Abstract
Water hyacinth is an invasive weed that can valorize through the production of hydrolytic enzymes via solid-state culture. This study explores the application of Trichoderma harzianum in producing xylanases and endoglucanases on water hyacinth beds. Laboratory-scale packed-bed column bioreactors (PBCBs) with a capacity of 8 grams of dry mass (gdm) were used to evaluate the effects of temperature (28–36 °C) and initial moisture content (65–80%) on microbial growth and enzyme production. High yields of biomass and enzymes were produced at 30 °C. Moreover, xylanase activity was enhanced in cultures with a moisture content of 65% (~71.24 U/gdm), and endoglucanase activity at 75–80% moisture (~20.13 U/gdm). The operational conditions identified for xylanase production were applied to 6 L bench-scale cross-flow internally stirred bioreactors, packed to 40% capacity with 450 gdm. Two stirring regimes were tested: intermittent and continuous. The results showed that continuous stirring promotes both microbial growth and xylanase activity. In fact, xylanase activity in continuous stirring conditions was comparable to that achieved in PBCBs. Consequently, continuous stirring enables a 56-fold increase in bioreactor capacity without compromising xylanase production. The approaches developed in this study can support the design of large-scale bioprocesses for the valorization of water hyacinth.
1. Introduction
Water hyacinth (Eichhornia crassipes) is an invasive aquatic weed native to the Amazon basin that has spread globally, causing various environmental, economic, and social issues [1]. This weed forms dense mats on water surfaces, blocking light and displacing wildlife, depleting oxygen levels, and reducing aquatic plant life, which negatively impacts fisheries. These mats also limit habitats for water birds and promote breeding grounds for mosquitoes and other disease vectors [2]. Economically, water hyacinth obstructs transport and fishing, damages infrastructure, raises service costs, and disrupts riparian communities. Once established, it is challenging to eradicate water hyacinth [3].
Controlling water hyacinth using chemical herbicides is generally prohibited in aquatic ecosystems due to their nonspecific action. The most common strategy for managing infested areas is mechanical removal [4]. While harvesting floating plants is effective, it requires significant investment in equipment and typically produces a large amount of waste. If this residual biomass is left to decay, it can lead to pollution [5]. However, water hyacinth has a high concentration of hemicellulose, ranging from 24.7% to 49.2% on a dry weight basis, and cellulose, ranging from 15.4% to 18.4%, as well as lignin, ranging from 1.1% to 7.0% [6,7,8], which allows for the integration of its biomass to produce cellulases and xylanases [9].
Cellulases are a complex of enzymes that hydrolyze cellulose, which includes different types of enzymes: Endoglucanases, cellobiohydrolases, and β-glucosidases [10]. Similarly, xylanases involve a complex that involves endo-1,4-β-xylanases, β-xylosidases, and accessory enzymes, such as α-arabinofuranosidases and α-glucuronidases, which degrade xylan [11]. These hydrolytic enzymes are valuable for many economically important processes, including the manufacturing of detergents, food and feed processing, paper and pulp production, and the generation of biofuels, among others [12].
The cost of the carbon source is a crucial factor influencing the final expenses of the enzymatic production process [13]. However, previous studies have demonstrated that efficient production of cellulases and xylanases can be obtained from lignocellulosic waste (straw, bran, bagasse, shoots, and forest waste) by solid-state culture (SSC) using filamentous fungi such as Aspergillus flavus [14], A. fumigatus [15], A. niger [16,17], Myceliophthora thermophila [18], Penicillium echinulatum [19], Trichoderma asperellum [20], T. reesei [21,22], and T. harzianum [23]. Since water hyacinth is abundant and low-cost [4], it is an excellent candidate for enzyme production, which has led to research focused on producing cellulases and xylanases via SSC.
Previous research indicates that fungi from the Trichoderma genus can produce endoglucanases and xylanases when using water hyacinth as a solid substrate. Deshpande et al. [24] utilized a tray bioreactor with a co-culture of Aspergillus niger and Trichoderma reesei growing on water hyacinth, supplemented with Toyoma Ogow medium, whey, and peptone, to achieve xylanase production of 57.20 U per gram of dry mass (gdm). In another study, Arana-Cuenca et al. [25] screened the enzymatic potential of 100 fungal strains using water hyacinth as the sole solid support. They showed that Trichoderma harzianum produced 149.30 U/gdm of xylanase at 108 h of culture and 16.40 U/gdm of endoglucanase at 84 h. Notably, both studies utilized static-bed bioreactors.
Static-bed SSC bioreactors can experience overheating and poor gas exchange as they scale up [26,27]. A viable alternative is the use of mixed-bed bioreactors, which can include rotatory drum and internal stirrer bioreactors (vertical or horizontal) [28]. Both types have been successfully employed in the production of xylanases and endoglucanases while using low agitation velocities (<2 rpm) to avoid critical damage to the mycelium [29,30]. The mixed action in these bioreactors prevents the agglomeration and shrinkage of substrates [31]. However, in internal stirred bioreactors (ISB), the movement of the propellers favors mixing within the bed, enhancing heat removal and gas exchange [28,32]. Moreover, the incorporation of forced aeration into the bioreactor further enhances heat and mass transfer [33]. These features make ISBs a suitable option for bioprocess intensification [34,35,36]. Therefore, in this study, a process to produce T. harzianum enzymes in bench-scale ISBs was established. To achieve this, the incubation temperature and initial moisture content of the bed were determined using laboratory-scale packed bed column bioreactors (PBCBs). The operating conditions established in the PBCBs were applied to evaluate the stirring regime (intermittent and continuous) in the bench-scale ISBs.
The approaches developed in this study may aid in designing large-scale bioprocesses that promote the valorization of water hyacinth or other lignocellulosic waste through the production of hydrolytic enzymes.
2. Materials and Methods
2.1. Microorganism
The PBLA strain of T. harzianum, from the Institute of Biotechnology at the National Autonomous University of Mexico (IBT-UNAM), was used in this study. The microorganism was preserved in cryoprotective beads (Technical Services Consultant Ltd., Heywood, England) at −20 °C.
2.2. Inoculum
The microorganism was propagated in 250 mL Erlenmeyer flasks containing 50 mL of potato dextrose agar (Becton Dickinson Bioxon, Franklin Lakes, NJ, USA) and incubated at 30 °C for 7 days. The conidia produced in each flask were suspended with magnetic stirring in 20 mL of sterile 0.05% (v/v) Tween 80 solution (Sigma-Aldrich, St. Louis, MO, USA) and used to produce mycelium in submerged culture (SmC). For this purpose, 250 mL Erlenmeyer flasks were prepared with 50 mL of a sterile medium composed of 20 g/L yeast extract, 20 g/L peptone, and 40 g/L glucose, adjusted to a pH of 6. The flasks were then inoculated with 1 × 106 conidia/mL and incubated at 30 °C for 72 h in a shaker set at 150 rpm. The biomass produced by SmC was used as inoculum for the SSC processes.
2.3. Solid Substrate
Water hyacinths (E. crassipes) used in this study were collected from the Cuemanco canals in Mexico City (19°16′40.109″ N, 99°6′20.336″ W). The collected plants were washed with tap water, stripped of their roots, and dried at 60 °C for 24 h, resulting in a moisture content of less than 10%. The dehydrated material was then ground using a hammer mill (Brabender Technologie GmbH & Co. KG, Duisburg, Germany) to reduce the particle size to between 1 and 1.5 mm.
This fragmented material was placed in polyethylene bags and impregnated at a mass ratio of 1:1 with a culture medium (IM) composed of 100 g/L glucose, 10 g/L NH4NO3, 4 g/L urea, 0.84 g/L MgSO4·7H2O, 2 g/L CaCl2, 10 g/L peptone, 10 g/L yeast extract, 0.1 g/L FeSO4·7H2O, 0.012 g/L MnSO4·7H2O, 0.02 g/L ZnSO4·7H2O, and 0.01 g/L CoCl2·H2O, enriched with 0.25 M H2SO4. After impregnation, the material was manually homogenized and sterilized at 120 °C for 15 min. Following sterilization, the material was inoculated, and the moisture content was adjusted. A mixture was prepared using a mycelium suspension produced in SmC (15 mL per 100 g of dry water hyacinth) and the IM (without H2SO4) to achieve the moisture content addressed in this study (65%, 70%, 75%, and 80%). This resulting suspension was carefully added to the pretreated water hyacinth, thoroughly mixed, and continuously stirred within the bag. Finally, the material was transferred to bioreactors for SSC determinations.
2.4. Packed-Bed Experiments
The effects of incubation temperature and initial moisture content on the growth and enzyme production (specifically xylanase and endoglucanase) of T. harzianum were evaluated using laboratory-scale packed bed column bioreactors (PBCBs). The PBCBs employed in this study were made of glass with an internal diameter of 2.3 cm and a bed height of 16 cm (Figure 1a). Each PCBB was packed with ~8 g of dry mass (gdm), which accounts for 75% of their total capacity.
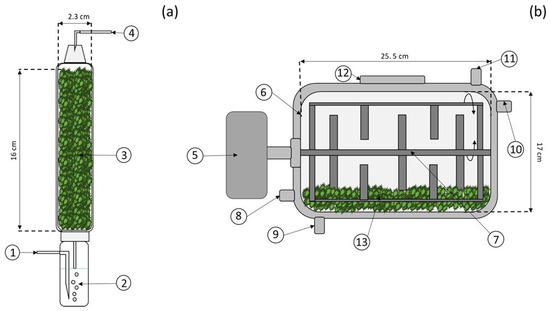
Figure 1.
Packed bed column bioreactor (a) and internal stirred bioreactor (b). The numbers symbolize the components of both bioreactors. Air-flow inlet (1), air humidifier (2), culture bed (3), air-flow outlet (4), motor and transmission system (5), water jacked (6), stirring device (7), air-flow inlet (8), water inlet (9), air-flow outlet (10), water outlet (11), filling and sampling door (12), and culture bed (13).
In the first stage, the incubation temperature was determined using a one-way experimental design that evaluated five levels: 28 °C, 30 °C, 32 °C, 34 °C, and 36 °C. To conduct this step, an inoculated water hyacinth, adjusted to a moisture content of 70%, was packed into the PBCBs. The bioreactors were then incubated in water baths maintained at the specified temperatures (±1 °C) for ~100 h. During the culture, each bioreactor was supplied with 2.80 L/min of saturated air per kgdm. Additionally, the CO2 and O2 contents in the gas exhaust from the bioreactor outlet were monitored. At the end of the culture, substrate moisture content and the activities of xylanase and endoglucanase were analyzed. The moisture content of the fermented material was determined using an Ohaus moisture analyzer (Ohaus, Model MB23, Parsippany, NJ, USA). Three 1-gram samples were taken from each bioreactor for this analysis.
Once the incubation temperature was selected, the initial moisture content was determined using a one-way experimental design to examine four different moisture content levels. At this second stage, water hyacinth batches adjusted to 65%, 70%, 75%, and 80% moisture content were used for packaging the PBCBs. The bioreactors were incubated in a water bath at 30 ± 1 °C (determined in the previous stage) for ~100 h. During the culture, saturated air was supplied to the bioreactors (2.80 L/kgdm min), while CO2 production and O2 uptake were monitored. At the end of the culture, moisture content and enzyme activity were analyzed.
2.5. Stirred-Bed Experiments
Bench-scale cross-flow internal stirred bioreactors (ISBs) were utilized to assess the effect of stirred regime (intermittent and continuous) on microbial growth and enzyme production through a one-way experimental design. Details of 6 L stainless steel ISBs are available in Figure 1b. Each bioreactor was filled to 40% capacity with 0.45 kgdm of inoculated water hyacinth, adjusted to a moisture content of 65% (determined in the laboratory-scale experiments). To evaluate both stirring regimes, an impeller rotation speed of 1 rpm was implemented. The frequency of intermittent stirring was 1 min every 2.3 h. The incubation temperature (30 °C) was regulated through a water jacket. During the culture (~100 h), the ISBs were supplied with saturated air (2.80 L/kgdm min), and the CO2 and O2 contents in the gas exhaust from the bioreactor outlet were monitored. At the end of the culture, moisture content and xylanase and endoglucanase activity were analyzed.
2.6. Respirometry Analysis
Carbon dioxide production and oxygen uptake were used to estimate fungal growth indirectly. Online measurements of O2 and CO2 concentrations were taken in the gas exhaust at the output of the bioreactor using a gas analyzer designed by Metropolitan Autonomous University [37]. This gas analyzer system is equipped with 15 solenoid valves, a flow meter (Honeywell, Model EW-32707-34, Charlotte, NC, USA), CO2 (Sensair, Model K33-ICB, Delsbo, Sweden) and O2 (Winsen, Model O2-A2, Shanghai, China) sensors, and a datalogger M6+ (PROTEC, Mexico City, Mexico).
The rates of O2 uptake (OUR) and CO2 production (CDPR) were calculated based on the concentration gradients of both gases in relation to the air supplied to the bioreactor. These rates were expressed in mg/gidm h (gidm, gram of initial dry mass). The area under the curve of the O2 uptake and CO2 production rates was estimated using the trapezoidal method to determine the total O2 uptake (TOU) and the total CO2 production (TCDP), both expressed in mg/gidm.
The kinetic parameters associated with CO2 production and O2 uptake were estimated from the equations and mathematical procedures described by Martínez-Ramírez et al. [30]. Briefly, assuming the yield between biomass growth and CO2 production is constant, the logistic model can be expressed in terms of CO2 (Equation (1)). The maximal specific CO2 production rate (), the initial CO2 production (), and the total CO2 production () were estimated to integrate Equation (1) (Equation (2)) and using the generalized reduced gradient method (GRG).
On the other hand, considering O2 as a substrate and is a constant, Pirt model can be expressed as an equation to describe the O2 uptake in function of CO2 production (Equation (3)). This equation was coupled with the logistic model to obtain an analytical solution (Equation (4)). Equation (4) was used to calculate the O2 uptake during the culture, estimating the terms and by multilinear regression.
2.7. Enzyme Assays
Enzymes were extracted by adding 10 mL of distilled water to 1 g of solid fermented material and mixing in a vortex for 1 min. The extract was collected and centrifuged at 10,000 rpm for 2 min. The resulting supernatant was considered the crude enzyme extract and was stored at −4 °C until the determination of xylanase and endoglucanase activities.
Enzyme activities were assayed in duplicate using at least two different biological samples. Endoglucanase and xylanase activities were measured using carboxymethylcellulose and birchwood xylan as substrates, respectively. For each reaction mixture, 0.1 mL of the crude enzyme extract was combined with 0.9 mL of the corresponding 1% (w/v) substrate dissolved in sodium citrate buffer (0.1 M, pH 5.2). The mixtures were incubated at 40 °C for 30 min for endoglucanase and 15 min for xylanase. The enzyme reaction was halted by adding 1.5 mL of 3,5-dinitrosalicylic acid, and the concentration of reducing sugars was measured as either glucose or xylose equivalents through the method developed by Miller et al. [38]. One unit of enzyme activity (U) is defined as the amount of enzyme required to liberate 1 μmol of reducing sugars from the corresponding substrate per minute. These measurements were expressed as U per gram dry mass (gdm).
2.8. Statistical Analysis
The adjusted coefficient of determination () and the residual sum of squares were used to determine the goodness of fit and dispersion (respectively) between the respirometry experimental data and the values estimated by mathematical models. Additionally, the typical error was calculated as a complement to the analysis.
The moisture content and enzyme activity (xylanase and endoglucanase) datasets were analyzed using the Shapiro–Wilk goodness of fit test. Datasets that followed a normal distribution were analyzed with ANOVA (α = 0.05), while those that did not fit a normal distribution were analyzed with the Kruskal–Wallis’s test. Following this, datasets that showed significant differences were further analyzed at the same significance level using either Student’s t-test or Tukey’s test.
3. Results
The results of this study are divided into two sections. The first section focuses on determining the optimal incubation temperature and initial moisture content using laboratory-scale PBCBs. After establishing these operational conditions, the second section examines the effect of the stirring regime employed in bench-scale ISBs on the growth and enzyme production of T. harzianum.
3.1. Packed-Bed Bioreactor Processes
The production of xylanases and endoglucanases is closely related to microbial growth and the consumption of substrates. These parameters were monitored indirectly through culture respirometry. The respirometry profile of T. harzianum was sensitive to changes in incubation temperature (Figure 2). The highest values of CDPR and TCDP were obtained in cultures incubated at 30 °C (5.41 mg/gidm h and 123.22 mg/gidm, respectively) and 32 °C (5.43 mg/gidm h and 124.76 mg/gidm, respectively). However, when the incubation temperature increased to 34 °C, both CDPR and TCDP decreased by approximately 5% and 8%. Further raising the temperature from 34 °C to 36 °C resulted in a drastic drop of almost 50% in both parameters (CDPR and TCDP). Additionally, it was noted that OUR and TOU are proportional to CDPR and TCPC, respectively. Consequently, the maximum values of OUR and TOU are also reached at 30 °C (3.78 mg/gidm h and 93.44 mg/gidm, respectively) and 32 °C (3.63 mg/gidm h and 97.87 mg/gidm, respectively).
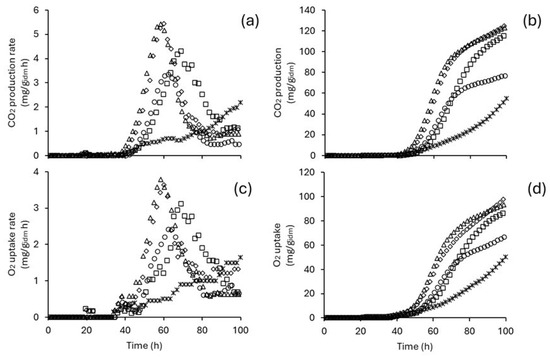
Figure 2.
Respirometry profile of T. harzianum as a function of incubation temperature. CO2 production rate (a), total CO2 production (b), O2 uptake rate (c), and total O2 uptake (d). Circles represent measurements at 28 °C, triangles at 30 °C, diamonds at 32 °C, squares at 34 °C, and asterisks at 36 °C.
To enhance the understanding of the bioprocess, kinetic parameters associated with CO2 production and O2 uptake were estimated through analytical solutions of the logistic model (Equation (2)) and the logistic-Pirt model (Equation (4)). The logistic model effectively described the Total Dissolved Carbon Production (TDCP) curve, achieving a goodness of fit () greater than 99.50%. The coupled logistic-Pirt model described the Total Oxygen Uptake (TOU) curve with a goodness of fit exceeding 97.80% (Table 1). The simulation of the TCDP curve indicates that the maximum CO2 production () is attained in all temperature treatments except at 36 °C. Additionally, the kinetic parameters , , and show a significant decrease in bioreactors incubated at 36 °C. Notably, the values of obtained at temperatures of 28 °C, 30 °C, 32 °C, and 34 °C are all greater than 1.

Table 1.
Kinetic and statistical parameters associated with CO2 production and O2 uptake as a function of incubation temperature.
At the end of the experiment, moisture content and enzyme activity were measured. The culture beds incubated at different temperatures achieved a final moisture content ranging from 68.84% to 73.46%. No significant differences were observed in the final moisture content among the treatments analyzed (Tukey, α = 0.95). Xylanase activity was observed at all incubation temperatures without any significant differences (Figure 3). In contrast, endoglucanase activity decreased by up to 50% when incubated at 36 °C. The results indicate that incubation temperatures between 30 °C and 32 °C enhance microbial growth and enzyme production. However, a temperature of 30 °C was chosen to minimize the risk of overheating in larger-scale bioreactors.
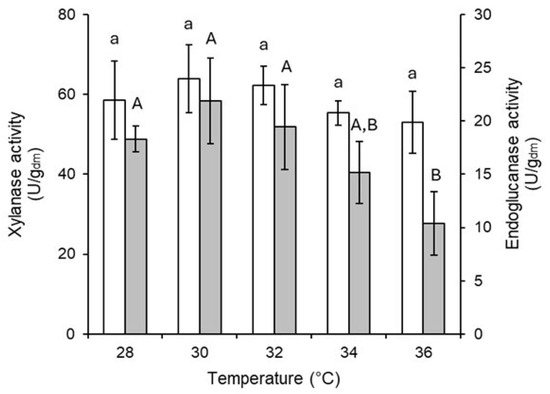
Figure 3.
Xylanase (white bars) and endoglucanase (grey bars) activity produced by T. harzianum as a function of incubation temperature. Different letters represent significant differences (Tukey’s test, α = 0.05%). Lowercase letters indicate significant differences in xylanase activity, while uppercase letters indicate significant differences in endoglucanase activity.
Once the incubation temperature was selected, the appropriate initial moisture content for enzyme formation was determined. The respirometry profile was sensitive to changes in the initial moisture content (Figure 4). Adding water to the substrate increased the values of CPDR, TCDP, OUR, and TOU. The highest values of CDPR and TCDP were obtained in substrates adjusted to a moisture content of 75% (7.53 mg/gidm h and 133.33 mg/gidm, respectively) and 80% (7.39 mg/gidm h and 127.57 mg/gidm, respectively). However, the maximum CDPR value was obtained 15 h earlier in cultures with a moisture content of 75%. The obtained OUR and TOU profiles were proportional to CDPR and TCPC. Consequently, the maximum values for OUR (5.46 mg/gidm·h) and TOU (96.72 mg/gidm) were also achieved in beds with an initial moisture content of 75%.
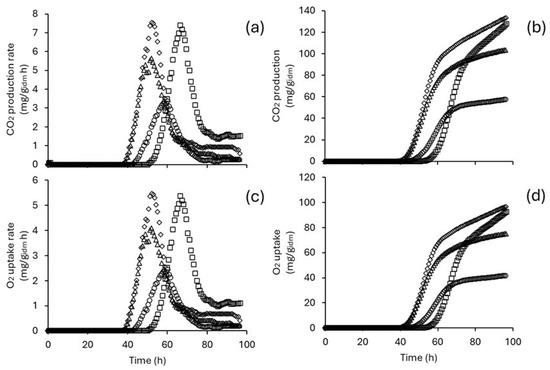
Figure 4.
Respirometry profile of T. harzianum as a function of initial moisture content. CO2 production rate (a), total CO2 production (b), O2 uptake rate (c), and total O2 uptake (d). Circles represent measurements at 65%, triangles at 70%, diamonds at 75%, and squares at 80%.
The TCDP and TOU curves were simulated mathematically, yielding values greater than 98.90% (Table 2). The modeling of the TCDP curve indicated that is consistent across all treatments. Similarly, the modeling of the TOU curve revealed similar values of , which are greater than 1. In contrast, increases up to 2.5 times in treatments with an initial moisture content of 75% and 80%.

Table 2.
Kinetic and statistical parameters associated with CO2 production and O2 uptake as a function of initial moisture content.
At the end of culture, treatments with initial moisture contents of 65%, 70%, 75%, and 80% reached final moisture contents of 67.96%, 73.84%, 77.86%, and 82.59%, respectively. The xylanase and endoglucanase activities measured at different initial moisture levels showed opposing patterns (Figure 5). Xylanase activity is favored by decreasing the moisture content, while endoglucanase activity is enhanced by higher moisture content. This result indicates that adjusting the initial moisture content can influence the production of either xylanases or endoglucanases. In this study, culture beds with an initial moisture content of 65% were used to evaluate the effect of the agitation regime on a process aimed at generating xylanases.
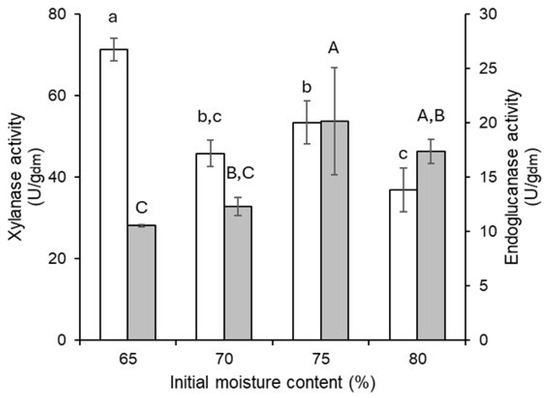
Figure 5.
Xylanase (white bars) and endoglucanase (grey bars) activity produced by T. harzianum as a function of initial moisture content. Different letters represent significant differences (Tukey’s test, α = 0.05%). Lowercase letters indicate significant differences in xylanase activity, while uppercase letters indicate significant differences in endoglucanase activity.
3.2. Stirred-Bed Bioreactor Processes
Bench-scale ISBs were used to address intermittent and continuous stirring regimes. The results showed that stirring enhanced CO2 production and O2 uptake (Figure 6). Maximum CDPR (8.42 mg/gidm h) and OUR (6.11 mg/gidm h) values were obtained operating under the continuous stirring regime. These values are up to 60% higher than those achieved in PBCBs (operated under the same temperature and moisture content conditions). Notably, the TDCP and TOU values were similar across both stirring regimes.
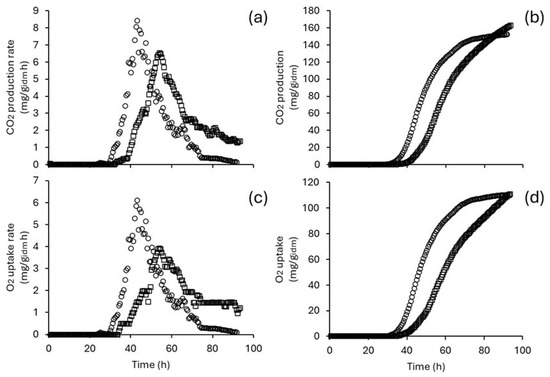
Figure 6.
Respirometry profile of T. harzianum as a function of stirring regime. CO2 production rate (a), total CO2 production (b), O2 uptake rate (c), and total O2 uptake (d). Circles indicate measurements with continuous stirring, while squares represent intermittent stirring.
The values obtained from the mathematical models describing the TCDP and TOU curves exceeded 99% (Table 3), indicating a goodness of fit comparable to that observed in laboratory-scale PBCBs. In cultures with continuous stirring, the value of increased by 33% compared to cultures that were stirred intermittently. Conversely, the values of and increased with intermittent stirring. In both shaking regimes, the values of were greater than 1.

Table 3.
Kinetic and statistical parameters associated with CO2 production and O2 uptake as a function of stirring regime.
Water hyacinth beds processed under intermittent and continuous stirring regimes achieved final moisture levels of 63.23% and 64.74%, respectively. No significant differences were observed in the final moisture content between the two stirred regimes (Student’s t-test, α = 0.95). In contrast, the stirring regime had a significant influence on enzyme production. Continuous stirring enhanced xylanase activity by 25%, whereas intermittent stirring increased endoglucanase activity by nearly 50% (Figure 7).
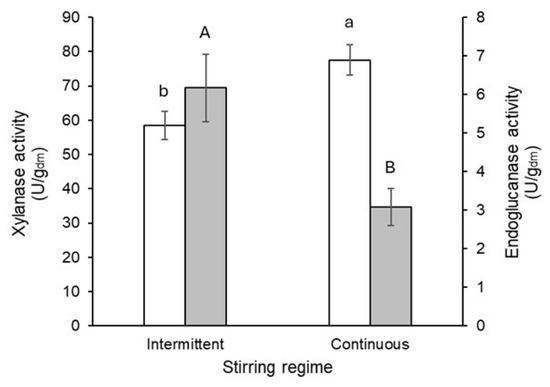
Figure 7.
Xylanase (white bars) and endoglucanase (grey bars) activity produced by T. harzianum as a function of stirring regime. Different letters represent significant differences (Student’s t-test, α = 0.05%). Lowercase letters indicate significant differences in xylanase activity, while uppercase letters indicate significant differences in endoglucanase activity.
4. Discussion
Xylanases and endoglucanases are enzymes associated with microbial growth [39], which in SSC can be monitored indirectly using respirometry [40]. The TCDP curves observed during cultivation displayed a sigmoid profile, which the logistic model can accurately represent [41]. This model effectively simulates the TCDP curves, achieving a goodness of fit () higher than 99%. By coupling the logistic equation with the Pirt model, a function was obtained to estimate O2 uptake associated with microbial growth and cell maintenance, expressed in terms of CO2 production [30]. This function successfully described the TOU curves, achieving a value exceeding 98%. The kinetic parameters included in Equations (2) and (4) provide a deeper understanding of the culture process, which helps determine operating conditions and formulate effective scale-up strategies [42,43].
The results demonstrate that the incubation temperature and the initial moisture content are variables linked to the respirometry profile of T. harzianum. Temperatures between 30 °C and 34 °C promote CO2 production and O2 uptake. In contrast, lower levels of CO2 production and O2 uptake are observed at 28 °C and 36 °C. Incubating at 36 °C, a decrease in the kinetic parameters , , and (Table 1) and in the endoglucanase activity (Figure 3) was noted. On the other hand, the endoglucanase activity is similar at all the temperatures evaluated. Therefore, to ensure good microbial growth and high enzymatic activity, it is advisable to maintain an operating temperature between 30 °C and 34 °C. This temperature range aligns with findings by López-Ramírez [44] in T. harzianum cultures.
After selecting the incubation temperature, the effect of the initial moisture content was evaluated. Adding water to the culture increased the values of CPDR, TCDP, OUR, and TOU (Figure 4) and, thereby, promoted microorganism growth. This improvement may be attributed to the fact that a higher moisture content enhances nutrient transport and reduces the surface tension of the aqueous film [45], generating better conditions for microbial growth.
Increasing the initial moisture content also benefited endoglucanase activity (Figure 5). However, this effect was opposite for xylanase activity. Kumar et al. [46] observed that in wheat bran beds, maximum endoglucanase production occurs at an initial moisture content higher (70%) than that required for maximum xylanase production (65%). Therefore, as demonstrated in this study, by modifying the initial moisture content, the culture can be directed towards the production of xylanases or endoglucanases. However, previous research has shown that endoglucanase activity is sensitive to stirring, resulting in reductions of up to 75% [44]. Thus, experiments in ISBs were conducted under operating conditions that favor the generation of xylanases. Under these conditions, the stirring regime was evaluated, and a of 1.03 was obtained; this value corresponds to the consumption of substrates with an oxidation grade ≥ 4 under aerobic conditions [47]. This suggests effective consumption of both i) the glucose added to the culture and ii) the glucose released from cellulose chains. In this type of culture, the energy destined to biomass formation is greater than that required for cell maintenance [48]. This fact was verified in terms of mass since the values estimated through mathematical modeling corroborate that .
In filamentous fungal cultures, limited oxygen availability affects microbial growth, resulting in reduced CO2 production [49]. In contrast, bench-scale ISB cultures have achieved up to 60% greater CO2 production and O2 uptake (Figure 6) compared to PBCBs (Figure 4). Stirring in SSC bioreactors reduces the agglomeration and contraction of substrate particles, thereby improving oxygen distribution and promoting microbial growth [30,50]. Previously, López-Ramírez et al. [44] demonstrated that T. harzianum does not present mechanical damage in cross-flow ISBs operating at stirring speeds up to 1 rpm and packed with pine sawdust. Similarly, the results obtained in this study indicate that the mycelium of T. harzianum was not affected in any of the agitation regimes, at a rotation speed of 1 rpm. Even operating the ISB under a continuous regime, the CDPR and OUR values were improved. This improvement may be attributed to the creation of void spaces within the bed, since implementing an intermittent regime, the aerial mycelium can lead to the agglomeration of solid particles, which restricts mass and heat transfer [51]. Moreover, implementing continuous agitation increased the bioreactor’s mass capacity 56-fold (from 8 to 450 gdm) without affecting xylanase production. The xylanase activity achieved by T. harzianum on water hyacinth in ISBs operated under continuous conditions (77.60 ± 4.49 U/gdm) is higher than that achieved by co-cultures of A. niger and T. ressei in conical flasks (~57.20 U/gdm) [24]. The differences in xylanase activity could be attributed to factors such as the specific types of microorganisms used, the culture conditions, and the composition of the water hyacinth [52]. In contrast, the xylanase activity reported by López-Ramírez et al. [44] on pine sawdust beds (109.32 ± 4.91 U/gdm) is slightly higher than what was achieved in this study. Despite using the same microorganism and similar operating conditions, the higher lignin content in pine sawdust likely plays a significant role in promoting the effective synthesis of xylanases. It is essential to note that the hemicellulose content can induce the synthesis of xylanases, while cellulose and its derivatives, such as cellobiose, act as inducers of cellulases [10,53]. Consequently, the varying composition of water hyacinth should be taken into account when implementing this bioprocess.
The process designed in this study could be scaled up and implemented to recover water hyacinth waste, helping to mitigate the harmful effects of its proliferation in water reservoirs.
5. Conclusions
The removal of water hyacinth generates large amounts of waste that can be utilized through solid-state culture for enzyme production. This study found that significant xylanase production can be achieved from water hyacinth beds with a moisture content of 65%, incubating at 30 °C. These optimal conditions were determined using packed-bed column bioreactors. However, scaling up these bioreactors presents challenges, as the larger size hinders heat removal and gas exchange. To address this issue, the process was transferred to bench-scale cross-flow stirred bioreactors, where two stirring regimes were evaluated: intermittent and continuous. The results indicated that stirring increased carbon dioxide production and oxygen uptake, demonstrating that internal stirring enhances growth conditions for Trichoderma harzianum. Furthermore, operating with continuous stirring promoted xylanase production, achieving levels comparable to those in static-bed bioreactors. As a result, continuous stirring enabled a 56-fold increase in bioreactor capacity without negatively impacting xylanase production. The approaches developed in this study can aid in designing large-scale bioprocesses that promote the valorization of water hyacinth, thereby mitigating its adverse effects on freshwater ecosystems.
Author Contributions
Conceptualization, N.L.-R. and E.F.-T.; methodology, E.F.-T. and F.M.-G.; software, F.M.-G.; validation, E.F.-T. and T.V.-S.; formal analysis, N.L.-R. and F.M.-G.; investigation, N.L.-R. and T.V.-S.; resources, E.F.-T.; data curation, N.L.-R. and F.M.-G.; writing—original draft preparation, F.M.-G.; writing—review and editing, N.L.-R., E.F.-T. and T.V.-S.; visualization, E.F.-T. and T.V.-S.; supervision, E.F.-T.; project administration, E.F.-T.; funding acquisition, E.F.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council for Science and Technology (CONACyT), project 247111.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
N.L.-R. thanks SECIHTI for financing her postdoctoral fellowship.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations and symbols are used in this manuscript:
| Maximal specific growth rate | |
| Carbon dioxide concentration | |
| Oxygen concentration | |
| Maximum carbon dioxide concentration | |
| Initial carbon dioxide concentration | |
| Initial oxygen concentration | |
| Carbon dioxide production rate (CDPR) | |
| Oxygen uptake rate (OUR) | |
| Oxygen-carbon dioxide mass yield | |
| Carbon dioxide-oxygen molar yield | |
| Maintenance coefficient associated with oxygen uptake | |
| SSC | Solid-state culture |
| PBCB | Packed bed column bioreactor |
| ISB | Internal stirred bioreactor |
| IM | Impregnated medium |
| TOU | Total oxygen uptake |
| TCDP | Total carbon dioxide production |
References
- Téllez, T.R.; López, E.; Granado, G.; Pérez, E.; López, R.; Guzmán, J. The Water Hyacinth, Eichhornia crassipes: An Invasive Plant in the Guadiana River Basin (Spain). Aquat. Invasion 2008, 3, 42–53. [Google Scholar] [CrossRef]
- Honlah, E.; Yao Segbefia, A.; Odame Appiah, D.; Mensah, M.; Atakora, P.O. Effects of Water Hyacinth Invasion on the Health of the Communities, and the Education of Children along River Tano and Abby-Tano Lagoon in Ghana. Cogent Soc. Sci. 2019, 5, 1619652. [Google Scholar] [CrossRef]
- Julien, M. Plant Biology and Other Issues That Relate to the Management of Water Hyacinth: A Global Perspective with Focus on Europe 1. EPPO Bull. 2008, 38, 477–486. [Google Scholar] [CrossRef]
- Gunnarsson, C.C.; Petersen, C.M. Water Hyacinths as a Resource in Agriculture and Energy Production: A Literature Review. Waste Manag. 2007, 27, 117–129. [Google Scholar] [CrossRef]
- Hermoso-López Araiza, J.P.; Quecholac-Piña, X.; Beltrán-Villavicencio, M.; Espinosa-Valdemar, R.M.; Vázquez-Morillas, A. Integral Valorization of the Water Hyacinth from the Canals of Xochimilco: Production of Edible Mushrooms and Forage. Waste Biomass Valoriz. 2016, 7, 1203–1210. [Google Scholar] [CrossRef]
- Carlini, M.; Castellucci, S.; Mennuni, A. Water Hyacinth Biomass: Chemical and Thermal Pre-Treatment for Energetic Utilization in Anaerobic Digestion Process. Energy Procedia 2018, 148, 431–438. [Google Scholar] [CrossRef]
- Lay, C.-H.; Sen, B.; Chen, C.-C.; Wu, J.-H.; Lee, S.-C.; Lin, C.-Y. Co-Fermentation of Water Hyacinth and Beverage Wastewater in Powder and Pellet Form for Hydrogen Production. Bioresour. Technol. 2013, 135, 610–615. [Google Scholar] [CrossRef]
- Ma, F.; Yang, N.; Xu, C.; Yu, H.; Wu, J.; Zhang, X. Combination of Biological Pretreatment with Mild Acid Pretreatment for Enzymatic Hydrolysis and Ethanol Production from Water Hyacinth. Bioresour. Technol. 2010, 101, 9600–9604. [Google Scholar] [CrossRef]
- Soccol, C.R.; da Costa, E.S.F.; Letti, L.A.J.; Karp, S.G.; Woiciechowski, A.L.; de Souza Vandenberghe, L.P. Recent Developments and Innovations in Solid State Fermentation. Biotechnol. Res. Innov. 2017, 1, 52–71. [Google Scholar] [CrossRef]
- Thapa, S.; Mishra, J.; Arora, N.; Mishra, P.; Li, H.; O’Hair, J.; Bhatti, S.; Zhou, S. Microbial Cellulolytic Enzymes: Diversity and Biotechnology with Reference to Lignocellulosic Biomass Degradation. Rev. Environ. Sci. Biotechnol. 2020, 19, 621–648. [Google Scholar] [CrossRef]
- Dahiya, S.; Rapoport, A.; Singh, B. Biotechnological Potential of Lignocellulosic Biomass as Substrates for Fungal Xylanases and Its Bioconversion into Useful Products: A Review. Fermentation 2024, 10, 82. [Google Scholar] [CrossRef]
- Bajaj, P.; Mahajan, R. Cellulase and Xylanase Synergism in Industrial Biotechnology. Appl. Microbiol. Biotechnol. 2019, 103, 8711–8724. [Google Scholar] [CrossRef]
- Klein-Marcuschamer, D.; Oleskowicz-Popiel, P.; Simmons, B.A.; Blanch, H.W. The Challenge of Enzyme Cost in the Production of Lignocellulosic Biofuels. Biotechnol. Bioeng. 2012, 109, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bajar, S.; Devi, A.; Bishnoi, N.R. Adding Value to Agro-Industrial Waste for Cellulase and Xylanase Production via Solid-State Bioconversion. Biomass Convers. Biorefin. 2023, 13, 7481–7490. [Google Scholar] [CrossRef]
- Legodi, L.M.; La Grange, D.C.; van Rensburg, E.L.J. Production of the Cellulase Enzyme System by Locally Isolated Trichoderma and Aspergillus Species Cultivated on Banana Pseudostem during Solid-State Fermentation. Fermentation 2023, 9, 412. [Google Scholar] [CrossRef]
- Saldaña-Mendoza, S.A.; Palacios-Ponce, A.S.; Ruiz, H.A.; Ascacio-Valdés, J.A.; Aguilar, C.N. Revalorization of Green Tea Waste through the Production of Cellulases by Solid-State Fermentation Using a Aspergillus niger 28A. Biomass Convers. Biorefin. 2024, 14, 16711–16724. [Google Scholar] [CrossRef]
- Afiqah Razali, S.; Rasit, N.; Kuan Ooi, C. Statistical Analysis of Xylanase Production from Solid State Fermentation of Rice Husk Associated Fungus Aspergillus niger. Mater. Today Proc. 2021, 39, 1082–1087. [Google Scholar] [CrossRef]
- Lopes-Perez, C.; Casciatori, F.P.; Thoméo, J.C. Improving Enzyme Production by Solid-State Cultivation in Packed-Bed Bioreactors by Changing Bed Porosity and Airflow Distribution. Bioprocess Biosyst. Eng. 2021, 44, 537–548. [Google Scholar] [CrossRef]
- Camassola, M.; Dillon, A.J.P. Production of Cellulases and Hemicellulases by Penicillium echinulatum Grown on Pretreated Sugar Cane Bagasse and Wheat Bran in Solid-State Fermentation. J. Appl. Microbiol. 2007, 103, 2196–2204. [Google Scholar] [CrossRef]
- Hamrouni, R.; Molinet, J.; Dupuy, N.; Taieb, N.; Carboue, Q.; Masmoudi, A.; Roussos, S. The Effect of Aeration for 6-Pentyl-Alpha-Pyrone, Conidia and Lytic Enzymes Production by Trichoderma asperellum Strains Grown in Solid-State Fermentation. Waste Biomass Valoriz. 2020, 11, 5711–5720. [Google Scholar] [CrossRef]
- Lodha, A.; Pawar, S.; Rathod, V. Optimised Cellulase Production from Fungal Co-Culture of Trichoderma reesei NCIM 1186 and Penicillium citrinum NCIM 768 under Solid State Fermentation. J. Environ. Chem. Eng. 2020, 8, 103958. [Google Scholar] [CrossRef]
- Outeiriño, D.; Costa-Trigo, I.; Pinheiro de Souza Oliveira, R.; Pérez Guerra, N.; Salgado, J.M.; Domínguez, J.M. Biorefinery of Brewery Spent Grain by Solid-State Fermentation and Ionic Liquids. Foods 2022, 11, 3711. [Google Scholar] [CrossRef]
- De la Cruz-Quiroz, R.; Robledo-Padilla, F.; Aguilar, C.N.; Roussos, S. Forced Aeration Influence on the Production of Spores by Trichoderma Strains. Waste Biomass Valoriz. 2017, 8, 2263–2270. [Google Scholar] [CrossRef]
- Deshpande, S.K.; Bhotmange, M.G.; Chakrabarti, T.; Shastri, P.N. Production of Cellulase and Xylanase by Trichoderma reesei (QM 9414 Mutant), Aspergillus niger and Mixed Culture by Solid State Fermentation (SSF) of Water Hyacinth (Eichhornia crassipes). Indian J. Chem. Technol. 2008, 15, 449–456. [Google Scholar]
- Arana-Cuenca, A.; Tovar-Jiménez, X.; Favela-Torres, E.; Perraud-Gaime, I.; González-Becerra, A.E.; Martínez, A.; Moss-Acosta, C.L.; Mercado-Flores, Y.; Téllez-Jurado, A. Use of Water Hyacinth as a Substrate for the Production of Filamentous Fungal Hydrolytic Enzymes in Solid-State Fermentation. 3 Biotech 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Finkler, A.T.J.; Weber, M.Z.; Fuchs, G.A.; Scholz, L.A.; de Lima Luz, L.F., Jr.; Krieger, N.; Mitchell, D.A.; de Matos Jorge, L.M. Estimation of Heat and Mass Transfer Coefficients in a Pilot Packed-Bed Solid-State Fermentation Bioreactor. Chem. Eng. J. 2021, 408, 127246. [Google Scholar] [CrossRef]
- Ge, X.; Vasco-Correa, J.; Li, Y. Solid-State Fermentation Bioreactors and Fundamentals. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 381–402. [Google Scholar]
- Durand, A. Bioreactor Designs for Solid State Fermentation. Biochem. Eng. J. 2003, 13, 113–125. [Google Scholar] [CrossRef]
- Grajales, L.M.; Wang, H.; Casciatori, F.P.; Thoméo, J.C. Intensified Rotary Drum Bioreactor for Cellulase Production from Agro-Industrial Residues by Solid-State Cultivation. Chem. Eng. Process.-Process Intensif. 2025, 210, 110223. [Google Scholar] [CrossRef]
- Martínez-Ramírez, C.; Esquivel-Cote, R.; Ferrera-Cerrato, R.; Martínez-Ruiz, J.A.; Rodríguez-Serrano, G.; Saucedo-Castañeda, G. Solid-State Culture of Azospirillum brasilense: A Reliable Technology for Biofertilizer Production from Laboratory to Pilot Scale. Bioprocess Biosyst. Eng. 2021, 44, 1525–1538. [Google Scholar] [CrossRef]
- Mattedi, A.; Sabbi, E.; Farda, B.; Djebaili, R.; Mitra, D.; Ercole, C.; Cacchio, P.; Del Gallo, M.; Pellegrini, M. Solid-State Fermentation: Applications and Future Perspectives for Biostimulant and Biopesticides Production. Microorganisms 2023, 11, 1408. [Google Scholar] [CrossRef]
- Brand, D.; Soccol, C.R.; Sabu, A.; Roussos, S. Production of Fungal Biological Control Agents through Solid State Fermentation: A Case Study on Paecelomyces lilacinus Againts Root-Knot Nematodes. Micol. Apl. Int. 2010, 22, 31–48. [Google Scholar]
- Desobgo, S.C.Z.; Mishra, S.S.; Behera, S.K.; Panda, S.K. Scaling-up and Modelling Applications of Solid-State Fermentation and Demonstration in Microbial Enzyme Production Related to Food Industries. In Microbial Enzyme Technology in Food Applications; Ray, R., Rosell, C., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 452–468. [Google Scholar]
- Xue, M.; Liu, D.; Zhang, H.; Qi, H.; Lei, Z. A Pilot Process of Solid State Fermentation from Sugar Beet Pulp for the Production of Microbial Protein. J. Ferment. Bioeng. 1992, 73, 203–205. [Google Scholar] [CrossRef]
- De Vrije, T.; Antoine, N.; Buitelaar, R.M.; Bruckner, S.; Dissevelt, M.; Durand, A.; Gerlagh, M.; Jones, E.E.; Lüth, P.; Oostra, J.; et al. The Fungal Biocontrol Agent Coniothyrium minitans: Production by Solid-State Fermentation, Application and Marketing. Appl. Microbiol. Biotechnol. 2001, 56, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Durand, A.; Chereau, D. A New Pilot Reactor for Solid-State Fermentation: Application to the Protein Enrichment of Sugar Beet Pulp. Biotechnol. Bioeng. 1988, 31, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Saucedo-Castañeda, G.; Favela-Torres, E.; Viniegra-González, G.; Torres-Mancera, M.T.; Figueroa-Montero, A.; Rosales-Zamora, G. Respirometry System with Remote Management for the Inline Monitoring of the Concentration of CO2 and O2 and Flow of the Exhausting Gases in Biological Processes; Instituto Mexicano de la Propiedad Industrial: Mexico City, Mexico, 2016. [Google Scholar]
- Miller, G.L.; Blum, R.; Glennon, W.E.; Burton, A.L. Measurement of Carboxymethylcellulase Activity. Anal. Biochem. 1960, 1, 127–132. [Google Scholar] [CrossRef]
- Kalogeris, E.; Iniotaki, F.; Topakas, E.; Christakopoulos, P.; Kekos, D.; Macris, B.J. Performance of an Intermittent Agitation Rotating Drum Type Bioreactor for Solid-State Fermentation of Wheat Straw. Bioresour. Technol. 2003, 86, 207–213. [Google Scholar] [CrossRef]
- Saucedo-Castañeda, G.; Trejo-Hernández, M.R.; Lonsane, B.K.; Navarro, J.M.; Roussos, S.; Dufour, D.; Raimbault, M. On-Line Automated Monitoring and Control Systems for CO2 and O2 in Aerobic and Anaerobic Solid-State Fermentations. Process Biochem. 1994, 29, 13–24. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; van ’t Riet, K. Modeling of the Bacterial Growth Curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef]
- Méndez-González, F.; Loera, O.; Saucedo-Castañeda, G.; Buenrostro-Figueroa, J.J.; Favela-Torres, E. Improved Packed Bed Column Bioreactor to Produce Fungal Conidia for Biological Control. Syst. Microbiol. Biomanufacturing 2025, 5, 783–794. [Google Scholar] [CrossRef]
- Pedraza-Segura, L.; Rodríguez-Durán, L.V.; Saucedo-Castañeda, G.; de Jesús Cázares-Marinero, J. Application of Microorganisms in Biosurfactant Production. In Bioprospecting of Microorganism-Based Industrial Molecules; Singh, S., Upadhyay, S.K., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 6–30. [Google Scholar]
- Lopez-Ramirez, N.; Volke-Sepulveda, T.; Gaime-Perraud, I.; Saucedo-Castañeda, G.; Favela-Torres, E. Effect of Stirring on Growth and Cellulolytic Enzymes Production by Trichoderma harzianum in a Novel Bench-Scale Solid-State Fermentation Bioreactor. Bioresour. Technol. 2018, 265, 291–298. [Google Scholar] [CrossRef]
- Oriol, E.; Raimbault, M.; Roussos, S.; Viniegra-Gonzales, G. Water and Water Activity in the Solid State Fermentation of Cassava Starch by Aspergillus niger. Appl. Microbiol. Biotechnol. 1988, 27, 498–503. [Google Scholar] [CrossRef]
- Kumar, B.; Kumar, A.; Kumar, A.; Dharm, D. Wheat Bran Fermentation for the Production of Cellulase and Xylanase by Aspergillus niger NFCCI 4113. Res. J. Biotechnol. 2018, 13, 11–18. [Google Scholar]
- Erickson, L.E.; Minkevich, I.G.; Eroshin, V.K. Application of Mass and Energy Balance Regularities in Fermentation. Biotechnol. Bioeng. 1978, 20, 1595–1621. [Google Scholar] [CrossRef]
- Ramírez-Esparza, U.; Ordoñez-Cano, A.J.; Ochoa-Reyes, E.; Méndez-González, F.; Baeza-Jimenez, R.; Alvarado-González, M.; Ascacio-Valdes, J.A.; Buenrostro-Figueroa, J.J. Development of a Bioprocess to Improve the Phenolic Compounds Content and Antioxidant Capacity in Blue Corn Grains. Fermentation 2025, 11, 122. [Google Scholar] [CrossRef]
- Méndez-González, F.; Figueroa-Montero, A.; Saucedo-Castañeda, G.; Loera, O.; Favela-Torres, E. Addition of Spherical-style Packing Improves the Production of Conidia by Metarhizium robertsii in Packed Column Bioreactors. J. Chem. Technol. Biotechnol. 2022, 97, 1517–1525. [Google Scholar] [CrossRef]
- Finkler, A.T.J.; de Lima Luz, L.F.; Krieger, N.; Mitchell, D.A.; Jorge, L.M. A Model-Based Strategy for Scaling-up Traditional Packed-Bed Bioreactors for Solid-State Fermentation Based on Measurement of O2 Uptake Rates. Biochem. Eng. J. 2021, 166, 107854. [Google Scholar] [CrossRef]
- Schutyser, M.A.I.; de Pagter, P.; Weber, F.J.; Briels, W.J.; Boom, R.M.; Rinzema, A. Substrate Aggregation Due to Aerial Hyphae during Discontinuously Mixed Solid-state Fermentation with Aspergillus Oryzae: Experiments and Modeling. Biotechnol. Bioeng. 2003, 83, 503–513. [Google Scholar] [CrossRef]
- Domínguez-Rivera, Á.; Bibián-León, M.E.; Saucedo-Castañeda, G.; Buenrostro-Figueroa, J.J.; Méndez-González, F. From Farm to Industry: Valorization of Agricultural Waste by Solid-State Culture for Sustainable Development. In Agroindustrial Waste Management and Natural Resources Conservation; Apple Academic Press: New York, NY, USA, 2025; pp. 25–50. [Google Scholar]
- Quiroz-Castañeda, R.E.; Pérez-Mejía, N.; Martínez-Anaya, C.; Acosta-Urdapilleta, L.; Folch-Mallol, J. Evaluation of Different Lignocellulosic Substrates for the Production of Cellulases and Xylanases by the Basidiomycete Fungi Bjerkandera adusta and Pycnoporus sanguineus. Biodegradation 2011, 22, 565–572. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).