Could the Identification of Skin Lesions Be Beneficial for the Differential Diagnosis of Viral Meningitis?
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cutaneous Manifestations in Bacterial Meningitis: Diagnostic Considerations
2.2. The Role of Telemedicine in the Diagnosis of Skin Lesions
3. Summary
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kohil, A.; Jemmieh, S.; Smatti, M.K.; Yassine, H.M. Viral meningitis: An overview. Arch. Virol. 2021, 166, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Mrozowska-Nyckowska, K.; Zbrzeźniak, J.; Paradowska-Stankiewicz, I. Meningitis and encephalitis in Poland in 2022. Epidemiol. Rev./Przegląd Epidemiol. 2024, 78, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Irani, D.N. Aseptic meningitis and viral myelitis. Neurol. Clin. 2008, 26, 635–655. [Google Scholar] [CrossRef]
- Kupila, L.; Vuorinen, T.; Vainionpaa, R.; Hukkanen, V.; Marttila, R.J.; Kotilainen, P. Etiology of aseptic meningitis and encephalitis in an adult population. Neurology 2006, 66, 75–80. [Google Scholar] [CrossRef]
- Logan, S.A.; MacMahon, E. Viral meningitis. BMJ 2008, 336, 36–40. [Google Scholar] [CrossRef]
- Moriguchi, T.; Harii, N.; Goto, J.; Harada, D.; Sugawara, H.; Takamino, J.; Ueno, M.; Sakata, H.; Kondo, K.; Myose, N.; et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020, 94, 55–58. [Google Scholar] [CrossRef]
- Rotbart, H.A. Viral meningitis. Semin. Neurol. 2000, 20, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, M.H. Enterovirus infections: Diagnosis and treatment. Semin. Pediatr. Infect. Dis. 2002, 13, 40–47. [Google Scholar] [CrossRef]
- De Crom, S.C.M.; Rossen, J.W.A.; Van Furth, A.M.; Obihara, C.C. Enterovirus and parechovirus infection in children: A brief overview. Eur. J. Pediatr. 2016, 175, 1023–1029. [Google Scholar] [CrossRef]
- Sridhar, A.; Karelehto, E.; Brouwer, L.; Pajkrt, D.; Wolthers, K.C. Parechovirus A Pathogenesis and the Enigma of Genotype A-3. Viruses 2019, 11, 1062. [Google Scholar] [CrossRef]
- Chen, W.; Dai, S.; Xu, L. Clinical characterization of benign enterovirus infection in neonates. Medicine 2021, 100, e25706. [Google Scholar] [CrossRef] [PubMed]
- Miyamura, K.; Yamashita, K.; Yamadera, S.; Kato, N.; Akatsuka, M.; Yamazaki, S. An epidemic of echovirus 18 in 1988 in Japan--high association with clinical manifestation of exanthem. A report of the National Epidemiological Surveillance of Infectious Agents in Japan. Jpn. J. Med. Sci. Biol. 1990, 43, 51–58. [Google Scholar] [CrossRef]
- Newman, C.R.; Smith, R.B. An outbreak of meningitis associated with ECHO virus type 9. J. Clin. Pathol. 1965, 18, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, D.R. Viral meningitis. Br. Med. Bull. 2005, 75–76, 1–14. [Google Scholar] [CrossRef]
- Kaewpoowat, Q.; Salazar, L.; Aguilera, E.; Wootton, S.H.; Hasbun, R. Herpes simplex and varicella zoster CNS infections: Clinical presentations, treatments and outcomes. Infection 2016, 44, 337–345. [Google Scholar] [CrossRef]
- Gonzales, N.; Tyler, K.L.; Gilden, D.H. Recurrent dermatomal vesicular skin lesions: A clue to diagnosis of herpes simplex virus 2 meningitis. Arch. Neurol. 2003, 60, 868–869. [Google Scholar] [CrossRef] [PubMed]
- Karpathios, T.; Moustaki, M.; Yiallouros, P.; Sarifi, F.; Tzanakaki, G.; Fretzayas, A. HSV-2 meningitis disseminated from a herpetic whitlow. Paediatr. Int. Child. Health 2012, 32, 121–122. [Google Scholar] [CrossRef]
- Heininger, U.; Seward, J.F. Varicella. Lancet 2006, 368, 1365–1376. [Google Scholar] [CrossRef]
- Kratlian, C.M.; Hopkins-Braddock, P.; Tristram, D. Case 4: Unexpected Rash in a 12-year-old Girl. Pediatr. Rev. 2020, 41, 256–258. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Johnson, R.W.; Breuer, J.; Gnann, J.W.; Levin, M.J.; Backonja, M.; Betts, R.F.; Gershon, A.A.; Haanpää, M.L.; McKendrick, M.W.; et al. Recommendations for the management of herpes zoster. Clin. Infect. Dis. 2007, 44 (Suppl. S1), S1–S26. [Google Scholar] [CrossRef]
- Liesegang, T.J. Herpes Zoster Ophthalmicus: Natural History, Risk Factors, Clinical Presentation, and Morbidity. Ophthalmology 2008, 115 (Suppl. S2), S3–S12. [Google Scholar] [CrossRef]
- Bienkowski, C.; Kowalczyk, M.; Talarek, E.; Pokorska-Spiewak, M.; Kierdaszuk, B.; Marczynska, M. Meningitis and Ramsay-Hunt syndrome in a 17-year old girl. Neuro Endocrinol. Lett. 2019, 40, 149–151. [Google Scholar] [PubMed]
- Bieńkowski, C.; Talarek, E.; Pokorska-Śpiewak, M. The clinical course of herpes zoster is similar in immunocompetent and immunocompromised paediatric patients. J. Paediatr. Child. Health 2023, 59, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, M.K.; Pluta, M.; Pokorska-Śpiewak, M.; Marczyńska, M. Meningitis in the Course of Herpes Zoster Ophthalmicus in an Immunocompetent Boy. Pediatr. Infect. Dis. J. 2023, 42, e361–e362. [Google Scholar] [CrossRef] [PubMed]
- Boroń-Kaczmarska, A.W.-D. Alicja Choroby zakaźne i pasożytnicze. T, 1st ed.; Boroń-Kaczmarska, A.W.-D., Ed.; PZWL Wydawnictwo Lekarskie: Warsaw, Poland, 2022; Volume 2. [Google Scholar]
- Rajapakse, S.; Rodrigo, C.; Rajapakse, A. Atypical manifestations of chikungunya infection. Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 89–96. [Google Scholar] [CrossRef]
- Javelle, E.; Tiong, T.H.; Leparc-Goffart, I.; Savini, H.; Simon, F. Inflammation of the external ear in acute chikungunya infection: Experience from the outbreak in Johor Bahru, Malaysia, 2008. J. Clin. Virol. 2014, 59, 270–273. [Google Scholar] [CrossRef]
- Meena, S.S.; Arya, S.; Meena, D.; Matlani, M.; Salhan, M. Neonatal Chikungunya: A case series. Trop. Doct. 2021, 51, 103–105. [Google Scholar] [CrossRef]
- Ritz, N.; Hufnagel, M.; Gérardin, P. Chikungunya in Children. Pediatr. Infect. Dis. J. 2015, 34, 789–791. [Google Scholar] [CrossRef]
- Samra, J.A.; Hagood, N.L.; Summer, A.; Medina, M.T.; Holden, K.R. Clinical Features and Neurologic Complications of Children Hospitalized With Chikungunya Virus in Honduras. J. Child. Neurol. 2017, 32, 712–716. [Google Scholar] [CrossRef]
- Ferguson, D.D.; Gershman, K.; LeBailly, A.; Petersen, L.R. Characteristics of the rash associated with West Nile virus fever. Clin. Infect. Dis. 2005, 41, 1204–1207. [Google Scholar] [CrossRef]
- Watson, J.T.; Pertel, P.E.; Jones, R.C.; Siston, A.M.; Paul, W.S.; Austin, C.C.; Gerber, S.I. Clinical characteristics and functional outcomes of West Nile Fever. Ann. Intern. Med. 2004, 141, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Huhn, G.D.; Dworkin, M.S. Rash as a prognostic factor in West Nile virus disease. Clin. Infect. Dis. 2006, 43, 388–389. [Google Scholar] [CrossRef]
- Derrington, S.M.; Cellura, A.P.; McDermott, L.E.; Gubitosi, T.; Sonstegard, A.M.; Chen, S.; Garg, A. Mucocutaneous Findings and Course in an Adult With Zika Virus Infection. JAMA Dermatol. 2016, 152, 691–693. [Google Scholar] [CrossRef]
- Cordel, N.; Birembaux, X.; Chaumont, H.; Delion, F.; Chosidow, O.; Tressieres, B.; Storck, C.H. Main Characteristics of Zika Virus Exanthema in Guadeloupe. JAMA Dermatol. 2017, 153, 326–328. [Google Scholar] [CrossRef]
- Yendell, S.J.; Fischer, M.; Staples, J.E. Colorado tick fever in the United States, 2002–2012. Vector Borne Zoonotic Dis. 2015, 15, 311–316. [Google Scholar] [CrossRef]
- Goodpasture, H.C.; Poland, J.D.; Francy, D.B.; Bowen, G.S.; HORN, K.A. Colorado tick fever: Clinical, epidemiologic, and laboratory aspects of 228 cases in Colorado in 1973–1974. Ann. Intern. Med. 1978, 88, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Klasco, R. Colorado tick fever. Med. Clin. North. Am. 2002, 86, 435–440. [Google Scholar] [CrossRef]
- Sahu, R.; Verma, R.; Jain, A.; Garg, R.K.; Singh, M.K.; Malhotra, H.S.; Sharma, P.K.; Parihar, A. Neurologic complications in dengue virus infection: A prospective cohort study. Neurology 2014, 83, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Cobra, C.; Rigau-Pérez, J.G.; Kuno, G.; Vomdam, V. Symptoms of dengue fever in relation to host immunologic response and virus serotype, Puerto Rico, 1990-1991. Am. J. Epidemiol. 1995, 142, 1204–1211. [Google Scholar] [CrossRef]
- Solomon, T.; Dung, N.M.; Vaughn, D.W.; Kneen, R.; Thao, L.T.T.; Raengsakulrach, B.; Loan, H.T.; Day, N.P.; Farrar, J.; Myint, K.S.; et al. Neurological manifestations of dengue infection. Lancet 2000, 355, 1053–1059. [Google Scholar] [CrossRef]
- Morrison, A. Oropouche Virus Disease Among U.S. Travelers—United States, 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 769–773. [Google Scholar] [CrossRef]
- Hjalmarsson, A.; Blomqvist, P.; Sköldenberg, B. Herpes simplex encephalitis in Sweden, 1990–2001: Incidence, morbidity, and mortality. Clin. Infect. Dis. 2007, 45, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Aboelezz, A.; Mahmoud, S.H. Acyclovir dosing in herpes encephalitis: A scoping review. J. Am. Pharm. Assoc. 2024, 64, 102040. [Google Scholar] [CrossRef]
- Kimberlin, D.W.; Lin, C.Y.; Jacobs, R.F.; Powell, D.A.; Corey, L.; Gruber, W.C.; Rathore, M.; Bradley, J.S.; Diaz, P.S.; Kumar, M.; et al. Safety and efficacy of high-dose intravenous acyclovir in the management of neonatal herpes simplex virus infections. Pediatrics 2001, 108, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Van de Beek, D.; De Gans, J.; Spanjaard, L.; Weisfelt, M.; Reitsma, J.B.; Vermeulen, M. Clinical features and prognostic factors in adults with bacterial meningitis. N. Engl. J. Med. 2004, 351, 1849–1859. [Google Scholar] [CrossRef]
- Heckenberg, S.G.; de Gans, J.; Brouwer, M.C.; Weisfelt, M.; Piet, J.R.; Spanjaard, L.; van der Ende, A.; van de Beek, D. Clinical features, outcome, and meningococcal genotype in 258 adults with meningococcal meningitis: A prospective cohort study. Medicine 2008, 87, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Sud, E.; Anjankar, A. Applications of Telemedicine in Dermatology. Cureus 2022, 14, e27740. [Google Scholar] [CrossRef]
- Palacholla, R.S.; Kvedar, J.C. Telemedicine for infectious disease care-how do we measure the true value? Ann. Transl. Med. 2019, 7 (Suppl. S6), S178. [Google Scholar] [CrossRef]
- Glines, K.R.; Haidari, W.; Ramani, L.; Akkurt, Z.M.; Feldman, S.R. Digital future of dermatology. Dermatol. Online J. 2020, 26. [Google Scholar] [CrossRef]
- Ihekwaba, U.K.; Kudesia, G.; McKendrick, M.W. Clinical features of viral meningitis in adults: Significant differences in cerebrospinal fluid findings among herpes simplex virus, varicella zoster virus, and enterovirus infections. Clin. Infect. Dis. 2008, 47, 783–789. [Google Scholar] [CrossRef]
- Lee, G.H.; Kim, J.; Kim, H.W.; Cho, J.W. Herpes simplex viruses (1 and 2) and varicella-zoster virus infections in an adult population with aseptic meningitis or encephalitis: A nine-year retrospective clinical study. Medicine 2021, 100, e27856. [Google Scholar] [CrossRef] [PubMed]
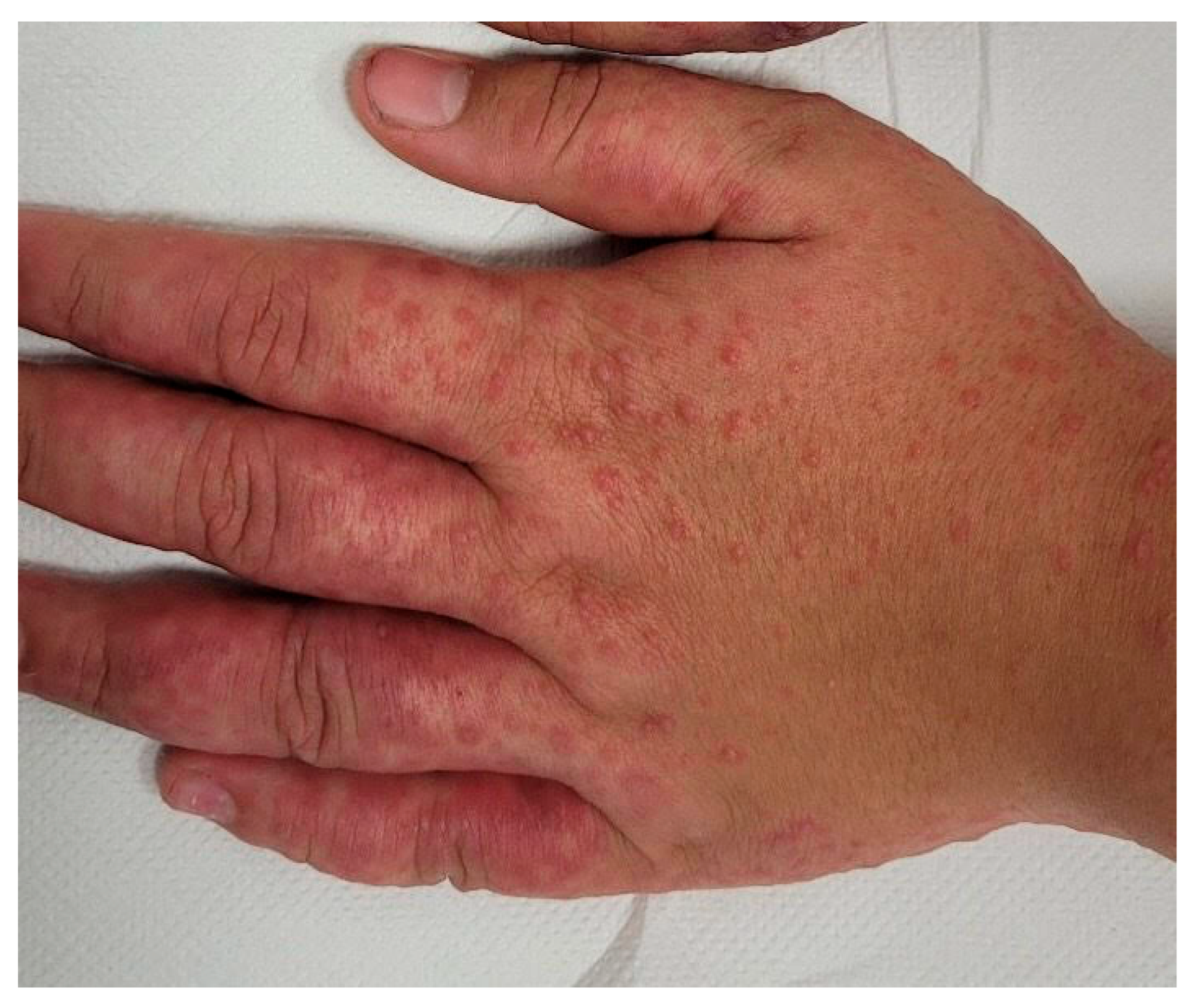
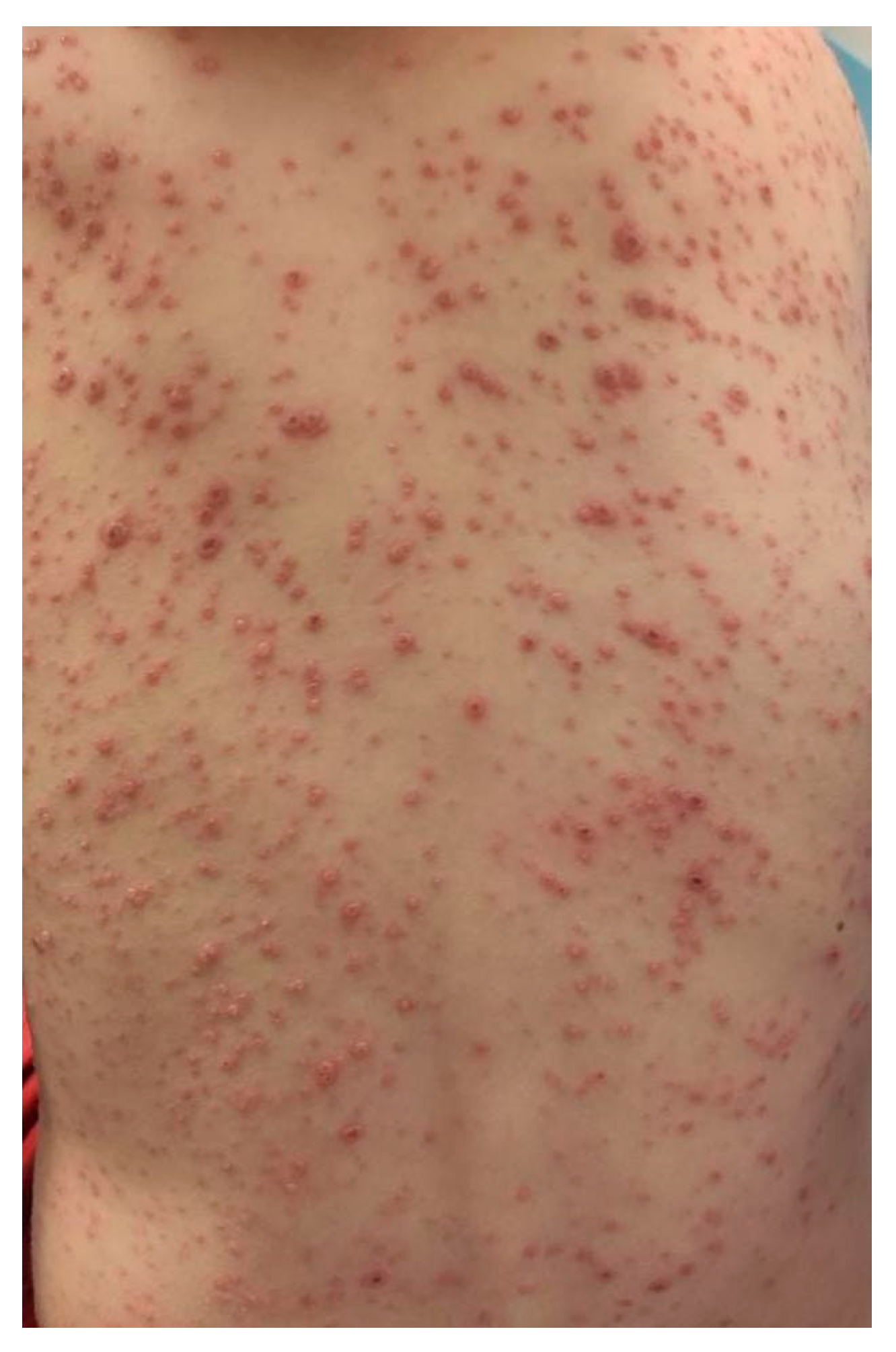
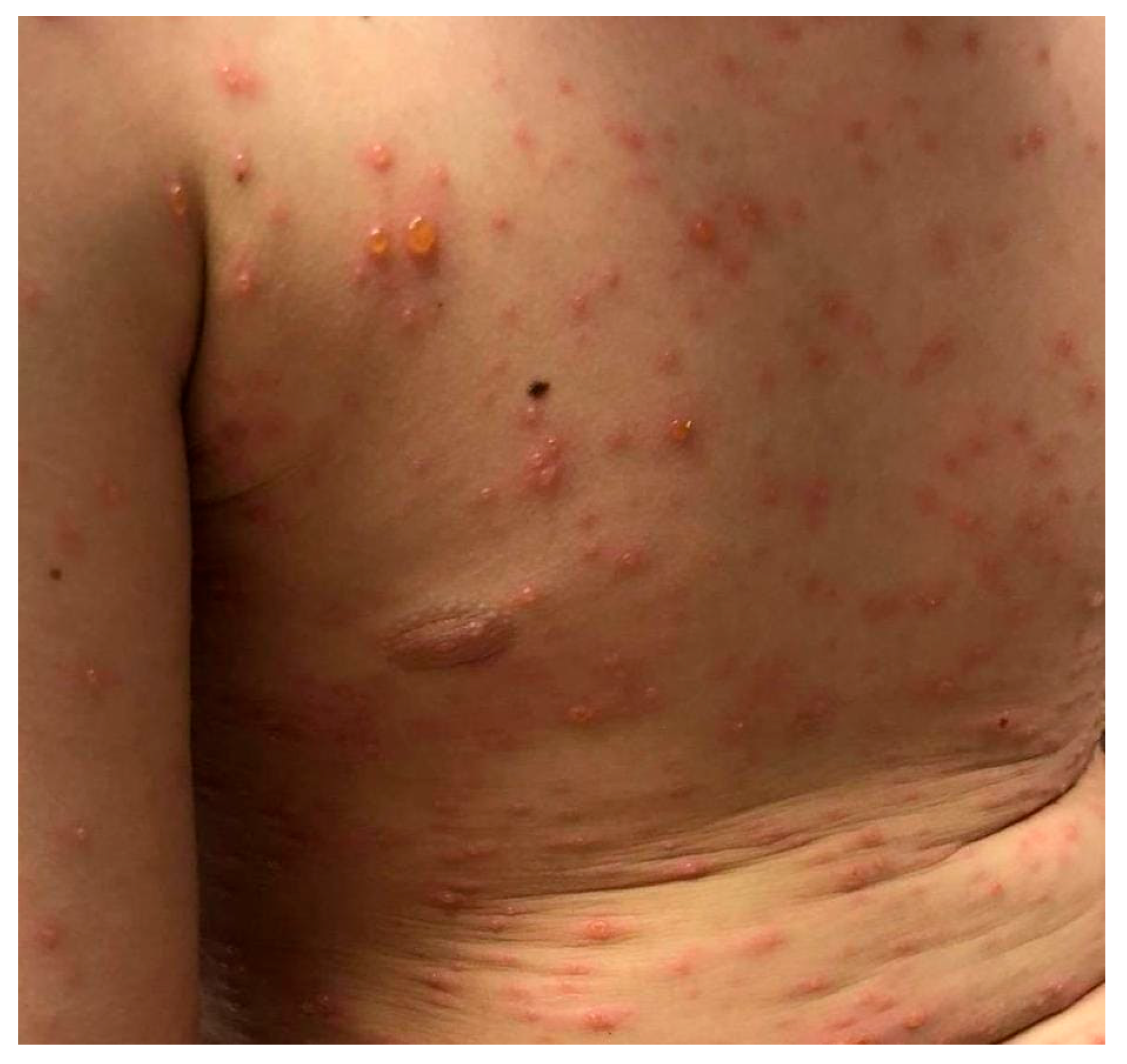
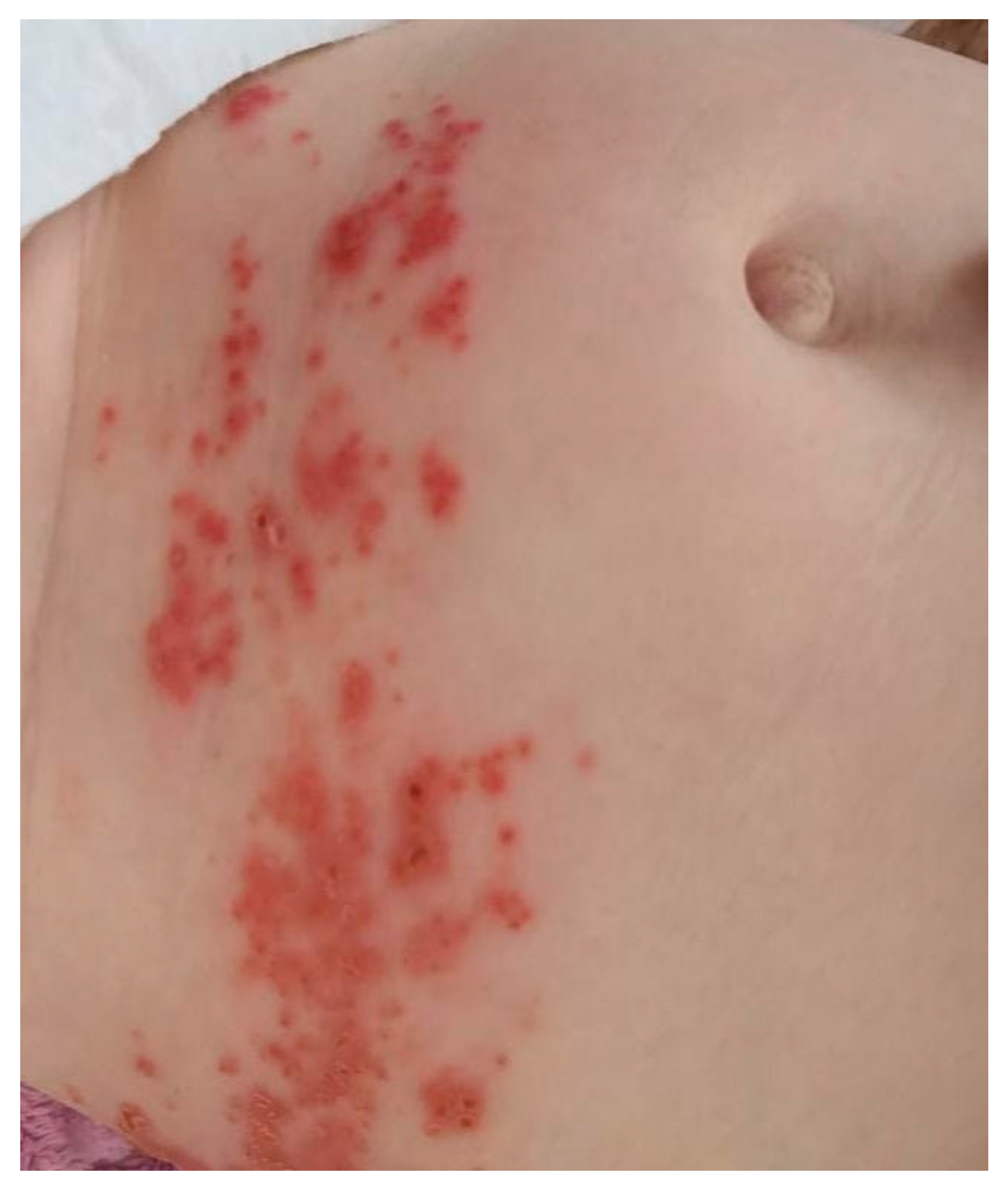
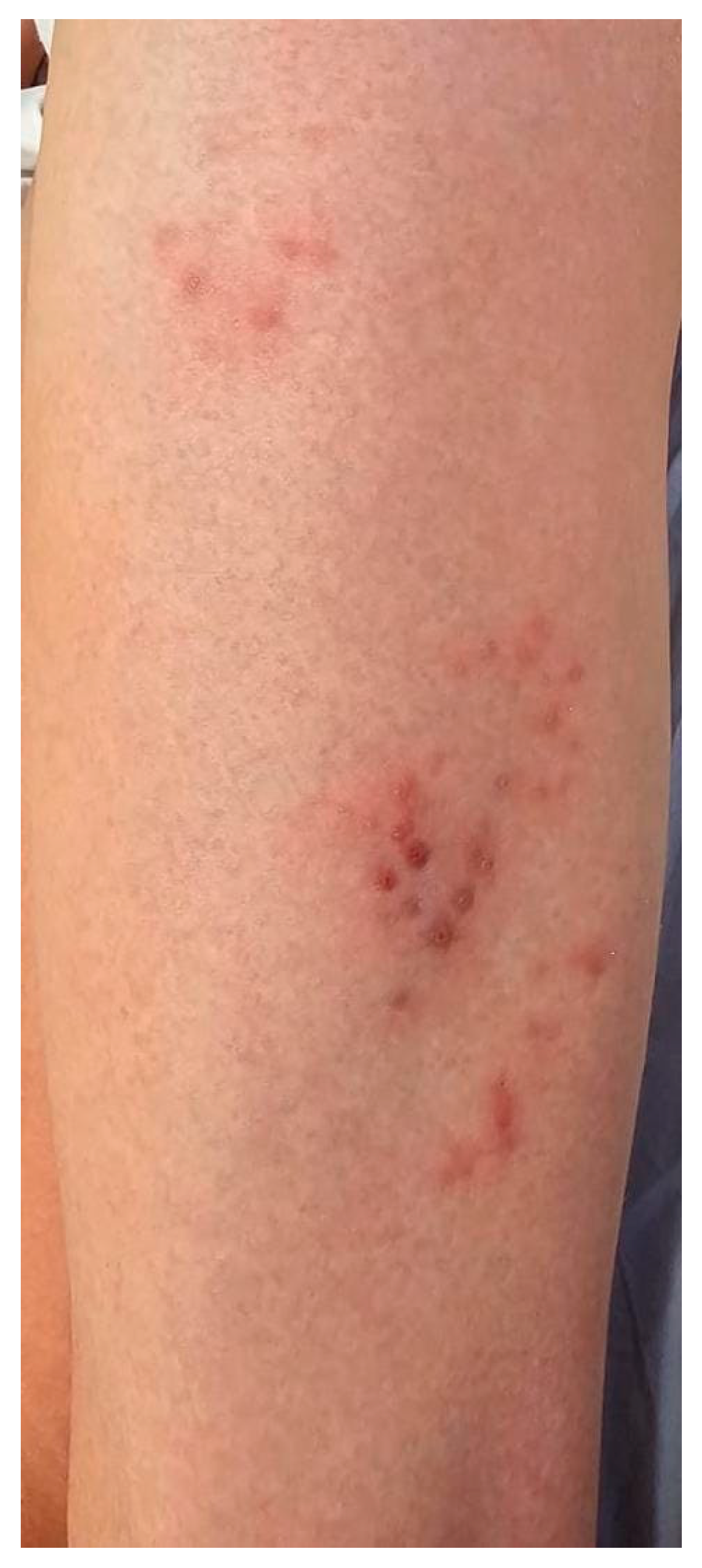
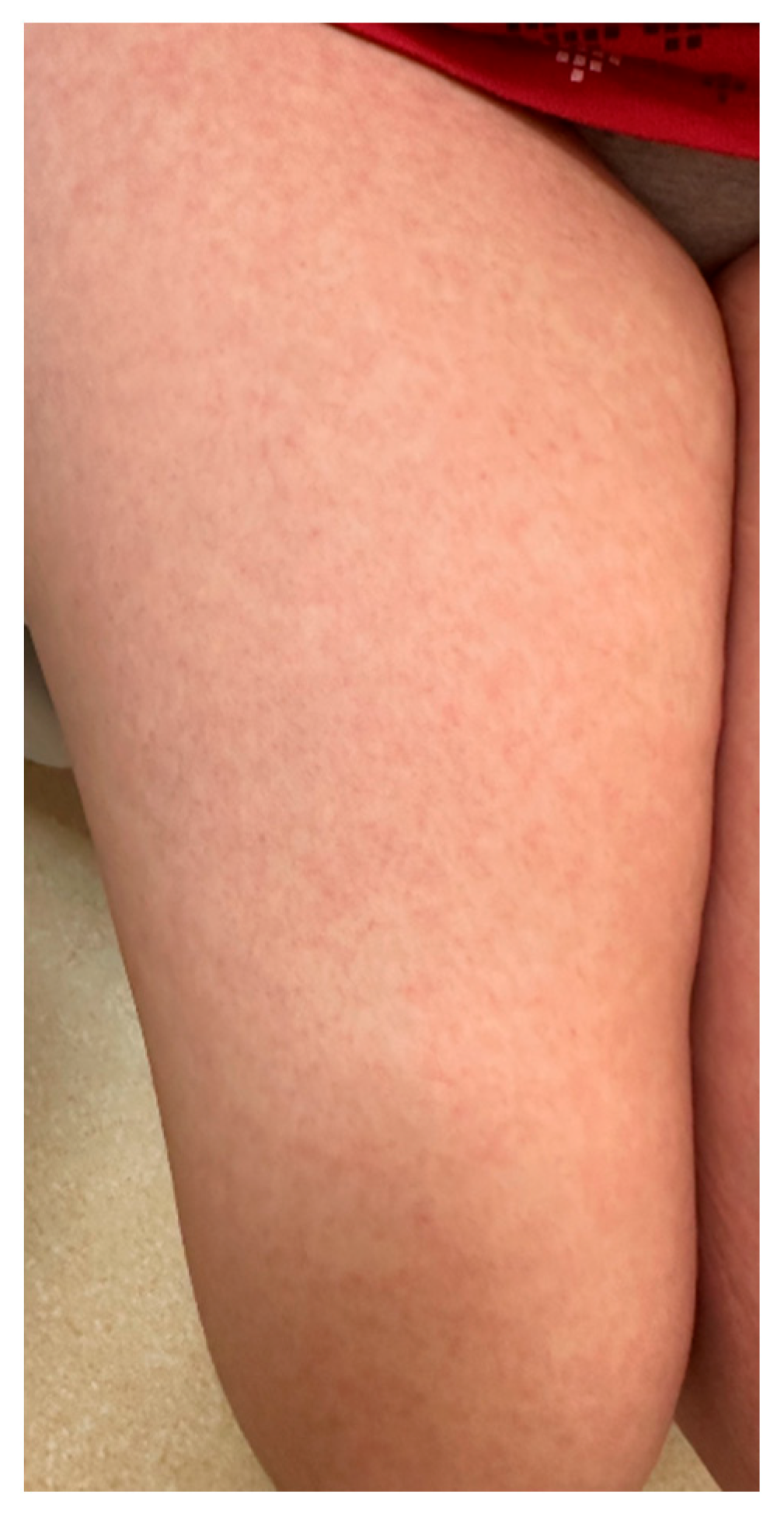
| Summary of Viruses and Skin Changes | ||||||||
|---|---|---|---|---|---|---|---|---|
| Enteroviruses (EVs) [10,11,12] | Herpes Simplex Viruses (HHV-1 and HHV-2) [15,16,17] | Varicella Zoster Virus (VZV/HHV-3) [18,51,52] | Chikungunya Virus (CHIKV) [26,27,29,30] | West Nile Virus (WNV) [31,32] | Zika Virus [34,35] | Colorado Tick Fever Virus [36,37,38] | Dengue Virus [40,41] | |
| Type of skin lesions | Rash, non-itchy, non-desquamating [10] | Recurrent dermatomal vesicular skin lesions, herpetic whitlow | Rash, vesicles, macules, papules | Macular, maculopapular rash, generalized erythematous blanchable rash, hyperpigmentation | Generalized, maculopapular rash | Exanthema or rash | Petechial or maculopapular rash (most frequently a red, haloed papule) | Macular rash, petechial rash and dengue recovery rash |
| Localization | Face, neck, chest, extremities | Non-specific | Singular dermatome with the affected ganglion | Face, neck, extremities, torso | Trunk, upper and lower extremities | Face, torso, limbs, palms, face, abdomen | Face, upper and lower limbs | Face, trunk and abdomen |
| Incidence of a rash (%) | 39.1–46.4 | 1.3 | 42.9–88 | 33–88 | 5–28 | 90 | 15 | 50 |
| Timing of onset | 1–3 days after fever onset | Concurrent with meningeal signs | 3–5 days before neurological symptoms | ~1 day after symptom onset | 5–12 days post-infection | 1–2 days after fever onset | 2–4 days after tick bite | 3–5 days after fever onset |
| Associated Symptoms | Fever, headache, neck stiffness, photophobia | Fever, headache, photophobia, oral/genital lesions | Fever, malaise, headache, pruritus | Fever, severe arthralgia/myalgia, headache | Fever, headache, myalgia, occasional pruritus | Fever, pruritus, arthralgia, conjunctivitis | Fever, myalgia, headache | Fever, severe myalgia, thrombocytopenia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marszałek, A.; Górska, W.; Łukawski, A.; Bieńkowski, C.; Pokorska-Śpiewak, M. Could the Identification of Skin Lesions Be Beneficial for the Differential Diagnosis of Viral Meningitis? Zoonotic Dis. 2025, 5, 16. https://doi.org/10.3390/zoonoticdis5020016
Marszałek A, Górska W, Łukawski A, Bieńkowski C, Pokorska-Śpiewak M. Could the Identification of Skin Lesions Be Beneficial for the Differential Diagnosis of Viral Meningitis? Zoonotic Diseases. 2025; 5(2):16. https://doi.org/10.3390/zoonoticdis5020016
Chicago/Turabian StyleMarszałek, Agata, Weronika Górska, Artur Łukawski, Carlo Bieńkowski, and Maria Pokorska-Śpiewak. 2025. "Could the Identification of Skin Lesions Be Beneficial for the Differential Diagnosis of Viral Meningitis?" Zoonotic Diseases 5, no. 2: 16. https://doi.org/10.3390/zoonoticdis5020016
APA StyleMarszałek, A., Górska, W., Łukawski, A., Bieńkowski, C., & Pokorska-Śpiewak, M. (2025). Could the Identification of Skin Lesions Be Beneficial for the Differential Diagnosis of Viral Meningitis? Zoonotic Diseases, 5(2), 16. https://doi.org/10.3390/zoonoticdis5020016








