Abstract
This study explores both pyrometallurgical and hydrometallurgical methods for decarbonizing and recovering valuable metals from bauxite residue, with hydrogen plasma reduction and direct acid leaching as the primary approaches. The goal is to offer innovative techniques for extracting metals from bauxite residue, a by-product of the Bayer process, which cannot be disposed of in an environmentally sustainable manner. Additionally, reducing the volume of bauxite residue through combined treatments is a key objective. In contrast to traditional carbon-based reductive melting, which generated significant CO2 emissions, hydrogen is now being investigated as a cleaner alternative. Through hydrogen plasma reduction, approximately 99.9% of iron is recovered as crude metallic iron, which can be easily separated from the slag containing other valuable metals. Thermochemical analysis was used to predict slag formation and chemical analysis of slag during hydrogen reduction. To further recover metals like aluminum and titanium, the slag is subjected to sulfuric acid leaching under high-pressure of oxygen in an autoclave avoiding silica gel formation. The results demonstrated a leaching efficiency of 93.21% for aluminum and 84.56% for titanium, using 5 mol/L sulfuric acid at 150 °C, with almost complete iron recovery. Assisted ultrasound leaching of slag with sulphuric acid under atmospheric pressure leads to 54% leaching efficiency of titanium.
1. Introduction
The Bayer Process is the established industrial process to produce alumina from bauxite ore [1]. Mining and metallurgical waste represents new potential secondary sources of metals for the West Balkan region [2,3,4]. The countries of the West Balkan have stagnated economically due to political developments in the previous century, but owing to their long history of mining, processing, and production of raw materials, they have enormous potential for metal recovery from secondary raw material deposits, which could additionally improve their socioeconomic position. There is growing interest in processing and utilizing the red mud by-product of the Bayer process from alumina extraction from bauxite [5,6,7,8,9,10]. The chemical quality of precipitated aluminum hydroxide, and consequently the final alumina product in the Bayer process directly depends of the level of impurities in a refinery’s Bayer liquor [11]. Under optimal reaction parameters (temperature and time) it is possible to remove iron, zinc and copper from Bayer liquor using precipitation agent such as calcium hydroxide with an efficiency of more than 90 %, in such a way that the treated solution is still economically usable in the following stages of processing while obtaining different types of aluminum trihydrate [12].
In Europe, alumina refineries operate in Bosnia and Herzegovina (Alumina, Zvornik), France, Hungary, Germany, Greece, Ireland (AAL), Romania (ALUM), Spain and Ukraine, while significant bauxite residue (BR) deposits from refineries that have stopped their operations exist in former Yugoslavia (Podgorica, Kidricevo, Mostar, Obrovac), Italy, France (RT), Germany, Hungary and other countries. The current BR production level in the EU is 6.8 million tonnes per year; while the cumulative stockpiled level is a staggering >250 million tonnes (dry matter) [13].
The typical mineralogical structure of bauxite residue in Alumina ltd (Bosnia and Herzegovina) consists of cancrinite (29–33%), hematite (27–29%), and sodalite (16–24%), which together form the major mineral components. Boehmite (5–6%), gibbsite (4–5%), and anatase (5%) are present in smaller amounts. Additionally, andradite (4%) and quartz (2%) contribute to the overall composition [14].
The bauxite residues contain scandium and gallium (approx. 50–150 ppm) and also much more elements such as vanadium and rare earths elements (0.05–0.5%). Since 1991, MYTILINEOS, Greece has been pioneering the research on BR handling and reuse, focusing initially on massive low value applications such as use as a raw material for geopolymer bricks, production of cement clinker, iron, bricks and tile, soil remediation (vegetation), extraction of rare earth elements and road substrate [15].
Due to the large amounts of bauxite residue (red mud) generated, the Pedersen Process was considered as an alternative to prevent its accumulation. In the conventional process, iron is separated as pig iron through carbothermic smelting-reduction, which has CO2 emissions similar to conventional iron production. To eliminate these emissions, this study focused on reducing iron oxides in bauxite ore using hydrogen gas before smelting, minimizing solid carbon usage. Thermogravimetric analysis, supported by microstructural and phase analysis, confirmed that hematite reduction begins below 560 °C at a slow rate, accelerating at higher temperatures. However, at 860–1060 °C, hercynite (FeAl2O4) formation hinders complete reduction to metallic iron [16].
The combined pyrometallurgical and hydrometallurgical treatment of bauxite residue included carbothermic reduction at 1500–1550 °C using coke and fluxes to produce conditioned slag. Additional conditioning with secondary resources like basic oxygen furnace slag and bottom ash facilitated valuable metal recovery. After pyrometallurgical processing, aluminum, titanium, rare earth elements, and scandium were extracted via hydrometallurgical leaching, filtration, and precipitation under atmospheric pressure [17,18,19].
Since 2013, the possibilities to recover rare earths from bauxite residues, which commonly contain only low concentrations of these elements, but are available in very large volumes, are the main research subject of the European funded projects (EURARE; REMOVAL, SCALE, REDMUD). They could provide significant amounts of rare earths to European countries. The extraction rate of the rare-earth recovery from these industrial waste streams is a part of a comprehensive, zero-waste, “product-centric” valorisation scheme, in which applications are found for the residual fractions that are obtained after removal of not only the rare earths but also other critical metals such as scandium, vanadium and gallium, and especially the base elements: aluminium, titanium and iron [16].
Unfortunately, the extraction of aluminium, iron and titanium from bauxite residue under acidic leaching is limited due to insufficient amount of acidic solution from leaching caused by the polymerization of silica [20,21]. Kinetic studies have demonstrated that at constant temperatures, silica dissolution increases with increasing acid concentration, but it decreases when the temperature is increased and the acid concentration is reduced. This is due to the enhancement in the solubility of monomeric silicic acid formed during acidic leaching. The control mechanisms of silica dissolution have been described according to the shrinking core model by a chemical reaction stage, i.e., silica polymerization, followed by a diffusion stage, because of the silica gel adsorbed on the surface of the particles that limits the metal extraction. Supressing the silica gel formation is crucial for a recovery process of iron, titanium, aluminium and rare earth elements from bauxite residues. Silica gel can be prevented with a dry digestion process with sulphuric acid [18] and hydrogen peroxide. Alkan et al. determine the optimal amount of hydrogen peroxide to be 2.5 M H2O2 into 2.5 M sulphuric acid to achieve the favoured quartz formation and supress rhomboclase precipitation. Since the leaching reactions mainly controlled by diffusion, no significant increase in the efficiencies were observed after 30 min of leaching. While silica gel was not formed in oxidative environment, high titanium extraction from bauxite residue was only achieved when hydrogen peroxide was introduced into the acidic solution under an atmospheric pressure at 90 °C. The leaching of red mud and its slag has already been intensively studied. The maximum achieved leaching efficiencies of titanium with inorganic acids in various studies are summarized in Table 1 together with the respective boundary conditions [22].

Table 1.
Maximum titanium leaching efficiency reported in various studies on the leaching of red mud and its slag.
A comparison of leaching with hydrochloric acid and sulphuric acid, as well as a 1:3 mixture of both acids with a concentration of 4 mol/L each, shows a slightly better leaching efficiency of titanium for sulphuric acid. It should be remembered that the normality of sulphuric acid is twice as high as the normality of hydrochloric acid at the same concentration. Both acids dissolve iron approximately equally well. The model by [7] based on leaching experiments shows that for the leaching of red mud with dilute sulfuric acid, a low S/L ratio, a high acid concentration and a high temperature have a positive effect on the leaching efficiency of titanium. Sayan et al. came to the same conclusions, but tested with a temperature range up to 90 °C instead of only up to 60 °C. The stirring speed has a small influence on the leaching efficiency. With higher concentration of sulfuric acid and a lower S/L ratio, higher yields are possible, for the leaching of red mud at 70 °C. Regardless of the concentration and S/L ratio, the selectivity is low. Generally, the obtained results have shown that maximal leaching of slag obtained by carbon reduction of bauxite residue is about 70% under an atmospheric pressure below 90 °C, what is not sufficient.
Ultrasound-assisted leaching (UAL) is previously tested in order to improve leaching efficiency. This is a method for improving leaching efficiency, reducing the amount of leaching agent and reducing the reaction time. The significant time savings can be illustrated, for example, by comparing germanium leaching with and without ultrasound [28,29]. The advantages of ultrasonic-assisted leaching are based on the cavitation it causes. Furthermore, the sonochemical effect ensures an increased autoprotolysis of the water, what is important for leaching process.
Using decarbonizing technologies instead carbothermic reduction including hydrogen reduction of red mud for extraction of valuable metals is of high importance for environmental strategy. Generally, high amount of iron oxide in red mud (approx. 50–55%) shall be firstly removed by reduction process in order to enable selective leaching process [24,30,31,32,33,34,35,36]. Agrawal and Drawan proposed hydrogen reduction of red mud for extraction of metallic values [9]. The key objective is to reduce the hematite to metallic iron and consequently alter the interlocked structure of red mud (“bauxite residue”). Reduction at 450 °C results in formation of magnetite with ~10% Fe metallization in 30 min, and increment in the temperature to 900 °C results in 97% Fe metallization in 120 min producing characteristic spherical morphology of iron. They concluded the hydrogen reduction is attractive compared to carbothermal reduction because of low temperature (600–900 °C), less reductant use (107 g H2/kg red mud) and restricting fayalite and hercynite formation.
Pilla et al. studied bauxite residue blended with NaOH and subsequently reduced with hydrogen, in order to develop an intermediate material rich in magnetite and sodium aluminate aiming the recovery of iron, aluminum as well as sodium [37]. The influence of sodium dosage, reduction temperature and soaking time, on the qualities of the final material, were studied in detail. The highest conversion degree from hematite to magnetite was 96% and it occurred for 20 wt% NaOH addition to BR, and reduction with 5 vol% H2, for 120 min at 500 °C. The aluminum oxides were transformed into water-soluble sodium aluminate phases, with a range of other interesting metals enriched in the resulting residue.
The selection of an eco-friendly reducing agent is also another crucial measure to lower the significant CO2 emissions released from the metallurgical industry. In fact, the iron and steelmaking industry alone accounts for nearly 34% and 7% of industrial and global CO2 emissions respectively [38] motivating the replacement of carbon with hydrogen as the reducing agent for the production of iron powder [39].
Generally, plasma hydrogen reduction is used for treatment of bauxite residue in order to remove iron oxide producing slag. Jovicević-Klug et al. [40]. proposed how this red mud can be turned into valuable and sustainable feedstock for ironmaking using hydrogen-plasma-based reduction at higher temperatures in electric arc furnace (more than 1600 °C), thus mitigating a part of the steel-production related carbon dioxide emissions by making it available for the production of several hundred million tonnes of green steel. The process proceeds through rapid liquid-state reduction, chemical partitioning, as well as density-driven and viscosity-driven separation between metals and oxides. They proposed mechanism of slag formation from bauxite residues and chemical and mineralogical composition of obtained slag was presented in detail.
Plasma pyrolysis is an emerging technology that has great potential for the large-scale production of low-carbon and affordable hydrogen [41]. It is known, that atomic hydrogen and hydrogen plasma are more powerful as reductant compared to molecular hydrogen. Hydrogen plasma can resolve the thermodynamic barrier where it can theoretically reduce unreducible oxides such as SiO2, CaO, and MgO [42,43]. However, studies report that some of the oxides (i.e., Al2O3, TiO2) were only reduced into suboxides. BR is mixture of (Fe2O3, Al2O3, TiO2, SiO2, CaO, and MgO). Therefore, reduction of dried bauxite with hydrogen plasma opens new field for studies.
The primary aim of this work is to provide initial insights into the characterization of bauxite residues from Alumina, Zvornik, and to investigate their behavior following combined pyrometallurgical and hydrometallurgical treatments. The novelty of this study lies in the use of a combined approach, where the pyrometallurgical treatment involves hydrogen plasma reduction of red mud, a method that has not been extensively studied in the literature. The hydrometallurgical part examines both ultrasound-assisted and autoclave leaching for the slag. Sulfuric acid is used as the leaching agent under high pressure of pure gaseous oxygen, as well as ultrasound-assisted leaching as an unconventional process, without the presence of hydrogen peroxide. This study focuses on the recovery of Ti, Al, and residual Fe from the treated bauxite residues.
2. Materials and Methods
2.1. Methods
Characterization
Mineralogical characterization of samples was carried out using X-ray diffraction technique—XRD. After the measurement, images have been processed with the help of Difrac software Version 7. EVA v 4.2.2. The obtained values d (2θ), which are characteristic for each mineralogical phase, were compared with the literature data in the existing database, and thus we identified the present crystal phases. Sample preparation was performed in single steps. The samples needed to be grinded to a granulation of about 50 μm in order to create a flat-surface pellet in polyethylene molds.
In order to determine the elements in ppm-range, samples are measured on the ICP-OES device, using an optical emission technique that uses inductive-coupled plasma as a source. This technique is intended for analysis trace elements and requires converting the sample into an acidic solution. Sample preparation was performed using method—ISO 6607-1985. The method involves the destruction of the sample with three concentrated acids (sulfuric, nitric and hydrochloric) at the beginning, and the treatment of the precipitate with hydrofluoric acid to convert residual elements (except SiO2) into solution. After this preparation, a complete dissolution was expected. Total dissolution was confirmed during treatment of solid residue obtained in leaching experiments at 90 °C.
Experimental design of this research is shown in Figure 1
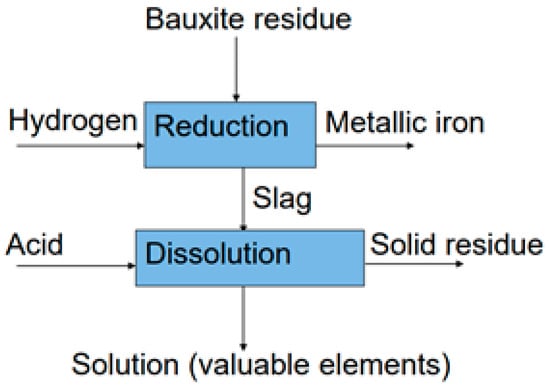
Figure 1.
Flow diagram of combined process.
2.2. Materials
Due to its properties such as high alkalinity, bauxite residue can be used as an input material in various neutralization processes. Three different types of bauxite residue were compared in this work, as shown in Table 2.

Table 2.
Chemical composition of BR.
This table shows that the bauxite residue from Zvornik, Bosnia and Herzegovina contain mostly iron oxide. The Greek bauxite residue contains more scandium, aluminium oxide, and chromium oxide but smaller content of sodium oxide, compared to the German and Zvornik one. Bauxite residue from Germany has higher alkalinity than other due to higher presence of the sodium hydroxide from the Bayer process. For this research, the bauxite residue was provided from Alumina ltd., Zvornik, Bosnia and Herzegovina as the starting raw material. The factory Alumina has been in continuous production mode since 6 October 1978 and continuously processes bauxite and produces alumina, aluminum hydroxide, zeolites and other related aluminosilicate products. The company Alumina currently has about 1500 employees, which is about 25% of all employees in Zvornik. Alumina owns a red mud disposal site located about 5 km from the factory. Transportation of the red mud suspension from the factory to the landfill is carried out by suitable pumps.
Depending on the quality of the bauxite, the amount of completely bauxite residues typically ranges from 0.8 to 2 tonnes of residue per tonne of alumina produced. The company Alumina doo from Zvornik uses bauxite with a silicon dioxide modulus (ratio of alumina (Al2O3) content to silica (SiO2) content in the bauxite) between 8.5 and 12. Accordingly, the amount of red mud that is separated and disposed of at the landfill is about 1.0–1.2 tonnes per tonne of Al2O3 produced, or approximately 400,000 t/per year.
The bauxite residue from Alumina was filtrated, washed and dried at 105 °C for 24 h. The chemical composition of bauxite residue is shown in Table 3.

Table 3.
Chemical composition of BR, Zvornik, Bosnia and Herzegovina.
One additional elemental ICP-OES analysis was performed in order to establish the content of rare earth elements (REE) presented in Table 4.

Table 4.
Content of rare earth elements in BR, Zvornik.
As shown at Figure 2, and Table 4, XRD-analysis for the red mud evidenced the following phases: hematite, calcium titanate perovskite, katoite, calcium hydroxide, silica and hydrogarnet.
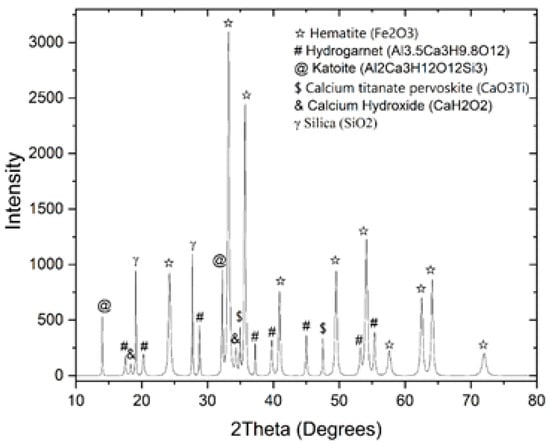
Figure 2.
XRD-analysis of BR from Zvornik.
As illustrated in Figure 2, iron is predominantly present in the structures of hematite, contributing significantly to the overall iron content of the red mud. Titanium occurs mainly within perovskite phases, indicating its close association with these minerals. Aluminum is distributed across multiple phases, including katoite, and hydrogarnet, reflecting the complexity of its occurrence in the red mud. The presence of these phases provides valuable insight into the mineralogical composition and behavior of the material during subsequent processing steps.
2.2.1. Hydrogen Plasma Reduction
Hydrogen plasma reduction of bauxite residue was performed in an electric arc furnace as an innovative technology to separate iron from bauxite residue at the Max Planck Institut for Sustainable Materials MPI SusMat, Duesseldorf, Germany.
An electric arc furnace (EAF) with a specialized design was used to heat materials via an electric arc. Industrial EAFs vary in size, from small units with capacities around one tonne, commonly used for producing cast iron, to large units of up to 400 tonnes employed in secondary steelmaking. Smaller EAFs, such as those used in research laboratories, can have capacities as low as a few dozen grams. Temperatures in industrial EAFs can reach up to 1800 °C [44] while laboratory furnaces can exceed 3000 °C. In an electric arc furnace, the material being heated (referred to as the charge) is directly exposed to the electric arc, with the current flowing from the furnace electrodes passing through the charge material.
The Electric Arc Furnace used for hydrogen reduction is operated under the following parameters: 20 g of bauxite residue (BR), total pressure 900 mbar, current 200 A, atmosphere of argon with 10% H2, and a temperature about 1800 °C (as shown at Figure 3).
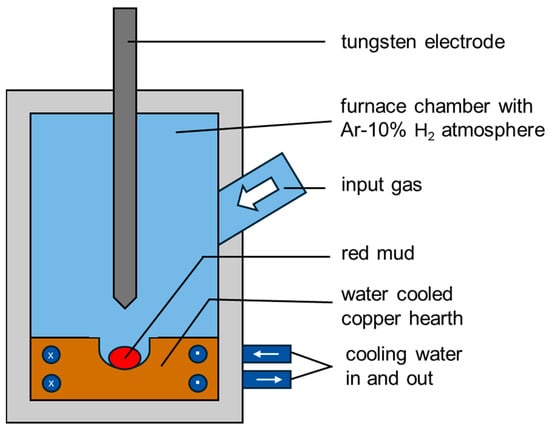
Figure 3.
Sketch of the Electric Arc Furnace used for plasma hydrogen reduction of BR.
To track the progress of both, the phase transformations and chemical composition over the course of the reduction process, the samples were submitted to interrupted reaction cycles. To obtain specimens in different partially reduced states, the bauxite residue pieces were subjected to sequential melting, solidification and remelting series at a pre-defined number of times (2, 5, 10 times) with a corresponding exposure time to the hydrogen plasma at 1 min for each cycle. During the 1-min cycles, complete melting of the samples was achieved with an average exposure time of 6 s. Once the plasma arc was switched off, rapid solidification conditions were achieved by means of the water-cooled copper hearth on which the bauxite residue pieces were molten and reduced. To ensure the proper stoichiometry conditions (i.e., a reductant atmosphere) and maintain the progressing reduction, the furnace chamber was replenished by a fresh mixture of Ar-10%H2 after the completion of every individual melting and solidification cycle. In summary, one single process cycle comprises the following steps: (1) filling the furnace with the bauxite residue sample and flooding the chamber with fresh Ar-10%H2 gas mixture; (2) simultaneous plasma arc melting and reduction of the sample; (3) intermediate interruption of the plasma arc operation and solidification of the (partially or fully reduced) sample, and (4) replenishing of the furnace’s chamber with fresh Ar-10%H2 gas mixture for the continuation of the process (i.e., succeeding cycle) [45,46].
2.2.2. Leaching of the Slag Using Unconventional Methods
This study investigates the extraction of valuable metals from reduced slag using sulfuric acid treatment. Key leaching parameters, including the solid-to-liquid ratio, pressure, and acid concentration, were maintained constant throughout the experiments. Variables such as temperature, reaction time, stirring speed, and particle size were optimized based on insights from prior experimental work.
High Pressure Leaching Method in an Autoclave
A 5 M sulfuric acid solution was prepared in a 1-L flask and introduced into a Buchi autoclave (as shown at Figure 4), containing slag derived from the hydrogen plasma reduction process.
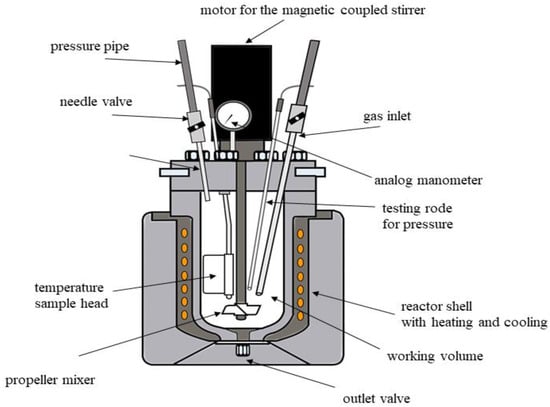
Figure 4.
Illustration of the the high pressure autoclave.
The system was pressurized to 9 bars with oxygen, the temperature was set to the desired level, and the magnetic stirrer was operated at a speed of 500 rpm. The leaching process was conducted for up to 120 min. The leaching process was performed in a Buchi autoclave, a specialized system designed for acid leaching with a capacity of 1.53 L, a maximum pressure of 200 bars, and a maximum temperature of 270 °C, and 2000 rpm.
The autoclave is equipped with a heat exchanger controlled by a thermostat, a mixer, pressure adjustment probes, and the capability for sample extraction during operation. The system is fully integrated with computer software, enabling precise control and real-time monitoring of operational parameters, with all data recorded for detailed post-experiment analysis.
The pressure within the system was monitored using both a manometer and digital sensors, with the total pressure comprising oxygen (9 bars) and water vapor (12–15 bars). Cooling was achieved using a dedicated cooling system, and the heating rate was controlled at 10 °C/min. Prior to each run, the autoclave was manually sealed with screws and subjected to a pressure integrity test to ensure safe and reliable operation. Due to very small amount of sample only one experiment has been conducted. Parameters were chosen considering experience from previous paper [47].
After the leaching process was completed, the autoclave was cooled to room temperature, and the system pressure was carefully released. The leachate solution was then subjected to filtration and neutralization with distilled water. Filtration was conducted using a vacuum-assisted filtration system integrated into the setup, ensuring effective separation of solid and liquid phases.
Ulltrasound Assisted Leaching Method
Additionally ultrasound assisted leaching process was used for slag and bauxite residues. The ultrasonic leaching setup is shown schematically in Figure 5. A flask with a capacity of 500 mL is immersed in a heatable ultrasonic bath. A gas supply is connected, which injects oxygen directly into the suspension at a flow rate of 1 L/min. The suspension is constantly swirled by a half-moon stirrer at 250 rpm. A laboratory cooler is also connected for condensing evaporating suspension. The water bath is kept at the desired temperature by the integrated heating plate of the ultrasonic device and by an external flow thermostat. The entire test setup is in a fume hood.
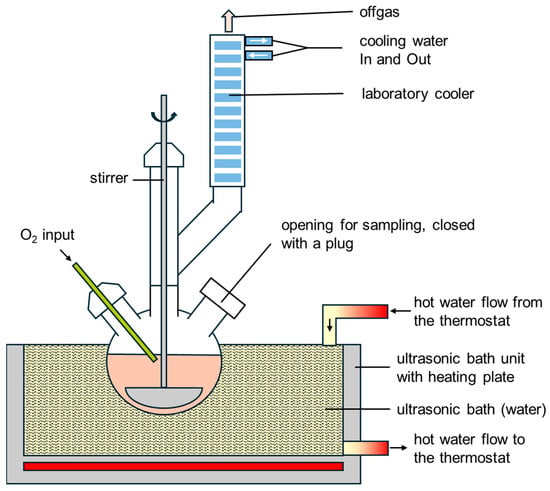
Figure 5.
Sketch of ultrasound assisted leaching process.
For an S/L ratio of 1/10, 5 g of solid, either red mud or the slag from plasma-thermally reduced red mud, were added to 50 mL of sulfuric acid with 2.7 mol/L. The temperature of the ultrasonic bath was constant within a test, at 50 °C, 65 °C or 80 °C, and was checked every half hour with a glass thermometer. The test lasted 120 min, and both ultrasound and oxygen supply were guaranteed throughout. Every 30 min, 2 mL of the suspension was removed. This amount was centrifuged and a 0.3 mL sample was taken from the liquid phase. The remainder was stirred again to form a suspension and added back to the test solution. The 0.3 mL samples were acidified with 1.5 mL concentrated nitric acid, diluted to 30 mL with distilled water and stored in a transparent plastic sample tube. After 120 min, a 0.3 mL sample was also taken using a centrifuge. The remaining suspension was filtered using fine filter paper in a plastic funnel. The solution was collected in a beaker and stored as a fifth sample, complete and undiluted, also in a transparent plastic sample tube.
3. Results and Discussion
3.1. Thermochemical Calculation
Thermochemical Analysis of Hydrogen Reduction
Equilibrium calculations were firstly done to identify relevant species and changes in chemical composition of red mud shown in Table 4. during hydrogen reduction at different temperatures until 1100 °C, as shown in Figure 6.
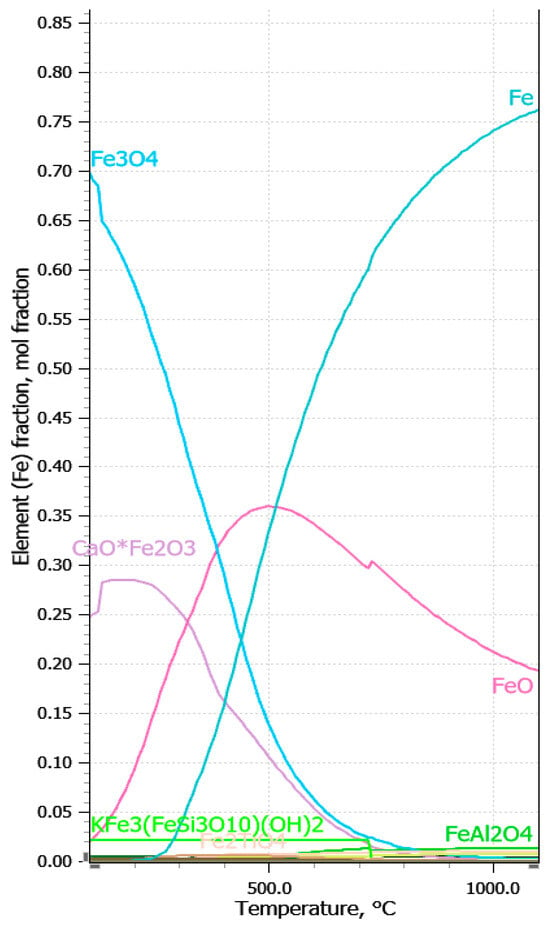
Figure 6.
Thermochemical calculation of reduction of iron oxides at different temperatures below 1000 °C.
Reduction by hydrogen, even with small ratios of H2/red mud leads to transformation of Fe2O3 to Fe. All these components can be easily recovered by magnetic separation.
Reduction of hematite by hydrogen proceeds in two or three steps, under and above 570 °C, respectively, via magnetite (Fe3O4) and wüstite (FeO) according to the following equations:
3Fe2O3 + H2 → 2Fe3O4 + H2O
Fe3O4 + H2 → 3FeO + H2O
FeO + H2 → Fe + H2O
Pineau et al., 2006 [48] found that reduction of iron ores with hydrogen leads to compact iron layers that could slow their reduction rate. Additionally, thermochemical analysis revealed the formation of Fe2TiO4 and FeAl2O4, which can lead to formation of passivation layer in reduction process. The formation of ferrite phases is expected for other elements such as calcium and sodium. Analysis of different phase during hydrogen reduction has performed using FactSage software 8.0 as shown at Figure 7 and Figure 8 [47].
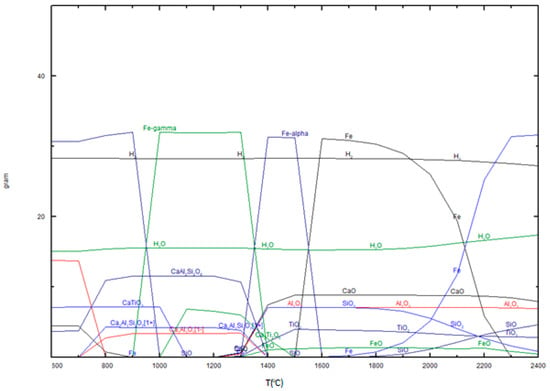
Figure 7.
Thermochemical prediction of hydrogen reduction of bauxite residues (49% Fe2O3, 8.0% CaO, 10% SiO2, 12% Al2O3).
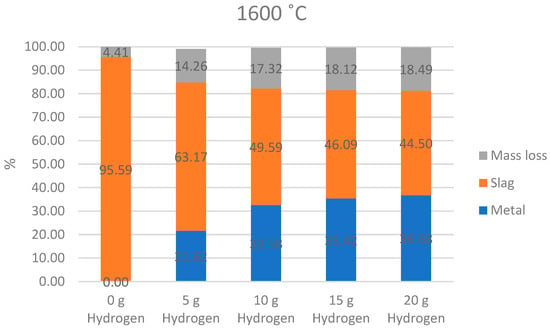
Figure 8.
Thermochemical prediction of formed slag, mass loss and metallic iron.
Figure 7 illustrates the thermodynamic simulation of phase transformations during heating in the presence of hydrogen. It shows that iron oxides undergo a series of reductions with increasing temperature, eventually forming metallic iron. At lower temperatures, iron exists in oxide forms, but as the temperature rises, hematite (Fe2O3) transitions to magnetite (Fe3O4), wustite (FeO), and finally to metallic iron (Fe-α and Fe-γ) at higher temperatures, with complete reduction occurring above 1400 °C.
Calcium compounds, such as calcium oxide (CaO), remain stable throughout the heating process, indicating their resistance to reduction under these conditions. Other calcium-containing phases, like calcium aluminosilicate (CaAl2Si2O8), appear and decompose at elevated temperatures, reflecting the redistribution of elements like calcium, aluminum, and silicon. Titanium dioxide (TiO2) remains relatively unaffected by the process, suggesting that it is not reduced or requires higher temperatures for reduction. Silicon dioxide (SiO2) is initially stable but partially reduces to silicon (Si) at very high temperatures, demonstrating the need for extreme conditions for significant reduction.
Aluminum oxide (Al2O3) shows high stability, indicating minimal reduction under these conditions. The water content in the system decreases with increasing temperature due to evaporation and reduction reactions, while hydrogen is consumed as it drives the reduction processes. The stability of refractory compounds like TiO2, Al2O3, and CaO suggests their incorporation into the slag phase, whereas iron reduction is highly effective under these conditions. This reduction of bauxite residues with hydrogen plasma at 1600 °C leads to formation of iron and slag and mass losses as shown at Figure 8.
The bar chart illustrates the distribution of mass loss, slag, and metallic products at 1600 °C under varying hydrogen inputs (0 g, 5 g, 10 g, 15 g, and 20 g).
At 0 g of hydrogen, the mass is almost entirely composed of slag (95.59%), with a small fraction (4.41%) attributed to mass loss. The introduction of hydrogen significantly alters the material distribution. At 5 g of hydrogen, the slag fraction decreases to 63.17%, while the metallic fraction (21.62%) emerges due to reduction reactions. The mass loss also increases to 14.26%, likely due to the release of gases such as H2O and oxygen during the reduction process.
As the hydrogen input increases, the metallic fraction continues to grow, reaching 32.58% at 10 g of hydrogen and 36.68% at 20 g. Correspondingly, the slag fraction declines, reaching 44.50% at 20 g. This trend indicates the progressive reduction of oxides to metallic phases with higher hydrogen mass. However, the mass loss stabilizes after an initial increase, remaining relatively constant between 17.32% and 18.49% for hydrogen inputs from 10 g to 20 g.
The data suggest that increasing hydrogen facilitates the reduction of oxides to metal, with diminishing returns in slag reduction and metal formation as the hydrogen mass exceeds 15 g. The process achieves optimal results in terms of metal yield and slag reduction at higher hydrogen inputs, though further increases beyond 20 g may not significantly improve outcomes.
As illustrated in Figure 9, the calculations (results based on the FactSage software 8.0 predictions) show a significant reduction in Fe2O3 concentration (represented by the red line and red y-axis), dropping from 46% in red mud to less than 1% mass fraction with just 5 g of hydrogen. The formation of FeO originates from the reduction of Fe2O3. At lower hydrogen masses, FeO constitutes a significant fraction, but its amount decreases as the hydrogen mass increases, due to further reduction to metallic Fe. By the time 30 g of hydrogen is used, the FeO fraction drops below 5%. Other compounds in red mud do not undergo reduction in the same manner; instead, they undergo phase changes, such as the release of CO2 or other gases, under high-temperature conditions. The mass fraction of TixOy (primarily TiO2 with a minor presence of Ti2O3) increases in the slag from 4.2% to nearly 15%. A hydrogen mass of 20 g raises it to 14%, with further increases in hydrogen showing negligible impact on the slag composition. An increase of reduction temperature from 1600 °C to 1700 °C does not show a significant influence on the compound mass fraction in slag.
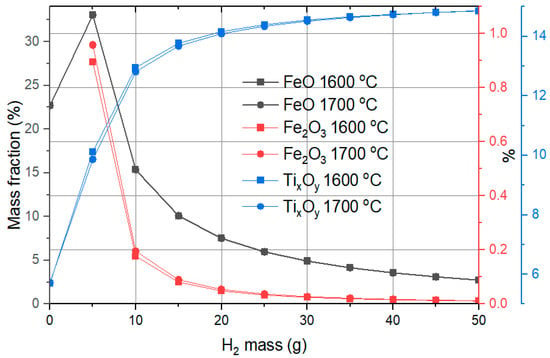
Figure 9.
Dependence of the compound mass fractions on hydrogen content in slag.
3.2. Hydrogen Plasma Reduction of BR
After plasma hydrogen reduction of the bauxite reside in furnace a slag and a metallic phase are formed. Figure 10a shows a clear picture of how the reddish-brown bauxite residue looks like after the reduction process. Figure 10b represents a metallic phase. Both slag and metallic phase are separated and measured separately for analysis, in order to know all the possible elements, present in both slag and metallic phase.

Figure 10.
(a) Slag phase separation and (b) Metallic phase in set 3-2.
The obtained results from three sets of experiments, each with three experiments are shown in Figure 11.
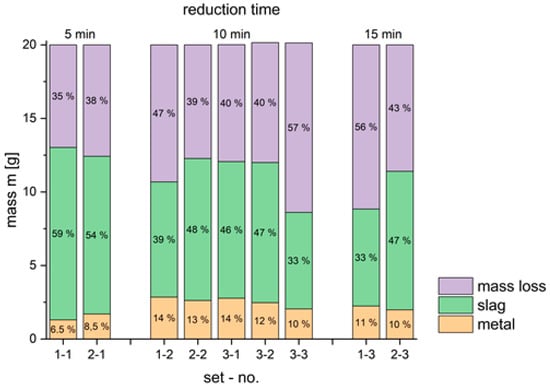
Figure 11.
Absolute mass and its distribution in metal and slag phase and mass loss.
The obtained ratio of formed materials from 20 g BR revealed that an increase of reduction time between 5 and 15 min increases the mass loss of BR between 34.8% and 55.9%. The same tendency is present in second set of experiments, e.g., the mass loss amounts between 37.9% and 43%. The mass loss contains evaporation, loss of oxygen during reduction of iron oxides and thermal decomposition of calcium carbonate. The expected chemical reactions for reduction of iron-oxides are shown below:
Because of the high amount of mass loss in the first step of the proposed process, the obtained results are very important for an environmentally friendly concept. Additionally, the produced metallic iron will be used in the steel industry. An increase in reduction time between 5 and 10 min leads to the production of metallic iron between 6.5% and 14.25% of the initial BR in the first set of experiments and between 8.5% and 13.1% in the second set of experiments. According to the chemical analysis of iron in Table 1, the chemical content of iron is 34.50%. Comparing the formed metallic iron and the initial mass of iron in 20 g of bauxite, the obtained maximal reduction efficiency amounts to 41.60%. A further increase in reduction time from 10 to 15 min decreases the mass of formed iron, indicating that 10 min provides the best results. This reduction in yield at longer times can be attributed to the significant mass loss at high temperatures, as well as possible interactions of the material with the reactor material, which may lead to mass and iron loss. The chemical analysis of the obtained metallic product has confirmed the presence of iron (99.5%). The content of impurities in metallic iron is shown in Table 5.

Table 5.
The chemical analysis of impurities in obtained metallic iron.
Table 5 presents the chemical analysis of impurities in the obtained metallic iron, highlighting the concentrations of aluminum (Al), cobalt (Co), gallium (Ga), and titanium (Ti) over three time intervals: 5 min, 10 min, and 15 min.
The concentration of Al exhibits a significant increase over time, rising from 218 ppm at 5 min to 2200 ppm at 10 min, and reaching 4000 ppm at 15 min, indicating a notable accumulation of this impurity. The content of Co shows slight variations, starting at 264 ppm at 5 min, slightly increasing to 287 ppm at 10 min, and decreasing to 244 ppm at 15 min, suggesting less consistent behavior during the reduction process. Ga content increases gradually from 89 ppm at 5 min to 106 ppm at 10 min and 112 ppm at 15 min, reflecting a steady but moderate rise. Ti content increases substantially, from 68 ppm at 5 min to 954 ppm at 10 min, and then to 1800 ppm at 15 min, indicating significant accumulation during the process, likely due to its high oxide stability.
The XRD analysis of the metallic iron, as illustrated in Figure 12, confirmed the predominant presence of BCC iron in the sample. Extending the reduction time from 5 min to 15 min results in a notable decrease in the quantity of slag produced. The maximum mass of slag generated accounts for 58.6% of the initial 20 g of bauxite residue, highlighting the efficiency of the reduction process in minimizing waste and concentrating metallic iron.
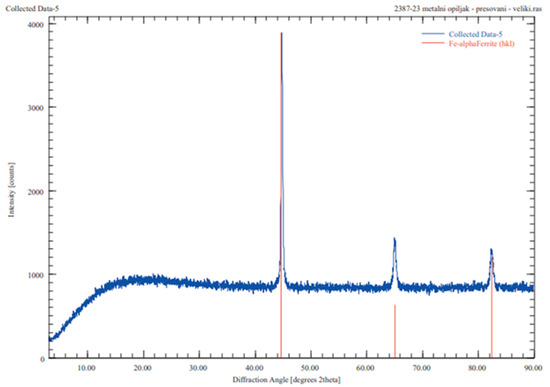
Figure 12.
XRD analysis of metallic iron phase.
After magnetic separation of metallic iron from slag, XRD analysis has shown different phases.
The XRD analysis of the plasma-reduced slag, shown in Figure 13, detects iron (II) and iron (III) oxide as well as elemental iron. This proves a partial reduction of the iron from the red mud, as well as an incomplete separation of the reduced, elemental iron from the slag. The iron titanium oxide FeTiO3 detected by the XRD analysis corresponds to ilmenite, the mineral from which titanium is primarily extracted. The last component detected by the XRD analysis is only crystalline silicon oxide. The XRD analysis shows no compounds for other elements such as aluminum or calcium, although they are detected by the ICP-IOS analysis in both the red mud and the plasma-reduced slag. Since the XRD analysis is based on X-ray diffraction at regular lattice spacings, this method can only detect crystalline substances. It can therefore be assumed that aluminum and calcium are present in a glassy compound, which cannot be detected using XRD. A glassy phase has already been observed in previous plasma reductions with red mud. A glassy phase in the slag is also plausible when considering the slag theory: In combination with basic compounds such as calcium oxide, aluminum oxide forms charge-deficit tetrahedra, which combine in a glassy structure. It is difficult to make quantitative statements using XRD analysis. It is therefore not possible to determine whether the reflections have captured the entire amount of silicon, iron and titanium and thus whether these elements are all present in crystalline compounds. It is therefore possible that titanium is also present in the glassy phase.
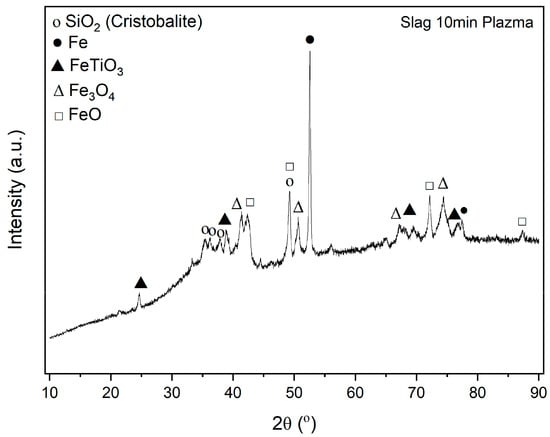
Figure 13.
XRD analysis of slag.
After hydrogen plasma reduction, hematite is not present. Hydrogen reduction started from hematite to magnetite and wustite with formation of FeAl2O4, franklinite and cuprospinel. An analysis of components during hydrogen reduction in 5 min, 10 min and 16 min is shown at Figure 14.
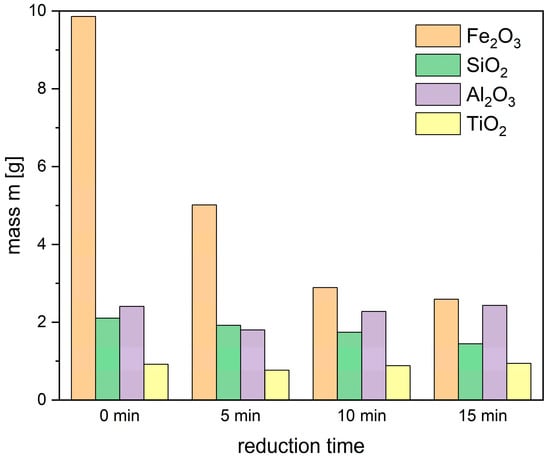
Figure 14.
Absolute mass of oxides in bauxite residues (0 min) and slag after 5-, 10- and 15-min reduction time (set 2).
As illustrated in Figure 14, the content of oxidic components decreases significantly during the reduction process between 5 and 15 min. The hydrogen reduction leads to a minimal Fe2O3 content in the slag after 15 min, as confirmed by the second set of experiments. This residual iron oxide represents the remnants following hydrogen reduction. Notably, some variations in the SiO2 content might be attributed to the partial reduction of SiO2 to SiO in the presence of hydrogen plasma species. In contrast, the high stability of oxides such as CaO, Al2O3, and TiO2 results in only minor changes to their content during the reduction process.
Analysis of distribition of rare elements offers a possibility to concentrate these elements, as shown in Table 6.

Table 6.
Content of rare earth elements in slag and metal after reduction, Zvornik.
The obtained results confirmed an increase of content of rare earth elements during plasma hydrogen reduction from 700 ppm to 1526 ppm (about 0.1%); as shown in Table 7.

Table 7.
Content of the main elements in slag and metal after reduction in 10 min.
The same case is with main elements in slag shown in Table 7.
The table compares the elemental composition of red mud, slag, and metal after processing. In slag, iron content decreases to 23.1%, while aluminum (12.2%) and titanium (5.76%) increase, indicating their concentration during reduction. Silicon (7.38%) and calcium (13.9%) also rise, reflecting mineral phase changes. Sodium remains stable, while vanadium and magnesium increase slightly, staying in the slag phase. In the metal sample, iron dominates at 99.5%, confirming efficient recovery. Other elements, including aluminum (0.22%) and titanium (0.10%), are present in trace amounts, while vanadium and magnesium are mostly retained in the slag. This composition demonstrates successful iron separation and concentration of valuable elements in the slag.
3.3. Leaching of the Slag
After the separation of reduced bauxite residue into metallic and non-metallic phases through magnetic separation, the slag (non-metallic phase) underwent a leaching process to extract valuable elements. The leaching process was conducted in an autoclave under controlled conditions to maximize efficiency and recovery.
3.3.1. Leaching in an Autoclave
A 5 M sulfuric acid solution was used as the leaching agent, ensuring sufficient acid concentration to dissolve targeted compounds in the slag. The process was carried out at a temperature of 150 °C, maintained consistently throughout the operation to enhance reaction kinetics. To provide an oxidizing environment conducive to effective leaching, 9 bar of oxygen pressure was applied within the autoclave. This high-pressure oxygen environment aids in breaking down refractory compounds and facilitates the dissolution of metal oxides.
The system was stirred continuously at 500 RPM to ensure homogeneity and proper contact between the slag particles and the leaching solution. This agitation helped in preventing sedimentation and promoting uniform reaction rates across the system. The total leaching duration was set to 120 min, allowing sufficient time for the reactions to reach completion while balancing operational efficiency. These optimized conditions aimed to achieve maximum dissolution of valuable elements from the slag while minimizing undesired side reactions and residual waste.
Upon completion of the leaching process, the suspension was subjected to filtration to separate the solid residue from the liquid phase. The solid residue was thoroughly washed with deionized water to remove any adhering leachate or impurities, thereby minimizing contamination and ensuring accurate analysis.
After washing, the solid residue was dried under controlled conditions to eliminate moisture content, preparing it for further characterization. The results of the analysis of the processed materials, including compositional and structural changes in the residue, are presented in Figure 15.
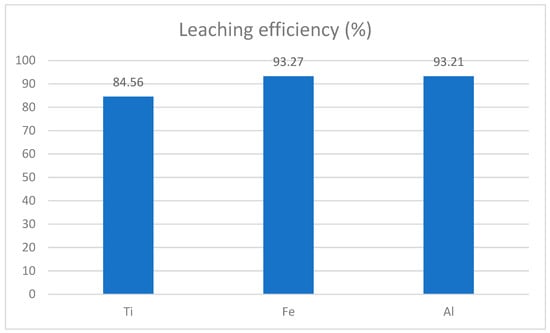
Figure 15.
Leaching efficiency of slag under high pressure in an autoclave at 150 °C.
The leaching efficiencies for titanium, iron, and aluminum demonstrate the effectiveness of the process. Titanium achieved a leaching efficiency of 84.56%, indicating that a substantial portion was dissolved into the leachate, though some remained in the solid residue, what is more than shown in the literature data shown in Table 2. Iron, with an efficiency of 93.27%, was extracted at a high rate, showing excellent dissolution under the applied conditions. Similarly, aluminum exhibited a leaching efficiency of 93.21%, comparable to iron, reflecting the suitability of the parameters for its recovery. These results highlight the overall optimization of the leaching process offering a detailed analysis of mechanism of process, as shown with chemical equations [7]:
To validate findings obtained from the chemical analysis of the leachate and solid residue, an XRD (X-ray diffraction) analysis was conducted on the solid residue following the leaching process.
The XRD (X-ray diffraction) analysis of the solid residue after leaching with sulfuric acid (Figure 16) indicates that the dominant crystalline phases present are CaSO4 (calcium sulfate) and SiO2 (silicon dioxide). This suggests that during the leaching process, sulfuric acid reacted with calcium-containing compounds in the slag, leading to the formation of CaSO4. The presence of SiO2 implies that silica remained largely unreacted under the applied leaching conditions, possibly due to its refractory nature.
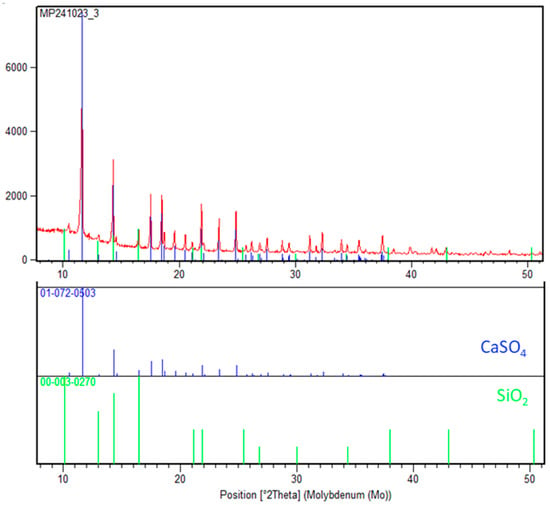
Figure 16.
XRD analysis of solid residue after leaching in an autoclave (green color SiO2, blue color CaSO4).
The absence of significant peaks for other crystalline phases indicates that most of the other elements (e.g., Fe, Ti, Al) were effectively dissolved during leaching or are present in an amorphous phase that does not produce prominent XRD signals. The crystalline nature of CaSO4 and SiO2 highlights their stability under the leaching conditions, with CaSO4 likely precipitating as a result of sulfate reactions and SiO2 being inert to sulfuric acid at the given parameters.
This analysis confirms the efficiency of the leaching process in removing targeted metals while leaving behind a residue dominated by inert or newly formed stable phases avoiding silica gel formation.
3.3.2. Ultrasound Assisted Leaching of Slag
Silica gel formation was observed in the test series of ultrasound assisted leaching of slag. All undiluted and unacidified samples formed a gel after standing for a few days. However, no gelation occurred in the diluted and acidified samples. The slag leachate exhibited the quickest gelation, with the solution leached at 50 °C forming a gel overnight, while the solution leached at 80 °C began to gel during filtration. The filter and beaker used to collect the silica gel residue are shown in Figure 17. Additionally, the consistency of the silica gel varied. The gel formed from red mud leaching could be broken down into a viscous, partially lumpy fluid by shaking. In contrast, the silica gel from the slag leached at 50 °C remained dimensionally stable even when shaken. The gel from the slag solution leached at 80 °C became solid within hours.
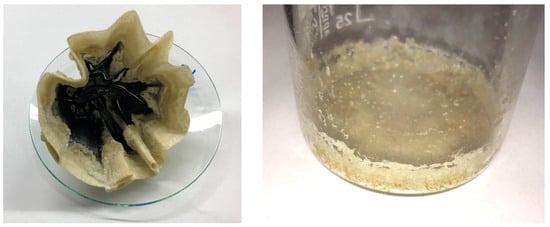
Figure 17.
Silica gel formation during the leaching of slag at 50 °C, (left) in filter paper, (right) in the beaker under the filter.
The observed difference in the adhesion of the residue to surfaces can be explained by different properties of the feed material. For example, a difference in the average particle size and grain size distribution, as well as a different porosity, can influence the physical behavior of the suspension. These differences are also an influencing factor for the leaching behavior, in particular for the leaching rate. Therefore, the concentrations in the solution obtained discussed below and the leaching efficiencies determined from them could also be influenced by the different physical properties of the feed materials. The white solid may be CaSO4, which is formed as a solid product in the reaction of CaO and sulphuric acid, as found at the Figure 18. It may not have been noticed in the red residue of the red mud leaching because it was colored by the iron oxide particles.
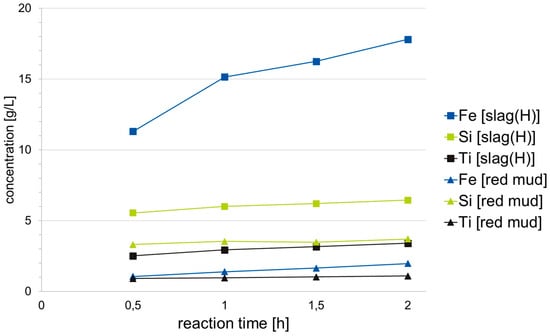
Figure 18.
Time-dependent concentration curve of Fe, Si and Ti from BR and slag, each at 50 °C.
The change in concentration of selected elements over the duration of the test can be seen in Figure 18 using the example of leaching at 50 °C. This shows that the leaching of silicon and titanium is not very time-dependent, but is almost completely completed after 30 min. The elements aluminum and calcium, which are not shown in Figure 19, are also not time-dependent. Small, steady increases in concentration can be explained by losses through evaporation despite exhaust gas condensation and by losses when taking samples. Since these increases in concentration can be traced primarily to errors in the test execution, the analysis after 30 min, which is the most precise, is primarily evaluated below. Only the concentration of iron, particularly when leaching slag, increases so much with the test time that a time dependency can be assumed.
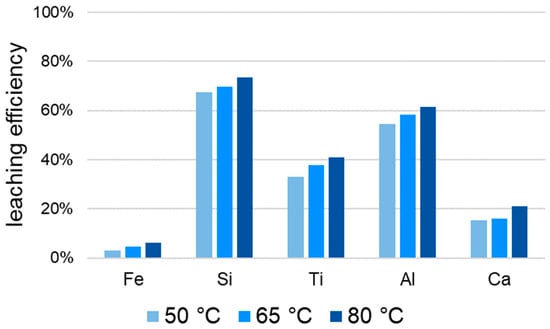
Figure 19.
Temperature-dependent leaching efficiency curve of Fe, Si and Ti from BR.
The leaching efficiency of silicon and the target metal titanium is increased by prior reduction. This corresponds to the goal of the greatest possible titanium mobilization, but the risk of silica gel formation is also increased by the improved leaching properties in addition to the concentration in the reduction. The leaching efficiency of aluminum and calcium is lower when leaching slag compared to BR (“red mud”). Since these two elements are not target metals, a low leaching efficiency is to be viewed positively. Temperature increases the leaching efficiency for all elements, both for BR and for slag as feedstock. The influence of a higher temperature in BR leaching can be seen in Figure 19. The low leaching efficiency of iron increases to 6% at 80 °C, so it is still suitable for separation. The maximum leaching efficiency of Titanium from BR is 40.7%.
Figure 20 shows the change in leaching efficiency of slag at elevated temperature. Due to the small amount of plasma-thermally reduced slag, the test was not carried out at 65 °C. The highest leaching efficiency of titanium is 54% at 80 °C.
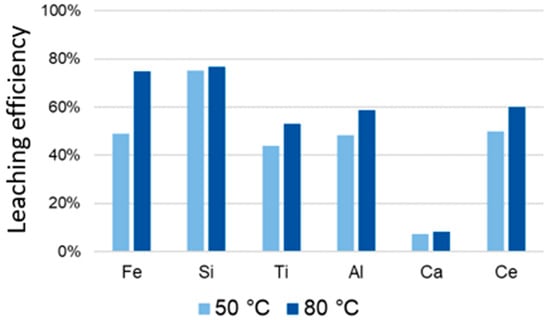
Figure 20.
Temperature dependence of ultrasound assisted added leaching efficiency from slag obtained by hydrogen plasma reduction of BR, after 30 min.
Generally, an increase of temperature from 50 °C to 80 °C increases the leaching efficiency for all elements such as Fe, Si, Ti, Al, Ca and Ce. An increase of solubility of Si is main reason for formation of siliga gel. The obtained results have shown technical limitations of ultrasound assisted leaching (leaching time of 2 h and very slow heating process), what leads un-sufficient leaching efficiency. Selectivity of leaching process is also big problem during UAL.
4. Conclusions
This study demonstrates the effective treatment of bauxite residue using plasma hydrogen reduction followed by leaching. Plasma hydrogen reduction efficiently separated the residue into metallic iron (with a recovery rate of up to 41.6%) and slag, concentrating valuable elements like aluminum, titanium, and rare earths in the slag phase. The metallic phase contained 99.5% pure iron, showcasing high recovery efficiency. Thermodynamic simulations confirmed the selective reduction of iron oxides, while refractory compounds remained in the slag.
Leaching the slag with sulfuric acid under high pressure achieved high extraction efficiencies for aluminum (93.21%), iron (93.27%), and titanium (84.56%). Ultrasonic assistance showed results with leaching efficiency lower than in autoclave with aslo silica gel formation. Leaching efficiency varied with time, with titanium and silicon leaching effectively in 30 min, while aluminum and calcium showed steady increases. X-ray diffraction (XRD) analysis confirmed the successful dissolution of iron, aluminum, and titanium, leaving stable phases like CaSO4 and SiO2 in the residue from autoclave leaching.
This approach offers significant environmental and economic benefits, including the high recovery of valuable elements, reduction of waste materials, and concentration of rare earth elements, enhancing the potential for industrial applications. The innovative combination of plasma hydrogen reduction, thermochemical optimization, and ultrasonic-assisted leaching provides a sustainable pathway for managing bauxite residue while enabling the recovery of essential resources for industries such as steel production and rare earth elements extraction.
Author Contributions
Conceptualization, S.S. and D.K.; methodology, S.S.; software, E.E.-K. and R.S.; validation, S.S., D.K. and I.R.S.F.; formal analysis, R.S. and I.R.S.F.; investigation, I.R.S.F., M.P. and D.K.; resources, S.S.; data curation, S.S.; writing—original draft preparation, D.K. and S.S.; writing—review and editing, I.R.S.F., M.P. and E.E.-K.; visualization, D.K.; supervision, B.F. and M.P.; project administration, S.S.; funding acquisition, S.S. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Commission, grant number 101135077 (EURO-TITAN project).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Živković, Ž.; Vračar, R. Ekstraktivna Metalurgija Aluminijuma. Available online: https://www.knjizara.com/Ekstraktivna-metalurgija-aluminijuma-Zivan-Zivkovic-3434 (accessed on 27 December 2024).
- Šajn, R.; Ristović, I.; Čeplak, B. Mining and Metallurgical Waste as Potential Secondary Sources of Metals—A Case Study for the West Balkan Region. Minerals 2022, 12, 547. [Google Scholar] [CrossRef]
- Alijagic, J.; Šajn, R.; Rokavec, D. Impact Assessment of Mining and Metallurgical Activities on the Distribution of Trace Elements in the Stavnja Valley. Int. J. Energy Environ. Econ. 2015, 23, 351–412. [Google Scholar]
- Paçarizi, M.; Stafilov, T.; Šajn, R.; Tašev, K.; Sopaj, F. Estimation of Elements’ Concentration in Air in Kosovo through Mosses as Biomonitors. Atmosphere 2021, 12, 415. [Google Scholar] [CrossRef]
- Piga, L.; Pochetti, F.; Stoppa, L. Recovering Metals from Red Mud Generated during Alumina Production. JOM 1993, 45, 54–59. [Google Scholar] [CrossRef]
- Bogatyrev, B.A.; Zhukov, V.V.; Tsekhovsky, Y.G. Formation Conditions and Regularities of the Distribution of Large and Superlarge Bauxite Deposits. Lithol. Miner. Resour. 2009, 44, 135–151. [Google Scholar] [CrossRef]
- Agatzini-Leonardou, S.; Oustadakis, P.; Tsakiridis, P.E.; Markopoulos, C. Titanium Leaching from Red Mud by Diluted Sulfuric Acid at Atmospheric Pressure. J. Hazard. Mater. 2008, 157, 579–586. [Google Scholar] [CrossRef]
- Kounalakis, P.; Aravossis, K.; Karayianni, C.H.S. Feasibility Study for an Innovative Industrial Red Mud Utilisation Method. Waste Manag. Res. 2016, 34, 171–175. [Google Scholar] [CrossRef]
- Agrawal, S.; Dhawan, N. Evaluation of Red Mud as a Polymetallic Source—A Review. Miner. Eng. 2021, 171, 107084. [Google Scholar] [CrossRef]
- Zinoveev, D.V.; Grudinskii, P.I.; Dyubanov, V.G.; Kovalenko, L.V.; Leont’ev, L.I. Oбзop Mиpoвoй Пpaктики Пepepaбoтки Kpacныx Шлaмoв. Чacть 1. Пиpoмeтaллуpгичecкиe Спocoбы. Изbecmuя Bыcmux Учeбных Зabeдeний. Чepнaя Meтaллуpгия 2018, 61, 843–858. [Google Scholar] [CrossRef]
- Park, N.K.; Choi, H.Y.; Kim, D.H.; Lee, T.J.; Kang, M.; Lee, W.G.; Kim, H.D.; Park, J.W. Purification of Al(OH)3 Synthesized by Bayer Process for Preparation of High Purity Alumina as Sapphire Raw Material. J. Cryst. Growth 2013, 373, 88–91. [Google Scholar] [CrossRef]
- Damjanovic, V.; Filipovic, R.; Obrenovic, Z.; Perusic, M.; Kostic, D.; Smiljanic, S.; Stopic, S. Influence of Process Parameters in Three-Stage Purification of Aluminate Solution and Aluminum Hydroxide. Metals 2023, 13, 1816. [Google Scholar] [CrossRef]
- Svobodova-Sedlackova, A.; Calderón, A.; Fernandez, A.I.; Chimenos, J.M.; Berlanga, C.; Yücel, O.; Barreneche, C.; Rodriguez, R. Mapping the Research Landscape of Bauxite By-Products (Red Mud): An Evolutionary Perspective from 1995 to 2022. Heliyon 2024, 10, e24943. [Google Scholar] [CrossRef] [PubMed]
- Castaldi, P.; Silvetti, M.; Santona, L.; Enzo, S.; Melis, P. XRD, FTIR, and Thermal Analysis of Bauxite Ore-Processing Waste (Red Mud) Exchanged with Heavy Metals. Clays Clay Miner. 2008, 56, 461–469. [Google Scholar] [CrossRef]
- Samouhos, M.; Taxiarchou, M.; Tsakiridis, P.E.; Potiriadis, K. Greek “Red Mud” Residue: A Study of Microwave Reductive Roasting Followed by Magnetic Separation for a Metallic Iron Recovery Process. J. Hazard. Mater. 2013, 254–255, 193–205. [Google Scholar] [CrossRef]
- Lazou, A.; van der Eijk, C.; Balomenos, E.; Kolbeinsen, L.; Safarian, J. On the Direct Reduction Phenomena of Bauxite Ore Using H2 Gas in a Fixed Bed Reactor. J. Sustain. Metall. 2020, 6, 227–238. [Google Scholar] [CrossRef]
- Xakalashe, B.; Friedrich, B. Combined Carbothermic Reduction of Bauxite Residue and Basic Oxygen Furnace Slag for Enhanced Recovery of Fe and Slag Conditioning. In Proceedings of the 2nd Conference of Bauxite Residue Valorisation and Best Practices, Athens, Greece, 7–10 May 2018. [Google Scholar]
- Davris, P.; Stopic, S.; Balomenos, E.; Panias, D.; Paspaliaris, I.; Friedrich, B. Leaching of Rare Earth Elements from Eudialyte Concentrate by Suppressing Silica Gel Formation. Miner. Eng. 2017, 108, 115–122. [Google Scholar] [CrossRef]
- Yagmurlu, B.; Alkan, G.; Xakalashe, B.; Schier, C.; Gronen, L.; Koiwa, I.; Dittrich, C.; Friedrich, B. Synthesis of Scandium Phosphate after Peroxide Assisted Leaching of Iron Depleted Bauxite Residue (Red Mud) Slags. Sci. Rep. 2019, 9, 11803. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Pontikes, Y. Towards Zero-Waste Valorisation of Rare-Earth-Containing Industrial Process Residues: A Critical Review. J. Clean. Prod. 2015, 99, 17–38. [Google Scholar] [CrossRef]
- Rivera, R.M.; Ulenaers, B.; Ounoughene, G.; Binnemans, K.; Gerven, T.V. Behaviour of Silica during Metal Recovery from Bauxite Residue by Acidic Leaching. In Proceedings of the International Committee for Study of Bauxite, Alumina & Aluminium, ICSOBA, Hamburg, Germany, 2–5 October 2017. [Google Scholar]
- Alkan, G.; Yagmurlu, B.; Cakmakoglu, S.; Hertel, T.; Kaya, Ş.; Gronen, L.; Stopic, S.; Friedrich, B. Novel Approach for Enhanced Scandium and Titanium Leaching Efficiency from Bauxite Residue with Suppressed Silica Gel Formation. Sci. Rep. 2018, 8, 5676. [Google Scholar] [CrossRef]
- Yagmurlu, B.; Alkan, G.; Xakalashe, B.; Friedrich, B.; Stopic, S.; Dittrich, C. Combined SAF Smelting and Hydrometallurgical Treatment of Bauxite Residue for Enhanced Valuable Metal Recovery. In Proceedings of the Travaux 46, Proceedings of 35th International ICSOBA Conference, Hamburg, Germany, 3 October 2017. [Google Scholar]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Recovery of Rare Earths and Other Valuable Metals From Bauxite Residue (Red Mud): A Review. J. Sustain. Metall. 2016, 2, 365–386. [Google Scholar] [CrossRef]
- Alkan, G.; Stopić, S. Titanium Extraction from Red Mud via Hydrometallurgical Treatment. In Proceedings of the European Metallurgical Conference, Leipzig, Germany, 25–28 June 2017. [Google Scholar]
- Alkan, G.; Schier, C.; Gronen, L.; Stopic, S.; Friedrich, B. A Mineralogical Assessment on Residues after Acidic Leaching of Bauxite Residue (Red Mud) for Titanium Recovery. Metals 2017, 7, 458. [Google Scholar] [CrossRef]
- Şayan, E.; Bayramoǧlu, M. Statistical Modeling of Sulfuric Acid Leaching of TiO2 from Red Mud. Hydrometallurgy 2000, 57, 181–186. [Google Scholar] [CrossRef]
- Rao, M.; Xia, H.; Xu, Y.; Jiang, G.; Zhang, Q.; Yuan, Y.; Zhang, L. Study on Ultrasonic Assisted Intensive Leaching of Germanium from Germanium Concentrate Using HCl/NaOCl. Hydrometallurgy 2024, 230, 106385. [Google Scholar] [CrossRef]
- Bao, S.; Chen, B.; Zhang, Y.; Ren, L.; Xin, C.; Ding, W.; Yang, S.; Zhang, W. A Comprehensive Review on the Ultrasound-Enhanced Leaching Recovery of Valuable Metals: Applications, Mechanisms and Prospects. Ultrason. Sonochem. 2023, 98, 106525. [Google Scholar] [CrossRef]
- Lucas, H.; Stopic, S.; Xakalashe, B.; Ndlovu, S.; Friedrich, B. Synergism Red Mud-Acid Mine Drainage as a Sustainable Solution for Neutralizing and Immobilizing Hazardous Elements. Metals 2021, 11, 620. [Google Scholar] [CrossRef]
- Liu, Y.; Naidu, R. Hidden Values in Bauxite Residue (Red Mud): Recovery of Metals. Waste Manag. 2014, 34, 2662–2673. [Google Scholar] [CrossRef]
- Cavaliere, P. Clean Ironmaking and Steelmaking Processes: Efficient Technologies for Greenhouse Emissions Abatement. In Clean Ironmaking and Steelmaking Processes; Springer: Cham, Switzerland, 2019; pp. 1–37. [Google Scholar] [CrossRef]
- Rukini, A.; Rhamdhani, M.A.; Brooks, G.A.; Van den Bulck, A. Metals Production and Metal Oxides Reduction Using Hydrogen: A Review. J. Sustain. Metall. 2022, 8, 1–24. [Google Scholar] [CrossRef]
- Khanna, R.; Konyukhov, Y.; Zinoveev, D.; Jayasankar, K.; Burmistrov, I.; Kravchenko, M.; Mukherjee, P.S. Red Mud as a Secondary Resource of Low-Grade Iron: A Global Perspective. Sustainability 2022, 14, 1258. [Google Scholar] [CrossRef]
- Teplov, O.A.; Lainer, Y.A. Rate of the Reduction of the Iron Oxides in Red Mud by Hydrogen and Converted Gas. Russ. Metall. 2013, 2013, 25–32. [Google Scholar] [CrossRef]
- Kapelari, S.; Gamaletsos, P.N.; Van Der Donck, T.; Pontikes, Y.; Blanpain, B. H2-Based Processes for Fe and Al Recovery from Bauxite Residue (Red Mud): Comparing the Options. Mater. Proc. 2021, 5, 45. [Google Scholar] [CrossRef]
- Pilla, G.; Kapelari, S.V.; Hertel, T.; Blanpain, B.; Pontikes, Y. Hydrogen Reduction of Bauxite Residue and Selective Metal Recovery. Mater. Today Proc. 2022, 57, 705–710. [Google Scholar] [CrossRef]
- Ma, Y.; Souza Filho, I.R.; Bai, Y.; Schenk, J.; Patisson, F.; Beck, A.; van Bokhoven, J.A.; Willinger, M.G.; Li, K.; Xie, D.; et al. Hierarchical Nature of Hydrogen-Based Direct Reduction of Iron Oxides. Scr. Mater. 2022, 213, 114571. [Google Scholar] [CrossRef]
- Patterer, L.; Mayer, E.B.; Mráz, S.; Pöllmann, P.J.; Hans, M.; Primetzhofer, D.; Souza Filho, I.R.; Springer, H.J.; Schneider, J.M. Effect of Si on the Hydrogen-Based Direct Reduction of Fe2O3 Studied by XPS of Sputter-Deposited Thin-Film Model Systems. Scr. Mater. 2023, 233, 115515. [Google Scholar] [CrossRef]
- Jovičević-Klug, M.; Souza Filho, I.R.; Springer, H.; Adam, C.; Raabe, D. Green Steel from Red Mud through Climate-Neutral Hydrogen Plasma Reduction. Nature 2024, 625, 703–709. [Google Scholar] [CrossRef]
- Chen, G.; Tu, X.; Homm, G.; Weidenkaff, A. Plasma Pyrolysis for a Sustainable Hydrogen Economy. Nat. Rev. Mater. 2022, 7, 333–334. [Google Scholar] [CrossRef]
- Gardner, R. The kinetics of silica reduction in hydrogen. J. Solid State Chem. 1974, 9, 336–344. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Ding, W.; Ding, W.; Lu, X.; Lu, X.; Guo, S.; Guo, S.; Xu, K.; Xu, K. Reduction of TiO2 with Hydrogen Cold Plasma in DC Pulsed Glow Discharge. Trans. Nonferrous Met. Soc. China 2005, 15, 594–599. [Google Scholar]
- Souza Filho, I.R.; da Silva, A.K.; Büyükuslu, Ö.K.; Raabe, D.; Springer, H. Sustainable Ironmaking Toward a Future Circular Steel Economy: Exploiting a Critical Oxygen Concentration for Metallurgical Cu Removal from Scrap-Based Melts. Steel Res. Int. 2024, 95, 2300785. [Google Scholar] [CrossRef]
- Souza Filho, I.R.; Ma, Y.; Raabe, D.; Springer, H. Fundamentals of Green Steel Production: On the Role of Gas Pressure During Hydrogen Reduction of Iron Ores. JOM 2023, 75, 2274–2286. [Google Scholar] [CrossRef]
- Souza Filho, I.R.; Ma, Y.; Kulse, M.; Ponge, D.; Gault, B.; Springer, H.; Raabe, D. Sustainable Steel through Hydrogen Plasma Reduction of Iron Ore: Process, Kinetics, Microstructure, Chemistry. Acta Mater. 2021, 213, 116971. [Google Scholar] [CrossRef]
- Stopic, S.; Kostić, D.; Schneider, R.; Sievers, M.; Wegmann, F.; Emil Kaya, E.; Perušić, M.; Friedrich, B. Recovery of Titanium from Red Mud Using Carbothermic Reduction and High Pressure Leaching of the Slag in an Autoclave. Minerals 2024, 14, 1151. [Google Scholar] [CrossRef]
- Pineau, A.; Kanari, N.; Gaballah, I. Kinetics of Reduction of Iron Oxides by H2: Part I: Low Temperature Reduction of Hematite. Thermochim Acta 2006, 447, 89–100. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).