Review on Aquatic Weeds as Potential Source for Compost Production to Meet Sustainable Plant Nutrient Management Needs
Abstract
1. Introduction
- Disturbance in navigation thereby influencing fisheries and water recreation [21].
- Blocking irrigation and water channels leading to flooding and bank erosion incidences [19].
- Noxious aquatic plants in water bodies reducing irrigation water for agriculture as it increases water loss by evapotranspiration [27].
- Influence on drinking and other household consumption, health-related issues such as insect-borne (primarily mosquito-infested) and poisonous incidences (plants, snakes, insects, and fish) of water bodies on the surrounding human community [29].
- Reduction of aesthetic appearance of water bodies as a result of eutrophication and foul smell [30].
- Economic losses related to social, ecological, and policy issues occurring mainly due to the eutrophication phenomenon in water bodies. This may cause the increasing purifying cost of polluted water and negative impacts on tourism [31].
- Challenges with the sustainability of aquatic ecosystems.
1.1. Preventive Management
1.2. Manual and Mechanical Management
1.3. Ecological Aquatic Weed Management
1.4. Chemical Management
1.5. Biological Management
1.6. Aquatic Weed Management through Utilization
2. Aquatic Weeds as a Potential Raw Material in Composting
| Potentially Toxic Element | EU Range (mg/kg) | USA Biosolids (mg/kg) | Sri Lankan Standards (SLS) (mg/kg Dry Basis, AOAC Testing Method) |
|---|---|---|---|
| Cadmium (Cd) | 0.7–10 | 39 | 1.5 |
| Chromium (Cr) | 70–200 | 1200 | 50 |
| Mercury (Hg) | 0.7–10 | 17 | 0.5 |
| Nickel (Ni) | 20–200 | 420 | 40 |
| Lead (Pb) | 70–1000 | 300 | 30 |
3. Methods for Compost Production with Aquatic Weeds
- Wind-row methods (turned wind-rows, passively aerated wind-rows, and aerated static pile)
- In-vessel composting methods (bin composting, passively aerated bin composting, rectangular agitated beds, silos, rotating drums, transportable containers)
- Traditional methods (anaerobic composting, aerobic composting through passive aeration/static composting)
- Rapid methods (aerobic high-temperature composting, aerobic high-temperature composting with inoculation, IBS rapid composting)
- Vermicomposting methods
4. Steps for Producing Compost with Aquatic Weeds
5. Potential Environmental Risks Associated with the Application of Aquatic Weed Composts
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dissanayaka, D.; Nuwarapaksha, T.; Udumann, S.; Dissanayake, D.; Atapattu, A.J. A Sustainable Way of Increasing Productivity of Coconut Cultivation Using Cover Crops: A Review. Circ. Agric. Syst. 2022, 2, 7. [Google Scholar] [CrossRef]
- Mengqi, Z.; Shi, A.; Ajmal, M.; Ye, L.; Awais, M. Comprehensive Review on Agricultural Waste Utilization and High-Temperature Fermentation and Composting. Biomass Convers. Biorefin. 2021, 1–25. [Google Scholar] [CrossRef]
- Zahra, M.B.; Fayyaz, B.; Aftab, Z.E.H.; Haider, M.S. Mitigation of Degraded Soils by Using Biochar and Compost: A Systematic Review. J. Soil Sci. Plant Nutr. 2021, 21, 2718–2738. [Google Scholar] [CrossRef]
- Mohammad, N.; Alam, M.Z.; Kabbashi, N.A.; Ahsan, A. Effective Composting of Oil Palm Industrial Waste by Filamentous Fungi: A Review. Resour. Conserv. Recycl. 2012, 58, 69–78. [Google Scholar] [CrossRef]
- Tuomela, M.; Vikman, M.; Hatakka, A.; Itavaara, M. Biodegradation of Lignin in a Compost Environment: A review. Bioresour. Technol. 2000, 72, 169–183. [Google Scholar] [CrossRef]
- Richard, T.L.; Hamelers, H.V.M.B.; Veeken, A.; Silva, T. Moisture Relationships in Composting Processes. Compos. Sci. Util. 2002, 10, 286–302. [Google Scholar] [CrossRef]
- Akyol, Ç.; Ince, O.; Ince, B. Crop-based Composting of Lignocellulosic Digestates: Focus on Bacterial and Fungal Diversity. Bioresour. Technol. 2019, 288, 121549. [Google Scholar] [CrossRef] [PubMed]
- Agnew, J.M.; Leonard, J.J. The Physical Properties of Compost. Compos. Sci. Util. 2003, 11, 238–264. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of Animal Manures and Chemical Criteria for Compost Maturity Assessment. A Review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Mia, S.; Uddin, M.E.; Kader, M.A.; Ahsan, A.; Mannan, M.A.; Hossain, M.M.; Solaiman, Z.M. Pyrolysis and Co-composting of Municipal Organic Waste in Bangladesh: A Quantitative Estimate of Recyclable Nutrients, Greenhouse Gas Emissions, and Economic Benefits. Waste Manag. 2018, 75, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Hu, B.; Wei, M.B.; Zhao, J.H.; Zhang, H.Z. Influence of Matured Compost Inoculation on Sewage Sludge Composting: Enzyme Activity, Bacterial and Fungal Community Succession. Bioresour. Technol. 2019, 294, 122165. [Google Scholar] [CrossRef]
- Ayilara, M.; Olanrewaju, O.; Babalola, O.; Odeyemi, O. Waste Management through Composting: Challenges and Potentials. Sustainability 2020, 12, 4456. [Google Scholar] [CrossRef]
- Irshad, M.; Eneji, A.E.; Hussain, Z.; Ashraf, M. Chemical Characterization of Fresh and Composted Livestock Manures. J. Soil Sci. Plant Nutr. 2013, 13, 115–121. [Google Scholar] [CrossRef]
- Wang, J.; Gu, J.; Wang, X.; Song, Z.; Dai, X.; Guo, H.; Yu, J.; Zhao, W.; Lei, L. Enhanced Removal of Antibiotic Resistance Genes and Mobile Genetic Elements during Swine Manure Composting Inoculated with Mature Compost. J. Hazard. Mater. 2021, 411, 125135. [Google Scholar] [CrossRef]
- Jain, M.S.; Kalamdhad, A.S. A Review on Management of Hydrilla verticillata and its Utilization as Potential Nitrogen-rich Biomass for Compost or Biogas Production. Bioresour. Technol. Rep. 2018, 1, 69–78. [Google Scholar] [CrossRef]
- Sarkar, S.; Sarkar, U.K.; Ali, S.; Kumari, S.; Puthiyotti, M. Status, ecological services and management of aquatic weeds of floodplain wetlands in India: An overview. Lakes Reserv. Sci. Policy Manag. Sustain. Use 2021, 26, 76–91. [Google Scholar] [CrossRef]
- McFadyen, R.E.C. Biological Control of Weeds. Annu. Rev. Entomol. 1998, 43, 369–393. [Google Scholar] [CrossRef] [PubMed]
- Ayanda, O.I.; Ajayi, T.; Asuwaju, F.P. Eichhornia crassipes (Mart.) Solms: Uses, Challenges, Threats, and Prospects. Sci. World J. 2020, 2020, 3452172. [Google Scholar] [CrossRef] [PubMed]
- Jayan, P.R.; Sathyanathan, N. Aquatic Weed Classification, Environmental Effects and the Management Technologies for its Effective Control in Kerala, India. Int. J. Agric. Biol. Eng. 2012, 5, 76–91. [Google Scholar] [CrossRef]
- Raney, F.C. Geobotany. Ecology 1966, 47, 173–174. [Google Scholar] [CrossRef]
- Holm, L.G.; Weldon, L.W.; Blackburn, R.D. Aquatic Weeds. Pest Artic. News Summ. (PANS) 1970, 16, 576–589. [Google Scholar] [CrossRef]
- Kariyawasam, C.S.; Kumar, L.; Kogo, B.K.; Ratnayake, S.S. Long-Term Changes of Aquatic Invasive Plants and Implications for Future Distribution: A Case Study Using a Tank Cascade System in Sri Lanka. Climate 2021, 9, 31. [Google Scholar] [CrossRef]
- Kaur, M.; Kumar, M.; Sachdeva, S.; Puri, S.K. Aquatic Weeds as the Next Generation Feedstock for Sustainable Bioenergy Production. Bioresour. Technol. 2018, 251, 390–402. [Google Scholar] [CrossRef]
- Ratnayake, S.S.; Khan, A.; Reid, M.; Dharmasena, P.B.; Hunter, D.; Kumar, L.; Herath, K.; Kogo, B.; Kadupitiya, H.K.; Dammalage, T.; et al. Land Use-Based Participatory Assessment of Ecosystem Services for Ecological Restoration in Village Tank Cascade Systems of Sri Lanka. Sustainability 2022, 14, 10180. [Google Scholar] [CrossRef]
- Perera, P.C.D.; Dahanayake, N. Review of Major Abundant Weeds of Cultivation in Sri Lanka. Int. J. Sci. Res. Publ. 2015, 5, 2250–3153. [Google Scholar]
- Mzuza, M.K.; Chapola, L.; Kapute, F.; Chikopa, I.; Gondwe, J. Analysis of the Impact of Aquatic Weeds in the Shire River on Generation of Electricity in Malawi: A Case of Nkula Falls Hydro-Electric Power Station in Mwanza District, Southern Malawi. Int. J. Geosci. 2015, 6, 636–643. [Google Scholar] [CrossRef]
- Howard, G.W.; Harley, K.L.S. How do Floating Aquatic Weeds Affect Wetland Conservation and Development? How Can These Effects be Minimised? Wetl. Ecol. Manag. 1997, 5, 215–225. [Google Scholar] [CrossRef]
- Samiei, J.; Mobaraki, R. Investigation of Effects of Control Method on Immersed Aquatic Weeds. Medbiotech J. 2019, 3, 88–92. [Google Scholar] [CrossRef]
- Honlah, E.; Yao Segbefia, A.; Odame Appiah, D.; Mensah, M.; Atakora, P.O. Effects of Water Hyacinth Invasion on the Health of the Communities, and the Education of Children along River Tano and Abby-Tano Lagoon in Ghana. Cogent Soc. Sci. 2019, 5, 1619652. [Google Scholar] [CrossRef]
- Ekwealor, K.U.; Echereme, C.B.; Ofobeze, T.N.; Okereke, C.N. Economic Importance of Weeds: A Review. Asian Plant Res. J. 2019, December, 1–11. [Google Scholar] [CrossRef]
- Pretty, J.N.; Mason, C.F.; Nedwell, D.B.; Hine, R.E.; Leaf, S.; Dils, R. Environmental Costs of Freshwater Eutrophication in England and Wales. Environ. Sci. Technol. 2003, 37, 201–208. [Google Scholar] [CrossRef]
- Sousa, W.T.Z. Hydrilla verticillata (Hydrocharitaceae), a Recent Invader Threatening Brazil’s Freshwater Environments: A Review of the Extent of the Problem. Hydrobiologia 2011, 669, 1–20. [Google Scholar] [CrossRef]
- Madsen, J.D. Methods for Management of Nonindigenous Aquatic Plants. In Assessment and Management of Plant Invasions; Luken, J.O., Thieret, J.W., Eds.; Springer Series on Environmental Management; Springer: New York, NY, USA, 1997; p. 27. [Google Scholar] [CrossRef]
- Hill, M.P.; Coetzee, J. The Biological Control of Aquatic Weeds in South Africa: Current Status and Future Challenges. Bothalia 2017, 47, 1–12. [Google Scholar] [CrossRef]
- Thomaz, S.M.; Pagioro, T.A.; Bini, L.M.; Murphy, K.J. Effect of Reservoir Drawdown on Biomass of Three Species of Aquatic Macrophytes in a Large Sub-Tropical Reservoir (Itaipu, Brazil). Hydrobiologia 2006, 570, 53–59. [Google Scholar] [CrossRef]
- Cilliers, C.J.; Hill, M.P.; Ogwang, J.A.; Ajuonu, O. Aquatic Weeds in Africa and Their Control. In Biological Control in IPM Systems in Africa; Neuenschwander, P., Borgemeister, C., Langewald, J., Eds.; CABI Publishing: Wallingford, UK, 2003; Volume 1991, pp. 161–178. [Google Scholar] [CrossRef]
- Goktogan, A.H.; Sukkarieh, S.; Bryson, M.; Randle, J.; Lupton, T.; Hung, C. A Rotary-wing Unmanned Air Vehicle for Aquatic Weed Surveillance and Management. J. Intell. Robot. Syst. 2010, 57, 467–484. [Google Scholar] [CrossRef]
- Dayan, F.E.; Netherland, M.D. Hydrilla, the Perfect Aquatic Weed, Becomes More Noxious Than Ever. Outlooks Pest Manag. 2005, 16, 277–282. [Google Scholar] [CrossRef]
- Mitchell, D.S. African Aquatic Weeds and Their Management. In The Ecology and Management of African Wetland Vegetation; Denny, P., Ed.; Springer: Dordrecht, The Netherlands, 1985; Volume 6, pp. 177–202. [Google Scholar] [CrossRef]
- Hill, M.P.; Coetzee, J.A.; Ueckermann, C. Toxic Effect of Herbicides Used for Water Hyacinth Control on Two Insects Released for Its Biological Control in South Africa. Biocontrol. Sci. Technol. 2012, 22, 1321–1333. [Google Scholar] [CrossRef]
- Lovell, S.J.; Stone, S.F.; Fernandez, L. The Economic Impacts of Aquatic Invasive Species: A Review of the Literature. Agric. Resour. Econ. Rev. 2006, 35, 195–208. [Google Scholar] [CrossRef]
- Coetzee, J.A.; Byrne, M.J.; Hill, M.P. Impact of Nutrients and Herbivory by Eccritotarsus catarinensis on the Biological Control of Water Hyacinth, Eichhornia crassipes. Aquat. Bot. 2007, 86, 179–186. [Google Scholar] [CrossRef]
- Barreto, R.; Charudattan, R.; Pomella, A.; Hanada, R. Biological Control of Neotropical Aquatic Weeds with Fungi. Crop Prot. 2000, 19, 697–703. [Google Scholar] [CrossRef]
- Purcell, M.; Harms, N.; Grodowitz, M.; Zhang, J.; Ding, J.; Wheeler, G.; Zonneveld, R.; de Chenon, R.D. Exploration for Candidate Biological Control Agents of the Submerged Aquatic Weed Hydrilla verticillata, in Asia and Australia 1996–2013. BioControl 2019, 64, 233–247. [Google Scholar] [CrossRef]
- Avault, J.W. Preliminary studies with grass carp for aquatic weed control. Progress. Fish-Cult. 1965, 27, 207–209. [Google Scholar] [CrossRef]
- Fu, Y.; Bhadha, J.H.; Rott, P.; Beuzelin, J.M.; Kanissery, R. Investigating the Use of Aquatic Weeds as Biopesticides towards Promoting Sustainable Agriculture. PLoS ONE 2020, 15, e0237258. [Google Scholar] [CrossRef] [PubMed]
- Tate, R.L.; Riemer, D.N. Aquatic Weed Biomass Disposal: Effect on Soil Organic Matter. J. Environ. Qual. 1988, 17, 163–168. [Google Scholar] [CrossRef]
- Nawaj Alam, S.; Singh, B.; Guldhe, A. Aquatic Weed as a Biorefinery Resource for Biofuels and Value-Added Products: Challenges and Recent Advancements. Clean. Eng. Technol. 2021, 4, 100235. [Google Scholar] [CrossRef]
- Bote, M.A.; Naik, V.R.; Jagdeeshgouda, K.B. Production of Biogas with Aquatic Weed Water Hyacinth and Development of Briquette Making Machine. Mater. Sci. Energy Technol. 2020, 3, 64–71. [Google Scholar] [CrossRef]
- Brouwer, P.; Schluepmann, H.; Nierop, K.G.; Elderson, J.; Bijl, P.K.; van der Meer, I.; de Visser, W.; Reichart, G.J.; Smeekens, S.; van der Werf, A. Growing Azolla to Produce Sustainable Protein Feed: The Effect of Differing Species and CO2 Concentrations on Biomass Productivity and Chemical Composition. J. Sci. Food Agric. 2018, 98, 4759–4768. [Google Scholar] [CrossRef]
- Das, M.; Rahim, F.; Hossain, M. Evaluation of Fresh Azolla pinnata as a Low-Cost Supplemental Feed for Thai Silver Barb Barbonymus gonionotus. Fishes 2018, 3, 15. [Google Scholar] [CrossRef]
- Aimvijarn, P.; Rodboon, T.; Payuhakrit, W.; Suwannalert, P. Nymphaea pubescens Induces Apoptosis, Suppresses Cellular Oxidants-Related Cell Invasion in B16 Melanoma Cells. Pharm. Sci. 2018, 24, 199–206. [Google Scholar] [CrossRef]
- Jafari, N. Ecological and Socio-Economic Utilization of Water Hyacinth (Eichhornia crassipes Mart Solms). J. Appl. Sci. Environ. Manag. 2010, 14, 43–49. [Google Scholar] [CrossRef]
- Wasagu, R.S.; Lawal, M.; Shehu, S.; Alfa, H.H.; Muhammad, C. Nutritive Values, Mineral and Antioxidant Properties Of Pistia stratiotes (Water lettuce). Niger. J. Basic Appl. Sci. 2014, 21, 253. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, M.; Guo, M. Research Advances in Traditional and Modern Use of Nelumbo nucifera : Phytochemicals, Health Promoting Activities and Beyond. Crit. Rev. Food Sci. Nutr. 2019, 59 (Suppl. 1), S189–S209. [Google Scholar] [CrossRef] [PubMed]
- Dhadse, S.; Alam, S.N.; Mallikarjuna Rao, M. Development of Nutrient Rich Biofertilizer by Co-vermistabilization of Aquatic Weeds Using Herbal Pharmaceutical Wastewater along with Sediment of Lake. Bioresour. Technol. Rep. 2021, 13, 100633. [Google Scholar] [CrossRef]
- Senarathne, S.H.S.; Dayananda, H.N.; Atapattu, A.A.A.J.; Raveendra, S.A.S.T. Feasibility of Using Problematic Aquatic Weeds in Productive Manner by Generating Vermicompost in Coconut Triangle Area of Sri Lanka. CORD 2017, 33, 15. [Google Scholar] [CrossRef]
- Larney, F.J.; Sullivan, D.M.; Buckley, K.E.; Eghball, B. The Role of Composting in Recycling Manure Nutrients. Can. J. Soil Sci. 2006, 86, 597–611. [Google Scholar] [CrossRef]
- Gusain, R.; Pandey, B.; Suthar, S. Composting as a Sustainable Option for Managing Biomass of Aquatic Weed Pistia: A Biological Hazard to Aquatic System. J. Clean. Prod. 2018, 177, 803–812. [Google Scholar] [CrossRef]
- Suthar, S.; Pandey, B.; Gusain, R.; Gaur, R.Z.; Kumar, K. Nutrient Changes and Biodynamics of Eisenia fetida during Vermicomposting of Water Lettuce (Pistia sp.) Biomass: A Noxious Weed of Aquatic System. Environ. Sci. Pollut. Res. 2017, 24, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Busato, J.G.; Lima, L.S.; Aguiar, N.O.; Canellas, L.P.; Olivares, F.L. Changes in Labile Phosphorus Forms during Maturation of Vermicompost Enriched with Phosphorus-solubilizing and Diazotrophic Bacteria. Bioresour. Technol. 2012, 110, 390–395. [Google Scholar] [CrossRef]
- Abhayawardhana, M.L.D.D.; Bandara, N.J.G.J.; Rupasinge, S.K.L.S. Removal of Heavy Metals and Nutrients from Municipal Wastewater using Salvinia molesta and Lemna gibba. J. Trop. For. Environ. 2019, 9, 65–77. [Google Scholar] [CrossRef]
- Brinton, W.F. Compost Quality Standards and Guidelines. 2000, p. 42. Available online: https://compost.css.cornell.edu/Brinton.pdf (accessed on 9 January 2023).
- Mesa, F.; Torres, J.; Sierra, O.; Escobedo, F.J. Enhanced Production of Compost from Andean Wetland Biomass Using a Bioreactor and Photovoltaic System. Biomass Bioenergy 2017, 106, 21–28. [Google Scholar] [CrossRef]
- SLS 1704:2021; Specification for Compost from Municipal Solid Waste and Agricultural Waste. Sri Lanka Standards Institute: Colombo, Sri Lanka, 2021. Available online: https://mfa.gov.lk/wp-content/uploads/2021/06/sls-1704_2021.pdf (accessed on 9 January 2023).
- Amarasinghe, S.R. Use of Invasive Water Hyacinth for Composting of Ordinary Leaf Litter. Sri Lankan J. Agric. Ecosyst. 2021, 3, 5. [Google Scholar] [CrossRef]
- Singh, J.; Kalamdhad, A.S. Reduction of Heavy Metals during Composting—A Review. Int. J. Environ. Prot. 2012, 2, 36–43. [Google Scholar]
- Milojkovic, J.V.; Stojanovic, M.D.; Mihajlovic, M.L.; Lopicic, Z.R.; Petrovic, M.S.; Sostaric, T.D.; Ristic, M.D. Compost of Aquatic Weed Myriophyllum spicatum as Low-Cost Biosorbent for Selected Heavy Metal Ions. Water Air Soil Pollut. 2014, 225, 1927. [Google Scholar] [CrossRef]
- Siles-Castellano, A.B.; Lopez, M.J.; Lopez-Gonzalez, J.A.; Suarez-Estrella, F.; Jurado, M.M.; Estrella-Gonzalez, M.J.; Moreno, J. Comparative Analysis of Phytotoxicity and Compost Quality in Industrial Composting Facilities Processing Different Organic Wastes. J. Clean. Prod. 2020, 252, 119820. [Google Scholar] [CrossRef]
- Ahmed, K.; Saqib, A.I.; Naseem, A.R.; Qadir, G.; Nawaz, M.Q.; Khalid, M.; Warraich, I.A.; Arif, M. Use of Hyacinth Compost in Salt-Affected Soils. Pak. J. Agric. Res. 2020, 33, 274. [Google Scholar] [CrossRef]
- Misra, R.V.; Roy, R.N.; Hiraoka, H. On-Farm Composting Methods; Land and Water Discussion Paper; UN-FAO: Rome, Italy, 2003; Volume 2, p. 51. [Google Scholar]
- Narayan, S.; Nabi, A.; Hussain, K.; Khan, F. Practical Aspects of Utilizing Aquatic Weeds in Compost Preparation. March 2017. Available online: https://www.researchgate.net/profile/Dr-Khan-11/publication/315100323_Practical_aspects_of_utilizing_aquatic_weeds_in_compost_preparation/links/58ca44eeaca27286b3b19644/Practical-aspects-of-utilizing-aquatic-weeds-in-compost-preparation.pdf (accessed on 9 January 2023). [CrossRef]
- Jayapal, A.; Mini, V.; Resmi, A.R.; Lovely, B. Composting Limnocharis flava Buchenau: A Comparative Analysis. J. Krishi Vigyan 2021, 10, 28–32. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, S.; Ahmed, N.; Nabi, S.U.; Singh, D.B.; Akbar, S.A. Managing Problematic Aquatic Macrophytes through Vermitechnology—Composting and Pelleting. Waste Biomass Valorization 2021, 12, 5561–5571. [Google Scholar] [CrossRef]
- Nsenga Kumwimba, M.; Dzakpasu, M.; Li, X. Potential of Invasive Watermilfoil (Myriophyllum spp.) to Remediate Eutrophic Waterbodies with Organic and Inorganic Pollutants. J. Environ. Manag. 2020, 270, 110919. [Google Scholar] [CrossRef]
- Sakthika, T.; Sornalaksmi, V. Nutrients Analysis of Vermicompost of Water Hyacinth Supplemented with Probiotics. Acta Sci. Agric. 2019, 3, 10–13. [Google Scholar] [CrossRef]
- Hussain, N.; Abbasi, T.; Abbasi, S.A. Vermiremediation of an Invasive and Pernicious Weed Salvinia (Salvinia molesta). Ecol. Eng. 2016, 91, 432–440. [Google Scholar] [CrossRef]
- Martinez-Nieto, P.; Bernal-Castillo, J.; Calixto-Díaz, M.; Del Basto-Riaño, M.A. Chaparro-Rico, B. Biofertilizers and Composting Accelerators of Polluting Macrophytes of a Colombian Lake. J. Soil Sci. Plant Nutr. 2011, 11, 47–61. [Google Scholar] [CrossRef]
- Dorahy, C.G.; Pirie, A.D.; McMaster, I.; Muirhead, L.; Pengelly, P.; Chan, K.Y.; Jackson, M.; Barchia, I.M. Environmental Risk Assessment of Compost Prepared from Salvinia, Egeria densa, and Alligator Weed. J. Environ. Qual. 2009, 38, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Petric, I.; Selimbasic, V. Composting of Poultry Manure and Wheat Straw in a Closed Reactor: Optimum Mixture Ratio and Evolution of Parameters. Biodegradation 2008, 19, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Alkarimiah, R. Effects of Technical Factors towards Achieving the Thermophilic Temperature Stage in Composting Process and the Benefits of Closed Rector System Compared to Conventional Method—A Mini Review. Appl. Ecol. Environ. Res. 2019, 17, 9979–9996. [Google Scholar] [CrossRef]
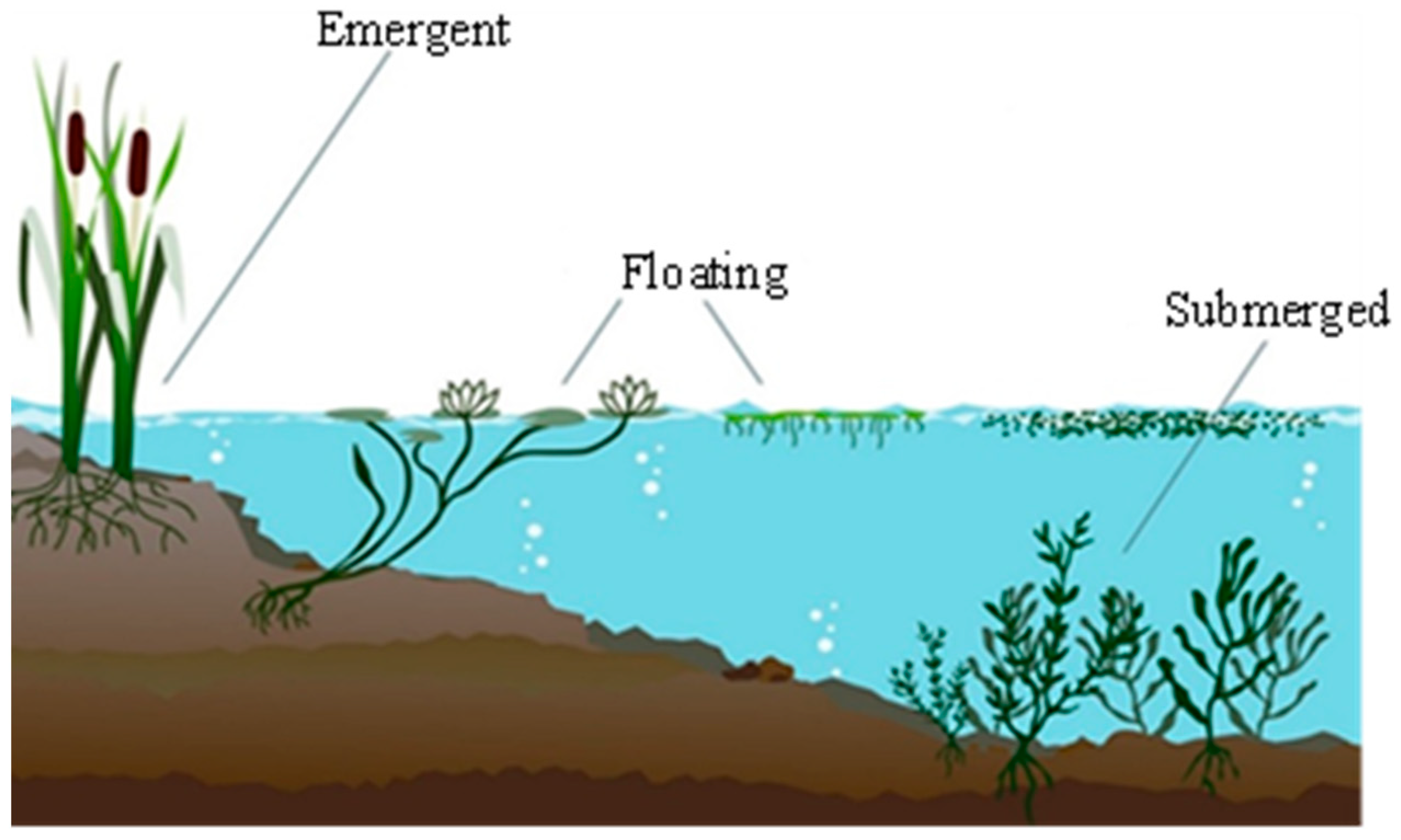
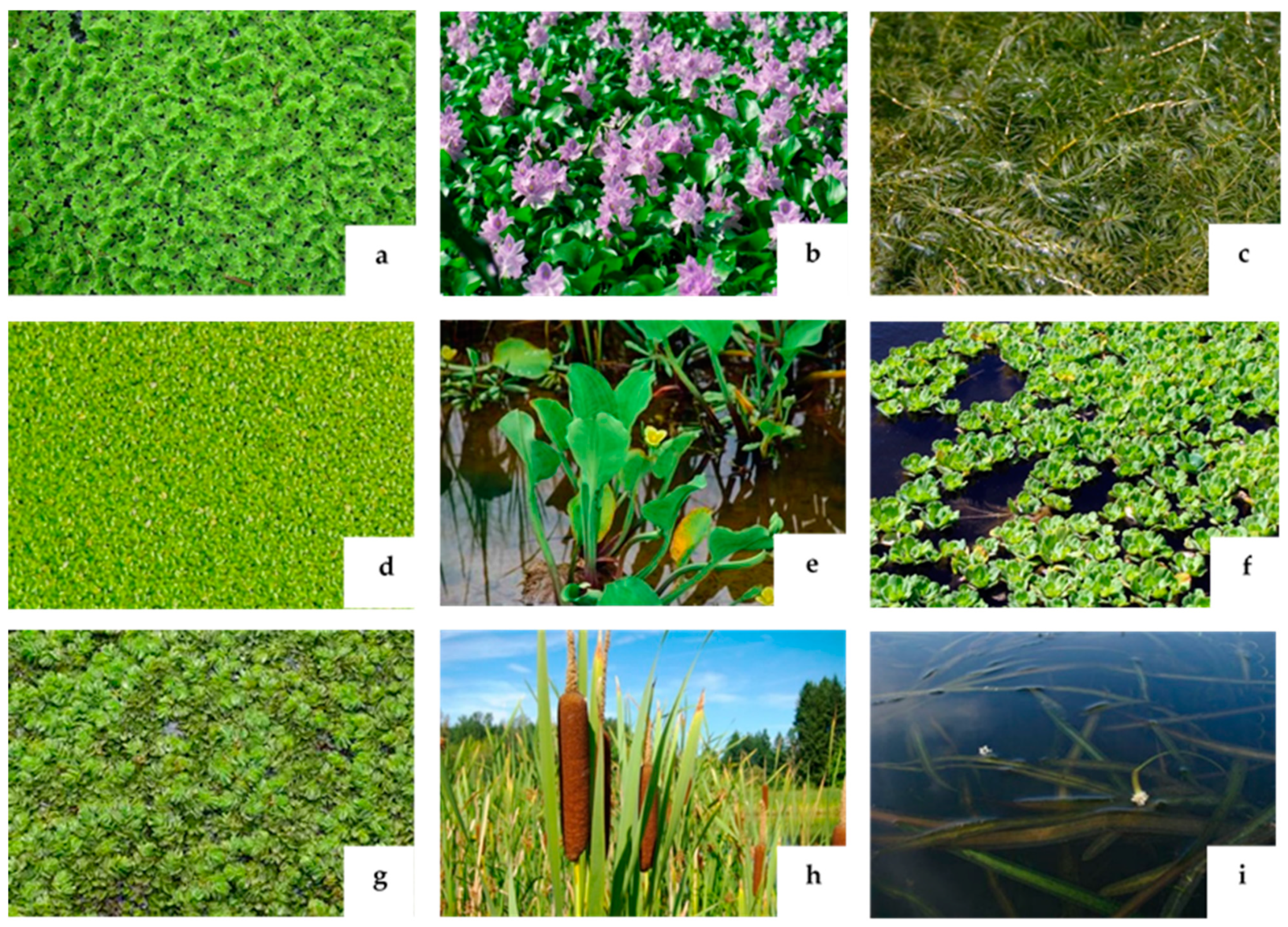

| Plant Type | Botanical Name | Family |
|---|---|---|
| Floating | Azolla spp. | Azollaceae |
| Eichhornia crassipes | Pontederiaceae | |
| Hydrilla verticillata | Hydrocharitaceae | |
| Lemna spp. | Lemnaceae | |
| Pistia stratiotes | Araceae | |
| Salvinia molesta | Salviniaceae | |
| Submersed | Cabomba caroliniana | Cabombaceae |
| Ceratophyllum demersum | Ceratophyllaceae | |
| Chara spp. | Characeae | |
| Crassula helmsii | Crassulaceae | |
| Egeria spp. | Hydrocharitaceae | |
| Lagarosiphon major | Hydrocharitaceae | |
| Nitella spp. | Characeae | |
| Potamogeton spp. | Potamogetonaceae | |
| Utricularia spp. | Lentibulariaceae | |
| Vallisneria spp. | Hydrocharitaceae | |
| Emersed | Brachiaria spp. | Poaceae |
| Ipomoea spp. | Convolvulaceae | |
| Limnocharis flava | Limnocharitaceae | |
| Ludwigia spp. | Onagraceae | |
| Lythrum salicaria | Lythraceae | |
| Monochoria spp. | Pontederiaceae | |
| Myriophyllum spp. | Haloragaceae | |
| Nelumbo nucifera | Nymphaeaceae | |
| Nuphar luteum (Nyphaea spp.) | Nymphaeaceae | |
| Nymphaea stellata | Nymphaeaceae | |
| Phragmites karka | Poaceae | |
| Polygonum spp. | Polygonaceae | |
| Sagittaria spp. | Alismataceae | |
| Scirpus spp. | Cyperaceae | |
| Spartina spp. | Poaceae | |
| Sphenoclea zeylanica | Sphenocleaceae | |
| Typha spp. | Typhaceae | |
| Vossia cuspidata | Poaceae | |
| Wetland tree | Melaleuca quinquenervia | Myrtaceae |
| Mat-forming | Alternanthera philoxeroides | Amaranthaceae |
| Potentially Toxic Metals (mg/kg Dry Basis) | Different Compost Mixing Methods | ||||||
|---|---|---|---|---|---|---|---|
| 50% WH + 25% DLL + 25% CM | 50% WH + 45% DLL + 5% WA | 50% WH + 45% DLL + 5% ERP | 50% WH + 50% DLL | 50% WH + 25% DLL + 5% ERP + 5% WA + 15% SLP | 50% WH + 25% DLL + 15% CM + 5% ERP + 5% WA | 100% WH | |
| Cu | 18.5 ± 0.14 | 13.83 ± 0.18 | 8.28 ± 0.08 | 6.44 ± 0.14 | 5.71 ± 0.08 | 17.24 ± 0.38 | 14.6 ± 0.0 |
| Cd | - | - | - | - | - | - | - |
| Pb | 19.59 ± 0.93 | 6.74 ± 0.26 | 10.58 ± 0.30 | 5.78 ± 0.51 | 5.10 ± 0.48 | 18.34 ± 0.13 | - |
| Zn | 25.16 ± 0.16 | 31.43 ± 0.70 | 5.77 ± 0.02 | 15.97 ± 0.25 | 26.51 ± 0.32 | 21.93 ± 0.19 | 32.47 ± 0.29 |
| Ni | - | - | - | - | - | - | - |
| As | 1.24 ± 0.03 | 0.28 ± 0.01 | 1.0 ± 0.05 | 0.28 ± 0.02 | 0.21 ± 0.03 | 1.35 ± 0.04 | 0.79 ± 0.01 |
| Organic Waste Category | Main Ingredients | pH | Electrical Conductivity (dS m−1) |
|---|---|---|---|
| Vegetal Residue | Cucumber and zucchini crop residues | 9.18 | 8.48 |
| Cucumber and zucchini crop residues | 8.08 | 17.36 | |
| Pepper crop residues | 9.68 | 9.97 | |
| Municipal Solid Waste | 8.66 | 4.97 | |
| Different sources | 7.50 | 5.58 | |
| 6.00 | 10.29 | ||
| Agri-food Waste | Citric sludge and palm tree pruning (1:3 v/v) | 6.64 | 7.24 |
| Cull tomatoes and tomato plant (stalks and leaves) | 7.83 | 5.1 | |
| Citric sludge, pig slurry, and pruning wastes (mainly palm tree) (3:1:1.5 v/v) | 6.67 | 2.72 | |
| Water hyacinth | 50% water hyacinth, 25% cattle manure, 25% dry leaf litter | 8.50 | 4.78 |
| 50% water hyacinth, 45% cattle manure, 5% Eppawala rock phosphate | 7.30 | 1.59 | |
| 50% water hyacinth, 25% cattle manure, 15% dry leaf litter, 5% Eppawala rock phosphate, 5% wood ash | 7.15 | 3.49 | |
| 100% water hyacinth | 7.60 | 5.28 | |
| Requirement set by Sri Lankan Standards Institution | 6.5–8.5 | 4.0 | |
| Treatment | Rice Yield (t ha−1) | Wheat Yield (t ha−1) |
|---|---|---|
| Control (no fertilizer application) | 1.85 | 1.52 |
| Application of 100% Gypsum requirement | 3.64 | 3.53 |
| Application of 50% Gypsum requirement with 10 t ha−1 Eichhornia crassipes compost | 3.71 | 3.58 |
| Application of 10 t ha−1 Eichhornia crassipes compost | 2.44 | 2.68 |
| Name of the Aquatic Weed | Reference | Remarks |
|---|---|---|
| Phragmites australis, Typha angustata, Azolla sp., Nymphoides peltatum, Nelumbo nucifera, Nymphaea sp., and Cerutophyllum demersum, Myriophyllum spicatum | [74] | Production of Vermipellet is more effective than the production of vermicomposting from these mentioned aquatic weeds due to less disease transmission potential, lower heavy metal concentrations, minimum weed growth ability, enhanced dispersal nature, high C/N ratio, and maximum nutrient composition in vermipellets. |
| Ceratophyllum demursum, Nelumbo nucifera, Ludwigia palustris | [56] | Even though the parameters of compost produced with these weeds are varied, they are generally recognized as good sources for nutrient-rich vermicomposting. |
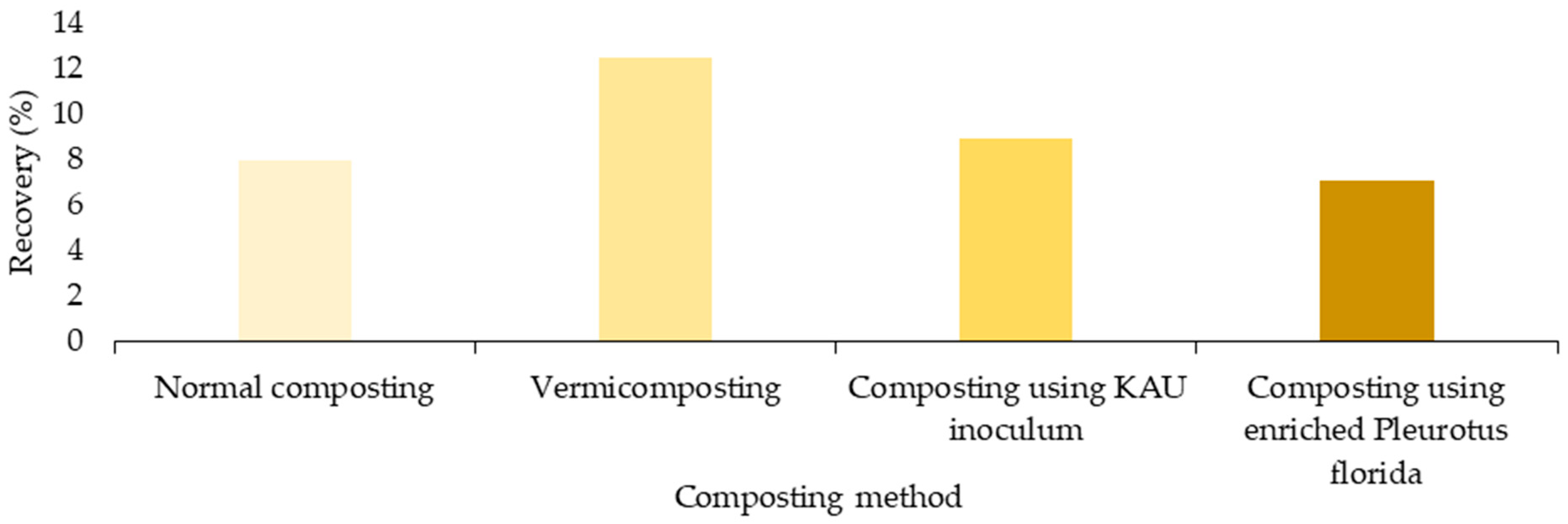
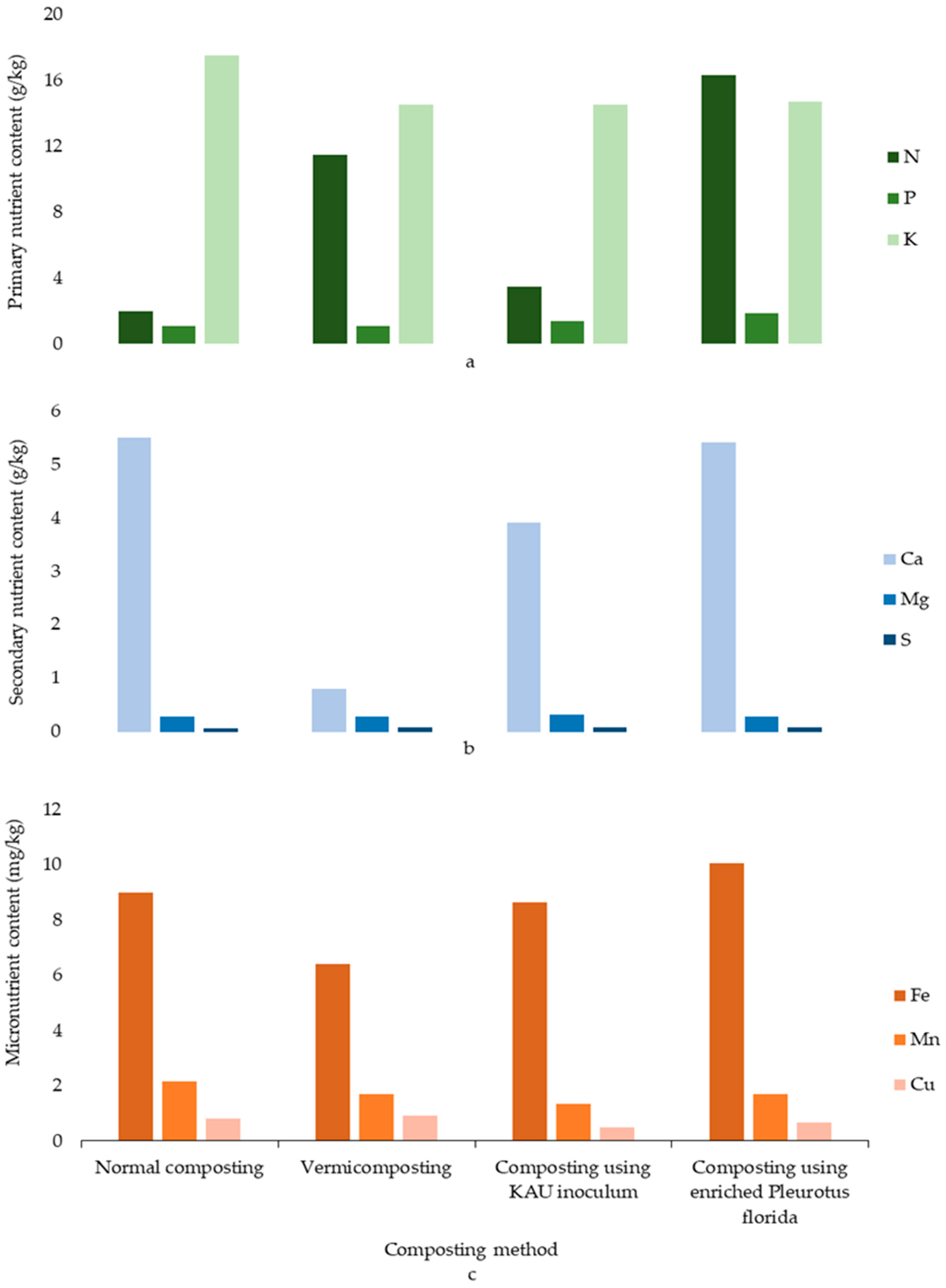
| Limnocharis flava | [73] | According to this research, the potential of Limnocharis flava for vermicomposting compared to normal composting and KAU inoculum composting was confirmed with the highest recovery percentage (the quantity of compost given by the unit amount of biomass) within 60–70 days. |
| Myriophyllum spp. | [68,75] | Compost made by Myriophyllum spp. (M. spicatum) had significantly higher bio-sorption capacity and ability, and thereby can be used to purify heavy metals from waste. Furthermore, herbicide application is not advisable after applying compost produced with Myriophyllum spp. |
| Eicchornia crassipis | [76] | Eicchornia crassipis can be transferred into nutrient-rich vermicompost materials within 60 days with the help of probiotics Lactobacillus sporogens. |
| Hydrilla verticilata | [15] | Since the whole plant is decomposable, shredding is not a must, and it can easily supply enough moisture (more than 60%) and plant growth nutrients such as P, K, Mg, and Ca. |
| Lagenandra toxicaria | [57] | Vermicomposting with L. toxicaria gives a better-quality, nutrient-rich end product than normal composting techniques. Since the end product has 6.75 dS m−1 of electrical conductivity, 13.21% organic carbon, 3.61% P content, 5.03% K content, and 6.12% Ca content with good microbial activities, it can be an excellent organic fertilizer source for coconut. |
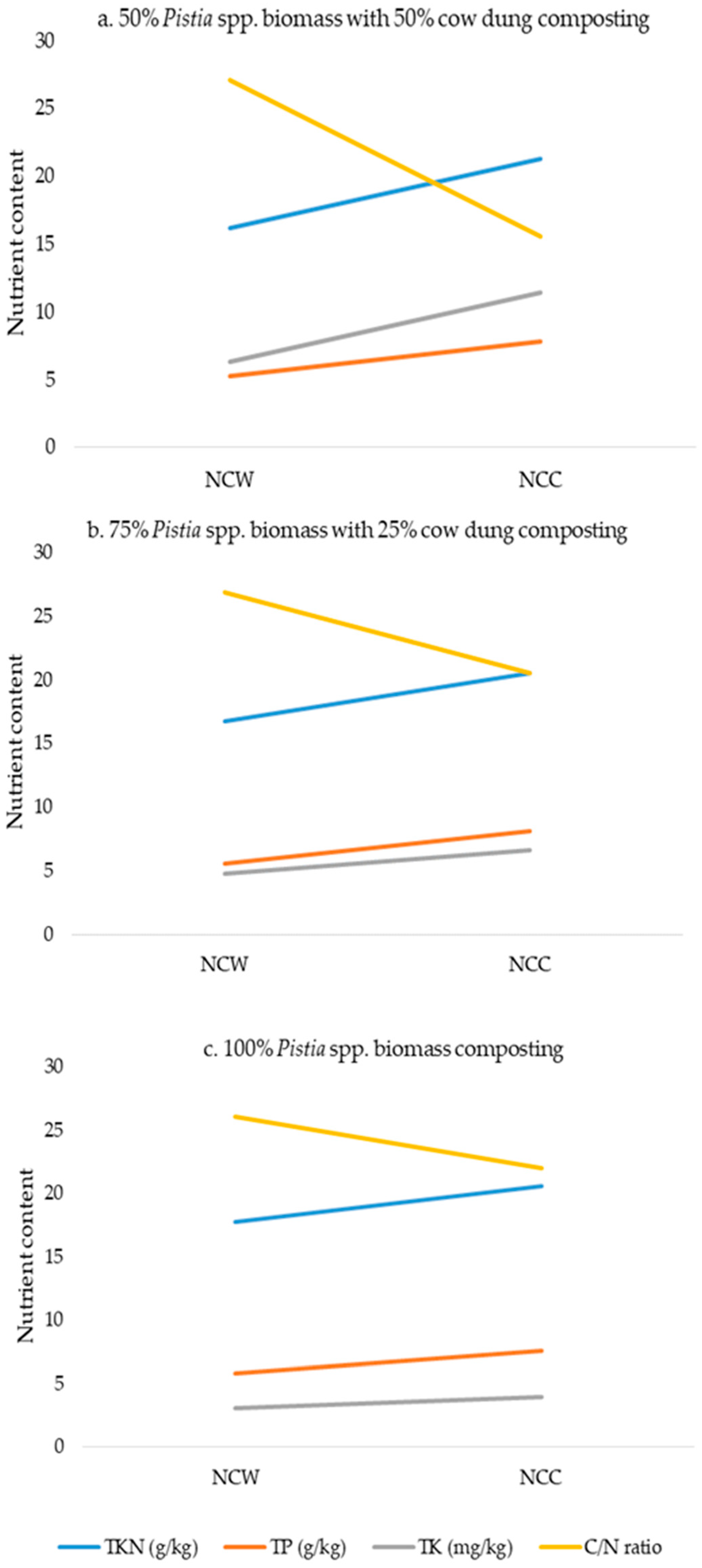
| Pistia spp. | [60] | After inoculating 60–80 % of cow dung and Eisenia fetida, Pistia spp. can be transferred into odor-free, nutrient-rich (N, P, K, Cu, Zn, Fe) vermicompost. |
| Azolla filiculoides and Typha latifolia | [64] | This research justified the suitability of compost produced from these plants for agronomic usage depending on its nutritional and physical properties. Here, an autonomous, self-powered fixed bed gasifier with a gyrating cylinder bioreactor was used to reduce the time of the composting process. |
| Salvinia molesta | [77] | S. molesta has a greater potential to be an excellent vermicomposting material resulting in an ideal reduction in C: N ratio, humification index, allelopathy, and other kinds of toxicity. |
| Egeria densa | [78] | Composting of Egeria densa can be induced by inoculating microbial bio-preparations with bacteria, fungi, and actinomycetes. |
| Alternanthera philoxeroides | [79] | Because of the higher survival rate of these seeds, the mixing should be done properly, and the whole mixture should be uniformly subjected to a temperature higher than 55 °C over three consecutive days to avoid spreading aquatic weeds on the terrestrial composing. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dissanayaka, D.M.N.S.; Udumann, S.S.; Dissanayake, D.K.R.P.L.; Nuwarapaksha, T.D.; Atapattu, A.J. Review on Aquatic Weeds as Potential Source for Compost Production to Meet Sustainable Plant Nutrient Management Needs. Waste 2023, 1, 264-280. https://doi.org/10.3390/waste1010017
Dissanayaka DMNS, Udumann SS, Dissanayake DKRPL, Nuwarapaksha TD, Atapattu AJ. Review on Aquatic Weeds as Potential Source for Compost Production to Meet Sustainable Plant Nutrient Management Needs. Waste. 2023; 1(1):264-280. https://doi.org/10.3390/waste1010017
Chicago/Turabian StyleDissanayaka, D. M. N. S., S. S. Udumann, D. K. R. P. L. Dissanayake, T. D. Nuwarapaksha, and Anjana J. Atapattu. 2023. "Review on Aquatic Weeds as Potential Source for Compost Production to Meet Sustainable Plant Nutrient Management Needs" Waste 1, no. 1: 264-280. https://doi.org/10.3390/waste1010017
APA StyleDissanayaka, D. M. N. S., Udumann, S. S., Dissanayake, D. K. R. P. L., Nuwarapaksha, T. D., & Atapattu, A. J. (2023). Review on Aquatic Weeds as Potential Source for Compost Production to Meet Sustainable Plant Nutrient Management Needs. Waste, 1(1), 264-280. https://doi.org/10.3390/waste1010017







