 | 0.14 | ||
| Ile | 0.009 | 0.009 | 0.02 | 0.03 | 0.03 | 0.09 | ||
| Thr | 0.01 | 0.01 | 0.02 | 0.03 | 0.03 | 0.07 | ||
| Ser | 0.02 | 0.02 | 0.02 | 0.03 | 0.03 | 0.09 | ||
| Pro | 0.01 | 0.01 | 0.02 | 0.03 | 0.02 | 0.07 | ||
| Asp | 0.38 | 0.42 | 0.44 | 0.86 | 0.89 | 1.29 | ||
| Met | 0.009 | 0.009 | 0.009 | 0.009 | 0.009 | 0.16 | ||
| Glu | 0.24 | 0.22 | 0.4 | 0.77 | 0.8 | 1.24 | ||
| Phe | 0.009 | 0.009 | 0.009 | 0.009 | 0.009 | 0.08 | ||
| Lys | 0.01 | 0.01 | 0.02 | 0.04 | 0.03 | 0.12 | ||
| His | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.08 | ||
| Tyr | 0.009 | 0.009 | 0.009 | 0.009 | 0.009 | 0.05 | ||
| Cys | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.14 | ||
| Amino acids | 1.086 | 0.836 | 1.117 | 2.097 | 2.117 | 4.08 | ||
| Crude Protein | 1.3 | 1.7 | 2.0 | 3.8 | 3.4 | 6.3 | Volumetric essay | |
| Evaporator | DNA (g/L) | RNA (g/L) |
|---|---|---|
| Vinasse | 0.0751 | 0.0238 |
| 1 | 0.0373 | 0.0306 |
| 2 | 0.0466 | 0.0368 |
| 3 | 0.0866 | 0.0690 |
| 4 | 0.0923 | 0.0732 |
| 5 | 0.1158 | 0.1031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Casiano, N.; Ortíz-Sánchez, C.A.; Díaz-Castellanos, K.; Velázquez-Herrera, L.A.; Pérez-Guzmán, S.M.; Hernández-Aguilar, E. Impact on the Rheological Properties and Amino Acid Compositions of the Industrial Evaporation of Waste Vinasse in the Production of Nutritional Supplements for Livestock. Waste 2025, 3, 34. https://doi.org/10.3390/waste3040034
Gutiérrez-Casiano N, Ortíz-Sánchez CA, Díaz-Castellanos K, Velázquez-Herrera LA, Pérez-Guzmán SM, Hernández-Aguilar E. Impact on the Rheological Properties and Amino Acid Compositions of the Industrial Evaporation of Waste Vinasse in the Production of Nutritional Supplements for Livestock. Waste. 2025; 3(4):34. https://doi.org/10.3390/waste3040034
Chicago/Turabian StyleGutiérrez-Casiano, Nayeli, Cesar Antonio Ortíz-Sánchez, Karla Díaz-Castellanos, Luis Antonio Velázquez-Herrera, Solmaría Mandi Pérez-Guzmán, and Eduardo Hernández-Aguilar. 2025. "Impact on the Rheological Properties and Amino Acid Compositions of the Industrial Evaporation of Waste Vinasse in the Production of Nutritional Supplements for Livestock" Waste 3, no. 4: 34. https://doi.org/10.3390/waste3040034
APA StyleGutiérrez-Casiano, N., Ortíz-Sánchez, C. A., Díaz-Castellanos, K., Velázquez-Herrera, L. A., Pérez-Guzmán, S. M., & Hernández-Aguilar, E. (2025). Impact on the Rheological Properties and Amino Acid Compositions of the Industrial Evaporation of Waste Vinasse in the Production of Nutritional Supplements for Livestock. Waste, 3(4), 34. https://doi.org/10.3390/waste3040034






