Unveiling the Potential of Apricot Residues: From Nutraceuticals to Bioenergy
Abstract
1. Introduction
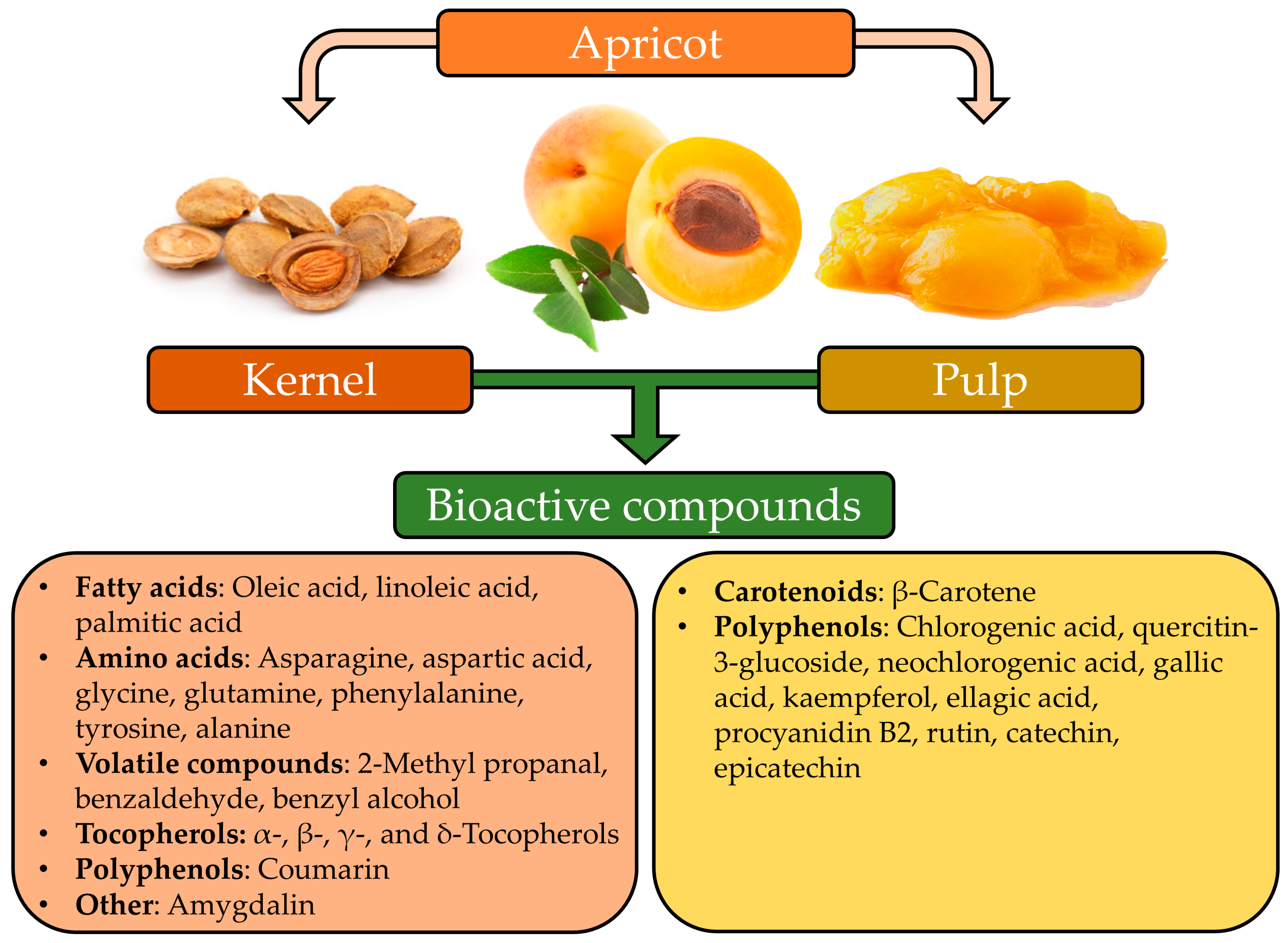
2. Apricot By-Products and Their Value
2.1. Apricot Kernel
2.1.1. Apricot Kernel Biomass
2.1.2. Apricot Kernel Oil
2.1.3. Apricot Kernel Shell
2.2. Apricot Pulp
2.2.1. Antioxidant Activity of Apricot Pulp
2.2.2. Other Applications
3. Nutritional Value
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AK | Apricot Kernel |
| AKO | Apricot Kernel Oil |
| AKS | Apricot Kernel Shell |
| AAE | Ascorbic acid Equivalents |
| CNC | Cellulose Nanocrystals |
| DES | Deep Eutectic Solvent |
| DM | Dry Mass |
| DW | Dry Weight |
| FRAP | Ferric-Reducing Antioxidant Power |
| FT-IR | Fourier Transform Infrared |
| FW | Fresh Weight |
| GAE | Gallic acid Equivalents |
| GC-MS | Gas Chromatography-Mass Spectrometry |
| HPLC | High-Performance Liquid Chromatography |
| NMR | Nuclear Magnetic Resonance |
| PEF | Pulsed Electric Field |
| SC-CO2 | Supercritical Carbon Dioxide |
| TPC | Total Polyphenol Content |
| TEAC | Trolox Equivalent Antioxidant Capacity |
References
- Hacıseferoğulları, H.; Gezer, İ.; Özcan, M.M.; MuratAsma, B. Post-Harvest Chemical and Physical–Mechanical Properties of Some Apricot Varieties Cultivated in Turkey. J. Food Eng. 2007, 79, 364–373. [Google Scholar] [CrossRef]
- Bruno, M.R.; Russo, D.; Faraone, I.; D’Auria, M.; Milella, L.; Todaro, L. Orchard Biomass Residues: Chemical Composition, Biological Activity and Wood Characterization of Apricot Tree (Prunus armeniaca L.). Biofuels Bioprod. Biorefining 2021, 15, 377–391. [Google Scholar] [CrossRef]
- Hormaza, J.I.; Yamane, H.; Rodrigo, J. Apricot. In Fruits and Nuts; Kole, C., Ed.; Genome Mapping and Molecular Breeding in Plants; Springer: Berlin/Heidelberg, Germany, 2007; pp. 171–187. ISBN 978-3-540-34533-6. [Google Scholar]
- Roussos, P.A.; Denaxa, N.-K.; Tsafouros, A.; Efstathios, N.; Intidhar, B. Apricot (Prunus armeniaca L.). In Nutritional Composition of Fruit Cultivars; Simmonds, M.S.J., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 19–48. ISBN 978-0-12-408117-8. [Google Scholar]
- Khadari, B.; Krichen, L.; Lambert, P.; Marrakchi, M.; Audergon, J.M. Genetic Structure in Tunisian Apricot, Prunus Armeniaca L., Populations Propagated by Grafting: A Signature of Bottleneck Effects and Ancient Propagation by Seedlings. Genet. Resour. Crop Evol. 2006, 53, 811–819. [Google Scholar] [CrossRef]
- Moustafa, K.; Cross, J. Production, Pomological and Nutraceutical Properties of Apricot. J. Food Sci. Technol. 2019, 56, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Dragovic-Uzelac, V.; Levaj, B.; Mrkic, V.; Bursac, D.; Boras, M. The Content of Polyphenols and Carotenoids in Three Apricot Cultivars Depending on Stage of Maturity and Geographical Region. Food Chem. 2007, 102, 966–975. [Google Scholar] [CrossRef]
- Alpaslan, M.; Hayta, M. Apricot Kernel: Physical and Chemical Properties. J. Am. Oil Chem. Soc. 2006, 83, 469–471. [Google Scholar] [CrossRef]
- Akhone, M.A.; Bains, A.; Tosif, M.M.; Chawla, P.; Fogarasi, M.; Fogarasi, S. Apricot Kernel: Bioactivity, Characterization, Applications, and Health Attributes. Foods 2022, 11, 2184. [Google Scholar] [CrossRef]
- Nagaraja, A.; Jalageri, M.D.; Puttaiahgowda, Y.M.; Raghava Reddy, K.; Raghu, A.V. A Review on Various Maleic Anhydride Antimicrobial Polymers. J. Microbiol. Methods 2019, 163, 105650. [Google Scholar] [CrossRef]
- Kasai, D.; Chougale, R.; Masti, S.; Gouripur, G.; Malabadi, R.; Chalannavar, R.; Raghu, A.V.; Radhika, D.; Shanavaz, H.; Dhanavant, S. Preparation, Characterization and Antimicrobial Activity of Betel-Leaf-Extract-Doped Polysaccharide Blend Films. Green Mater. 2021, 9, 49–68. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Spencer, J.P.E.; Mohsen, M.M.A.E.; Minihane, A.-M.; Mathers, J.C. Biomarkers of the Intake of Dietary Polyphenols: Strengths, Limitations and Application in Nutrition Research. Br. J. Nutr. 2008, 99, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Sójka, M.; Kołodziejczyk, K.; Milala, J.; Abadias, M.; Viñas, I.; Guyot, S.; Baron, A. Composition and Properties of the Polyphenolic Extracts Obtained from Industrial Plum Pomaces. J. Funct. Foods 2015, 12, 168–178. [Google Scholar] [CrossRef]
- World Population Prospects—Population Division—United Nations. Available online: https://population.un.org/wpp/ (accessed on 28 November 2023).
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Woerden, F.V. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank Publications: Washington, DC, USA, 2018; ISBN 978-1-4648-1347-4. [Google Scholar]
- Takhar, S.S.; Liyanage, K. The Impact of Industry 4.0 on Sustainability and the Circular Economy Reporting Requirements. Int. J. Integr. Supply Manag. 2020, 13, 107–139. [Google Scholar] [CrossRef]
- Ghisellini, P.; Cialani, C.; Ulgiati, S. A Review on Circular Economy: The Expected Transition to a Balanced Interplay of Environmental and Economic Systems. J. Clean. Prod. 2016, 114, 11–32. [Google Scholar] [CrossRef]
- Circular Economy: Definition, Importance and Benefits. Available online: https://www.europarl.europa.eu/news/en/headlines/economy/20151201STO05603/circular-economy-definition-importance-and-benefits (accessed on 29 November 2023).
- Jouhara, H.; Malinauskaite, J.; Spencer, N. Waste Prevention and Technologies in the Context of the EU Waste Framework Directive: Lost in Translation? Eur. Energy Environ. Law Rev. 2017, 26, 60–80. [Google Scholar] [CrossRef]
- Cheng, Y.-S. Insect Biorefinery: Sustainable Application of Insects in Circular Economy. Appl. Sci. Eng. Prog. 2023, 16, 6772. [Google Scholar] [CrossRef]
- Dincer, I.; Ezan, M.A. Heat Storage: A Unique Solution For Energy Systems; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-319-91893-8. [Google Scholar]
- Halysh, V.; Romero-García, J.M.; Vidal, A.M.; Kulik, T.; Palianytsia, B.; García, M.; Castro, E. Apricot Seed Shells and Walnut Shells as Unconventional Sugars and Lignin Sources. Molecules 2023, 28, 1455. [Google Scholar] [CrossRef]
- Yildiz, M.J.; Kalinowska, M.; Kalinowska-Wichrowska, K.; Gołębiewska, E.; Tarasewicz, P.; Bobin, T.; Tarapata, D.; Szatyłowicz, E.; Piekut, J. A Short Overview of the Possibilities of Using Waste from the Agri-Food Industry. Adv. Sci. Technol. Res. J. 2023, 17, 342–352. [Google Scholar] [CrossRef]
- Harja, M.; Teodosiu, C.; Isopescu, D.N.; Gencel, O.; Lutic, D.; Ciobanu, G.; Cretescu, I. Using Fly Ash Wastes for the Development of New Building Materials with Improved Compressive Strength. Materials 2022, 15, 644. [Google Scholar] [CrossRef]
- Jamil, U.; Husain Khoja, A.; Liaquat, R.; Raza Naqvi, S.; Nor Nadyaini Wan Omar, W.; Aishah Saidina Amin, N. Copper and Calcium-Based Metal Organic Framework (MOF) Catalyst for Biodiesel Production from Waste Cooking Oil: A Process Optimization Study. Energy Convers. Manag. 2020, 215, 112934. [Google Scholar] [CrossRef]
- Özbay, N.; Uzun, B.B.; Varol, E.A.; Pütün, A.E. Comparative Analysis of Pyrolysis Oils and Its Subfractions under Different Atmospheric Conditions. Fuel Process. Technol. 2006, 87, 1013–1019. [Google Scholar] [CrossRef]
- Demirbas, E.; Kobya, M.; Sulak, M.T. Adsorption Kinetics of a Basic Dye from Aqueous Solutions onto Apricot Stone Activated Carbon. Bioresour. Technol. 2008, 99, 5368–5373. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, S.; Liu, S.; Yang, C.; Lu, Q. Fast Pyrolysis of Biomass in Free-Fall Reactor for Hydrogen-Rich Gas. Fuel Process. Technol. 2004, 85, 1201–1211. [Google Scholar] [CrossRef]
- Savova, D.; Apak, E.; Ekinci, E.; Yardim, F.; Petrov, N.; Budinova, T.; Razvigorova, M.; Minkova, V. Biomass Conversion to Carbon Adsorbents and Gas. Biomass Bioenergy 2001, 21, 133–142. [Google Scholar] [CrossRef]
- Petrova, B.; Budinova, T.; Tsyntsarski, B.; Kochkodan, V.; Shkavro, Z.; Petrov, N. Removal of Aromatic Hydrocarbons from Water by Activated Carbon from Apricot Stones. Chem. Eng. J. 2010, 165, 258–264. [Google Scholar] [CrossRef]
- Kancabas Kilinc, A.; Karakaya, S. The Behavior of Apricot Kernel Oil Body and Proteins during in Vitro Gastric and Intestinal Digestion. Ital. J. Food Sci. 2022, 34, 33–43. [Google Scholar] [CrossRef]
- Karsavuran, N.; Charehsaz, M.; Celik, H.; Asma, B.M.; Yakıncı, C.; Aydın, A. Amygdalin in Bitter and Sweet Seeds of Apricots. Toxicol. Amp Environ. Chem. 2014, 96, 1564. [Google Scholar] [CrossRef]
- Lolli, V.; Viscusi, P.; Bonzanini, F.; Conte, A.; Fuso, A.; Larocca, S.; Leni, G.; Caligiani, A. Oil and Protein Extraction from Fruit Seed and Kernel By-Products Using a One Pot Enzymatic-Assisted Mild Extraction. Food Chem. X 2023, 19, 100819. [Google Scholar] [CrossRef]
- Makrygiannis, I.; Athanasiadis, V.; Bozinou, E.; Chatzimitakos, T.; Makris, D.P.; Lalas, S.I. Combined Effects of Deep Eutectic Solvents and Pulsed Electric Field Improve Polyphenol-Rich Extracts from Apricot Kernel Biomass. Biomass 2023, 3, 66–77. [Google Scholar] [CrossRef]
- Jose, D.; Tawai, A.; Divakaran, D.; Bhattacharyya, D.; Venkatachalam, P.; Tantayotai, P.; Sriariyanun, M. Integration of Deep Eutectic Solvent in Biorefining Process of Lignocellulosic Biomass Valorization. Bioresour. Technol. Rep. 2023, 21, 101365. [Google Scholar] [CrossRef]
- Makrygiannis, I.; Athanasiadis, V.; Chatzimitakos, T.; Bozinou, E.; Mantzourani, C.; Chatzilazarou, A.; Makris, D.P.; Lalas, S.I. Exploring the Chemical Composition and Antioxidant Properties of Apricot Kernel Oil. Separations 2023, 10, 332. [Google Scholar] [CrossRef]
- Pop, E.A.; Diaconeasa, Z.M.; Fetea, F.; Bunea, A.; Dulf, F.; Pintea, A.; Socaciu, C. Carotenoids, Tocopherols and Antioxidant Activity of Lipophilic Extracts from Sea Buckthorn Berries (Hippophae rhamnoides), Apricot Pulp and Apricot Kernel (Prunus armeniaca). Bull. UASVM Food Sci. Technol. 2015, 72, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in Antioxidant Effects and Their Relationship to Phytonutrients in Fruits of Sea Buckthorn (Hippophae rhamnoides L.) during Maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, P.C.; Tilakratne, B.M.K.S.; Verma, A.K. Studies on Physico-Chemical Characteristics and Fatty Acid Composition of Wild Apricot (Prunus Armeniaca Linn.) Kernel Oil. Indian J. Nat. Prod. Resour. 2012, 3, 366–370. [Google Scholar]
- Alajil, O.; Sagar, V.R.; Kaur, C.; Rudra, S.G.; Sharma, R.R.; Kaushik, R.; Verma, M.K.; Tomar, M.; Kumar, M.; Mekhemar, M. Nutritional and Phytochemical Traits of Apricots (Prunus armeniaca L.) for Application in Nutraceutical and Health Industry. Foods 2021, 10, 1344. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, H.-D.; Zhang, L.; Tian, Z.-J.; Zhang, Z.-Q.; Shi, X.-C.; Ma, W.-H. Protective Effects of Apricot Kernel Oil on Myocardium against Ischemia–Reperfusion Injury in Rats. Food Chem. Toxicol. 2011, 49, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Karaboğa, İ.; Ovalı, M.A.; Yılmaz, A.; Alpaslan, M. Gastroprotective Effect of Apricot Kernel Oil in Ethanol-Induced Gastric Mucosal Injury in Rats. Biotech. Histochem. 2018, 93, 601–607. [Google Scholar] [CrossRef]
- Pavlović, N.; Vidović, S.; Vladić, J.; Popović, L.; Moslavac, T.; Jakobović, S.; Jokić, S. Recovery of Tocopherols, Amygdalin, and Fatty Acids From Apricot Kernel Oil: Cold Pressing Versus Supercritical Carbon Dioxide. Eur. J. Lipid Sci. Technol. 2018, 120, 1800043. [Google Scholar] [CrossRef]
- Stryjecka, M.; Kiełtyka-Dadasiewicz, A.; Michalak, M.; Rachoń, L.; Głowacka, A. Chemical Composition and Antioxidant Properties of Oils from the Seeds of Five Apricot (Prunus armeniaca L.) Cultivars. J. Oleo Sci. 2019, 68, 729–738. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, J.; Qi, L.; Qiu, Y.; Liu, H.; Zhang, Y.; Wang, X. A Comparative Study of Apricot Kernel Oil Yield Using Different Extraction Methods. BioResources 2022, 17, 5146–5163. [Google Scholar] [CrossRef]
- Demiral, İ.; Kul, Ş.Ç. Pyrolysis of Apricot Kernel Shell in a Fixed-Bed Reactor: Characterization of Bio-Oil and Char. J. Anal. Appl. Pyrolysis 2014, 107, 17–24. [Google Scholar] [CrossRef]
- Hrichi, S.; Rigano, F.; Chaabane-Banaoues, R.; Oulad El Majdoub, Y.; Mangraviti, D.; Di Marco, D.; Babba, H.; Dugo, P.; Mondello, L.; Mighri, Z.; et al. Identification of Fatty Acid, Lipid and Polyphenol Compounds from Prunus armeniaca L. Kernel Extracts. Foods 2020, 9, 896. [Google Scholar] [CrossRef]
- Manić, N.; Janković, B.; Pijović, M.; Waisi, H.; Dodevski, V.; Stojiljković, D.; Jovanović, V. Apricot Kernel Shells Pyrolysis Controlled by Non-Isothermal Simultaneous Thermal Analysis (STA). J. Therm. Anal. Calorim. 2020, 142, 565–579. [Google Scholar] [CrossRef]
- Hekimoğlu, G.; Sarı, A.; Gencel, O.; Önal, Y.; Ustaoğlu, A.; Erdogmus, E.; Harja, M.; Tyagi, V.V. Thermal Energy Storage Performance Evaluation of Bio-Based Phase Change Material/Apricot Kernel Shell Derived Activated Carbon in Lightweight Mortar. J. Energy Storage 2023, 73, 109122. [Google Scholar] [CrossRef]
- Khodadadi, B.; Bordbar, M.; Nasrollahzadeh, M. Green Synthesis of Pd Nanoparticles at Apricot Kernel Shell Substrate Using Salvia Hydrangea Extract: Catalytic Activity for Reduction of Organic Dyes. J. Colloid Interface Sci. 2017, 490, 1–10. [Google Scholar] [CrossRef]
- Jin, F.; Wang, J.M.; Regenstein, J.; Wang, F. Effect of Roasting Temperatures on the Properties of Bitter Apricot (Armeniaca sibirica L.) Kernel Oil. J. Oleo Sci. 2018, 67, 813–822. [Google Scholar] [CrossRef]
- Cheaib, D.; El Darra, N.; Rajha, H.N.; El-Ghazzawi, I.; Maroun, R.G.; Louka, N. Effect of the Extraction Process on the Biological Activity of Lyophilized Apricot Extracts Recovered from Apricot Pomace. Antioxidants 2018, 7, 11. [Google Scholar] [CrossRef]
- Cheaib, D.; El Darra, N.; Rajha, H.N.; El-Ghazzawi, I.; Mouneimne, Y.; Jammoul, A.; Maroun, R.G.; Louka, N. Study of the Selectivity and Bioactivity of Polyphenols Using Infrared Assisted Extraction from Apricot Pomace Compared to Conventional Methods. Antioxidants 2018, 7, 174. [Google Scholar] [CrossRef]
- Cheaib, D.; El Darra, N.; Rajha, H.N.; Ghazzawi, I.E.; Maroun, R.G.; Louka, N. Biological Activity of Apricot Byproducts Polyphenols Using Solid–Liquid and Infrared-Assisted Technology. J. Food Biochem. 2018, 42, e12552. [Google Scholar] [CrossRef]
- Vorobyova, V.; Skiba, M.; Miliar, Y.; Frolenkova, S. Enhanced Phenolic Compounds Extraction From Apricot Pomace By Natural Deep Eutectic Solvent Combined With Ultrasonic-Assisted Extraction. J. Chem. Technol. Metall. 2021, 56, 919–931. [Google Scholar]
- Hong, Y.; Wang, Z.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. High-Throughput Screening and Characterization of Phenolic Compounds in Stone Fruits Waste by LC-ESI-QTOF-MS/MS and Their Potential Antioxidant Activities. Antioxidants 2021, 10, 234. [Google Scholar] [CrossRef]
- Jan, N.; Anjum, S.; Wani, S.M.; Mir, S.A.; Malik, A.R.; Wani, S.A.; Hussein, D.S.; Rasheed, R.A.; Gatasheh, M.K. Influence of Canning and Storage on Physicochemical Properties, Antioxidant Properties, and Bioactive Compounds of Apricot (Prunus armeniaca L.) Wholes, Halves, and Pulp. Front. Nutr. 2022, 9, 850730. [Google Scholar] [CrossRef] [PubMed]
- Makrygiannis, I.; Athanasiadis, V.; Bozinou, E.; Chatzimitakos, T.; Makris, D.; Lalas, S. An Investigation into Apricot Pulp Waste as a Source of Antioxidant Polyphenols and Carotenoid Pigments. Biomass 2022, 2, 334–347. [Google Scholar] [CrossRef]
- Suo, A.; Fan, G.; Wu, C.; Li, T.; Cong, K. Green Extraction of Carotenoids from Apricot Flesh by Ultrasound Assisted Corn Oil Extraction: Optimization, Identification, and Application. Food Chem. 2023, 420, 136096. [Google Scholar] [CrossRef]
- Stramarkou, M.; Oikonomopoulou, V.; Panagiotopoulou, M.; Papadaki, S.; Krokida, M. Sustainable Valorisation of Peach and Apricot Waste Using Green Extraction Technique with Conventional and Deep Eutectic Solvents. Resources 2023, 12, 72. [Google Scholar] [CrossRef]
- Tsiaka, T.; Fotakis, C.; Lantzouraki, D.Z.; Tsiantas, K.; Siapi, E.; Sinanoglou, V.J.; Zoumpoulakis, P. Expanding the Role of Sub-Exploited DOE-High Energy Extraction and Metabolomic Profiling towards Agro-Byproduct Valorization: The Case of Carotenoid-Rich Apricot Pulp. Molecules 2020, 25, 2702. [Google Scholar] [CrossRef]
- Demiray, E.; Karatay, S.E.; Dönmez, G. Determination of Bioethanol Production from Apricot (Prunus armeniaca) Pomace. Braz. Arch. Biol. Technol. 2021, 64, e21200781. [Google Scholar] [CrossRef]
- Baississe, S.; Ghannem, H.; Fahloul, D.; Lekbir, A. Comparison of Structure and Emulsifying Activity of Pectin Extracted from Apple Pomace and Apricot Pulp. World J. Dairy. Amp Food Sci. 2010, 5, 79–84. [Google Scholar]
- Dinçel Kasapoğlu, E.; Kahraman, S.; Tornuk, F. Extraction Optimization and Characterization of Cellulose Nanocrystals from Apricot Pomace. Foods 2023, 12, 746. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Anwar, S.; Yunusa, B.M.; Nayik, G.A.; Mousavi Khaneghah, A. The Potential of Apricot Seed and Oil as Functional Food: Composition, Biological Properties, Health Benefits & Safety. Food Biosci. 2023, 51, 102336. [Google Scholar] [CrossRef]
- Jaafar, H.J. Effects of Apricot and Apricot Kernels on Human Health and Nutrition: A Review of Recent Human Research. Tech. Biochem. 2021, 2, 139–162. [Google Scholar] [CrossRef]
- Femenia, A.; Rossello, C.; Mulet, A.; Canellas, J. Chemical Composition of Bitter and Sweet Apricot Kernels. J. Agric. Food Chem. 1995, 43, 356–361. [Google Scholar] [CrossRef]
- Worwood, V.A. The Complete Book of Essential Oils and Aromatherapy, Revised and Expanded: Over 800 Natural, Nontoxic, and Fragrant Recipes to Create Health, Beauty, and Safe Home and Work Environments; New World Library: Novato, CA, USA, 2016; ISBN 978-1-60868-426-7. [Google Scholar]
- Bishop, J. Naturally Sweet Summer Desserts: Ten Easy Ways to Turn Fresh Summer Fruit into Tarts, Crisps, and Betties without Refined Sugar or Dairy Products. Nat. Health 1996, 26, 58–64. [Google Scholar]
- Kiralan, M.; Özkan, G.; Kucukoner, E.; Ozcelik, M.M. Apricot (Prunus armeniaca L.) Oil. In Fruit Oils: Chemistry and Functionality; Ramadan, M.F., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 505–519. ISBN 978-3-030-12473-1. [Google Scholar]
- Gezer, C.; Buratti, C. A ZigBee Smart Energy Implementation for Energy Efficient Buildings. In Proceedings of the 2011 IEEE 73rd Vehicular Technology Conference (VTC Spring), Budapest, Hungary, 15–18 May 2011; pp. 1–5. [Google Scholar]
- Fan, X.; Zhao, H.; Wang, X.; Cao, J.; Jiang, W. Sugar and Organic Acid Composition of Apricot and Their Contribution to Sensory Quality and Consumer Satisfaction. Sci. Hortic. 2017, 225, 553–560. [Google Scholar] [CrossRef]
- Mesarović, J.; Trifković, J.; Tosti, T.; Fotirić Akšić, M.; Milatović, D.; Ličina, V.; Milojković-Opsenica, D. Relationship between Ripening Time and Sugar Content of Apricot (Prunus armeniaca L.) Kernels. Acta Physiol. Plant. 2018, 40, 157. [Google Scholar] [CrossRef]
- Mosa, Z.M.; Kkalil, A.F. Comparative Study between the Effects of Mango and Orange Peels Preparations on the Total Dietary Fiber. IOSR J. Environ. Sci. Toxicol. Food Technol. 2015, 9, 129–136. [Google Scholar] [CrossRef]
- Khedr, M.A.; Abdelgaleel, M.A.; Bessar, B.A.; Salama, A.A. Effect of Using Tomato Peels as a Fat Replacer on the Sensory, Nutritional and Physical Properties of Beef Burger and Sausages. J. Sustain. Agric. Sci. 2016, 42, 469–490. [Google Scholar] [CrossRef]
- Mahde, N.F.; Fayed, M.I.A. Using Apricot Seed Kernels for the Development of Supplemented Cakes. CASP. J. Environ. Sci. 2022, 20, 911–918. [Google Scholar] [CrossRef]
- Tareen, A.K.; Panezai, M.A.; Sajjad, A.; Achakzai, J.K.; Kakar, A.M.; Khan, N.Y. Comparative Analysis of Antioxidant Activity, Toxicity, and Mineral Composition of Kernel and Pomace of Apricot (Prunus armeniaca L.) Grown in Balochistan, Pakistan. Saudi J. Biol. Sci. 2021, 28, 2830–2839. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Grāvīte, I.; Soliven, A.; Kaufmane, E.; Segliņa, D. Tocochromanols Composition in Kernels Recovered from Different Apricot Varieties: RP-HPLC/FLD and RP-UPLC-ESI/MSn Study. Nat. Prod. Res. 2015, 29, 1222–1227. [Google Scholar] [CrossRef]
- Matthaus, B.; Özcan, M.M.; Al Juhaimi, F. Fatty Acid Composition and Tocopherol Content of the Kernel Oil from Apricot Varieties (Hasanbey, Hacihaliloglu, Kabaasi and Soganci) Collected at Different Harvest Times. Eur. Food Res. Technol. 2016, 242, 221–226. [Google Scholar] [CrossRef]
- Uluata, S. Effect of Extraction Method on Biochemical Properties and Oxidative Stability of Apricot Seed Oil. Akad. Gıda 2016, 14, 333–340. [Google Scholar]
- Al-Soufi, M.H.; Alshwyeh, H.A.; Alqahtani, H.; Al-Zuwaid, S.K.; Al-Ahmed, F.O.; Al-Abdulaziz, F.T.; Raed, D.; Hellal, K.; Mohd Nani, N.H.; Zubaidi, S.N.; et al. A Review with Updated Perspectives on Nutritional and Therapeutic Benefits of Apricot and the Industrial Application of Its Underutilized Parts. Molecules 2022, 27, 5016. [Google Scholar] [CrossRef]
- Göttingerová, M.; Kumšta, M.; Rampáčková, E.; Kiss, T.; Nečas, T. Analysis of Phenolic Compounds and Some Important Analytical Properties in Selected Apricot Genotypes. HortScience 2021, 56, 1446–1452. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Dulf, E.-H.; Pintea, A. Phenolic Compounds, Flavonoids, Lipids and Antioxidant Potential of Apricot (Prunus armeniaca L.) Pomace Fermented by Two Filamentous Fungal Strains in Solid State System. Chem. Cent. J. 2017, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.M.; Hussain, P.R.; Masoodi, F.A.; Ahmad, M.; Wani, T.A.; Gani, A.; Rather, S.A.; Suradkar, P. Evaluation of the Composition of Bioactive Compounds and Antioxidant Activity in Fourteen Apricot Varieties of North India. J. Agric. Sci. 2017, 9, 66. [Google Scholar] [CrossRef]
- Amina, B.; Kirkin, C.; Chatterjee, R.; Elmaliklis, I.-N.; Rao, G.M.N.; Skott, E.; Xagoraris, M.; Datta, J.; Ratnasekera, D.; Iqbal, M.A.; et al. Mediterranean Fruits and Berries with Bioactive and Toxic Components. A Review. Curr. Top. Nutraceut. Res. 2022, 20, 1–16. [Google Scholar] [CrossRef]
- Santulli, G. Epidemiology of Cardiovascular Disease in the 21st Century: Updated Updated Numbers and Updated Facts. J. Cardiovasc. Dis. Res. 2013, 1, 1. [Google Scholar]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef]
- Kitic, D.; Miladinovic, B.; Randjelovic, M.; Szopa, A.; Sharifi-Rad, J.; Calina, D.; Seidel, V. Anticancer Potential and Other Pharmacological Properties of Prunus armeniaca L.: An Updated Overview. Plants 2022, 11, 1885. [Google Scholar] [CrossRef]
- Ramadan, A.; Kamel, G.; Awad, N.E.; Shokry, A.A.; Fayed, H.M. The Pharmacological Effect of Apricot Seeds Extracts and Amygdalin in Experimentally Induced Liver Damage and Hepatocellular Carcinoma. J. Herbmed Pharmacol. 2020, 9, 400–407. [Google Scholar] [CrossRef]
- Vahedi-Mazdabadi, Y.; Karimpour-Razkenari, E.; Akbarzadeh, T.; Lotfian, H.; Toushih, M.; Roshanravan, N.; Saeedi, M.; Ostadrahimi, A. Anti-Cholinesterase and Neuroprotective Activities of Sweet and Bitter Apricot Kernels (Prunus armeniaca L.). Iran. J. Pharm. Res. IJPR 2020, 19, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Young, K.A. Of Poops and Parasites: Unethical FDA Overregulation. Food Drug Law. J. 2014, 69, 555. [Google Scholar] [PubMed]
- Camadan, Y.; Akkemik, E. Searching for New Natural Inhibitors of Acetylcholinesterase Enzyme. Cumhur. Sci. J. 2022, 43, 66–71. [Google Scholar] [CrossRef]
- Guo, J.; Wu, W.; Sheng, M.; Yang, S.; Tan, J. Amygdalin Inhibits Renal Fibrosis in Chronic Kidney Disease. Mol. Med. Rep. 2013, 7, 1453–1457. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Rahavian, A.; Pereira Carneiro, J.N.; Rocha, J.E.; Alves Borges Leal, A.L.; Bezerra Morais Braga, M.F.; Melo Coutinho, H.D.; Ansari Djafari, A.; Alarcón-Zapata, P.; et al. Bioactive Compounds as Potential Agents for Sexually Transmitted Diseases Management: A Review to Explore Molecular Mechanisms of Action. Front. Pharmacol. 2021, 12, 674682. [Google Scholar] [CrossRef]
- Fratianni, F.; d’Acierno, A.; Ombra, M.N.; Amato, G.; De Feo, V.; Ayala-Zavala, J.F.; Coppola, R.; Nazzaro, F. Fatty Acid Composition, Antioxidant, and in Vitro Anti-Inflammatory Activity of Five Cold-Pressed Prunus Seed Oils, and Their Anti-Biofilm Effect Against Pathogenic Bacteria. Front. Nutr. 2021, 8, 775751. [Google Scholar] [CrossRef]
- Nafis, A.; Kasrati, A.; Jamali, C.A.; Custódio, L.; Vitalini, S.; Iriti, M.; Hassani, L. A Comparative Study of the in Vitro Antimicrobial and Synergistic Effect of Essential Oils from Laurus nobilis L. and Prunus armeniaca L. from Morocco with Antimicrobial Drugs: New Approach for Health Promoting Products. Antibiotics 2020, 9, 140. [Google Scholar] [CrossRef]
- Amiran, F.; Shafaghat, A.; Shafaghatlonbar, M. Omega-6 Content, Antioxidant and Antimicrobial Activities of Hexanic Extract from Prunus armeniaca L. Kernel from North-West Iran. Natl. Acad. Sci. Lett. 2015, 38, 107–111. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Liu, R.; Shen, X. Chinese Medicine Amygdalin and β-Glucosidase Combined with Antibody Enzymatic Prodrug System As A Feasible Antitumor Therapy. Chin. J. Integr. Med. 2018, 24, 237–240. [Google Scholar] [CrossRef]
- Liczbiński, P.; Bukowska, B. Molecular Mechanism of Amygdalin Action in Vitro: Review of the Latest Research. Immunopharmacol. Immunotoxicol. 2018, 40, 212–218. [Google Scholar] [CrossRef]
- Jaszczak-Wilke, E.; Polkowska, Ż.; Koprowski, M.; Owsianik, K.; Mitchell, A.E.; Bałczewski, P. Amygdalin: Toxicity, Anticancer Activity and Analytical Procedures for Its Determination in Plant Seeds. Molecules 2021, 26, 2253. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, Q.-A.; Fan, X.-H.; Zhang, X.-Y. Effect of Debitterizing Treatment on the Quality of the Apricot Kernels in the Industrial Processing. J. Food Process. Preserv. 2018, 42, e13556. [Google Scholar] [CrossRef]
- Saadi, S.; Saari, N.; Ariffin, A.A.; Ghazali, H.M.; Hamid, A.A.; Abdulkarim, S.M.; Anwar, F.; Nacer, N.E. Novel Emulsifiers and Stabilizers from Apricot (Prunus armeniaca L.): Their Potential Therapeutic Targets and Functional Properties. Appl. Food Res. 2022, 2, 100085. [Google Scholar] [CrossRef]
- Liu, M.-J.; Zhang, Q.-A. Valorization of the Under-Utilized Apricot Kernels Protein Based on the Rheology and Texture Properties of Dough. LWT 2022, 169, 114019. [Google Scholar] [CrossRef]
- Dhen, N.; Ben Rejeb, I.; Boukhris, H.; Damergi, C.; Gargouri, M. Physicochemical and Sensory Properties of Wheat- Apricot Kernels Composite Bread. LWT 2018, 95, 262–267. [Google Scholar] [CrossRef]
- Gunel, Z.; Parlak, A.; Adsoy, M.; Topuz, A. Physicochemical Properties and Storage Stability of Turkish Coffee Fortified with Apricot Kernel Powder. J. Food Process. Preserv. 2022, 46, e16453. [Google Scholar] [CrossRef]
- El-safy, S.; Salem, R.; Abd El-Ghany, M. Chemical and Nutritional Evaluation of Different Seed Flours as Novel Sources of Protein. World J. Dairy Food Sci. 2012, 7, 59–65. [Google Scholar]
- Munekata, P.E.S.; Yilmaz, B.; Pateiro, M.; Kumar, M.; Domínguez, R.; Shariati, M.A.; Hano, C.; Lorenzo, J.M. Valorization of By-Products from Prunus Genus Fruit Processing: Opportunities and Applications. Crit. Rev. Food Sci. Nutr. 2022, 63, 7795–7810. [Google Scholar] [CrossRef]

| By-Product | Application | Type of Solvent | Results | Ref. |
|---|---|---|---|---|
| Kernel | Preparation of a suspension of AK milk | – | Primary acids: palmitic acid, oleic acid, and linoleic acid, low amygdalin concentration on 250 mL milk | [32] |
| Kernel | Oil extraction | One-pot protease in aqueous medium | ~47% protein yield 179 mg/g asparagine & aspartic acid 172 mg/g glycine & glutamine 86 mg/g phenylalanine & tyrosine 70 mg/g alanine Oleic acid > 25% & linoleic acid ~40% | [34] |
| Kernel | Polyphenol extraction, DES, and PEF utilization to improve extraction yield | Glycerol:choline chloride 2:1 (w/w) (DES) PEF treatment: 1 kV/cm, 10 μs pulse duration & 1000 μs pulse period | PEF prior to extraction: 88% TPC 1 increase DES: 70% TPC increase PEF and DES: 173% TPC increase | [35] |
| Kernel | Oil extraction, analysis, and antioxidant properties | Hexane solvent | Oleic acid and palmitoleic acid in abundance, volatile compounds 2-methyl propanal, benzaldehyde, and benzyl alcohol, benzaldehyde in essential oil, antioxidant activity decreased through time (TPC ~3%, TFC 2 ~18.7%, FRAP ~4.5%, DPPH ~5.2%) | [37] |
| Kernel and pulp | Compounds quantification and antioxidant properties | Methylene, petroleum ether, and acetate, 1:1:1 (v/v/v) | γ-Tocopherol main compound, 3.51 mg of carotenoids/100 g pulp Antioxidant activity pulp 0.51 μM TEAC 3/g FW and kernel 0.05 μM TEAC/g FW | [38] |
| Kernel and kernel oil | Determination of principal characteristics of the fruit | – | Fruit weight: 8–15 g Stone recovery 12.7–22.2% Stone weight: 1.78–1.92 g Kernel oil recovery: 30.7–33.7% Vitamin E: 72–107 mg/100 g | [40] |
| Kernel oil | In vivo potential cardioprotective effects on myocardial IR | – | Significant cardioprotective properties, a potential dietary supplement | [42] |
| Kernel oil | Gastroprotective properties | – | Anti-inflammatory, antioxidative, and antiapoptotic properties | [43] |
| Kernel oil | Fatty acid, tocopherol, and amygdalin levels | – | 5.93% Palmitic acid, 57.33% oleic acid, and 33.81% linoleic acid, 0.20 mg/g amygdalin | [44] |
| Kernel oil | Chemical and biological characterization | n-hexane | Iodine value 99.2 g of I2/100 g of oil, saponification value 189 mg KOH/g oil, peroxide value 1.40 meq O2/kg, 70.70% oleic acid, 22.41% linoleic acid, 3.14% palmitic acid, 1.40% stearic acid, 0.90% linolenic acid, and 0.70% palmitoleic acid, FRAP value 1.07–1.38 mM Fe2+/L, TPC 0.85–1.22 mM GAE 4/L and β-carotene content 42.3–66.8 μg/g | [45] |
| Kernel oil | Comparative analysis of AKO | Ultrasonication & Sohxlet applied petroleum ether solvent | Soxhlet was a more effective technique | [46] |
| Apricot kernel shell | Investigation of primary characteristics and quantities of liquid and solid products from pyrolysis | – | Bio-oil yield 26.3% at 500 °C and 150 cm3/min flow rate | [47] |
| Kernel extracts | Analysis of the fatty acid, lipid, and polyphenolic composition | Dichloromethane, chloroform, ethyl acetate, ethanol | 19.0–32.8% dilinoleyl-olein, 20.3–23.6% dioleoyl-linolein, 12.1–20.1% triolein, ethyl acetate and ethanol had higher TPCs | [48] |
| Kernel oil | Correlation between slow pyrolysis mechanism and its primary constituents | – | An increased yield of biochar when CO and CO2 were released | [49] |
| Kernel shell | Novel material with enhanced properties | Mixture of lauric acid and capric acid to a ratio of 36:64 | Melting temperature of 21.58 °C and a latent heat capacity of 126.8 J/g | [50] |
| Kernel shell | Green synthesis of Pd-nanoparticles | – | High reduction of organic dyes, multiple recoveries and reusable material, catalytic activity | [51] |
| Kernel oil | Impact of various roasting temperatures | – | Peroxide values 0.46–0.82 meq/kg, acid values 0.60–1.40 mg KOH/g, phenol content 54.1–71.5 μg GAE/g, 53 volatile compounds | [52] |
| By-Product | Application | Solvent | Results | Ref. |
|---|---|---|---|---|
| Pomace | Evaluation of two pretreatment extraction techniques | – | Infrared had a lower TPC 1, antiradical activity, and antibacterial activity compared to the heat-assisted one | [53] |
| Pomace | Enhancement of the value of industrial food by-products | – | IR 2 extraction: TPC 10 mg GAE 3/g DM, TFC 6 mg CE/g DM, tannins 3.6 mg/L | [54] |
| Pomace and Kernels | Evaluation of antiradical, antioxidant, and antimicrobial properties | – | IR pomace: TPC 10.8 mg GAE/g DM, TFC 4 6.3 mg CE 5/g DM, tannins 3.6 mg/L, Inhibitory activity against Gram-positive bacteria and Escherichia coli | [55] |
| Pomace | Polyphenol extraction | Choline chlorine and lactic acid at a 1:2 ratio with 30% v/v water | TPC 80.75 ± 1.85 mg GAE/g, TFC 47.41 ± 1.20 mg QE/g, DPPH 70% scavenging, ABTS 60% scavenging | [56] |
| Fruit waste | – | – | TFC 0.19 ± 0.03 mg CE/g, DPPH activity 1.47 ± 0.12 mg AAE/g | [57] |
| Wholes, halves, and pulp | Impact of canning and storage on physicochemical, mineral, and antioxidant properties | – | Pulp: total soluble solids 37.15%, titratable acidity 1.39%, total sugars 20.74%, ascorbic acid 7.21 mg/100 g fw, TPC 13.76 GAE mg/100 g FW, DPPH 92.23 TEAC μg/g DW, ABTS+ 92.33 TEAC μg/g DW, MCA 33.80 TEAC μg/g DW, and BCBA 68.40 TEAC μg/g DW | [58] |
| Pulp | Examination of the extraction process in antioxidant polyphenols and carotenoid pigments | DES glycerol, citric acid, and L-proline at a 2:1:1 ratio | TPC 28.6 mg GAE/g dw, carotenoids 171.2 mg β-carotene equivalents/100 g dw | [59] |
| Waste flesh | Carotenoid extraction | Corn oil as a solvent | Total carotenoid content 42.75 mg/100 g dw | [60] |
| Kernel and pulp | Optimization of the extraction process | Water and ethanol 8:2 v/v, DESs of choline chloride and urea at a 1:2 v/v ratio & choline chloride and lactic acid at a 1:2 v/v ratio | Extraction yields: 25.65% kernel 26.83% pulp | [61] |
| Pulp | Establishment of an integrated analytical platform for plant metabolomics and phytochemical profiling | Ethanol, chloroform, and methanol at 1:1 v/v, methanol | Ethanol as a solvent on microwave-assisted extraction yielded the highest TPC | [62] |
| Pomace | Bioethanol production | Ethanol | 15 FPU/g, theoretical yield 94.7%, ethanol yields 0.50 g/g | [63] |
| Pulp | Analysis of pectins | 0.5 M Hydrochloric acid | Pectins ranged from 10 to 13%, protein content from 3.06 to 3.93%, phenolic compounds from 2.75 to 6.20 μg/mg | [64] |
| Pomace | Optimization of extraction conditions of cellulose nanocrystals from apricot pomace | – | 34.56% CNC yield | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makrygiannis, I.; Athanasiadis, V.; Chatzimitakos, T.; Mantiniotou, M.; Bozinou, E.; Lalas, S.I. Unveiling the Potential of Apricot Residues: From Nutraceuticals to Bioenergy. Waste 2024, 2, 1-28. https://doi.org/10.3390/waste2010001
Makrygiannis I, Athanasiadis V, Chatzimitakos T, Mantiniotou M, Bozinou E, Lalas SI. Unveiling the Potential of Apricot Residues: From Nutraceuticals to Bioenergy. Waste. 2024; 2(1):1-28. https://doi.org/10.3390/waste2010001
Chicago/Turabian StyleMakrygiannis, Ioannis, Vassilis Athanasiadis, Theodoros Chatzimitakos, Martha Mantiniotou, Eleni Bozinou, and Stavros I. Lalas. 2024. "Unveiling the Potential of Apricot Residues: From Nutraceuticals to Bioenergy" Waste 2, no. 1: 1-28. https://doi.org/10.3390/waste2010001
APA StyleMakrygiannis, I., Athanasiadis, V., Chatzimitakos, T., Mantiniotou, M., Bozinou, E., & Lalas, S. I. (2024). Unveiling the Potential of Apricot Residues: From Nutraceuticals to Bioenergy. Waste, 2(1), 1-28. https://doi.org/10.3390/waste2010001











