Isolation and Characterization of a Novel Orthomyxovirus from a Bothriocroton hydrosauri Tick Removed from a Blotched Blue-Tongued Skink (Tiliqua nigrolutea) in Tasmania, Australia
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Viral Isolation and Culturing
2.2. PCR Screening
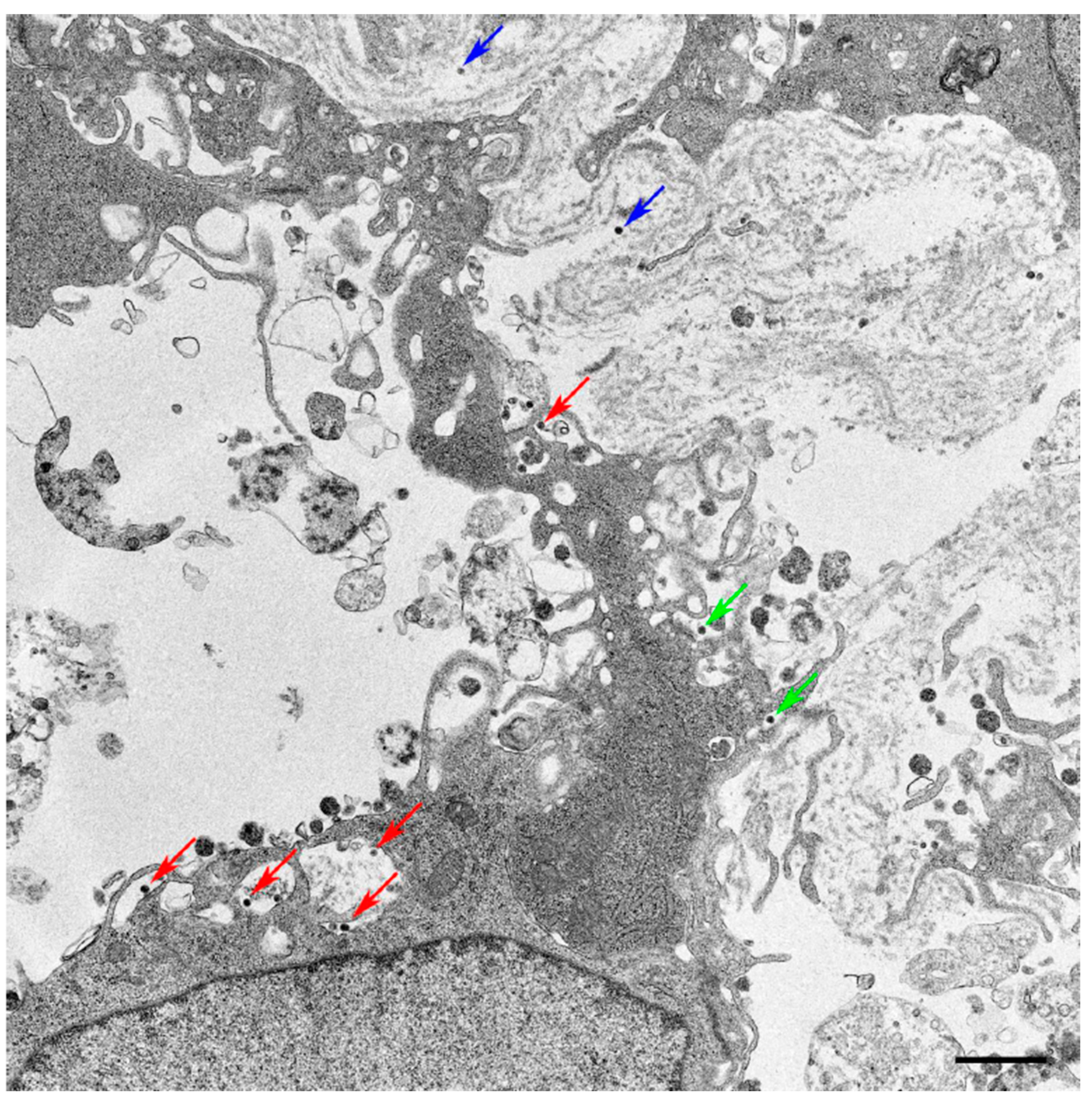
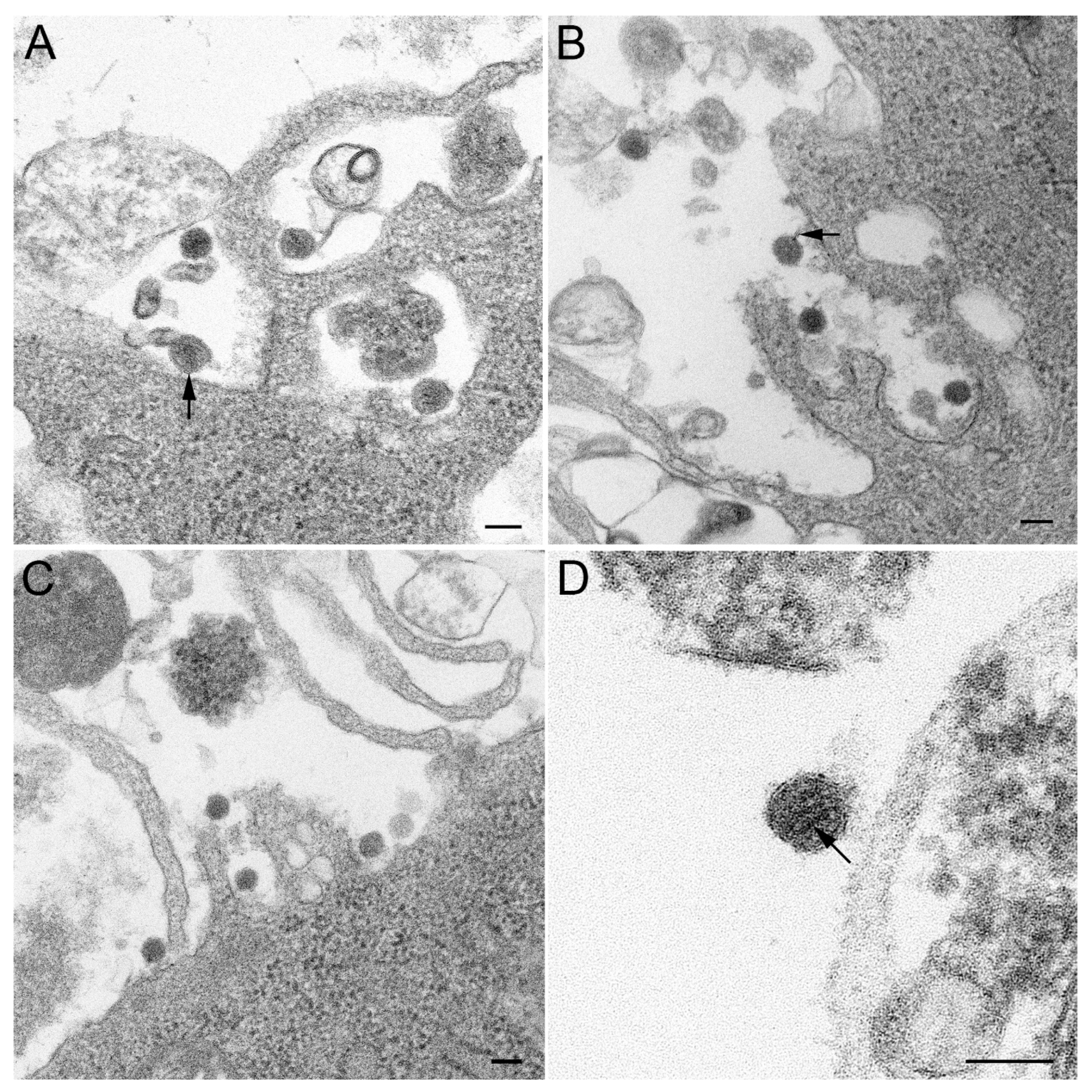
2.3. Transmission Electron Microscopy (TEM)
2.4. Genome Sequencing
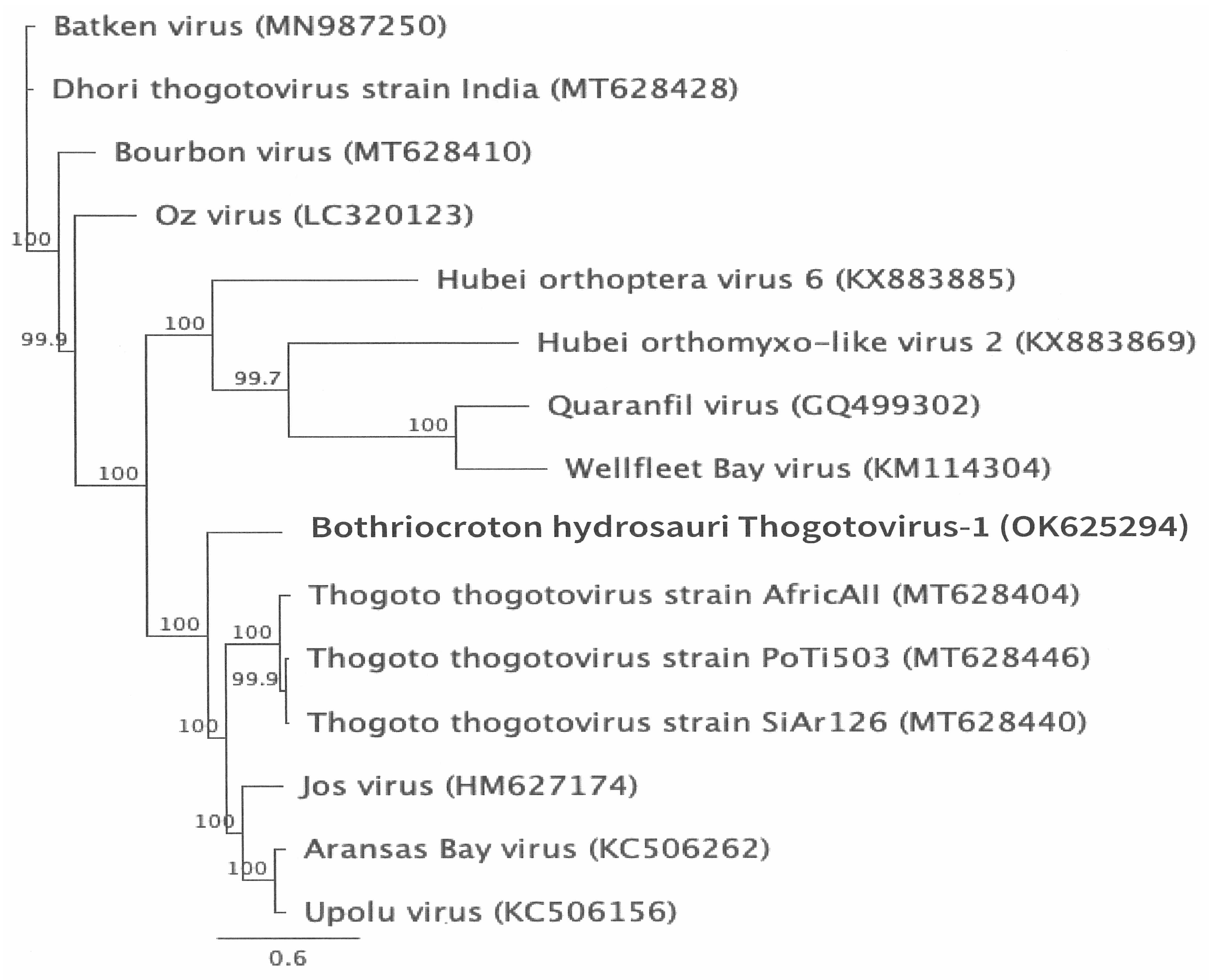
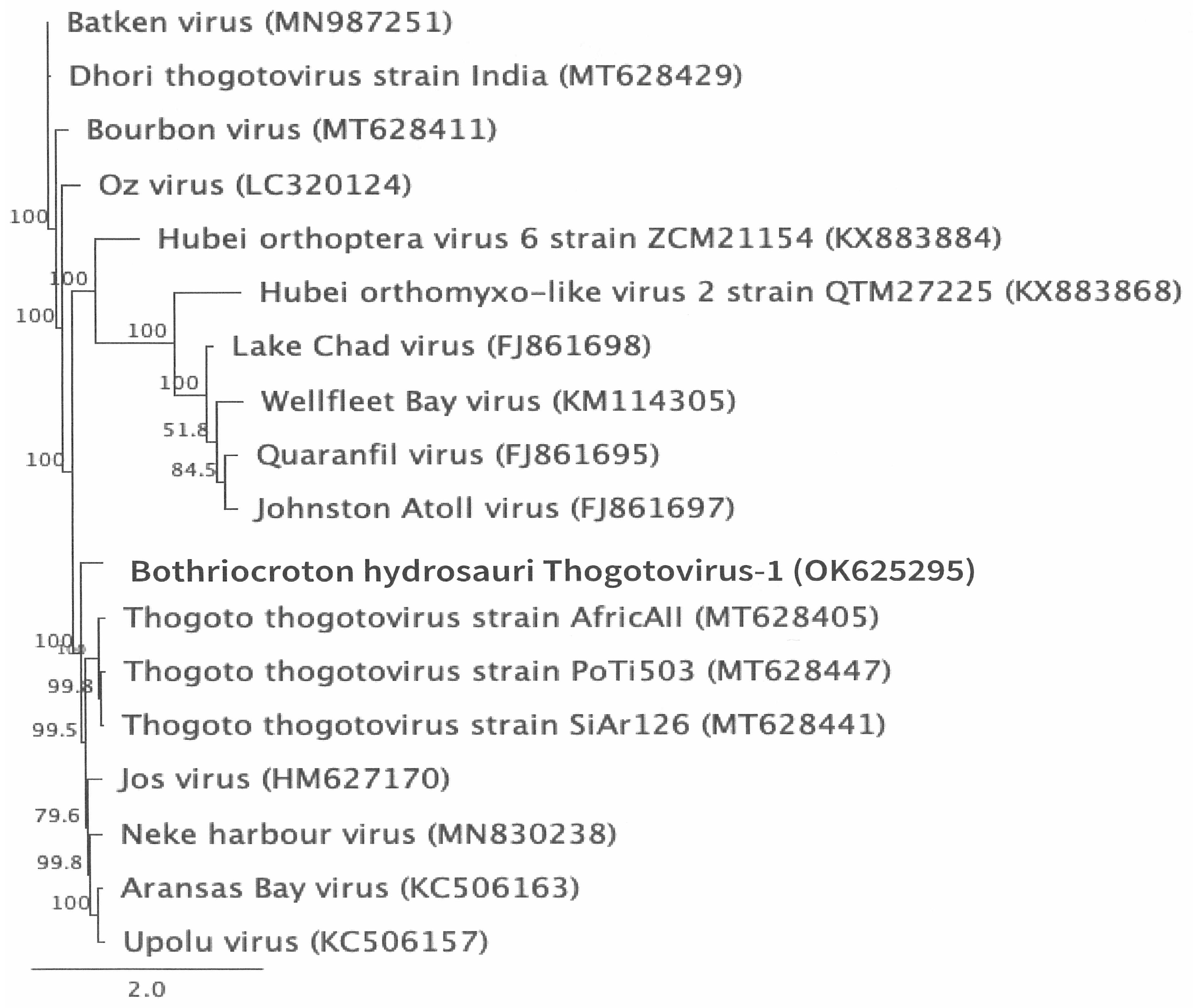
2.5. Genome Annotation, Alignment, and Phylogenetic Analyses
3. Results
3.1. Isolation and Morphology of Novel Tick Virus
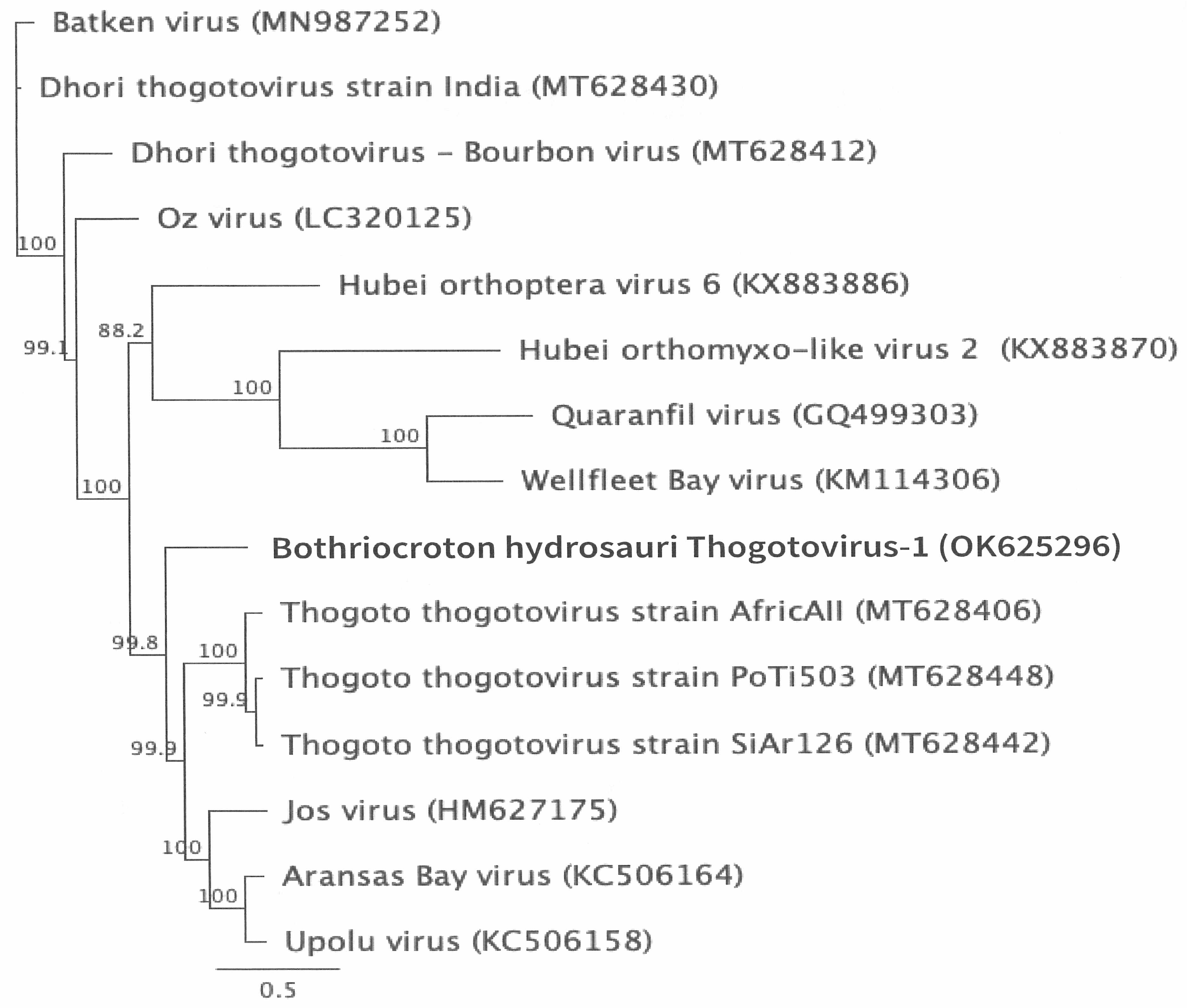
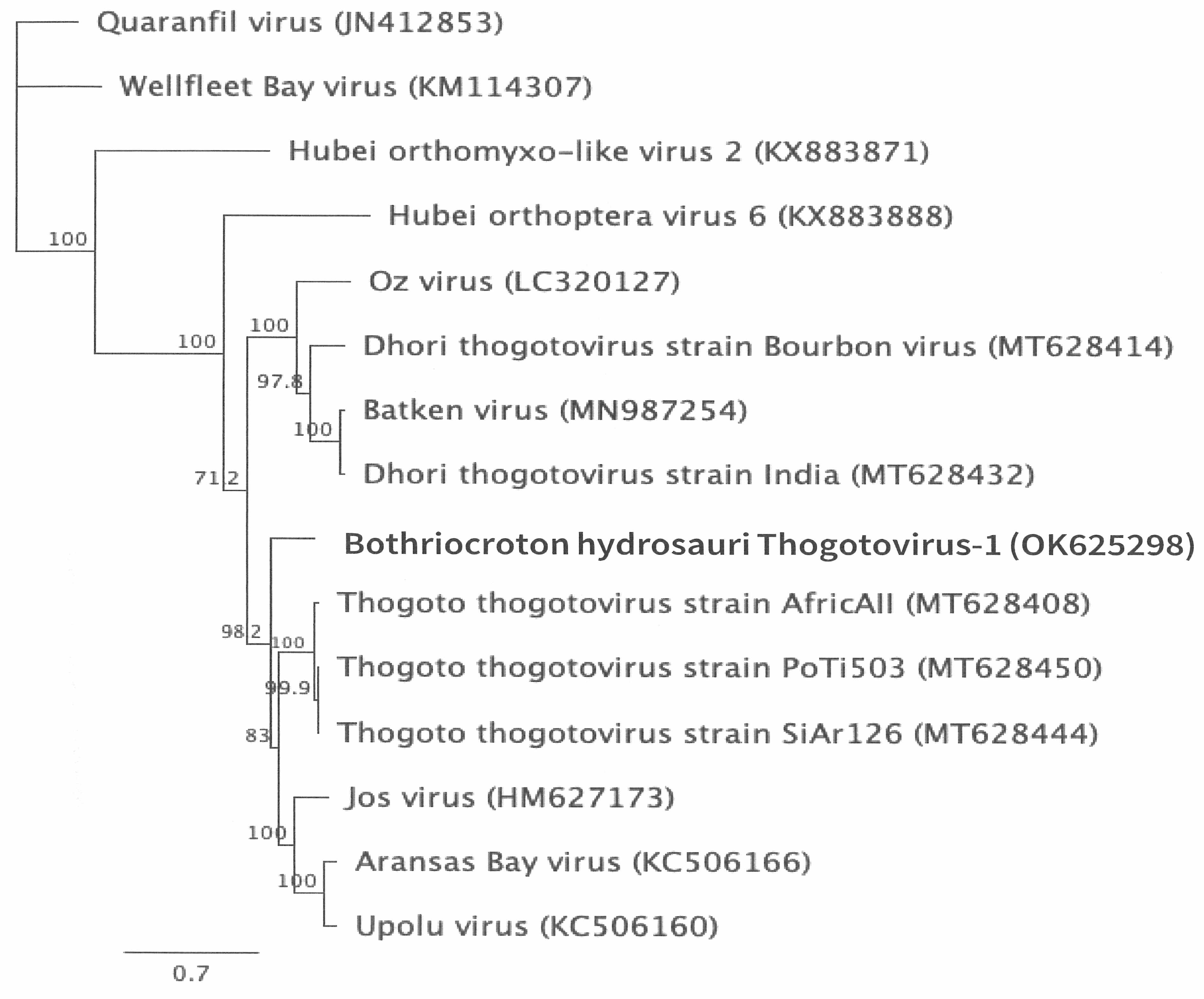
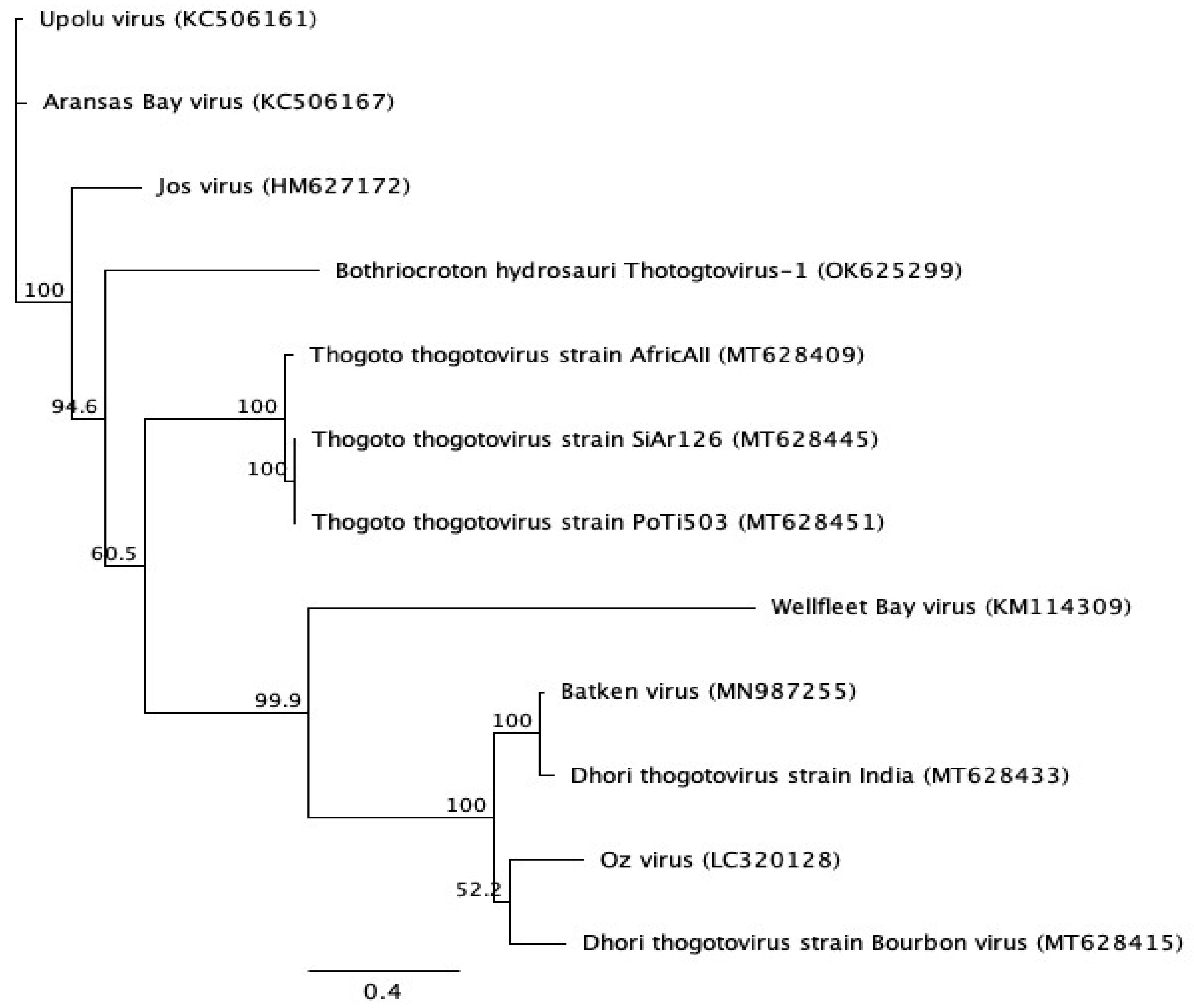
3.2. Genomic Characterization and Phylogenetic Analysis of the Novel Tick Virus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rochlin, I.; Toledo, A. Emerging tick-borne pathogens of public health importance: A mini-review. J. Med. Microbiol. 2020, 69, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.C.; Walker, A.R.; Campelo, D. A list of the 70 species of Australian ticks; diagnostic guides to and species accounts of Ixodes holocyclus (paralysis tick), Ixodes cornuatus (southern paralysis tick) and Rhipicephalus australis (Australian cattle tick); and consideration of the place of Australia in the evolution of ticks with comments on four controversial ideas. Int. J. Parasitol. 2014, 44, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Dehhaghi, M.; Kazemi Shariat Panahi, H.; Holmes, E.C.; Hudson, B.J.; Schloeffel, R.; Guillemin, G.J. Human Tick-Borne Diseases in Australia. Front. Cell. Infect. Microbiol. 2019, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Graves, S.R.; Stenos, J. Tick-borne infectious diseases in Australia. Med. J. Aust. 2017, 206, 320–324. [Google Scholar] [CrossRef]
- O’Brien, C.A.; Hall-Mendelin, S.; Hobson-Peters, J.; Deliyannis, G.; Allen, A.; Lew-Tabor, A.; Rodriguez-Valle, M.; Barker, D.; Barker, S.C.; Hall, R.A. Discovery of a novel iflavirus sequence in the eastern paralysis tick Ixodes holocyclus. Arch. Virol. 2018, 163, 2451–2457. [Google Scholar] [CrossRef]
- Doherty, R.; Carley, J.; Filippich, C.; Kay, B. Isolation of virus strains related to Kao Shuan virus from Argas robertsi in Northern Territory, Australia. Search 1976, 7, 484–486. [Google Scholar]
- St George, T.; Cybinski, D.; Jmain, A.; Mckilligan, N.; Kemp, D. Isolation of a new arbovirus from the tick Argas robertsi from a cattle egret (Bubulcus ibis coromandus) colony in Australia. Aust. J. Biol. Sci. 1984, 37, 85–90. [Google Scholar] [CrossRef]
- Gauci, P.J.; McAllister, J.; Mitchell, I.R.; Cybinski, D.; St George, T.; Gubala, A.J. Genomic Characterisation of Vinegar Hill Virus, An Australian Nairovirus Isolated in 1983 from Argas Robertsi Ticks Collected from Cattle Egrets. Viruses 2017, 9, 373. [Google Scholar] [CrossRef]
- Doherty, R.; Whitehead, R.H.; Wetters, E.J. Isolation of viruses from Ornithodoros capensis Neumann from a tern colony on the Great Barrier Reef, North Queensland. Aust. J. Sci. 1968, 31, 363–364. [Google Scholar]
- George, T.S.; Standfast, H.; Doherty, R.; Carley, J.; Fillipich, C.; Brandsma, J. The isolation of Saumarez Reef virus, a new flavivirus, from bird ticks Ornithodoros capensis and Ixodes eudyptidis in Australia. Immun. Cell Biol. 1977, 55, 493. [Google Scholar] [CrossRef]
- Briese, T.; Chowdhary, R.; Travassos da Rosa, A.; Hutchison, S.K.; Popov, V.; Street, C.; Tesh, R.B.; Lipkin, W.I. Upolu virus and Aransas Bay virus, two presumptive bunyaviruses, are novel members of the family Orthomyxoviridae. J. Virol. 2014, 88, 5298–5309. [Google Scholar] [CrossRef] [PubMed]
- Madison-Antenucci, S.; Kramer, L.D.; Gebhardt, L.L.; Kauffman, E. Emerging Tick-Borne Diseases. Clin. Microbiol. Rev. 2020, 33. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.E.; et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef] [PubMed]
- Stenos, J.; Graves, S.; Popov, V.L.; Walker, D.H. Aponomma hydrosauri, the reptile-associated tick reservoir of Rickettsia honei on Flinders Island, Australia. Am. J. Trop. Med. Hyg. 2003, 69, 314–317. [Google Scholar] [CrossRef]
- Whiley, H.; Custance, G.; Graves, S.; Stenos, J.; Taylor, M.; Ross, K.; Gardner, M.G. Rickettsia Detected in the Reptile Tick Bothriocroton hydrosauri from the Lizard Tiliqua rugosa in South Australia. Pathogens 2016, 5, 41. [Google Scholar] [CrossRef]
- Tadepalli, M.; Hii, S.F.; Vincent, G.; Watharow, S.; Graves, S.; Stenos, J. Molecular evidence of novel spotted fever group Rickettsia species in Amblyomma albolimbatum ticks from the shingleback skink, Tiliqua rugosa, in southern Western Australia. Pathogens 2020, 10, 35. [Google Scholar] [CrossRef]
- Vilcins, I.M.; Fournier, P.E.; Old, J.M.; Deane, E. Evidence for the presence of Francisella and spotted fever group rickettsia DNA in the tick Amblyomma fimbriatum (Acari: Ixodidae), Northern Territory, Australia. J. Med. Entomol. 2009, 46, 926–933. [Google Scholar] [CrossRef]
- Edwards, U.; Rogall, T.; Blocker, H.; Emde, M.; Bottger, E.C. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 1989, 17, 7843–7853. [Google Scholar] [CrossRef] [PubMed]
- Stenos, J.; Graves, S.R.; Unsworth, N.B. A highly sensitive and specific real-time PCR assay for the detection of spotted fever and typhus group Rickettsiae. Am. J. Trop. Med. Hyg. 2005, 73, 1083–1085. [Google Scholar] [CrossRef]
- Unsworth, N.; Stenos, J.; Graves, S.; Faa, A.; Cox, E.; Dyer, J.; Boutlis, C.; Lane, A.; Shaw, M.; Robson, J.; et al. Flinders Island Spotted Fever rickettsioses caused by “marmionii” strain of Rickettsia honei, Eastern Australia. Emerg. Infect. Dis. 2007, 13, 566–573. [Google Scholar] [CrossRef]
- Kumar, S.; Tamura, K.; Nei, M. MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief. Bioinform. 2004, 5, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Bussetti, A.V.; Palacios, G.; Travassos da Rosa, A.; Savji, N.; Jain, K.; Guzman, H.; Hutchison, S.; Popov, V.L.; Tesh, R.B.; Lipkin, W.I. Genomic and antigenic characterization of Jos virus. J. Gen. Virol. 2012, 93, 293–298. [Google Scholar] [CrossRef]
- Karabatsos, N. International Catalogue of Arboviruses including certain other viruses of vertebrates, 1985. Am. J. Trop. Med. Hyg. 1978, 27, 372. [Google Scholar] [CrossRef] [PubMed]
- Ogen-Odoi, A.; Miller, B.R.; Happ, C.M.; Maupin, G.O.; Burkot, T.R. Isolation of thogoto virus (Orthomyxoviridae) from the banded mongoose, Mongos mungo (Herpestidae), in Uganda. Am. J. Trop. Med. Hyg. 1999, 60, 439–440. [Google Scholar] [CrossRef]
- Hubalek, Z.; Rudolf, I.; Nowotny, N. Arboviruses pathogenic for domestic and wild animals. Adv. Virus Res. 2014, 89, 201–275. [Google Scholar] [CrossRef] [PubMed]
- Lutomiah, J.; Musila, L.; Makio, A.; Ochieng, C.; Koka, H.; Chepkorir, E.; Mutisya, J.; Mulwa, F.; Khamadi, S.; Miller, B.R.; et al. Ticks and tick-borne viruses from livestock hosts in arid and semiarid regions of the eastern and northeastern parts of Kenya. J. Med. Entomol. 2014, 51, 269–277. [Google Scholar] [CrossRef]
- Komar, N.; Hamby, N.; Palamar, M.B.; Staples, J.E.; Williams, C. Indirect Evidence of Bourbon Virus (Thogotovirus, Orthomyxoviridae) Infection in North Carolina. N. C. Med. J. 2020, 81, 214–215. [Google Scholar] [CrossRef]
- Lambert, A.J.; Velez, J.O.; Brault, A.C.; Calvert, A.E.; Bell-Sakyi, L.; Bosco-Lauth, A.M.; Staples, J.E.; Kosoy, O.I. Molecular, serological and in vitro culture-based characterization of Bourbon virus, a newly described human pathogen of the genus Thogotovirus. J. Clin. Virol. 2015, 73, 127–132. [Google Scholar] [CrossRef]
- Kosoy, O.I.; Lambert, A.J.; Hawkinson, D.J.; Pastula, D.M.; Goldsmith, C.S.; Hunt, D.C.; Staples, J.E. Novel thogotovirus associated with febrile illness and death, United States, 2014. Emerg. Infect. Dis. 2015, 21, 760–764. [Google Scholar] [CrossRef]
- Savage, H.M.; Burkhalter, K.L.; Godsey, M.S., Jr.; Panella, N.A.; Ashley, D.C.; Nicholson, W.L.; Lambert, A.J. Bourbon Virus in Field-Collected Ticks, Missouri, USA. Emerg. Infect. Dis. 2017, 23, 2017–2022. [Google Scholar] [CrossRef]
- Ejiri, H.; Lim, C.K.; Isawa, H.; Fujita, R.; Murota, K.; Sato, T.; Kobayashi, D.; Kan, M.; Hattori, M.; Kimura, T.; et al. Characterization of a novel thogotovirus isolated from Amblyomma testudinarium ticks in Ehime, Japan: A significant phylogenetic relationship to Bourbon virus. Virus Res. 2018, 249, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Stenos, J.; Roux, V.; Walker, D.; Raoult, D. Rickettsia honei sp. nov., the aetiological agent of Flinders Island spotted fever in Australia. Int. J. Syst. Bacteriol. 1998, 48 Pt 4, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, N.; Guzman, H.; Sbrana, E.; Yoshikawa, T.; Tseng, C.T.; Tesh, R.B.; Xiao, S.Y. Dhori virus (Orthomyxoviridae: Thogotovirus) infection of mice produces a disease and cytokine response pattern similar to that of highly virulent influenza A (H5N1) virus infection in humans. Am. J. Trop. Med. Hyg. 2008, 78, 675–680. [Google Scholar] [CrossRef] [PubMed]

| ORF (Size bp) | % Identity | Genbank ID | Accession Description |
|---|---|---|---|
| ORF1 (2346 bp) | 54.4% | AED98375.1 (95% coverage) | Jos virus PB2 |
| ORF2 (2139 bp) | 70.1% | AED98371.1 (96% coverage) | Jos virus PB1 |
| ORF3 (1890 bp) | 48.9% | AHB34062.1 (96% coverage) | Aransas Bay virus PA |
| ORF4 (1582 bp) | 41.1% | AHB34057.1 (89% coverage) | Upolu virus GP |
| ORF5 (1365 bp) | 59.6% | YP_145809.1 (95% coverage) | Thogotovirus NP |
| ORF6 (921 bp) | 45.7% | AED98373.1 (78% coverage) | Jos virus ML |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selleck, P.; Vincent, G.; Tachedjian, M.; Crameri, S.; Marsh, G.; Graves, S.; Stenos, J. Isolation and Characterization of a Novel Orthomyxovirus from a Bothriocroton hydrosauri Tick Removed from a Blotched Blue-Tongued Skink (Tiliqua nigrolutea) in Tasmania, Australia. Zoonotic Dis. 2025, 5, 9. https://doi.org/10.3390/zoonoticdis5020009
Selleck P, Vincent G, Tachedjian M, Crameri S, Marsh G, Graves S, Stenos J. Isolation and Characterization of a Novel Orthomyxovirus from a Bothriocroton hydrosauri Tick Removed from a Blotched Blue-Tongued Skink (Tiliqua nigrolutea) in Tasmania, Australia. Zoonotic Diseases. 2025; 5(2):9. https://doi.org/10.3390/zoonoticdis5020009
Chicago/Turabian StyleSelleck, Paul, Gemma Vincent, Mary Tachedjian, Sandra Crameri, Glenn Marsh, Stephen Graves, and John Stenos. 2025. "Isolation and Characterization of a Novel Orthomyxovirus from a Bothriocroton hydrosauri Tick Removed from a Blotched Blue-Tongued Skink (Tiliqua nigrolutea) in Tasmania, Australia" Zoonotic Diseases 5, no. 2: 9. https://doi.org/10.3390/zoonoticdis5020009
APA StyleSelleck, P., Vincent, G., Tachedjian, M., Crameri, S., Marsh, G., Graves, S., & Stenos, J. (2025). Isolation and Characterization of a Novel Orthomyxovirus from a Bothriocroton hydrosauri Tick Removed from a Blotched Blue-Tongued Skink (Tiliqua nigrolutea) in Tasmania, Australia. Zoonotic Diseases, 5(2), 9. https://doi.org/10.3390/zoonoticdis5020009







