Geospatial Model Suggests Sterilizing Free-Roaming Domestic Cats Reduces Potential Risk of Toxoplasma gondii Infection
Simple Summary
Abstract
1. Introduction
1.1. Environmental Contamination
1.2. The Role of Domestic Cats
1.3. Mitigation Measures
1.4. TNR as Harm Reduction
2. Materials and Methods
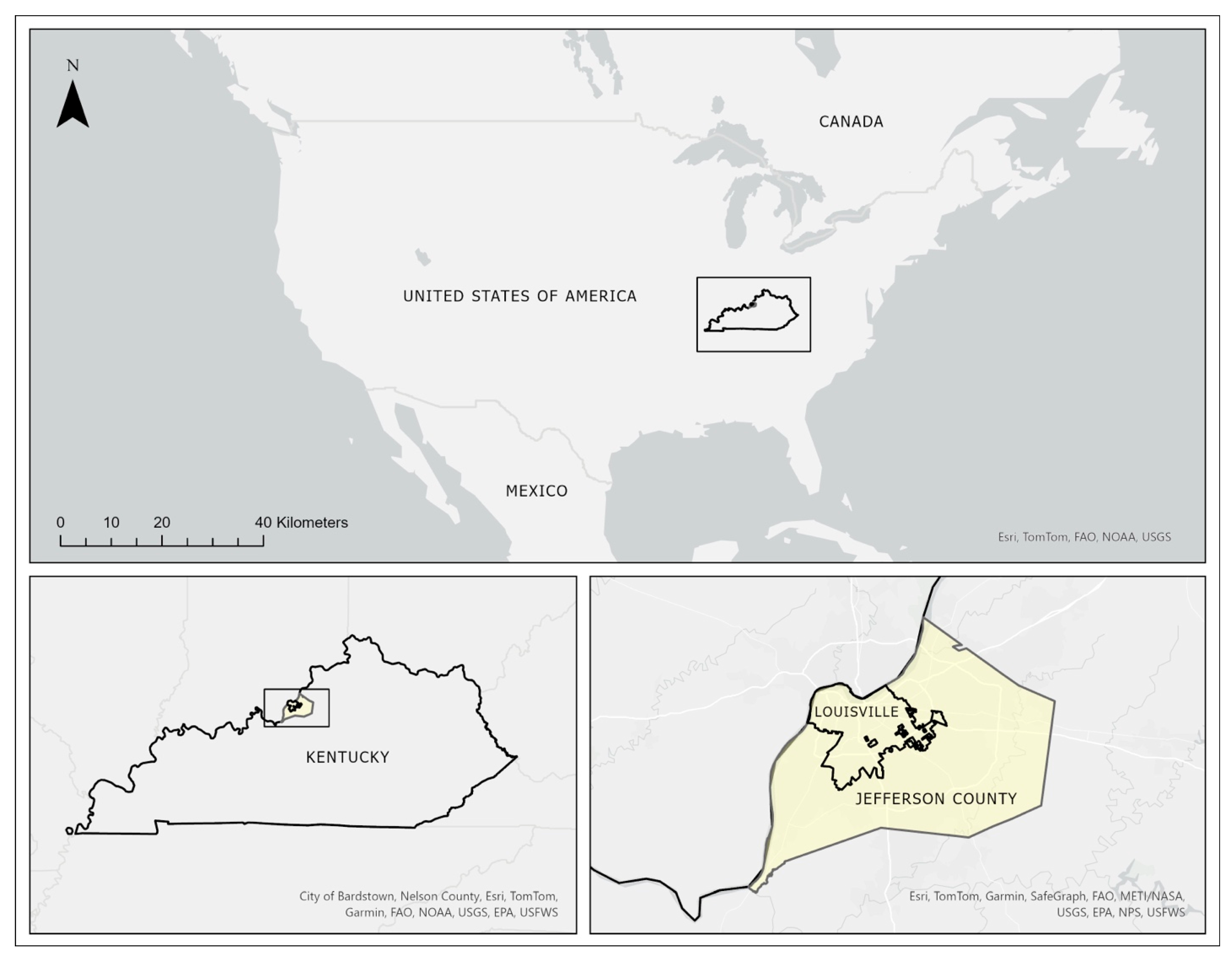
2.1. Study Area
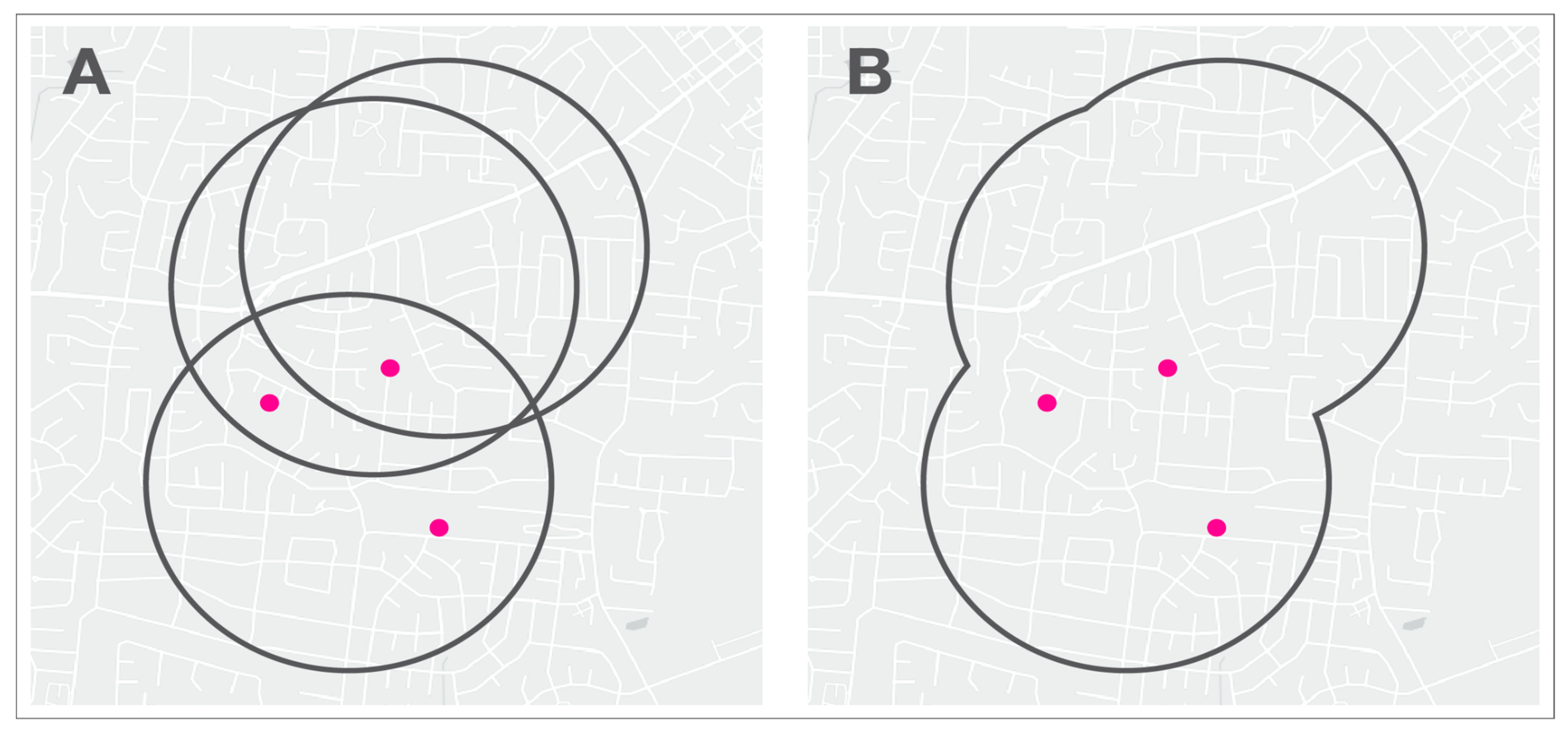
2.2. Home Range
2.3. Points of Interest
2.4. Cat Data
2.5. Spatial Analysis
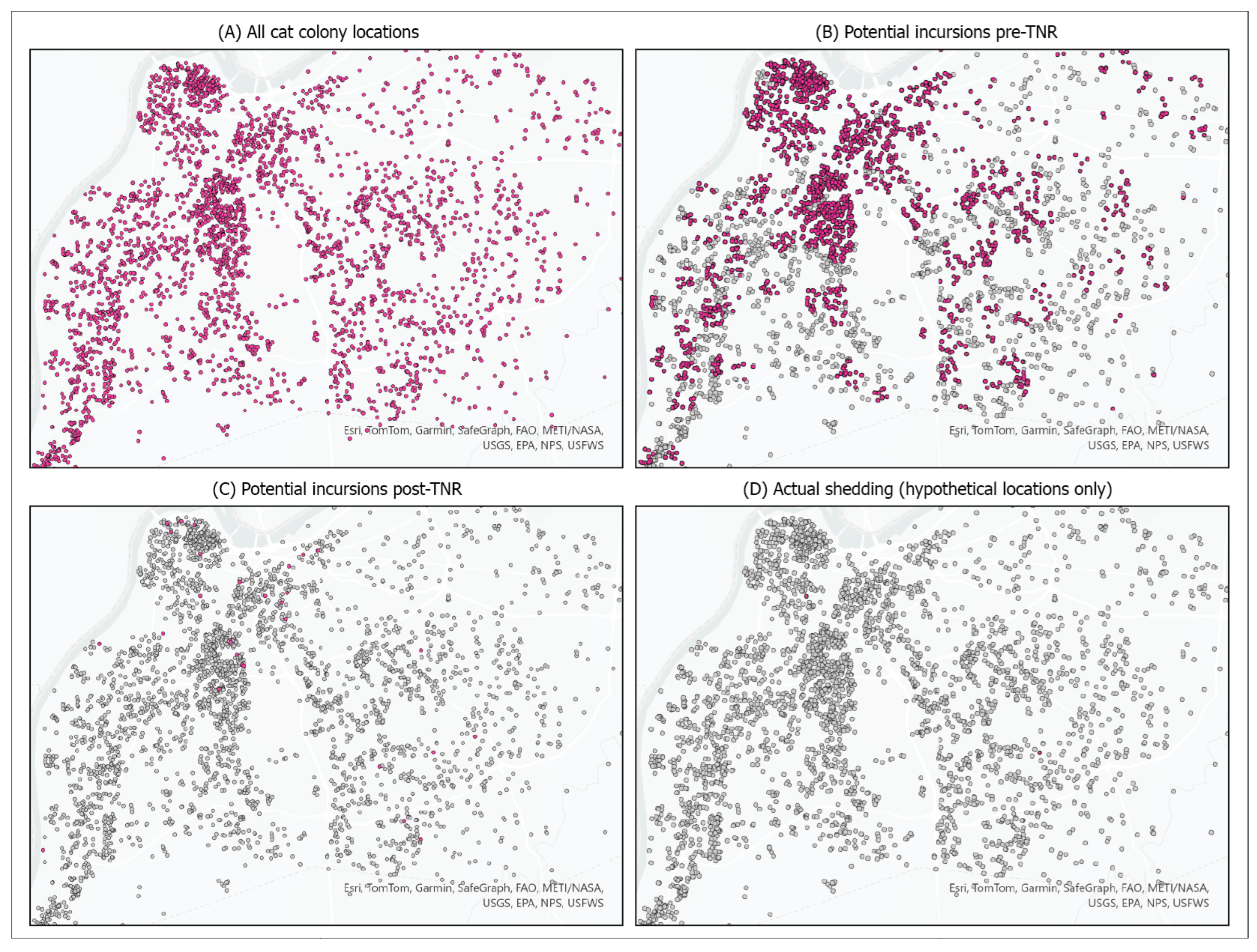
3. Results
4. Discussion
4.1. TNR Reduced Incursion Rates
4.2. The Impacts of Infection Risk and Shedding Prevalence
4.3. Additional Factors Leading to Reduced Infection Risk
4.4. TNR as Harm Reduction Strategy
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACA | Alley Cat Advocates |
| CDC | Centers for Disease Control and Prevention |
| LMAS | Louisville Metro Animal Services |
| MCP | Minimum convex polygon |
| NHANES | National Health and Nutrition Examination Survey |
| POI | Point of interest |
| TE | Toxoplasmic encephalitis |
| TNR | Trap-neuter-return |
References
- Dubey, J.P. Toxoplasmosis of Animals and Humans, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2022; ISBN 978-0-367-54312-9. [Google Scholar]
- Pal, M.; Berhanu, G.; Steinmetz, C.H.D.; Durglishvili, N. Toxoplasmosis: An Emerging and Re-Emerging Zoonosis of Global Public Health Concern. Am. J. Infect. Dis. Microbiol. 2021, 9, 32–38. [Google Scholar]
- Uttah, E.; Ogban, E.; Okonofua, C. Toxoplasmosis: A Global Infection, so Widespread, so Neglected. Int. J. Sci. Res. Publ. 2013, 3, 6. [Google Scholar]
- Molan, A.; Nosaka, K.; Hunter, M.; Wang, W. Global Status of Toxoplasma gondii Infection: Systematic Review and Prevalence Snapshots. Trop. Biomed. 2019, 36, 898–925. [Google Scholar]
- Jones, J.L.; Kruszon-Moran, D.; Rivera, H.N.; Price, C.; Wilkins, P.P. Toxoplasma gondii Seroprevalence in the United States 2009-2010 and Comparison with the Past Two Decades. Am. J. Trop. Med. Hyg. 2014, 90, 1135–1139. [Google Scholar] [CrossRef]
- Owusu-Dommey, A.; Pogreba-Brown, K.; Villa-Zapata, L. Seroprevalence of Toxoplasma gondii in the U.S.: Evidence from a Representative Cross-Sectional Survey. Parasitol. Int. 2020, 79, 102175. [Google Scholar] [CrossRef]
- Wiener, R.C.; Waters, C.; Bhandari, R. The Association of Toxoplasma gondii IgG and Cognitive Function Scores: NHANES 2013–2014. Parasitol. Int. 2020, 78, 102123. [Google Scholar] [CrossRef]
- Jones, J.L.; Kruszon-Moran, D.; Elder, S.; Rivera, H.N.; Press, C.; Montoya, J.G.; McQuillan, G.M. Toxoplasma gondii Infection in the United States, 2011–2014. Am. J. Trop. Med. Hyg. 2018, 98, 551–557. [Google Scholar] [CrossRef]
- CDC. CDC—Parasites—Neglected Parasitic Infections (NPIs) in the United States. Available online: https://www.cdc.gov/parasites/ (accessed on 7 February 2024).
- McCall, J. Public Health Surveillance and Reporting for Human Toxoplasmosis—Six States, 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 889–893. [Google Scholar] [CrossRef]
- Luft, B.J.; Hafner, R.; Korzun, A.H.; Leport, C.; Antoniskis, D.; Bosler, E.M.; Bourland, D.D.; Uttamchandani, R.; Fuhrer, J.; Jacobson, J.; et al. Toxoplasmic Encephalitis in Patients with the Acquired Immunodeficiency Syndrome. N. Engl. J. Med. 1993, 329, 995–1000. [Google Scholar] [CrossRef]
- Khan, A.; Su, C.; German, M.; Storch, G.A.; Clifford, D.B.; Sibley, L.D. Genotyping of Toxoplasma gondii Strains from Immunocompromised Patients Reveals High Prevalence of Type I Strains. J. Clin. Microbiol. 2005, 43, 5881–5887. [Google Scholar] [CrossRef]
- Kodym, P.; Malý, M.; Beran, O.; Jilich, D.; Rozsypal, H.; Machala, L.; Holub, M. Incidence, Immunological and Clinical Characteristics of Reactivation of Latent Toxoplasma gondii Infection in HIV-Infected Patients. Epidemiol. Infect. 2015, 143, 600–607. [Google Scholar] [CrossRef]
- Moncada, P.A.; Montoya, J.G. Toxoplasmosis in the Fetus and Newborn: An Update on Prevalence, Diagnosis and Treatment. Expert Rev. Anti-Infect. Ther. 2012, 10, 815–828. [Google Scholar] [CrossRef]
- Shapiro, K.; Bahia-Oliveira, L.; Dixon, B.; Dumètre, A.; de Wit, L.A.; VanWormer, E.; Villena, I. Environmental Transmission of Toxoplasma gondii: Oocysts in Water, Soil and Food. Food Waterborne Parasitol. 2019, 15, e00049. [Google Scholar] [CrossRef]
- Frenkel, J.K.; Ruiz, A.; Chinchilla, M. Soil Survival of Toxoplasma Oocysts in Kansas and Costa Rica. Am. J. Trop. Med. Hyg. 1975, 24, 439–443. [Google Scholar] [CrossRef]
- Dumètre, A.; Dardé, M.-L. How to Detect Toxoplasma gondii Oocysts in Environmental Samples? FEMS Microbiol. Rev. 2003, 27, 651–661. [Google Scholar] [CrossRef]
- Silva, A.L.P.; Lima, B.A.; Formiga, V.H.A.S.; Lima, E.F.; Silva Filho, G.M.; Silva, W.I.; Silva, J.O.; Alvares, F.B.V.; Vilela, V.L.R.; Feitosa, T.F. Survival and Viability of Toxoplasma gondii Oocysts under Natural Dry Season Conditions in the Brazilian Semi-Arid Region. Vet. Res. Commun. 2025, 49, 191. [Google Scholar] [CrossRef]
- Wallace, G.D. Intermediate and Transport Hosts in the Natural History of Toxoplasma gondii. Am. J. Trop. Med. Hyg. 1973, 22, 456–464. [Google Scholar] [CrossRef]
- Wallace, G.D. Isolation of Toxoplasma gondii from the Feces of Naturally Infected Cats. J. Infect. Dis. 1971, 124, 227–228. [Google Scholar] [CrossRef]
- Ruiz, A.; Frenkel, J.K. Intermediate and Transport Hosts of Toxoplasma gondii in Costa Rica. Am. J. Trop. Med. Hyg. 1980, 29, 1161–1166. [Google Scholar] [CrossRef]
- Etheredge, G.D.; Michael, G.; Muehlenbein, M.P.; Frenkel, J.K. The Roles of Cats and Dogs in the Transmission of Toxoplasma Infection in Kuna and Embera Children in Eastern Panama. Rev. Panam. Salud Publica 2004, 16, 176–186. [Google Scholar] [CrossRef][Green Version]
- Frenkel, J.K.; Parker, B.B. An Apparent Role of Dogs in the Transmission of Toxoplasma gondii: The Probable Importance of Xenosmophilia. Ann. N. Y. Acad. Sci. 1996, 791, 402–407. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Dubey, J.P.; Butler, J.M.; Blagburn, B.L. Mechanical Transmission of Toxoplasma gondii Oocysts by Dogs. Vet. Parasitol. 1997, 73, 27–33. [Google Scholar] [CrossRef]
- Schares, G.; Pantchev, N.; Barutzki, D.; Heydorn, A.O.; Bauer, C.; Conraths, F.J. Oocysts of Neospora caninum, Hammondia heydorni, Toxoplasma gondii and Hammondia hammondi in Faeces Collected from Dogs in Germany. Int. J. Parasitol. 2005, 35, 1525–1537. [Google Scholar] [CrossRef]
- de Wit, L.A.; Kilpatrick, A.M.; VanWormer, E.; Croll, D.A.; Tershy, B.R.; Kim, M.; Shapiro, K. Seasonal and Spatial Variation in Toxoplasma gondii Contamination in Soil in Urban Public Spaces in California, United States. Zoonoses Public Health 2020, 67, 70–78. [Google Scholar] [CrossRef]
- Cornelissen, J.B.W.J.; van der Giessen, J.W.B.; Takumi, K.; Teunis, P.F.M.; Wisselink, H.J. An Experimental Toxoplasma gondii Dose Response Challenge Model to Study Therapeutic or Vaccine Efficacy in Cats. PLoS ONE 2014, 9, e104740. [Google Scholar] [CrossRef]
- Poulle, M.-L.; Forin-Wiart, M.-A.; Josse-Dupuis, E.; Villena, I.; Aubert, D. Detection of Toxoplasma gondii DNA by qPCR in the Feces of a Cat That Recently Ingested Infected Prey Does Not Necessarily Imply Oocyst Shedding. Parasite 2016, 23, 29. [Google Scholar] [CrossRef]
- Taetzsch, S.J.; Bertke, A.S.; Gruszynski, K.R. Zoonotic Disease Transmission Associated with Feral Cats in a Metropolitan Area: A Geospatial Analysis. Zoonoses Public Health 2018, 65, 412–419. [Google Scholar] [CrossRef]
- Pacheco-Ortega, G.A.; Chan-Pérez, J.I.; Ortega-Pacheco, A.; Guzmán-Marín, E.; Edwards, M.; Brown, M.A.; Jiménez-Coello, M.; Hernández-Cortazar, I.B. Screening of Zoonotic Parasites in Playground Sandboxes of Public Parks from Subtropical Mexico. J. Parasitol. Res. 2019, 2019, e7409076. [Google Scholar] [CrossRef]
- Di Genova, B.M.; Wilson, S.K.; Dubey, J.P.; Knoll, L.J. Intestinal Delta-6-Desaturase Activity Determines Host Range for Toxoplasma Sexual Reproduction. PLOS Biol. 2019, 17, e3000364. [Google Scholar] [CrossRef]
- Marshall, P.A.; Hughes, J.M.; Williams, R.H.; Smith, J.E.; Murphy, R.G.; Hide, G. Detection of High Levels of Congenital Transmission of Toxoplasma gondii in Natural Urban Populations of Mus Domesticus. Parasitology 2004, 128, 39–42. [Google Scholar] [CrossRef]
- Hide, G. Role of Vertical Transmission of Toxoplasma gondii in Prevalence of Infection. Expert Rev. Anti-Infect. Ther. 2016, 14, 335–344. [Google Scholar] [CrossRef]
- Oksanen, A.; Åsbakk, K.; Prestrud, K.W.; Aars, J.; Derocher, A.E.; Tryland, M.; Wiig, Ø.; Dubey, J.P.; Sonne, C.; Dietz, R.; et al. Prevalence of Antibodies Against Toxoplasma gondii in Polar Bears (Ursus maritimus) From Svalbard and East Greenland. J. Parasitol. 2011, 95, 89–94. [Google Scholar] [CrossRef]
- Prestrud, K.W.; Dubey, J.P.; Åsbakk, K.; Fuglei, E.; Su, C. First Isolate of Toxoplasma gondii from Arctic Fox (Vulpes lagopus) from Svalbard. Vet. Parasitol. 2008, 151, 110–114. [Google Scholar] [CrossRef]
- Dubey, J.P.; Cerqueira-Cézar, C.K.; Murata, F.H.A.; Kwok, O.C.H.; Yang, Y.R.; Su, C. All about Toxoplasmosis in Cats: The Last Decade. Vet. Parasitol. 2020, 283, 109145. [Google Scholar] [CrossRef]
- Zhu, S.; Shapiro, K.; VanWormer, E. Dynamics and Epidemiology of Toxoplasma gondii Oocyst Shedding in Domestic and Wild Felids. Transbound. Emerg. Dis. 2021, 69, 2412–2423. [Google Scholar] [CrossRef]
- VanWormer, E.; Conrad, P.A.; Miller, M.A.; Melli, A.C.; Carpenter, T.E.; Mazet, J.A.K. Toxoplasma gondii, Source to Sea: Higher Contribution of Domestic Felids to Terrestrial Parasite Loading Despite Lower Infection Prevalence. EcoHealth 2013, 10, 277–289. [Google Scholar] [CrossRef]
- Lappin, M.R. Update on the Diagnosis and Management of Toxoplasma gondii Infection in Cats. Top. Companion Anim. Med. 2010, 25, 136–141. [Google Scholar] [CrossRef]
- Zulpo, D.L.; Sammi, A.S.; dos Santos, J.R.; Sasse, J.P.; Martins, T.A.; Minutti, A.F.; Cardim, S.T.; de Barros, L.D.; Navarro, I.T.; Garcia, J.L. Toxoplasma gondii: A Study of Oocyst Re-Shedding in Domestic Cats. Vet. Parasitol. 2018, 249, 17–20. [Google Scholar] [CrossRef]
- Ding, H.; Gao, Y.-M.; Deng, Y.; Lamberton, P.H.L.; Lu, D.-B. A Systematic Review and Meta-Analysis of the Seroprevalence of Toxoplasma gondii in Cats in Mainland China. Parasites Vectors 2017, 10, 27. [Google Scholar] [CrossRef]
- Gauss, C.B.L.; Almeria, S.; Ortuno, A.; Garcia, F.; Dubey, J.P. Seroprevalence of Toxoplasma gondii Antibodies in Domestic Cats from Barcelona, Spain. J. Parasitol. 2003, 89, 1067–1068. [Google Scholar] [CrossRef]
- Must, K.; Lassen, B.; Jokelainen, P. Seroprevalence of and Risk Factors for Toxoplasma gondii Infection in Cats in Estonia. Vector-Borne Zoonotic Dis. 2015, 15, 597–601. [Google Scholar] [CrossRef]
- Zhu, S.; Camp, L.; Patel, A.; VanWormer, E.; Shapiro, K. High Prevalence and Diversity of Toxoplasma gondii DNA in Feral Cat Feces from Coastal California. PLOS Negl. Trop. Dis. 2023, 17, e0011829. [Google Scholar] [CrossRef]
- Aguirre, A.A.; Longcore, T.; Barbieri, M.; Dabritz, H.; Hill, D.; Klein, P.N.; Lepczyk, C.; Lilly, E.L.; McLeod, R.; Milcarsky, J.; et al. The One Health Approach to Toxoplasmosis: Epidemiology, Control, and Prevention Strategies. EcoHealth 2019, 16, 378–390. [Google Scholar] [CrossRef]
- Gerhold, R.W.; Jessup, D.A. Zoonotic Diseases Associated with Free-Roaming Cats. Zoonoses Public Health 2012, 60, 189–195. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; Lohr, C.A.; Duffy, D.C. A Review of Cat Behavior in Relation to Disease Risk and Management Options. Appl. Anim. Behav. Sci. 2015, 173, 29–39. [Google Scholar] [CrossRef]
- Marra, P.P.; Santella, C. Cat Wars: The Devastating Consequences of a Cuddly Killer; Princeton University Press: Princeton, NJ, USA, 2016; ISBN 978-0-691-16741-1. [Google Scholar]
- Branco, P.P.; Hashem, M.J.; Ilagan, G. Relating to Invasive Species. 2022. Available online: https://www.capitol.hawaii.gov/session/archives/measure_indiv_Archives.aspx?billtype=HB&billnumber=1987&year=2022 (accessed on 16 August 2025).
- Wolf, P.J.; Weedon, G.R. An Inconvenient Truth: Targeted TNR Enjoys a Track Record Unmatched by Lethal Methods for Managing Free-Roaming Cats. J. Shelter Med. Community Anim. Health 2023, 2, 68. [Google Scholar] [CrossRef]
- Single, E. Defining Harm Reduction. Drug Alcohol Rev. 1995, 14, 287–290. [Google Scholar] [CrossRef]
- Reuter, P.; Caulkins, J.P. Redefining the Goals of National Drug Policy: Recommendations from a Working Group. Am. J. Public Health 1995, 85, 1059–1063. [Google Scholar] [CrossRef]
- Marlatt, G.A. Harm Reduction: Come as You Are. Addict. Behav. 1996, 21, 779–788. [Google Scholar] [CrossRef]
- APPA. 2021–2022 APPA National Pet Owners Survey; American Pet Products Association, Inc.: Stamford, CT, USA, 2022. [Google Scholar]
- Carme, B.; Demar, M.; Ajzenberg, D.; Dardé, M.L. Severe Acquired Toxoplasmosis Caused by Wild Cycle of Toxoplasma gondii, French Guiana. Emerg. Infect. Dis. J. 2009, 15, 656–658. [Google Scholar] [CrossRef]
- Aramini, J.J.; Stephen, C.; Dubey, J.P.; Engelstoft, C.; Schwantje, H.; Ribble, C.S. Potential Contamination of Drinking Water with Toxoplasma gondii Oocysts. Epidemiol. Infect. 1999, 122, 305–315. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; Longcore, T.; Rich, C. Misunderstanding the Free-Ranging Cat Issue: Response to Debrot et al. 2022. Conserv. Sci. Pract. 2022, 4, e12817. [Google Scholar] [CrossRef]
- Silva-Rodríguez, E.A.; Sieving, K.E. Influence of Care of Domestic Carnivores on Their Predation on Vertebrates. Conserv. Biol. 2011, 25, 808–815. [Google Scholar] [CrossRef]
- Cove, M.V.; Gardner, B.; Simons, T.R.; Kays, R.; O’Connell, A.F. Free-Ranging Domestic Cats (Felis catus) on Public Lands: Estimating Density, Activity, and Diet in the Florida Keys. Biol. Invasions 2018, 20, 333–344. [Google Scholar] [CrossRef]
- Simon, J.A.; Pradel, R.; Aubert, D.; Geers, R.; Villena, I.; Poulle, M.-L. A Multi-Event Capture-Recapture Analysis of Toxoplasma gondii Seroconversion Dynamics in Farm Cats. Parasites Vectors 2018, 11, 339. [Google Scholar] [CrossRef] [PubMed]
- Eppink, D.M.; Wisselink, H.J.; Krijger, I.M.; van der Giessen, J.W.B.; Swanenburg, M.; van Wagenberg, C.P.A.; van Asseldonk, M.A.P.M.; Bouwknegt, M. Effectiveness and Costs of Interventions to Reduce the Within-Farm Toxoplasma gondii Seroprevalence on Pig Farms in the Netherlands. Porc. Health Manag. 2021, 7, 44. [Google Scholar] [CrossRef]
- Spehar, D.D.; Wolf, P.J. Back to School: An Updated Evaluation of the Effectiveness of a Long-Term Trap-Neuter-Return Program on a University’s Free-Roaming Cat Population. Animals 2019, 9, 768. [Google Scholar] [CrossRef]
- Cafazzo, S.; Bonanni, R.; Natoli, E. Neutering Effects on Social Behaviour of Urban Unowned Free-Roaming Domestic Cats. Animals 2019, 9, 1105. [Google Scholar] [CrossRef]
- Ferreira, G.A.; Machado, J.C.; Nakano-Oliveira, E.; Andriolo, A.; Genaro, G. The Effect of Castration on Home Range Size and Activity Patterns of Domestic Cats Living in a Natural Area in a Protected Area on a Brazilian Island. Appl. Anim. Behav. Sci. 2020, 230, 105049. [Google Scholar] [CrossRef]
- USA Census Bureau. QuickFacts: Jefferson County, Kentucky. Available online: https://www.census.gov/quickfacts/fact/table/jeffersoncountykentucky/PST045222 (accessed on 22 July 2023).
- LMAS. Animal Ordinance–Louisville-Jefferson, KY. Available online: https://codelibrary.amlegal.com/codes/louisvillemetro/latest/loukymetro/0-0-0-7177 (accessed on 30 November 2024).
- Neal, S.M.; Wolf, P.J. A Cat Is a Cat: Attachment to Community Cats Transcends Ownership Status. J. Shelter Med. Community Anim. Health 2023, 2, 62. [Google Scholar] [CrossRef]
- Spehar, D.D.; Wolf, P.J. The Impact of Return-to-Field and Targeted Trap-Neuter-Return on Feline Intake and Euthanasia at a Municipal Animal Shelter in Jefferson County, Kentucky. Animals 2020, 10, 1395. [Google Scholar] [CrossRef]
- Turner, D.C. Social Organisation and Behavioural Ecology of Free-Ranging Domestic Cats. In The Domestic Cat: The Biology of its Behaviour; Turner, D.C., Bateson, P., Eds.; Cambridge University Press: Cambridge, UK, 2014; pp. 63–70. [Google Scholar]
- Horn, J.A.; Mateus-Pinilla, N.; Warner, R.E.; Heske, E.J. Home Range, Habitat Use, and Activity Patterns of Free-Roaming Domestic Cats. J. Wildl. Manag. 2011, 75, 1177–1185. [Google Scholar] [CrossRef]
- Schmidt, P.M.; Lopez, R.R.; Collier, B.A. Survival, Fecundity, and Movements of Free-Roaming Cats. J. Wildl. Manag. 2007, 71, 915–919. [Google Scholar] [CrossRef]
- JCPS. School Profile Pages. Available online: https://www.jefferson.kyschools.us/o/jcps/page/school-profile-pages (accessed on 30 November 2024).
- ArcGIS Hub. ArcGIS Hub: Louisville KY Metro Parks. Available online: https://hub.arcgis.com/datasets/3ce3306260a448d2afc707961ed3f339/explore (accessed on 30 November 2024).
- JCES. Community Gardens. Available online: https://www.jcmgaky.org/community-gardens (accessed on 30 November 2024).
- Liberg, O.; Sandell, M.; Pontier, D.; Natoli, E. Density, Spatial Organisation and Reproductive Tactics in the Domestic Cat and Other Felids. In The Domestic Cat: The Biology of Its Behaviour; Turner, D.C., Bateson, P.P.G., Eds.; Cambridge University Press: Cambridge, UK, 2000; pp. 119–147. [Google Scholar]
- Rowan, A.N.; Kartal, T.; Hadidian, J. Cat Demographics & Impact on Wildlife in the USA, the UK, Australia and New Zealand: Facts and Values. J. Appl. Anim. Ethics Res. 2019, 2, 7–37. [Google Scholar]
- Hall, C.M.; Bryant, K.A.; Haskard, K.; Major, T.; Bruce, S.; Calver, M.C. Factors Determining the Home Ranges of Pet Cats: A Meta-Analysis. Biol. Conserv. 2016, 203, 313–320. [Google Scholar] [CrossRef]
- Guttilla, D.A.; Stapp, P. Effects of Sterilization on Movements of Feral Cats at a Wildland-Urban Interface. J. Mammal. 2010, 91, 482–489. [Google Scholar] [CrossRef]
- Meek, P.D. Home Range of House Cats Felis Catus Living within a National Park. Aust. Mammal. 2003, 25, 51–60. [Google Scholar] [CrossRef]
- Nutter, F.B. Evaluation of a Trap-Neuter-Return Management Program for Feral Cat Colonies: Population Dynamics, Home Ranges, and Potentially Zoonotic Diseases; North Carolina State University: Raleigh, NC, USA, 2005. [Google Scholar]
- Johnson, K.L.; Cicirelli, J. Study of the Effect on Shelter Cat Intakes and Euthanasia from a Shelter Neuter Return Project of 10,080 Cats from March 2010 to June 2014. PeerJ 2014, 2, e646. [Google Scholar] [CrossRef]
- Spehar, D.D.; Wolf, P.J. Integrated Return-to-Field and Targeted Trap-Neuter-Vaccinate-Return Programs Result in Reductions of Feline Intake and Euthanasia at Six Municipal Animal Shelters. Front. Vet. Sci. 2019, 6, 77. [Google Scholar] [CrossRef]
- Gunther, I.; Finkler, H.; Terkel, J. Demographic Differences between Urban Feeding Groups of Neutered and Sexually Intact Free-Roaming Cats Following a Trap-Neuter-Return Procedure. J. Am. Vet. Med. Assoc. 2011, 238, 1134–1140. [Google Scholar] [CrossRef]
- Aeluro, S.; Buchanan, J.M.; Boone, J.D.; Rabinowitz, P.M. “State of the Mewnion”: Practices of Feral Cat Care and Advocacy Organizations in the United States. Front. Vet. Sci. 2021, 8, 791134. [Google Scholar] [CrossRef]
- Houser, S.K. Prodigal Pets: A History of Animal Sheltering in America and the Origin of the No-Kill Movement; WBI Studies Repository: Potomac, MD, USA, 2018. [Google Scholar]
- Neal, S.M.; Kremer, T. Who Cares? Exploring the Demographics and Proportion of People Providing Care for Community Cats in Seven Study Communities in the United States. J. Shelter Med. Community Anim. Health 2024, 3, 71. [Google Scholar] [CrossRef]
- Dubey, J.P. Duration of Immunity to Shedding of Toxoplasma gondii Oocysts by Cats. J. Parasitol. 1995, 81, 410–415. [Google Scholar] [CrossRef]
- Davis, A.A.; Lepczyk, C.A.; Haman, K.H.; Morden, C.W.; Crow, S.E.; Jensen, N.; Lohr, M.T. Toxoplasma gondii Detection in Fecal Samples from Domestic Cats (Felis Catus) in Hawai’i. Pasc 2018, 72, 501–511. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; Haman, K.H.; Sizemore, G.C.; Farmer, C. Quantifying the Presence of Feral Cat Colonies and Toxoplasma gondii in Relation to Bird Conservation Areas on O’ahu, Hawai’i. Conserv. Sci. Pract. 2020, 2, e179. [Google Scholar] [CrossRef]
- Zhu, S.; VanWormer, E.; Shapiro, K. More People, More Cats, More Parasites: Human Population Density and Temperature Variation Predict Prevalence of Toxoplasma gondii Oocyst Shedding in Free-Ranging Domestic and Wild Felids. PLoS ONE 2023, 18, e0286808. [Google Scholar] [CrossRef]
- CDC. About Toxoplasmosis. Available online: https://www.cdc.gov/toxoplasmosis/about/index.html (accessed on 16 August 2025).
- AVMA. Toxoplasmosis. Available online: https://www.avma.org/resources-tools/pet-owners/petcare/toxoplasmosis (accessed on 15 December 2024).
- Meneses, A.M.C.; Negrão, K.A.; Miranda, C.F.; Bastos, R.K.G.; de Souza, N.F.; dos Santos Kuroda, R.B.; de Moraes, C.C.G.; Benigno, R.N.M. Ensaio imunoenzimático indireto para detecção de anticorpos igg anti-toxoplasma gondii em gatos na cidade de Belém do Pará, Brasil. Rev. Ciênc. Agrár. Amazonian J. Agric. Environ. Sci. 2009, 52, 99–105. [Google Scholar]
- Dubey, J.P.; Hoover, E.A.; Walls, K.W. Effect of Age and Sex on the Acquisition of Immunity to Toxoplasmosis in Cats. J. Protozool. 1977, 24, 184–186. [Google Scholar] [CrossRef]
- Afonso, E.; Thulliez, P.; Gilot-Fromont, E. Local Meteorological Conditions, Dynamics of Seroconversion to Toxoplasma gondii in Cats (Felis Catus) and Oocyst Burden in a Rural Environment. Epidemiol. Infect. 2010, 138, 1105–1113. [Google Scholar] [CrossRef]
- Hostetler, M.; Wisely, S.M.; Johnson, S.; Pienaar, E.F.; Main, M. How Effective and Humane Is Trap-Neuter-Release (TNR) for Feral Cats? Institute of Food and Agricultural Sciences Extension, University of Florida: Gainesville, FL, USA, 2020. [Google Scholar]
- Crawford, H.M.; Calver, M.C.; Fleming, P.A. A Case of Letting the Cat out of The Bag—Why Trap-Neuter-Return Is Not an Ethical Solution for Stray Cat (Felis catus) Management. Animals 2019, 9, 171. [Google Scholar] [CrossRef]
- Longcore, T.; Rich, C.; Sullivan, L.M. Critical Assessment of Claims Regarding Management of Feral Cats by Trap-Neuter-Return. Conserv. Biol. 2009, 23, 887–894. [Google Scholar] [CrossRef]
- Kreisler, R.E.; Cornell, H.N.; Levy, J.K. Decrease in Population and Increase in Welfare of Community Cats in a Twenty-Three Year Trap-Neuter-Return Program in Key Largo, FL: The ORCAT Program. Front. Vet. Sci. 2019, 6, 7. [Google Scholar] [CrossRef]
- Gallagher, C.A.; Keehner, J.R.; Hervé-Claude, L.P.; Stephen, C. Health Promotion and Harm Reduction Attributes in One Health Literature: A Scoping Review. One Health 2021, 13, 100284. [Google Scholar] [CrossRef]
- Makov, T.; Newman, G.E.; Zauberman, G. Inconsistent Allocations of Harms versus Benefits May Exacerbate Environmental Inequality. Proc. Natl. Acad. Sci. USA 2020, 117, 8820–8824. [Google Scholar] [CrossRef]
- Gallagher, C.A.; Hervé-Claude, L.P.; Cruz-Martinez, L.; Stephen, C. Understanding Community Perceptions of the St. Kitts’ “Monkey Problem” by Adapting Harm Reduction Concepts and Methods. Front. Ecol. Evol. 2022, 10, 904797. [Google Scholar] [CrossRef]
- Stephen, C.; Wittrock, J.; Wade, J. Using a Harm Reduction Approach in an Environmental Case Study of Fish and Wildlife Health. EcoHealth 2018, 15, 4–7. [Google Scholar] [CrossRef]
- Hurley, K.F.; Levy, J.K. Rethinking the Animal Shelter’s Role in Free-Roaming Cat Management. Front. Vet. Sci. 2022, 9, 847081. [Google Scholar] [CrossRef]
- Lazenby, B.T.; Mooney, N.J.; Dickman, C.R. Effects of Low-Level Culling of Feral Cats in Open Populations: A Case Study from the Forests of Southern Tasmania. Wildl. Res. 2015, 41, 407–420. [Google Scholar] [CrossRef]
- Palmas, P.; Gouyet, R.; Oedin, M.; Millon, A.; Cassan, J.-J.; Kowi, J.; Bonnaud, E.; Vidal, E. Rapid Recolonisation of Feral Cats Following Intensive Culling in a Semi-Isolated Context. NeoBiota 2020, 63, 177–200. [Google Scholar] [CrossRef]
- Taggart, P.L.; Caraguel, C.G.B.; McAllister, M.M. Fractional Seroprevalence Rates in Common Prey Species Can Cause More than Half of Feral Cats to Be Exposed to Toxoplasma gondii Annually. Vet. Parasitol. 2020, 288, 109306. [Google Scholar] [CrossRef] [PubMed]
- Lepczyk, C.A.; Duffy, D.C.; Bird, D.M.; Calver, M.; Cherkassky, D.; Cherkassky, L.; Dickman, C.R.; Hunter, D.; Jessup, D.; Longcore, T.; et al. A Science-Based Policy for Managing Free-Roaming Cats. Biol. Invasions 2022, 24, 3693–3701. [Google Scholar] [CrossRef]
- Wolf, P.J.; Schaffner, J.E. The Road to TNR: Examining Trap-Neuter-Return Through the Lens of Our Evolving Ethics. Front. Vet. Sci. 2019, 5, 341. [Google Scholar] [CrossRef] [PubMed]

| Ownership Status | Sex | No. of Cats Tracked | Estimated Home Range (95% MCP, ha) | |||
|---|---|---|---|---|---|---|
| Intact | Sterilized | Total | Mean | Median | ||
| Owned | M | 0 | 3 | 3 | 1.83 | 0.68 |
| Owned | F | 0 | 8 | 8 | 1.92 | 0.42 |
| Unowned | M | 5 | 1 | 6 | 157.01 | 48.30 |
| Unowned | F | 9 | 1 | 10 | 56.59 | 34.31 |
| Age Class * | Sex | Totals (%) | |
|---|---|---|---|
| Male (%) | Female (%) | ||
| Kittens | 1414 (26.9) | 1550 (28.2) | 2964 (27.6) |
| Sub-adults | 25 (0.5) | 5 (0.1) | 30 (0.3) |
| Adults | 3817 (72.6) | 3939 (71.7) | 7756 (72.2) |
| Totals | 5256 (100) | 5494 (100) | 10,750 (100) |
| Points of Interest | Jefferson County, Kentucky (%) | “Central Virginia” * |
|---|---|---|
| Elementary schools | 108 (40.0) | 32 (19.5) |
| Preschools | 7 (2.6) | |
| Public parks | 143 (53.0) | 125 (76.2) |
| Community gardens | 12 (4.4) | 7 (4.3) |
| Total | 270 (100) | 164 (100) |
| POI | Count (%) |
|---|---|
| Elementary schools | 108 (40.0) |
| Preschools | 7 (2.6) |
| Public parks | 143 (53.0) |
| Community gardens | 12 (4.4) |
| Total | 270 (100) |
| Free-Roaming Cat Classification | Model Results | POI Category | |||
|---|---|---|---|---|---|
| Elementary Schools | Preschools | Community Gardens | Public Parks | ||
| Intact | Sites with potential incursions (%) | 100 | 100 | 91.7 | 87.7 |
| No. of cats | 3598 | 193 | 440 | 5979 | |
| Mean no. of cats per site (range) | 33.3 (1–280) | 27.6 (8–46) | 36.7 (0–122) | 41.8 (0–335) | |
| Median no. of cats per site | 21 | 29 | 30 | 21 | |
| Sterilized | Sites with potential incursions (%) | 0.03 | 0.00 | 33.3 | 12.6 |
| No. of cats | 7 | 0 | 12 | 78 | |
| Mean no. of cats per site (range) | 0.06 (0–2) | 0 (0–0) | 1 (0–0) | 0.54 (0–0) | |
| Median no. of cats per site | 0 | 0 | 0 | 0 | |
| Free-Roaming Cat Classification | Model Results | Points of Interest | ||||
|---|---|---|---|---|---|---|
| Elementary Schools | Preschools | Community Gardens | Public Parks | Any Point of Interest | ||
| Intact | No. of cats (%) | 3351 (31.16) | 165 (1.53) | 440 (4.09) | 3688 (34.30) | 5766 (53.62) |
| Sterilized | No. of cats (%) | 7 (0.06) | 0 (0) | 12 (0.11) | 78 (0.72) | 97 (0.90) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neal, S.M.; Wolf, P.J.; Anderson, M.E. Geospatial Model Suggests Sterilizing Free-Roaming Domestic Cats Reduces Potential Risk of Toxoplasma gondii Infection. Zoonotic Dis. 2025, 5, 24. https://doi.org/10.3390/zoonoticdis5030024
Neal SM, Wolf PJ, Anderson ME. Geospatial Model Suggests Sterilizing Free-Roaming Domestic Cats Reduces Potential Risk of Toxoplasma gondii Infection. Zoonotic Diseases. 2025; 5(3):24. https://doi.org/10.3390/zoonoticdis5030024
Chicago/Turabian StyleNeal, Sue M., Peter J. Wolf, and Melanie E. Anderson. 2025. "Geospatial Model Suggests Sterilizing Free-Roaming Domestic Cats Reduces Potential Risk of Toxoplasma gondii Infection" Zoonotic Diseases 5, no. 3: 24. https://doi.org/10.3390/zoonoticdis5030024
APA StyleNeal, S. M., Wolf, P. J., & Anderson, M. E. (2025). Geospatial Model Suggests Sterilizing Free-Roaming Domestic Cats Reduces Potential Risk of Toxoplasma gondii Infection. Zoonotic Diseases, 5(3), 24. https://doi.org/10.3390/zoonoticdis5030024







