A Review of Pretreatment Strategies for Anaerobic Digestion: Unlocking the Biogas Generation Potential of Wastes in Ghana
Abstract
1. Introduction
1.1. Methods
1.1.1. Comprehensive Review
- Search Strategy
- Inclusion and Exclusion Criteria
- Data Extraction and Quality Assessment
- Statistical Analysis
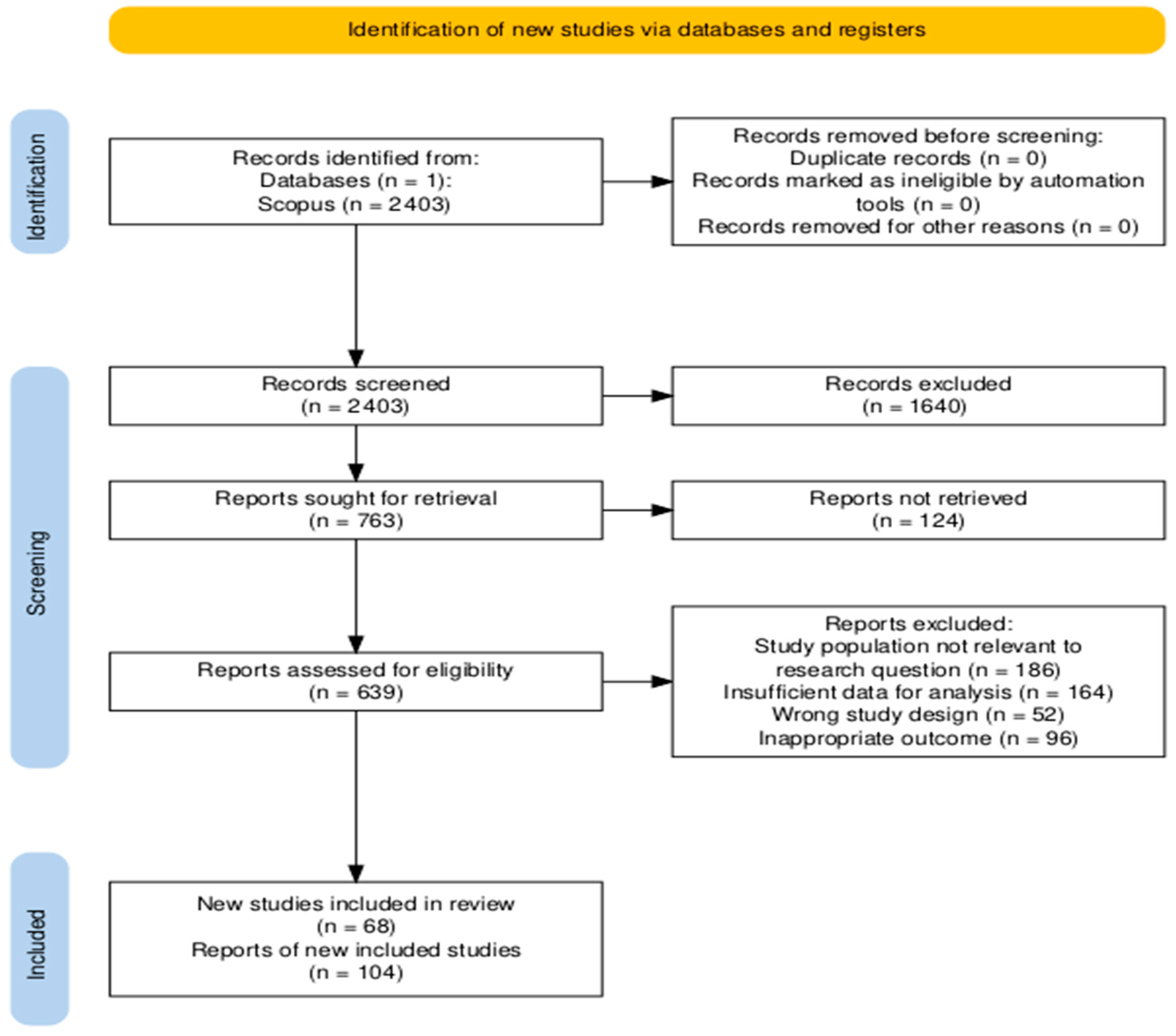
- Results
- Search Results
1.1.2. Bibliometric Review
- Search Strategy
- Bibliometric Data Processing and Coverage
2. Mechanical Pretreatment
3. Thermal Pretreatment
3.1. Steam Explosion
3.2. Hydrothermal/Liquid Hot Water (LHW)
3.3. Comparative Performance of Steam Explosion and Hydrothermal Pretreatment
4. Chemical Pretreatment
4.1. Acid Pretreatment
4.2. Alkaline Pretreatment
4.3. Organosolvs
4.4. Oxidative Pretreatment
4.5. Hydrogen Peroxide
4.6. Ozone Treatment
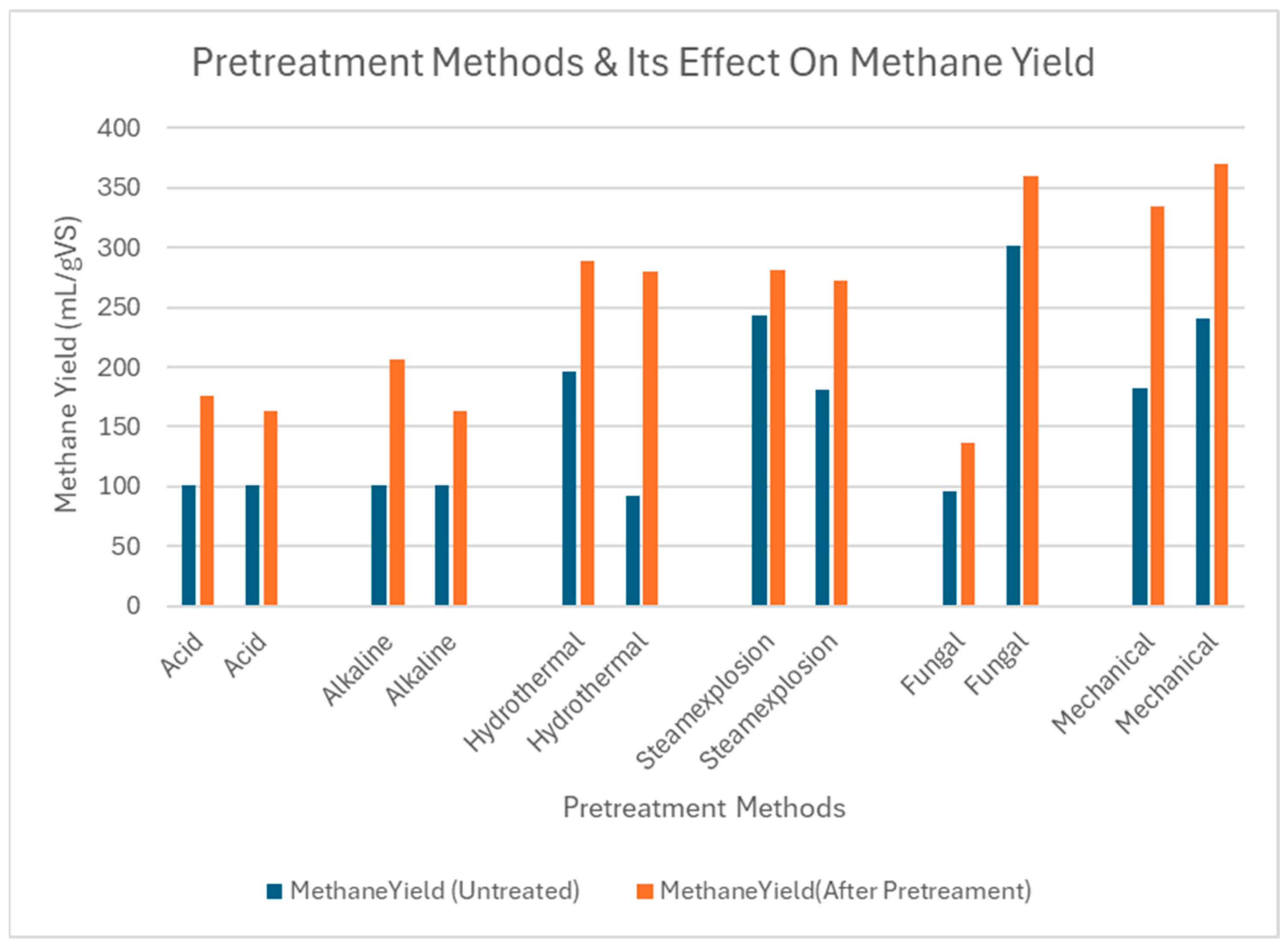
4.7. Comparative Effects of Chemical Pretreatment on Methane Yield
5. Biological Pretreatment
5.1. Fungal Pretreatment
5.2. Enzymatic Pretreatment
5.3. Laccase
6. Combined Pretreatment
7. Bibliometric Analysis of Pretreatment of Precursors for Biogas Production
- Rationale for Bibliometric Analysis
- Methodology
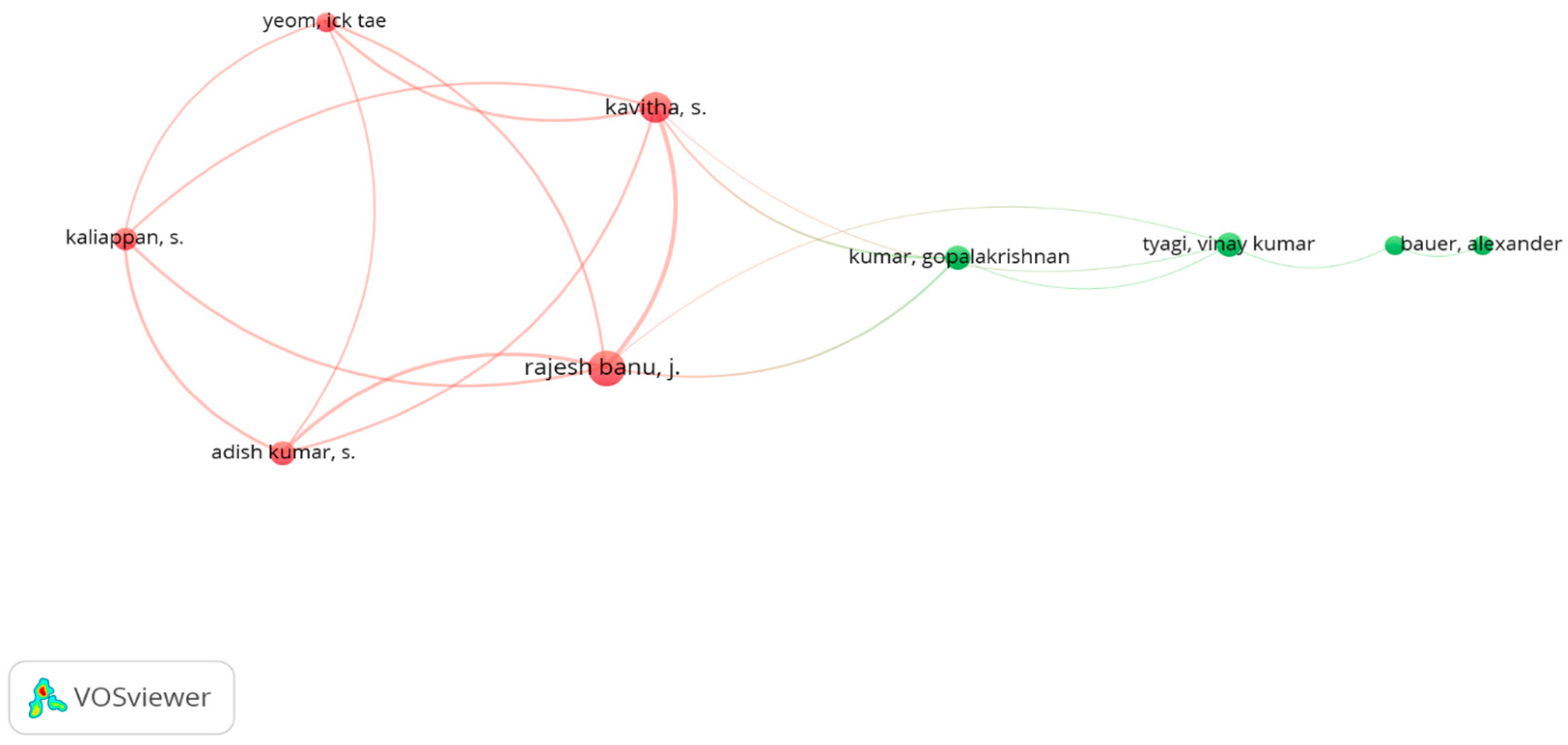
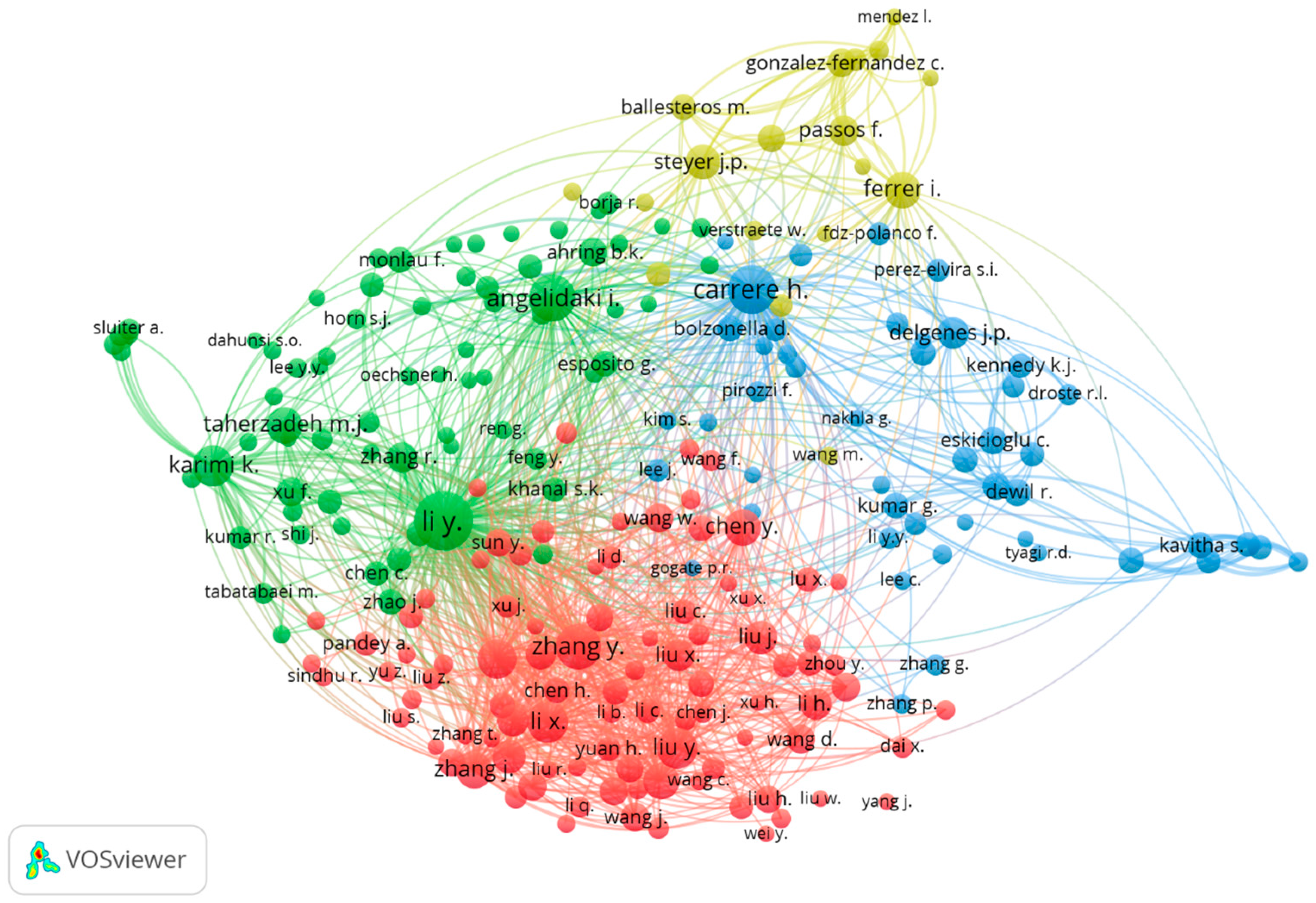
7.1. Analysis of Co-Authorship Clusters Using VOSviewer
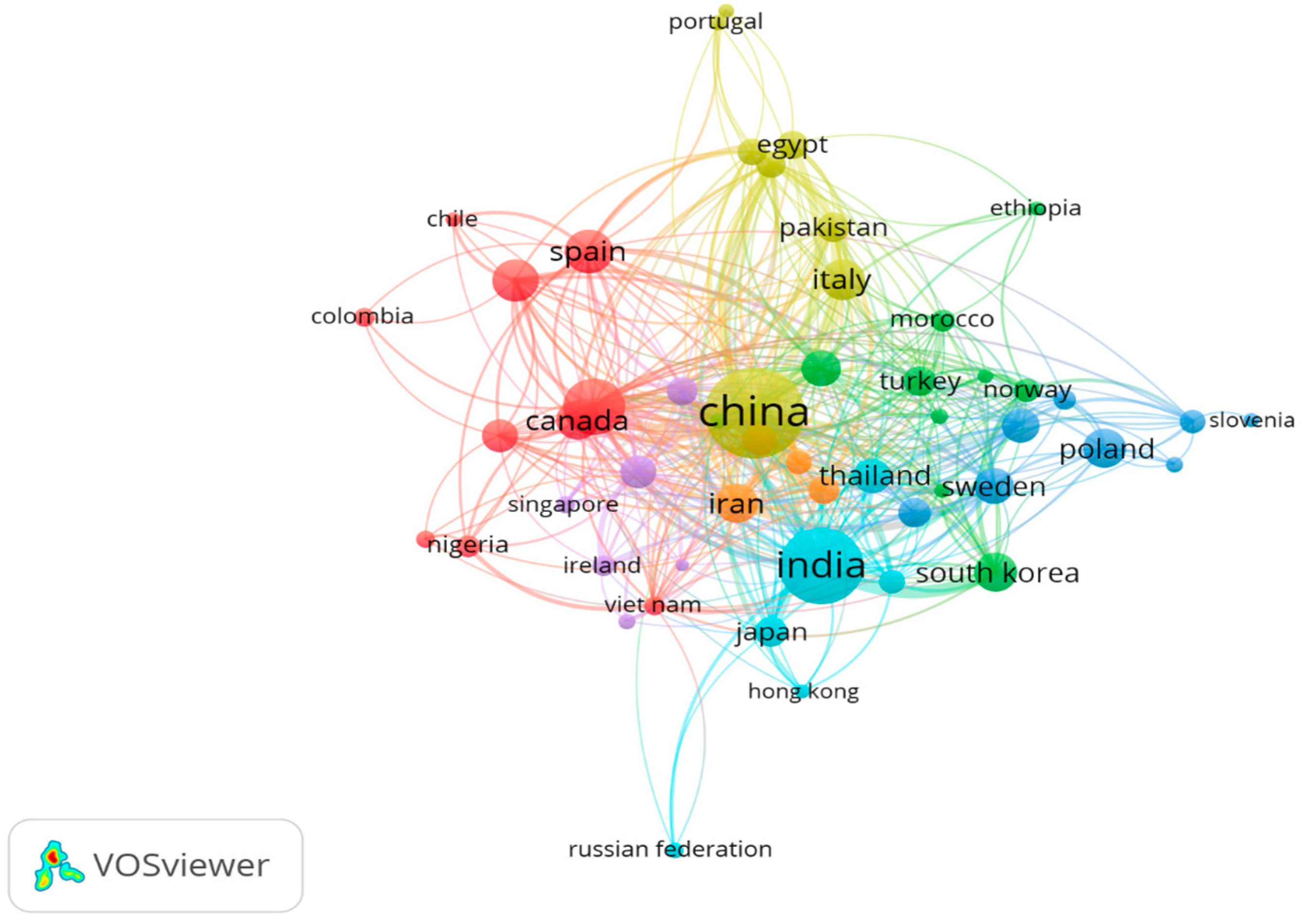
7.2. Co-Authorship Clusters Across Countries: A Global Perspective
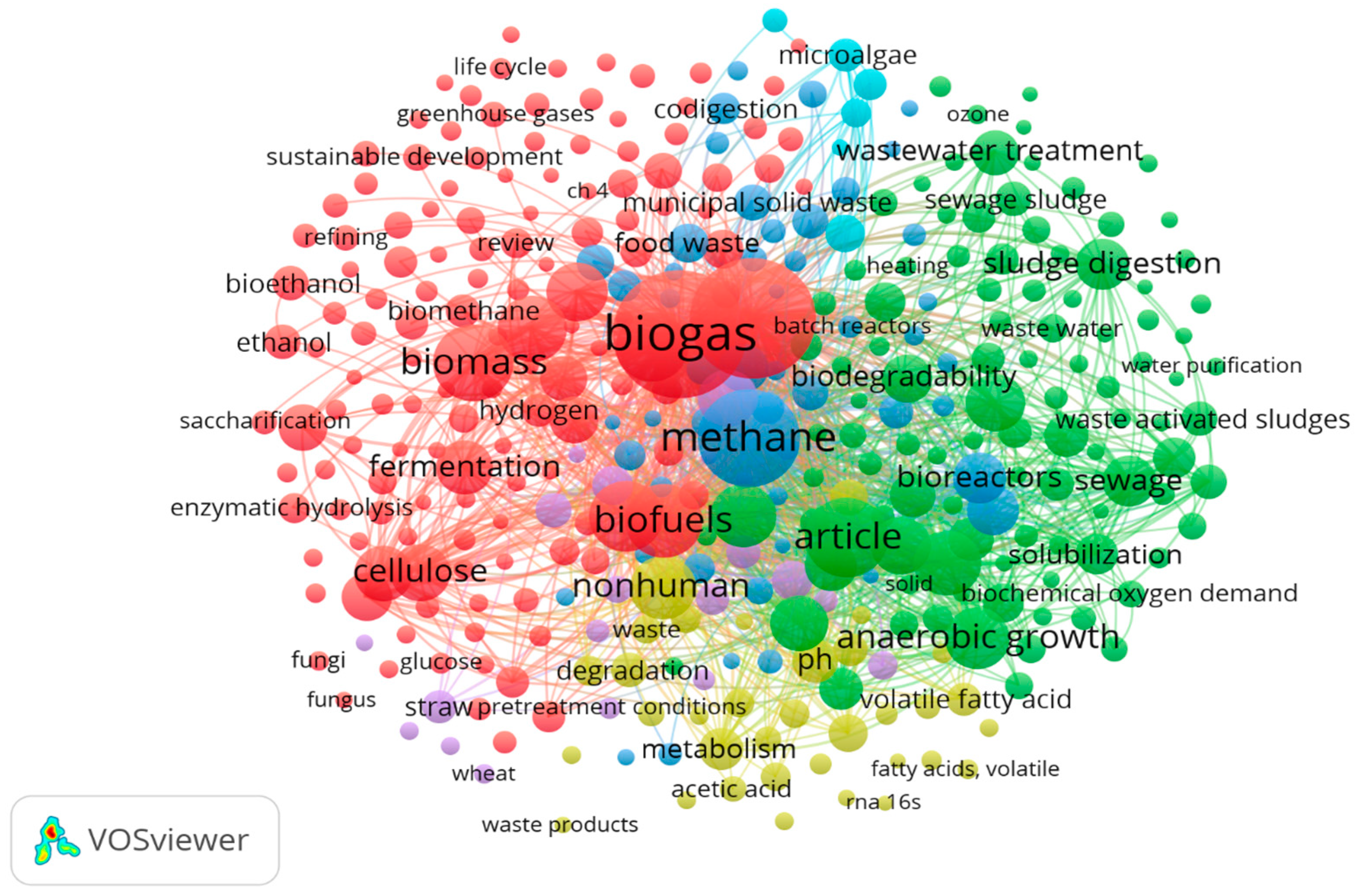
7.3. Co-Occurrence Analysis of Keywords
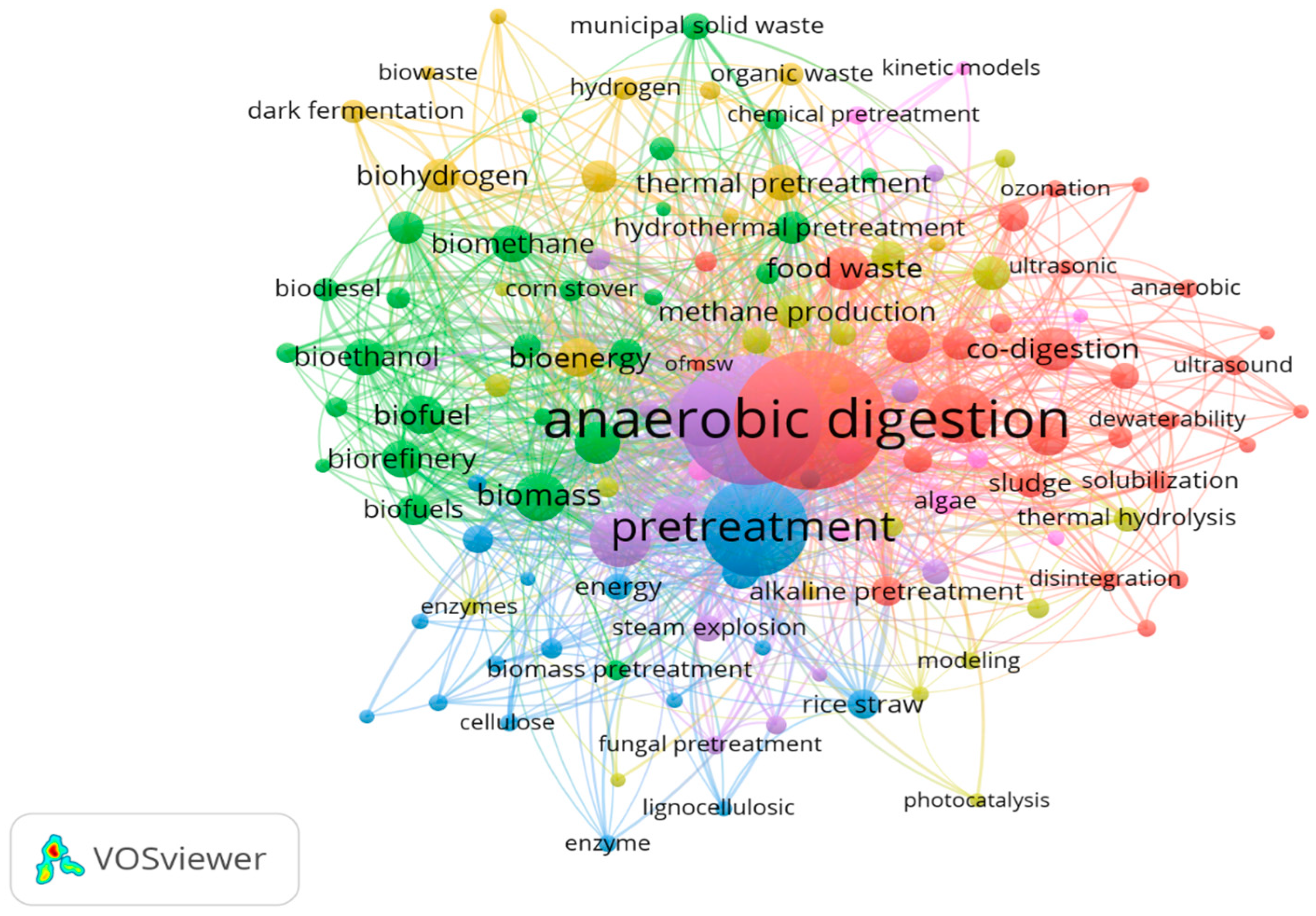
7.4. Co-Occurrence Analysis of Author Keywords
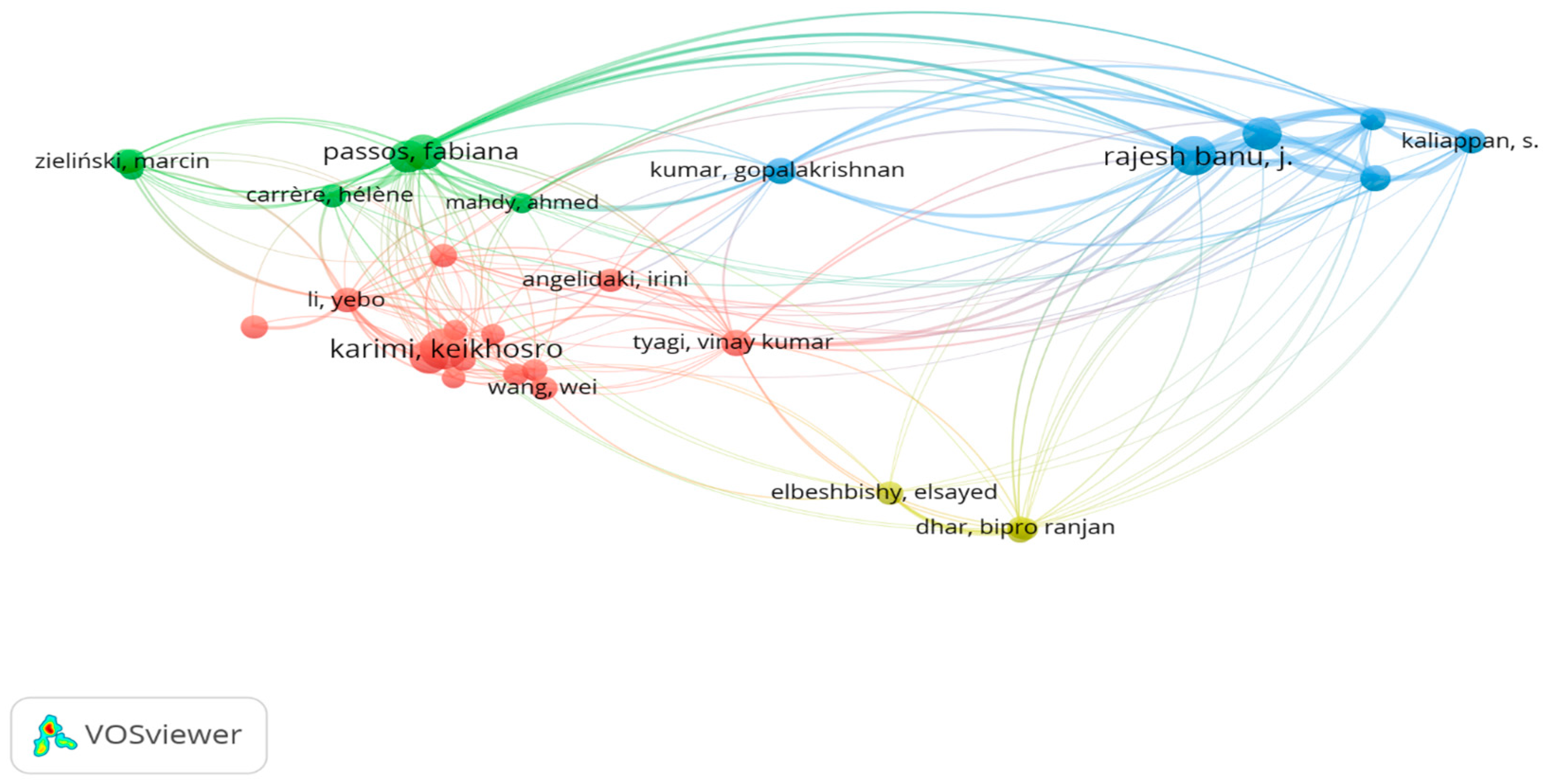
7.5. Citation Against Authors
7.6. Citation Analysis Across Documents: Insights from VOSviewer
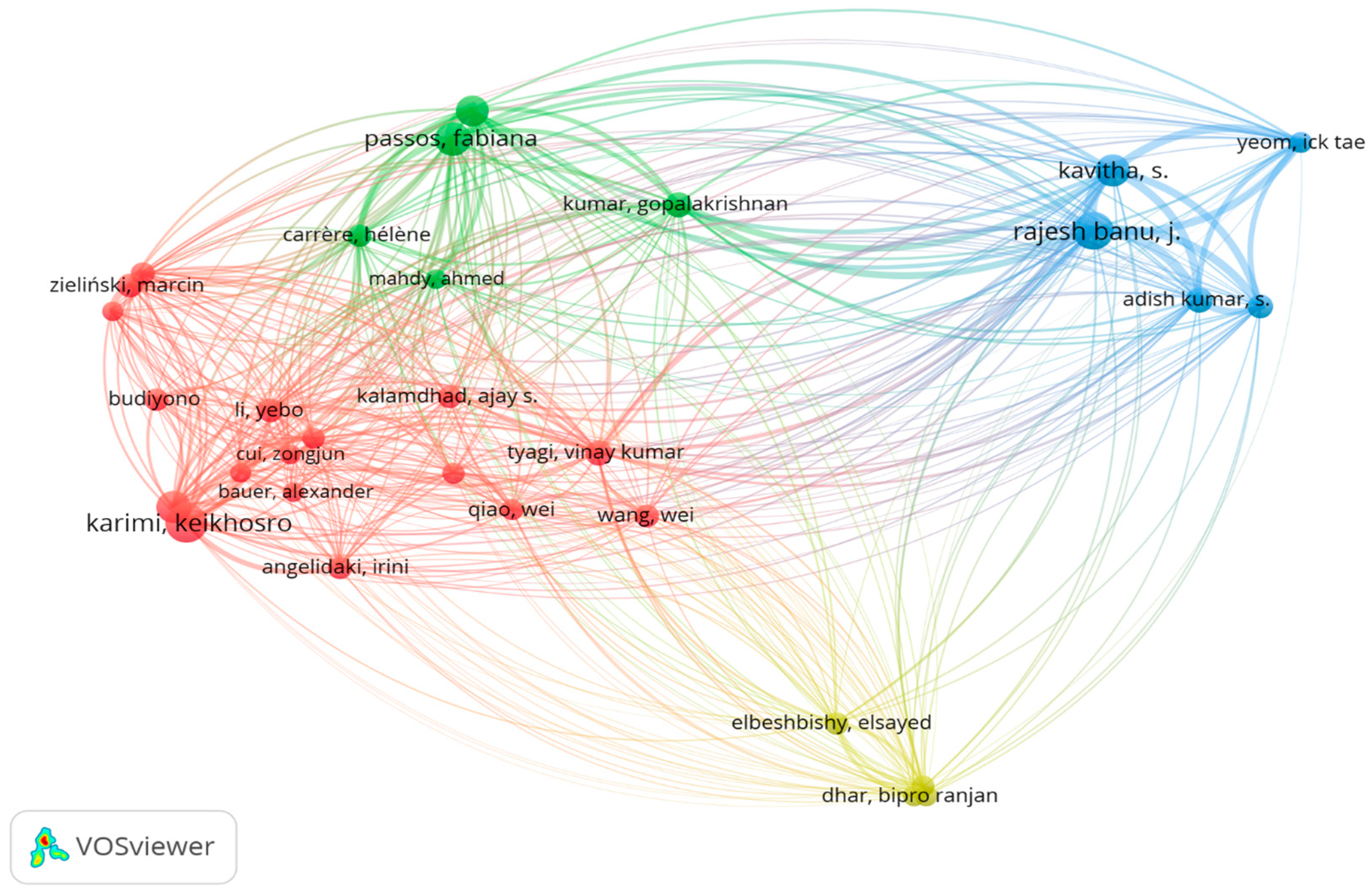
7.7. Bibliographic Coupling Analysis of Authors Using VOSviewer
7.8. Cross-Cluster Connections
7.9. Co-Citation Analysis of Cited Authors Using VOSviewer
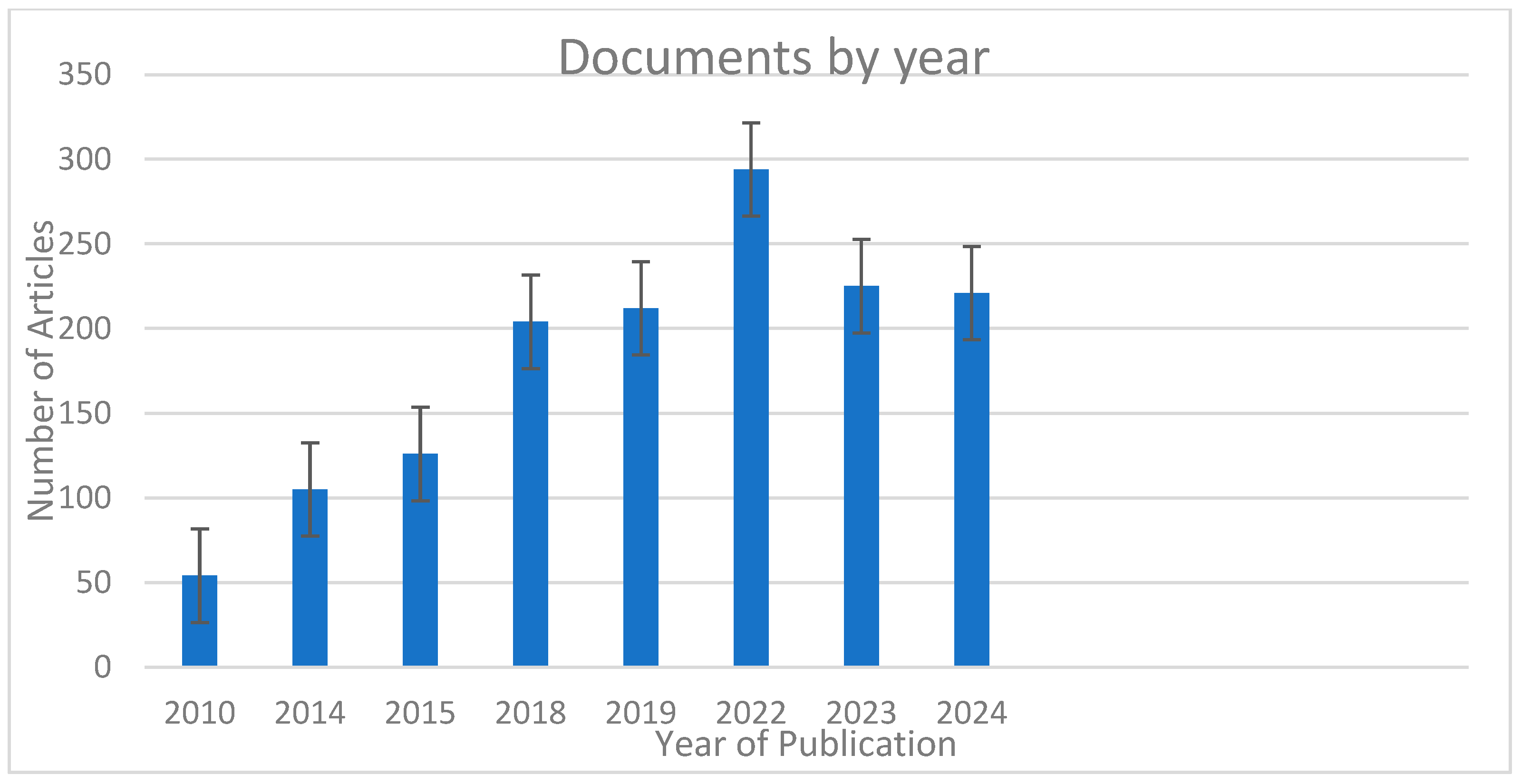
7.10. Documents by Year
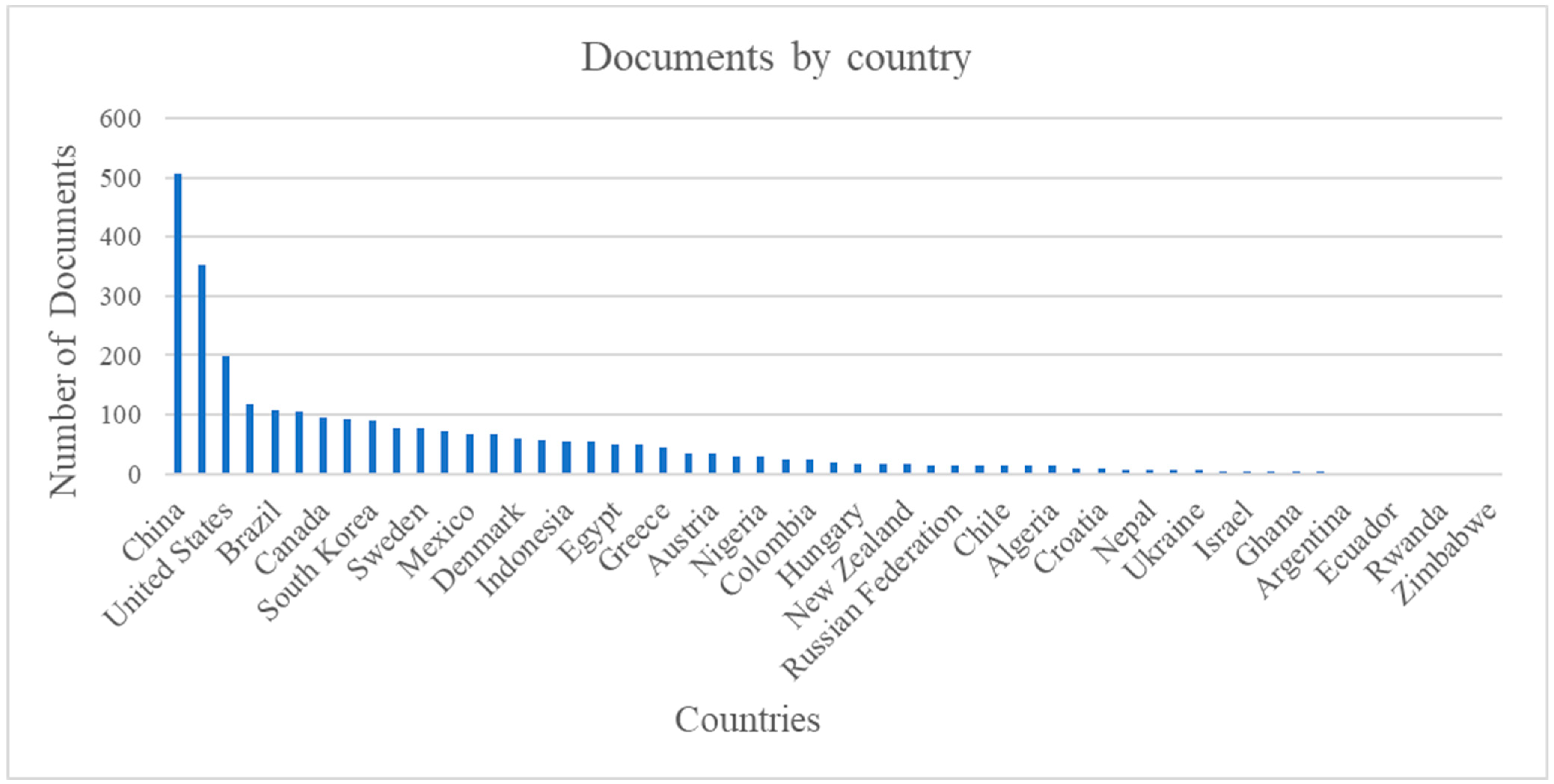
7.11. Documents by Country
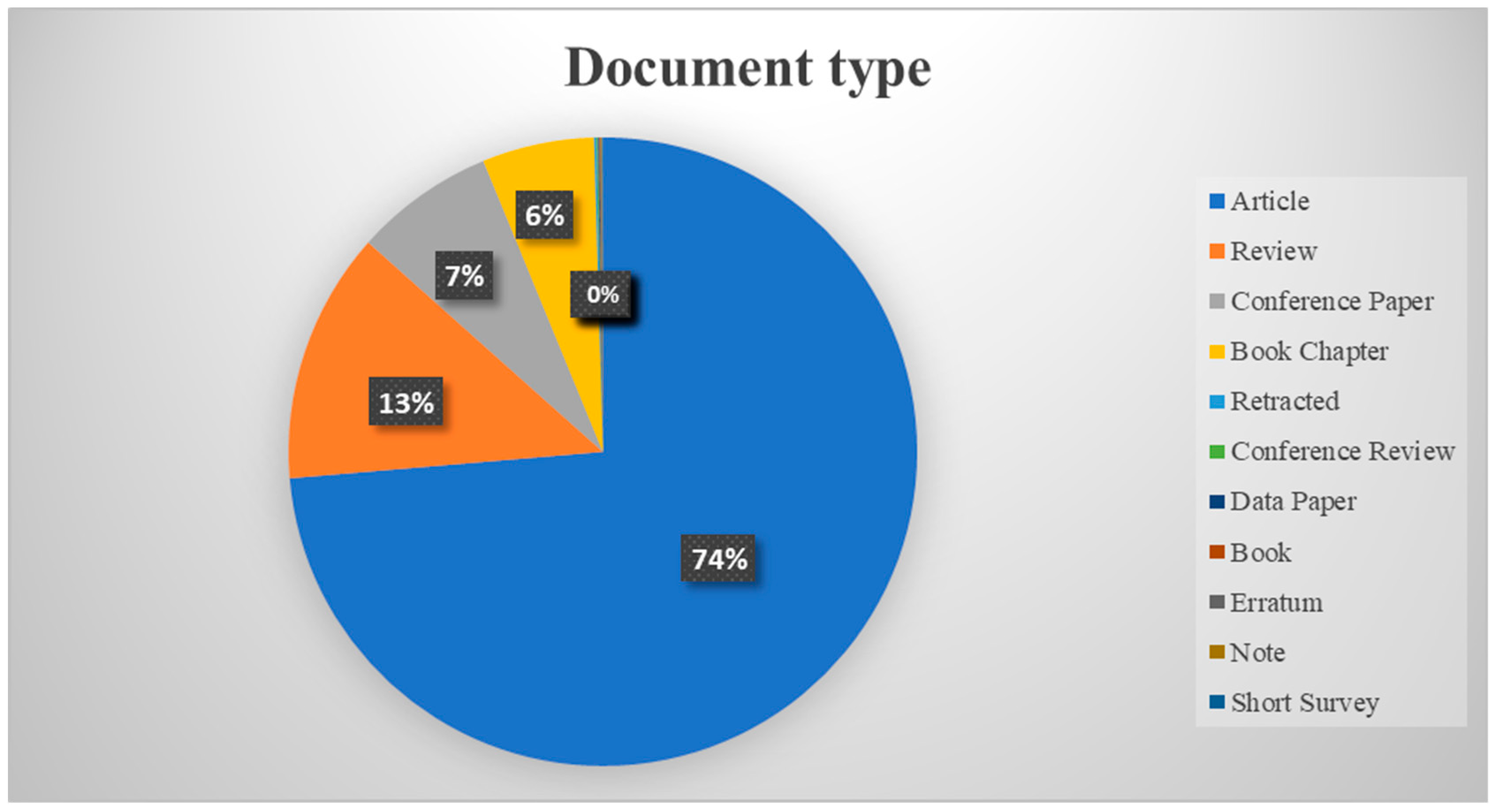
7.12. Document Types
8. Research Prospect in Ghana
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ofori-Boateng, C.; Lee, K.T.; Mensah, M. The prospects of electricity generation from municipal solid waste (MSW) in Ghana: A better waste management option. Fuel Process. Technol. 2013, 110, 94–102. [Google Scholar] [CrossRef]
- Miezah, K.; Obiri-Danso, K.; Kádár, Z.; Fei-Baffoe, B.; Mensah, M.Y. Municipal solid waste characterization and quantification as a measure towards effective waste management in Ghana. Waste Manag. 2015, 46, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Darmey, J.; Ahiekpor, J.C.; Narra, S.; Achaw, O.W.; Ansah, H.F. Municipal solid waste generation trend and bioenergy recovery potential: A review. Energies 2023, 16, 7753. [Google Scholar] [CrossRef]
- Nelson, N.; Darkwa, J.; Calautit, J. Prospects of Bioenergy Production for Sustainable Rural Development in Ghana. J. Sustain. Bioenergy Syst. 2021, 11, 227–259. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, M.; Bolan, N.S.; Kapley, A.; Kumar, R.; Singh, L. Multidimensional approaches of biogas production and up-gradation: Opportunities and challenges. Bioresour. Technol. 2021, 338, 125514. [Google Scholar] [CrossRef]
- Morya, R.; Kumar, M.; Thakur, I.S. Utilization of glycerol by Bacillus sp. ISTVK1 for production and characterization of Polyhydroxyvalerate. Bioresour. Technol. Rep. 2018, 2, 1–6. [Google Scholar] [CrossRef]
- Amin, F.R.; Khalid, H.; Zhang, H.; Rahman, S.; Zhang, R.; Liu, G.; Chen, C. Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express 2017, 7, 72. [Google Scholar] [CrossRef]
- Jain, S.; Jain, S.; Wolf, I.T.; Lee, J.; Tong, Y.W. A comprehensive review on operating parameters and different pretreatment methodologies for anaerobic digestion of municipal solid waste. Renew. Sustain. Energy Rev. 2015, 52, 142–154. [Google Scholar] [CrossRef]
- Plugge, C.M. Biogas. Microb. Biotechnol. 2017, 10, 1128–1130. [Google Scholar] [CrossRef]
- Darmey, J.; Narra, S.; Achaw, O.-W.; Ahiekpor, J.C.; Cougouais, B.A.N. Comparative Analysis of Lignocellulosic Composition Characteristics of Organic Municipal Solid Wastes: Insights from Kumasi and Global Cities for Biogas Feedstock. In Proceedings of the VENICE 2024, Venice, Italy, 25–27 November 2024; CISA Publisher, 2024. Available online: https://www.cisapublisher.com (accessed on 10 July 2025).
- Carrere, H.; Antonopoulou, G.; Affes, R.; Passos, F.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of feedstock pretreatment strategies for improved anaerobic digestion: From lab-scale research to full-scale application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef]
- Abraham, A.; Mathew, A.K.; Park, H.; Choi, O.; Sindhu, R.; Parameswaran, B.; Pandey, A.; Park, J.H.; Sang, B.I. Pretreatment strategies for enhanced biogas production from lignocellulosic biomass. Bioresour. Technol. 2020, 301, 122725. [Google Scholar] [CrossRef]
- Melville, L.; Weger, A.; Wiesgickl, S.; Franke, M. Anaerobic digestion. In Transformation of Biomass: Theory to Practice; Hornung, A., Ed.; John Wiley & Sons: Chichester, UK, 2014; pp. 31–59. [Google Scholar]
- Ziemiński, K.; Romanowska, I.; Kowalska, M. Enzymatic pretreatment of lignocellulosic wastes to improve biogas production. Waste Manag. 2012, 32, 1131–1137. [Google Scholar] [CrossRef]
- Atelge, M.R.; Atabani, A.E.; Banu, J.R.; Krisa, D.; Kaya, M.; Eskicioglu, C.; Kumar, G.; Lee, C.; Yildiz, Y.; Unalan, S.; et al. A critical review of pretreatment technologies to enhance anaerobic digestion and energy recovery. Fuel 2020, 270, 117494. [Google Scholar] [CrossRef]
- Rajendran, K.; Drielak, E.; Sudarshan Varma, V.; Muthusamy, S.; Kumar, G. Updates on the pretreatment of lignocellulosic feedstocks for bioenergy production—A review. Biomass Convers. Biorefinery 2018, 8, 471–483. [Google Scholar] [CrossRef]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Alexandropoulou, M.; Antonopoulou, G.; Fragkou, E.; Ntaikou, I.; Lyberatos, G. Fungal pretreatment of willow sawdust and its combination with alkaline treatment for enhancing biogas production. J. Environ. Manag. 2017, 203, 704–713. [Google Scholar] [CrossRef]
- Antwi, E.; Engler, N.; Nelles, M.; Schüch, A. Anaerobic digestion and the effect of hydrothermal pretreatment on the biogas yield of cocoa pods residues. Waste Manag. 2019, 88, 131–140. [Google Scholar] [CrossRef]
- Hernández-Beltrán, J.U.; Hernández-De Lira, I.O.; Cruz-Santos, M.M.; Saucedo-Luevanos, A.; Hernández-Terán, F.; Balagurusamy, N. Insight into pretreatment methods of lignocellulosic biomass to increase biogas yield: Current state, challenges, and opportunities. Appl. Sci. 2019, 9, 3721. [Google Scholar] [CrossRef]
- Menardo, S.; Airoldi, G.; Balsari, P. The effect of particle size and thermal pre-treatment on the methane yield of four agricultural by-products. Bioresour. Technol. 2012, 104, 708–714. [Google Scholar] [CrossRef]
- Karthikeyan, P.K.; Bandulasena, H.C.H.; Radu, T. A comparative analysis of pre-treatment technologies for enhanced biogas production from anaerobic digestion of lignocellulosic waste. Ind. Crops Prod. 2024, 215, 118591. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Sharma, H.K.; Xu, C.; Qin, W. Biological Pretreatment of Lignocellulosic Biomass for Biofuels and Bioproducts: An Overview. Waste Biomass Valorization 2019, 10, 235–251. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Esposito, G.; Frunzo, L.; Panico, A.; Pirozzi, F. Modelling the effect of the OLR and OFMSW particle size on the performances of an anaerobic co-digestion reactor. Process Biochem. 2011, 46, 557–565. [Google Scholar] [CrossRef]
- Khanh Nguyen, V.; Kumar Chaudhary, D.; Hari Dahal, R.; Hoang Trinh, N.; Kim, J.; Chang, S.W.; Hong, Y.; Duc La, D.; Nguyen, X.C.; Hao Ngo, H.; et al. Review on pretreatment techniques to improve anaerobic digestion of sewage sludge. Fuel 2021, 285, 119105. [Google Scholar] [CrossRef]
- Bochmann, G.; Montgomery, L.F.R. Storage and pre-treatment of substrates for biogas production. In The Biogas Handbook: Science, Production and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 85–103. [Google Scholar] [CrossRef]
- Shah, F.A.; Mahmood, Q.; Rashid, N.; Pervez, A.; Raja, I.A.; Shah, M.M. Co-digestion, pretreatment and digester design for enhanced methanogenesis. Renew. Sustain. Energy Rev. 2015, 42, 627–642. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Rodriguez, C.; Alaswad, A.; Benyounis, K.Y.; Olabi, A.G. Pretreatment techniques used in biogas production from grass. Renew. Sustain. Energy Rev. 2017, 68, 1193–1204. [Google Scholar] [CrossRef]
- Kratky, L.; Jirout, T. Biomass Size Reduction Machines for Enhancing Biogas Production. Chem. Eng. Technol. 2011, 34, 391–399. [Google Scholar] [CrossRef]
- Kang, X.; Zhang, Y.; Song, B.; Sun, Y.; Li, L.; He, Y.; Kong, X.; Luo, X.; Yuan, Z. The effect of mechanical pretreatment on the anaerobic digestion of Hybrid Pennisetum. Fuel 2019, 252, 469–474. [Google Scholar] [CrossRef]
- Pommier, S.; Llamas, A.M.; Lefebvre, X. Analysis of the outcome of shredding pretreatment on the anaerobic biodegradability of paper and cardboard materials. Bioresour. Technol. 2010, 101, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, B.; Sarker, S.; Lamb, J.J.; Lien, K.M. Yield improvements in anaerobic digestion of lignocellulosic feedstocks. J. Clean. Prod. 2021, 288, 125447. [Google Scholar] [CrossRef]
- Morales-Polo, C.; del Mar Cledera-Castro, M.; Yolanda Moratilla Soria, B. Reviewing the anaerobic digestion of food waste: From waste generation and anaerobic process to its perspectives. Appl. Sci. 2018, 8, 1804. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, W.; Wong, J.W.C.; Yong, X.; Yan, B.; Zhang, X.; Jia, H. Ultrasonic and thermal pretreatments on anaerobic digestion of petrochemical sludge: Dewaterability and degradation of PAHs. PLoS ONE 2015, 10, e0136162. [Google Scholar] [CrossRef]
- Panigrahi, S.; Dubey, B.K. A critical review on operating parameters and strategies to improve the biogas yield from anaerobic digestion of organic fraction of municipal solid waste. Renew. Energy 2019, 143, 779–797. [Google Scholar] [CrossRef]
- Sayara, T.; Sánchez, A. A review on anaerobic digestion of lignocellulosic wastes: Pretreatments and operational conditions. Appl. Sci. 2019, 9, 4655. [Google Scholar] [CrossRef]
- Paudel, S.R.; Banjara, S.P.; Choi, O.K.; Park, K.Y.; Kim, Y.M.; Lee, J.W. Pretreatment of agricultural biomass for anaerobic digestion: Current state and challenges. Bioresour. Technol. 2017, 245, 1194–1205. [Google Scholar] [CrossRef]
- Jo, H.; Parker, W.; Kianmehr, P. Comparison of the impacts of thermal pretreatment on waste activated sludge using aerobic and anaerobic digestion. Water Sci. Technol. 2018, 78, 1772–1781. [Google Scholar] [CrossRef]
- Nazari, L.; Yuan, Z.; Santoro, D.; Sarathy, S.; Ho, D.; Batstone, D.; Xu, C.C.; Ray, M.B. Low-temperature thermal pre-treatment of municipal wastewater sludge: Process optimization and effects on solubilization and anaerobic degradation. Water Res. 2017, 113, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Lu, X.; Kato, H.; Zhao, Y.; Li, Y.Y. Overview of pretreatment strategies for enhancing sewage sludge disintegration and subsequent anaerobic digestion: Current advances, full-scale application and future perspectives. Renew. Sustain. Energy Rev. 2017, 69, 559–577. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Donoso-Bravo, A.; Nilsen, P.J.; Fdz-Polanco, F.; Pérez-Elvira, S.I. Influence of thermal pretreatment on the biochemical methane potential of wheat straw. Bioresour. Technol. 2013, 143, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Koupaie, E.H.; Johnson, T.; Eskicioglu, C. Comparison of different electricity-based thermal pretreatment methods for enhanced bioenergy production from municipal sludge. Molecules 2018, 23, 2006. [Google Scholar] [CrossRef]
- Pielhop, T.; Amgarten, J.; Von Rohr, P.R.; Studer, M.H. Steam explosion pretreatment of softwood: The effect of the explosive decompression on enzymatic digestibility. Biotechnol. Biofuels 2016, 9, 152. [Google Scholar] [CrossRef]
- Behera, S.; Arora, R.; Nandhagopal, N.; Kumar, S. Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2014, 36, 91–106. [Google Scholar] [CrossRef]
- Bondesson, P.M.; Galbe, M.; Zacchi, G. Ethanol and biogas production after steam pretreatment of corn stover with or without the addition of sulphuric acid. Biotechnol. Biofuels 2013, 6, 1–11. [Google Scholar] [CrossRef]
- Wang, J.; Ma, D.; Lou, Y.; Ma, J.; Xing, D. Optimization of biogas production from straw wastes by different pretreatments: Progress, challenges, and prospects. Sci. Total Environ. 2023, 905, 166992. [Google Scholar] [CrossRef]
- Khan, M.U.; Usman, M.; Ashraf, M.A.; Dutta, N.; Luo, G.; Zhang, S. A review of recent advancements in pretreatment techniques of lignocellulosic materials for biogas production: Opportunities and Limitations. Chem. Eng. J. Adv. 2022, 10, 100263. [Google Scholar] [CrossRef]
- Luo, T.; Ge, Y.; Yang, Y.; Fu, Y.; Awasthi, M.K.; Pan, J.; Zhai, L.; Mei, Z.; Liu, H. The impact of immersed liquid circulation on anaerobic digestion of rice straw bale and methane generation improvement. Bioresour. Technol. 2021, 337, 125368. [Google Scholar] [CrossRef]
- Ren, T.; Yu, X.; Liao, J.; Du, Y.; Zhu, Y.; Jin, L.; Wang, B.; Xu, H.; Xiao, W.; Chen, H.Y.; et al. Application of biogas slurry rather than biochar increases soil microbial functional gene signal intensity and diversity in a poplar plantation. Soil Biol. Biochem. 2020, 146, 107825. [Google Scholar] [CrossRef]
- Ji, X.; Liu, Y.; Meng, J.; Wu, X. Global supply chain of biomass use and the shift of environmental welfare from primary exploiters to final consumers. Appl. Energy 2020, 276, 115484. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Ahmed, N.A.; Ogunkunle, O. Optimization of biogas yield from lignocellulosic materials with different pretreatment methods: A review. Biotechnol. Biofuels 2021, 14, 159. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.A.; Zeshan; Visvanathan, C. Effect of thermal pretreatment on chemical composition, physical structure and biogas production kinetics of wheat straw. J. Environ. Manag. 2018, 221, 45–52. [Google Scholar] [CrossRef]
- Granström, K.M.; Montelius, J. Pre-treatment to enhance biogas yield from pulp and paper mill sludge. J. Chem. Chem. Eng. 2014, 8, 825–833. [Google Scholar]
- Li, C.; Liu, G.; Nges, I.A.; Liu, J. Enhanced biomethane production from Miscanthus lutarioriparius using steam explosion pretreatment. Fuel 2016, 179, 267–273. [Google Scholar] [CrossRef]
- Gunes, B.; Stokes, J.; Davis, P.; Connolly, C.; Lawler, J. Pre-treatments to enhance biogas yield and quality from anaerobic digestion of whiskey distillery and brewery wastes: A review. Renew. Sustain. Energy Rev. 2019, 113, 109281. [Google Scholar] [CrossRef]
- Mancini, G.; Papirio, S.; Lens, P.N.L.; Esposito, G. Increased biogas production from wheat straw by chemical pretreatments. Renew. Energy 2018, 119, 608–614. [Google Scholar] [CrossRef]
- Sarto, S.; Hildayati, R.; Syaichurrozi, I. Effect of chemical pretreatment using sulfuric acid on biogas production from water hyacinth and kinetics. Renew. Energy 2019, 132, 335–350. [Google Scholar] [CrossRef]
- Vats, N.; Khan, A.A.; Ahmad, K. Options for Enhanced Anaerobic Digestion of Waste and Biomass—A Review. J. Biosyst. Eng. 2020, 45, 1–15. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Huzaifah, M.R.M.; Farid, M.A.A.; Shazleen, S.S.; Misenan, M.S.M.; Yasim-Anuar, T.A.T.; Naveen, J.; Nurazzi, N.M.; Rani, M.S.A.; Hakimi, M.I.; et al. Greener pretreatment approaches for the valorisation of natural fibre biomass into bioproducts. Polymers 2021, 13, 2971. [Google Scholar] [CrossRef]
- You, F.; Zhang, L.; Ye, J.; Huang, L. Microbial decomposition of biomass residues mitigated hydrogeochemical dynamics in strongly alkaline bauxite residues. Sci. Total Environ. 2019, 663, 216–226. [Google Scholar] [CrossRef]
- Poddar, B.J.; Nakhate, S.P.; Gupta, R.K.; Chavan, A.R.; Singh, A.K.; Khardenavis, A.A.; Purohit, H.J. A comprehensive review on the pretreatment of lignocellulosic wastes for improved biogas production by anaerobic digestion. Int. J. Environ. Sci. Technol. 2022, 19, 3429–3456. [Google Scholar] [CrossRef]
- Zhu, J.; Wan, C.; Li, Y. Enhanced solid-state anaerobic digestion of corn stover by alkaline pretreatment. Bioresour. Technol. 2010, 101, 7523–7528. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Capecchi, L.; Galbe, M.; Barbanti, L.; Wallberg, O. Combined ethanol and methane production using steam pretreated sugarcane bagasse. Ind. Crops Prod. 2015, 74, 255–262. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Verma, P. An overview of key pretreatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value added products. 3 Biotech 2013, 3, 415–431. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef]
- Hu, Z.; Wen, Z. Enhancing enzymatic digestibility of switchgrass by microwave-assisted alkali pretreatment. Biochem. Eng. J. 2008, 38, 369–378. [Google Scholar] [CrossRef]
- Venturin, B.; Bonatto, C.; Damaceno, F.M.; Mulinari, J.; Fongaro, G.; Treichel, H. Physical, Chemical, and Biological Substrate Pretreatments to Enhance Biogas Yield. In Microbial Resources and Bioenergies; Singh, O.V., Ed.; Springer: Cham, Switzerland, 2019; pp. 25–44. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Orlando, M.Q.; Borja, V.M. Pretreatment of animal manure biomass to improve biogas production: A review. Energies 2020, 13, 3573. [Google Scholar] [CrossRef]
- Suárez-Forero, S.J.; Candela-Soto, A.M.; Henao-Martínez, J.A.; Bayona-Ayala, O.L. Evaluation of the performance of the preteretment with the hydrogen peroxide on sugar cane bagasse for removing lignin. ITECKNE 2019, 16, 21–28. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Cesaro, A.; Belgiorno, V. Sonolysis and ozonation as pretreatment for anaerobic digestion of solid organic waste. Ultrason. Sonochem. 2013, 20, 931–936. [Google Scholar] [CrossRef]
- Syaichurrozi, I.; Villta, P.K.; Nabilah, N.; Rusdi, R. Effect of sulfuric acid pretreatment on biogas production from Salvinia molesta. J. Environ. Chem. Eng. 2019, 7, 102857. [Google Scholar] [CrossRef]
- Song, Z.; Yang, G.; Liu, X.; Yan, Z.; Yuan, Y.; Liao, Y. Comparison of seven chemical pretreatments of corn straw for improving methane yield by anaerobic digestion. PLoS ONE 2014, 9, e101617. [Google Scholar] [CrossRef]
- Dahunsi, S.O.; Adesulu-Dahunsi, A.T.; Osueke, C.O.; Lawal, A.I.; Olayanju, T.M.A.; Ojediran, J.O.; Izebere, J.O. Biogas generation from Sorghum bicolor stalk: Effect of pretreatment methods and economic feasibility. Energy Rep. 2019, 5, 584–593. [Google Scholar] [CrossRef]
- Hosseini Koupaie, E.; Dahadha, S.; Bazyar Lakeh, A.A.; Azizi, A.; Elbeshbishy, E. Enzymatic pretreatment of lignocellulosic biomass for enhanced biomethane production—A review. J. Environ. Manag. 2019, 233, 774–784. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Supriya, R.D.; Sindhu, R.; Binod, P.; Nair, R.B.; Pandey, A.; Gnansounou, E. Biological pretreatment of lignocellulosic biomass-current trends and future perspectives. In Second and Third Generation of Feedstocks: The Evolution of Biofuels; Gnansounou, E., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 197–212. [Google Scholar] [CrossRef]
- Gurung, N.; Ray, S.; Bose, S.; Rai, V. A broader view: Microbial enzymes and their relevance in industries, medicine, and beyond. BioMed Res. Int. 2013, 2013, 329121. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.O.; Lackner, N.; Mutschlechner, M.; Prem, E.M.; Markt, R.; Illmer, P. Biological pretreatment strategies for second-generation lignocellulosic resources to enhance biogas production. Energies 2018, 11, 1797. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Singh, P.K.; Dash, S.; Pattnaik, R. Microbial pretreatment of lignocellulosic biomass for enhanced biomethanation and waste management. 3 Biotech 2018, 8, 458. [Google Scholar] [CrossRef] [PubMed]
- Kamperidou, V.; Terzopoulou, P. Anaerobic digestion of lignocellulosic waste materials. Sustainability 2021, 13, 12810. [Google Scholar] [CrossRef]
- Zhao, X.; Luo, K.; Zhang, Y.; Zheng, Z.; Cai, Y.; Wen, B.; Cui, Z.; Wang, X. Improving the methane yield of maize straw: Focus on the effects of pretreatment with fungi and their secreted enzymes combined with sodium hydroxide. Bioresour. Technol. 2018, 250, 204–213. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Poulsen, T.G.; Sheng, K. Fungal pretreatment of rice straw with Pleurotus ostreatus and Trichoderma reesei to enhance methane production under solid-state anaerobic digestion. Appl. Energy 2016, 180, 661–671. [Google Scholar] [CrossRef]
- Rouches, E.; Zhou, S.; Steyer, J.P.; Carrere, H. White-Rot Fungi pretreatment of lignocellulosic biomass for anaerobic digestion: Impact of glucose supplementation. Process Biochem. 2016, 51, 1784–1792. [Google Scholar] [CrossRef]
- Krishna, D.; Kalamdhad, A.S. Pre-treatment and anaerobic digestion of food waste for high rate methane production—A review. J. Environ. Chem. Eng. 2014, 2, 1821–1830. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Kulupa, T.; Kubiak, A.; Wolna-Maruwka, A.; Pilarski, K.; Niewiadomska, A. Anaerobic Digestion of Food Waste—A Short Review. Energies 2023, 16, 5742. [Google Scholar] [CrossRef]
- Zhao, J.; Ge, X.; Vasco-Correa, J.; Li, Y. Fungal pretreatment of unsterilized yard trimmings for enhanced methane production by solid-state anaerobic digestion. Bioresour. Technol. 2014, 158, 248–252. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Li, Y. Solid-state anaerobic digestion of fungal pretreated Miscanthus sinensis harvested in two different seasons. Bioresour. Technol. 2015, 185, 211–217. [Google Scholar] [CrossRef]
- Ge, X.; Matsumoto, T.; Keith, L.; Li, Y. Fungal pretreatment of albizia chips for enhanced biogas production by solid-state anaerobic digestion. Energy Fuels 2015, 29, 200–204. [Google Scholar] [CrossRef]
- Weide, T.; Baquero, C.D.; Schomaker, M.; Brügging, E.; Wetter, C. Effects of enzyme addition on biogas and methane yields in the batch anaerobic digestion of agricultural waste (silage, straw, and animal manure). Biomass Bioenergy 2020, 132, 105442. [Google Scholar] [CrossRef]
- Rafique, R.; Poulsen, T.G.; Nizami, A.S.; Asam, Z.-u.-Z.; Murphy, J.D.; Kiely, G. Effect of thermal, chemical and thermo-chemical pre-treatments to enhance methane production. Energy 2010, 35, 4556–4561. [Google Scholar] [CrossRef]
- Ferdeş, M.; Dincă, M.N.; Moiceanu, G.; Zabava, B.Ş.; Paraschiv, G. Microorganisms and enzymes used in the biological pretreatment of the substrate to enhance biogas production: A review. Sustainability 2020, 12, 7205. [Google Scholar] [CrossRef]
- Hansen, G.H.; Lübeck, M.; Frisvad, J.C.; Lübeck, P.S.; Andersen, B. Production of cellulolytic enzymes from ascomycetes: Comparison of solid state and submerged fermentation. Process Biochem. 2015, 50, 1327–1341. [Google Scholar] [CrossRef]
- Wei, S. The application of biotechnology on the enhancing of biogas production from lignocellulosic waste. Appl. Microbiol. Biotechnol. 2016, 100, 9821–9836. [Google Scholar] [CrossRef]
- Ometto, F.; Quiroga, G.; Pšenička, P.; Whitton, R.; Jefferson, B.; Villa, R. Impacts of microalgae pre-treatments for improved anaerobic digestion: Thermal treatment, thermal hydrolysis, ultrasound and enzymatic hydrolysis. Water Res. 2014, 65, 350–361. [Google Scholar] [CrossRef]
- Plácido, J.; Capareda, S. Ligninolytic enzymes: A biotechnological alternative for bioethanol production. Bioresour. Bioprocess. 2015, 2, 23. [Google Scholar] [CrossRef]
- Damasceno, F.R.C.; Cammarota, M.C.; Freire, D.M.G. The combined use of a biosurfactant and an enzyme preparation to treat an effluent with a high fat content. Colloids Surf. B Biointerfaces 2012, 95, 241–246. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Aghbashlo, M.; Valijanian, E.; Kazemi Shariat Panahi, H.; Nizami, A.S.; Ghanavati, H.; Sulaiman, A.; Mirmohamadsadeghi, S.; Karimi, K. A comprehensive review on recent biological innovations to improve biogas production, Part 1: Upstream strategies. Renew. Energy 2020, 146, 1204–1220. [Google Scholar] [CrossRef]
- Huang, X.; Shen, C.; Liu, J.; Lu, L. Improved volatile fatty acid production during waste activated sludge anaerobic fermentation by different bio-surfactants. Chem. Eng. J. 2015, 264, 280–290. [Google Scholar] [CrossRef]
- Shraddha; Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial sources, production, purification, and potential biotechnological applications. Enzym. Res. 2011, 2011, 217861. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Couto, S.; Toca Herrera, J.L. Industrial and biotechnological applications of laccases: A review. Biotechnol. Adv. 2006, 24, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Ehimen, E.A.; Holm-Nielsen, J.B.; Poulsen, M.; Boelsmand, J.E. Influence of different pre-treatment routes on the anaerobic digestion of a filamentous algae. Renew. Energy 2013, 50, 476–480. [Google Scholar] [CrossRef]
- Shrestha, B.; Hernandez, R.; Fortela, D.L.B.; Sharp, W.; Chistoserdov, A.; Gang, D.; Revellame, E.; Holmes, W.; Zappi, M.E. A review of pretreatment methods to enhance solids reduction during anaerobic digestion of municipal wastewater sludges and the resulting digester performance: Implications to future urban biorefineries. Appl. Sci. 2020, 10, 9141. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, R.; Li, K.; Ma, R. A review of crop straw pretreatment methods for biogas production by anaerobic digestion in China. Renew. Sustain. Energy Rev. 2019, 107, 51–58. [Google Scholar] [CrossRef]
- Abudi, Z.N.; Hu, Z.; Sun, N.; Xiao, B.; Rajaa, N.; Liu, C.; Guo, D. Batch anaerobic co-digestion of OFMSW (organic fraction of municipal solid waste), TWAS (thickened waste activated sludge) and RS (rice straw): Influence of TWAS and RS pretreatment and mixing ratio. Energy 2016, 107, 131–140. [Google Scholar] [CrossRef]
- Ma, J.; Duong, T.H.; Smits, M.; Verstraete, W.; Carballa, M. Enhanced biomethanation of kitchen waste by different pre-treatments. Bioresour. Technol. 2011, 102, 592–599. [Google Scholar] [CrossRef]
- Vavouraki, A.I.; Angelis, E.M.; Kornaros, M. Optimization of thermo-chemical hydrolysis of kitchen wastes. Waste Manag. 2013, 33, 740–745. [Google Scholar] [CrossRef]
- Bruni, E.; Jensen, A.P.; Angelidaki, I. Comparative study of mechanical, hydrothermal, chemical and enzymatic treatments of digested biofibers to improve biogas production. Bioresour. Technol. 2010, 101, 8713–8717. [Google Scholar] [CrossRef]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Hydrothermal pretreatment of rice straw biomass: A potential and promising method for enhanced methane production. Appl. Energy 2012, 94, 129–140. [Google Scholar] [CrossRef]
- Sajad Hashemi, S.; Karimi, K.; Majid Karimi, A. Ethanolic ammonia pretreatment for efficient biogas production from sugarcane bagasse. Fuel 2019, 248, 196–204. [Google Scholar] [CrossRef]
- Cesaro, A.; Belgiorno, V. Pretreatment methods to improve anaerobic biodegradability of organic municipal solid waste fractions. Chem. Eng. J. 2014, 240, 24–37. [Google Scholar] [CrossRef]
- Izumi, K.; Okishio, Y.-k.; Nagao, N.; Niwa, C.; Yamamoto, S.; Toda, T. Effects of particle size on anaerobic digestion of food waste. Int. Biodeterior. Biodegrad. 2010, 64, 601–608. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Fdez-Güelfo, L.A.; Zhou, Y.; Álvarez-Gallego, C.J.; Garcia, L.I.R.; Ng, W.J. Anaerobic co-digestion of organic fraction of municipal solid waste (OFMSW): Progress and challenges. Renew. Sustain. Energy Rev. 2018, 93, 380–399. [Google Scholar] [CrossRef]
- Zamri, M.F.M.A.; Hasmady, S.; Akhiar, A.; Ideris, F.; Shamsuddin, A.H.; Mofijur, M.; Fattah, I.M.R.; Mahlia, T.M.I. A comprehensive review on anaerobic digestion of organic fraction of municipal solid waste. Renew. Sustain. Energy Rev. 2021, 137, 408–415. [Google Scholar] [CrossRef]
- Anjum, M.; Khalid, A.; Mahmood, T.; Aziz, I. Anaerobic co-digestion of catering waste with partially pretreated lignocellulosic crop residues. J. Clean. Prod. 2016, 117, 56–63. [Google Scholar] [CrossRef]
- Carlsson, B. (Ed.) Technological Systems and Economic Performance: The Case of Factory Automation; Springer Science & Business Media: New York, NY, USA, 2012; Volume 5. [Google Scholar]
- Bhatia, S.K.; Kim, S.H.; Yoon, J.J.; Yang, Y.H. Current status and strategies for second generation biofuel production using microbial systems. Energy Convers. Manag. 2017, 148, 1142–1156. [Google Scholar] [CrossRef]
- Pilli, S.; Bhunia, P.; Yan, S.; LeBlanc, R.J.; Tyagi, R.D.; Surampalli, R.Y. Ultrasonic pretreatment of sludge: A review. Ultrason. Sonochem. 2011, 18, 1–18. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Lo, S.L. Application of physico-chemical pretreatment methods to enhance the sludge disintegration and subsequent anaerobic digestion: An up to date review. Rev. Environ. Sci. Biotechnol. 2011, 10, 215–242. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Patel, A.K.; Pant, D.; Banu, J.R.; Rao, C.V.; Kim, Y.-G.; Yang, Y.H. Recent developments in pretreatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresour. Technol. 2020, 300, 122724. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Shi, J.; Li, Y. Comparison of solid-state to liquid anaerobic digestion of lignocellulosic feedstocks for biogas production. Bioresour. Technol. 2012, 124, 379–386. [Google Scholar] [CrossRef]
- Yang, L.; Xu, F.; Ge, X.; Li, Y. Challenges and strategies for solid-state anaerobic digestion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2015, 44, 824–834. [Google Scholar] [CrossRef]
- Barakat, A.; Monlau, F.; Steyer, J.P.; Carrere, H. Effect of lignin-derived and furan compounds found in lignocellulosic hydrolysates on biomethane production. Bioresour. Technol. 2012, 104, 90–99. [Google Scholar] [CrossRef]
- Rabelo, S.C.; Carrere, H.; Maciel Filho, R.; Costa, A.C. Production of bioethanol, methane and heat from sugarcane bagasse in a biorefinery concept. Bioresour. Technol. 2011, 102, 7887–7895. [Google Scholar] [CrossRef]
- Anto, S.; Mukherjee, S.S.; Muthappa, R.; Mathimani, T.; Deviram, G.; Kumar, S.S.; Verma, T.N.; Pugazhendhi, A. Algae as green energy reserve: Technological outlook on biofuel production. Chemosphere 2020, 242, 125079. [Google Scholar] [CrossRef]
- Kaur, K.; Phutela, U.G. Morphological and structural changes in paddy straw influenced by alkali and microbial pretreatment. Detritus 2018, 3, 30–36. [Google Scholar] [CrossRef]
- Passos, F.; García, J.; Ferrer, I. Impact of low temperature pretreatment on the anaerobic digestion of microalgal biomass. Bioresour. Technol. 2013, 138, 79–86. [Google Scholar] [CrossRef]
- Passos, F.; Ferrer, I. Microalgae conversion to biogas: Thermal pretreatment contribution on net energy production. Environ. Sci. Technol. 2014, 48, 7171–7178. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K.; Kumar, R. Cellulose Solvent-Based Pretreatment for Enhanced Second-Generation Biofuel Production: A Review. Sustain. Energy Fuels 2019, 3, 11–22. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A review of the processes, parameters, and optimization of anaerobic digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef]
- Chang, H.N.; Kim, N.J.; Kang, J.; Jeong, C.M. Biomass-derived volatile fatty acid platform for fuels and chemicals. Biotechnol. Bioprocess. Eng. 2010, 15, 1–10. [Google Scholar] [CrossRef]
- Liu, X.; Lendormi, T.; Lanoisellé, J. A Review of Hygienization Technology of Biowastes for Anaerobic Digestion: Effect on Pathogen Inactivation and Methane Production. Chem. Eng. Trans. 2018, 70, 529–534. [Google Scholar] [CrossRef]
- Strazzera, G.; Battista, F.; Garcia, N.H.; Frison, N.; Bolzonella, D. Volatile fatty acids production from food wastes for biorefinery platforms: A review. J. Environ. Manag. 2018, 226, 278–288. [Google Scholar] [CrossRef]
- Rastogi, M.; Shrivastava, S. Recent advances in second generation bioethanol production: An insight to pretreatment, saccharification and fermentation processes. Renew. Sustain. Energy Rev. 2017, 80, 330–340. [Google Scholar] [CrossRef]
- Zhao, S.; Thakur, N.; Salama, E.S.; Zhang, P.; Zhang, L.; Xing, X.; Yue, J.; Song, Z.; Nan, L.; Yujun, S.; et al. Potential applications of protein-rich waste: Progress in energy management and material recovery. Resour. Conserv. Recycl. 2022, 182, 106315. [Google Scholar] [CrossRef]
- Appels, L.; Degrève, J.; Van der Bruggen, B.; Van Impe, J.; Dewil, R. Influence of low temperature thermal pre-treatment on sludge solubilisation, heavy metal release and anaerobic digestion. Bioresour. Technol. 2010, 101, 5743–5748. [Google Scholar] [CrossRef]
- Dhar, B.R.; Nakhla, G.; Ray, M.B. Techno-economic evaluation of ultrasound and thermal pretreatments for enhanced anaerobic digestion of municipal waste activated sludge. Waste Manag. 2012, 32, 542–549. [Google Scholar] [CrossRef]
- Vannarath, A.; Thalla, A.K. Evaluation, ranking, and selection of pretreatment methods for the conversion of biomass to biogas using multi-criteria decision-making approach. Environ. Syst. Decis. 2020, 40, 510–525. [Google Scholar] [CrossRef]

| Substrates | Particle Size | Methane Yield (Untreated) | Methane Yield (After Treatment) | Reaction System | References |
|---|---|---|---|---|---|
| Barley Straw | 50 mm | 240 mL/g VS | 286 mL/g VS | Glass reactor 2 L | [34] |
| 20 mm | 339 mL/g VS | ||||
| 5 mm | 370 mL/g VS | ||||
| Maize stalks | 20 mm | 246 mL/g VS | 254 mL/g VS | Glass reactor 2 L | |
| 2 mm | 272 mL/g VS | ||||
| Wheat straw | 50 mm | 182 mL/g VS | 285 mL/g VS | Glass reactor 2 L | |
| 2 mm | 334 mL/g VS | ||||
| Water hyacinth | 2.5 mm | Increase 10% (from 50 to 55%) | Digester 0.45 L | [35] | |
| 1.0 mm | Increase 20% (from 50 to 60%) | ||||
| 0.05 mm | Increase 32% (from 50 to 66%) | ||||
| 0.001 mm | Increase 40% (from 50 to 70%) | ||||
| Rice straw | 0.4 mm | 320 mL/g TS | 487 mL/g TS | 5 L batch AD bottle, 37 °C. | [36] |
| Mirabilis leaves | 355 mL/g TS | 418 mL/g TS | |||
| Dhub grass | 170 mL/g TS | 282 mL/g TS | |||
| Banana peeling | 460 mL/g TS | 510 mL/g TS |
| Substrates | Pre-Treatment | Conditions | Methane Yield (Untreated) | Methane Yield (After Treatment) | Reaction System | References |
|---|---|---|---|---|---|---|
| Wheat straw | Hydrothermal | 120 °C; 60 min | 388.9 mL/g VS | 483.3 mL/g VS | Serum bottle 0.3 L | [57] |
| 140 °C; 60 min | 511.2 mL/g VS | |||||
| 160 °C; 60 min | 552.7 mL/g VS | |||||
| 180 °C; 60 min | 611.7 mL/g VS | |||||
| Pulp and paper Sludge | 140 °C, 60 min | 225 mL/g VS | 603 mL/g VS | [58] | ||
| 70 °C, 60 min | 231 mL/g VS | |||||
| Miscanthus lutarioriparius | Steam explosion | 0.5 MPa; 153 °C; 5 min | 181.2 mL/g VS | 192.3 mL/g VS | Reactor 0.5 L | [59] |
| 1.0 MPa; 180 °C; 5 min | 217.9 mL/g VS | |||||
| 1.5 MPa; 198 °C; 3 min | 274.1 mL/g VS | |||||
| 1.5 MPa; 198 °C; 5 min | 272.0 mL/g VS | |||||
| 1.5 MPa; 198 °C; 10 min | 272.2 mL/g VS |
| Feedstock | Pretreatment Type | Temperature (°C) | Time (min) | Untreated Yield (mL/g VS) | Treated Yield (mL/g VS) | % Increase | Reference |
|---|---|---|---|---|---|---|---|
| Wheat Straw | Steam Explosion | 200 | 15 | 180 | 280 | 56% | [59] |
| Wheat Straw | Hydrothermal | 160 | 45 | 180 | 309.6 | 72% | [36] |
| Corn Stover | Steam Explosion | 200 | 10 | 170 | 250 | 47% | [59] |
| Corn Stover | Hydrothermal | 175 | 60 | 170 | 260 | 53% | [36] |
| Substrates | Pretreatment Conditions | Methane Yield (Untreated) | Methane Yield (After Pretreatment) | Reaction System | References |
|---|---|---|---|---|---|
| Salvinia Molesta | Sulfuric acid 2% v/v | 11.2 mL/g VS | 16.6 mL/g VS | Bottle 0.6 L | [81] |
| Sulfuric acid 4% v/v | 17.4 mL/g VS | ||||
| Sulfuric acid 6% v/v | 17.8 mL/g VS | ||||
| Corn Straw | Sulfuric acid 2% v/v | 100.6 mL/g VS | 175.6 mL/g VS | Flask 1 L | [82] |
| Hydrochloric acid 2% v/v | 163.4 mL/g VS | ||||
| Acetic acid 4% v/v | 145.1 mL/g VS | ||||
| Hydrogen peroxide 3% v/v | 216.7 mL/g VS | ||||
| Sodium hydroxide 8% v/v | 163.5 mL/g VS | ||||
| Calcium hydroxide 8% v/v | 206.6 mL/g VS | ||||
| Ammonia 10% v/v | 168.3 mL/g VS | ||||
| Sorghum bicolor Stalk | H2SO4 | 55% CH4 | 54.5% CH4 | 250 mL batch reactors at 37 °C | [83] |
| H2O2 | 61.5% CH4 |
| Pretreatment Method | Chemical Agent | Concentration (% v/v) | Methane Yield (mL/g VS) | % Increase Over Untreated | Industrial Context Suitability | Reference |
|---|---|---|---|---|---|---|
| Acid Pretreatment | Sulfuric Acid (H2SO4) | 2 | 175.6 | 74.6% | Suitable for large-scale hydrolysis plants | [82] |
| Acid Pretreatment | Hydrochloric Acid (HCl) | 2 | 163.4 | 62.4% | Effective in municipal waste treatment setups | [82] |
| Organosolv Pretreatment | Acetic Acid | 4 | 145.1 | 44.2% | Applicable in integrated biorefineries | [82] |
| Oxidative Pretreatment | Hydrogen Peroxide (H2O2) | 3 | 216.7 | 115.4% | Ideal for enzymatic enhancement in digesters | [82] |
| Alkaline Pretreatment | Sodium Hydroxide (NaOH) | 8 | 163.5 | 62.5% | Common in agro-industrial waste processing | [82] |
| Alkaline Pretreatment | Calcium Hydroxide (Ca(OH)2) | 8 | 206.6 | 105.4% | Suitable for decentralized rural biogas units | [82] |
| Alkaline Pretreatment | Ammonia (NH3) | 10 | 168.3 | 67.3% | Effective in low-energy, low-cost setups | [82] |
| Feedstocks | Fungal Pretreatment | Methane Yield (Untreated) | Methane Yield (After Pretreatment) | References |
|---|---|---|---|---|
| Willow sawdust | Leiotrametes menziesii, 30 days | 95.5 mL/g VS | 62.4 mL/g VS | [94] |
| Abortiporus biennis, 30 days | 136.7 mL/g VS | |||
| Rice straw | Pleurotus ostreatus, 28 °C/20 d | 120% higher methane yield | [90] | |
| Trichoderma reesei, 28 °C/20 d | 78.3% higher methane yield | |||
| Yard trimming | Ceriporiopsis subvermispora | 106% higher methane yield | [18] | |
| Mycelium grown, 30 Days | 20 mL/g VS | 40 mL/g VS | ||
| Miscanthus | C. subvermispora (ATCC 96608) | +25% (v/w VS) CH4 | [95] | |
| Albizia chips | +370% (v/w VS) CH4 | [96] |
| Substrates | Enzymes | Pretreatment Conditions | Result of Pretreatment on Methane Yield | References |
|---|---|---|---|---|
| Scenedesmus obliquus | cellulase and endo-galactouronase | 50 °C/24 h | 403% higher yield | [103] |
| esterase and protease | 273% higher yield | |||
| cellulase, esterase and protease, endogalactouronase, | 485% higher yield | |||
| Rhizoclonium | Lipase | 115 mL CH4/g TS | [110] | |
| Xylanase | 118 mL CH4/g TS | |||
| a-amylase | 121 mL CH4/g TS | |||
| Protease | 116 mL CH4/g TS | |||
| Cellulase | 133 mL CH4/g TS | |||
| lipase, xylanase, a-amylase, protease and cellulase | 145 mL CH4/g TS | |||
| Corn stover | Bjerkandera adusta (Versatile peroxidase) | 1 day | 15% higher than untreated | [66] |
| Ensilaged maize | 6% higher than untreated | |||
| Flax | 14% higher than untreated |
| Pre-Treatment | Feedstock | Untreated | Effect of Pretreatment on Biogas | Reference |
|---|---|---|---|---|
| Thermo-acid (HCl + 120 °C) | FW | Increased by 18% | [114] | |
| Thermo-acid (HCl at 100 °C) | OFMSW | Increased by 120% | [115] | |
| Steam+ NaOH + laccase | Bio-fibers | Increased by 49% | [116] | |
| NaOH + hydrothermal | Rice straw | 59.8 L/kg VS (CH4) | 132.7 L/kg VS (CH4) | [117] |
| Ethanol+ NH3 | Sugar cane bagasse | 105.6 mL/g VS | 299.3 mL/g VS | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darmey, J.; Narra, S.; Achaw, O.-W.; Stinner, W.; Ahiekpor, J.C.; Ansah, H.F.; N’guessan, B.A.; Agyekum, T.O.; Nutakor, E.M.K. A Review of Pretreatment Strategies for Anaerobic Digestion: Unlocking the Biogas Generation Potential of Wastes in Ghana. Waste 2025, 3, 24. https://doi.org/10.3390/waste3030024
Darmey J, Narra S, Achaw O-W, Stinner W, Ahiekpor JC, Ansah HF, N’guessan BA, Agyekum TO, Nutakor EMK. A Review of Pretreatment Strategies for Anaerobic Digestion: Unlocking the Biogas Generation Potential of Wastes in Ghana. Waste. 2025; 3(3):24. https://doi.org/10.3390/waste3030024
Chicago/Turabian StyleDarmey, James, Satyanarayana Narra, Osei-Wusu Achaw, Walter Stinner, Julius Cudjoe Ahiekpor, Herbert Fiifi Ansah, Berah Aurelie N’guessan, Theophilus Ofori Agyekum, and Emmanuel Mawuli Koku Nutakor. 2025. "A Review of Pretreatment Strategies for Anaerobic Digestion: Unlocking the Biogas Generation Potential of Wastes in Ghana" Waste 3, no. 3: 24. https://doi.org/10.3390/waste3030024
APA StyleDarmey, J., Narra, S., Achaw, O.-W., Stinner, W., Ahiekpor, J. C., Ansah, H. F., N’guessan, B. A., Agyekum, T. O., & Nutakor, E. M. K. (2025). A Review of Pretreatment Strategies for Anaerobic Digestion: Unlocking the Biogas Generation Potential of Wastes in Ghana. Waste, 3(3), 24. https://doi.org/10.3390/waste3030024