Ticks and Tick-Borne Zoonotic Pathogens from Wild Birds in Northwestern Coastal Spain
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
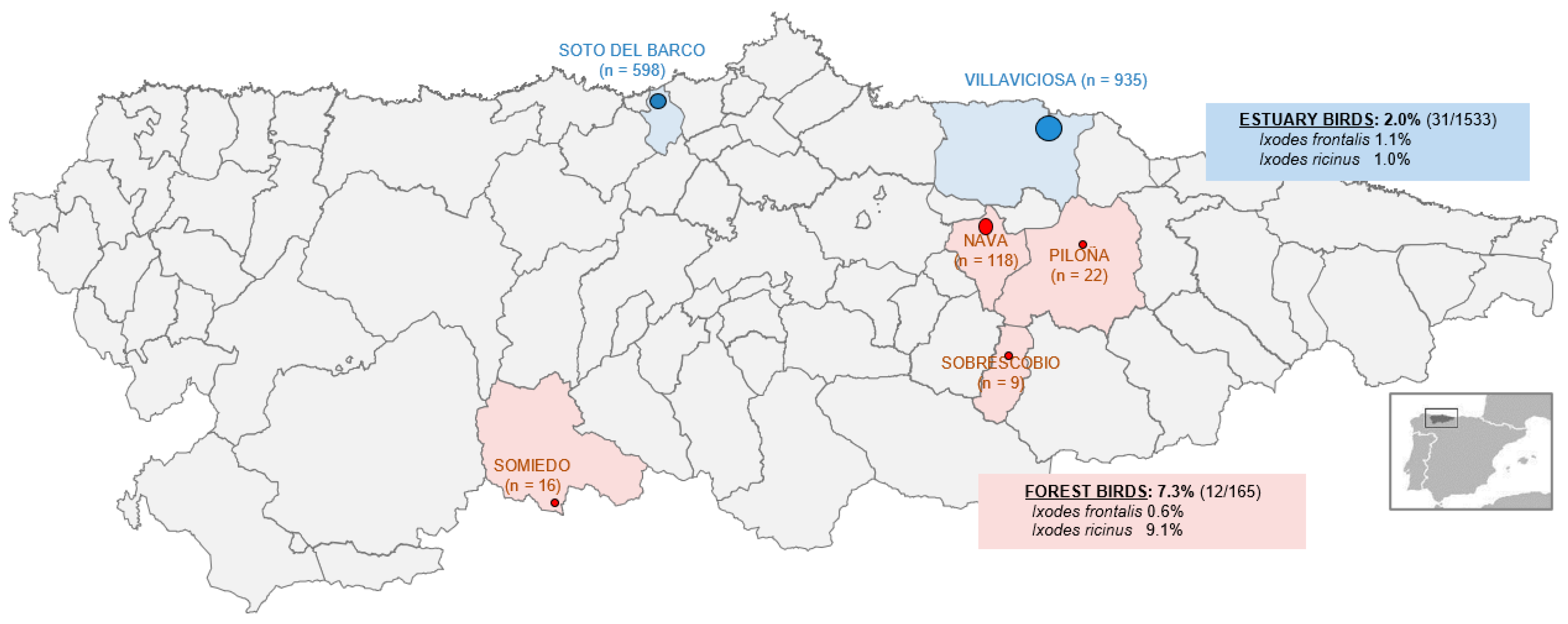
2.2. Site Selection
2.3. Bird Trapping
2.4. Ticks Collection
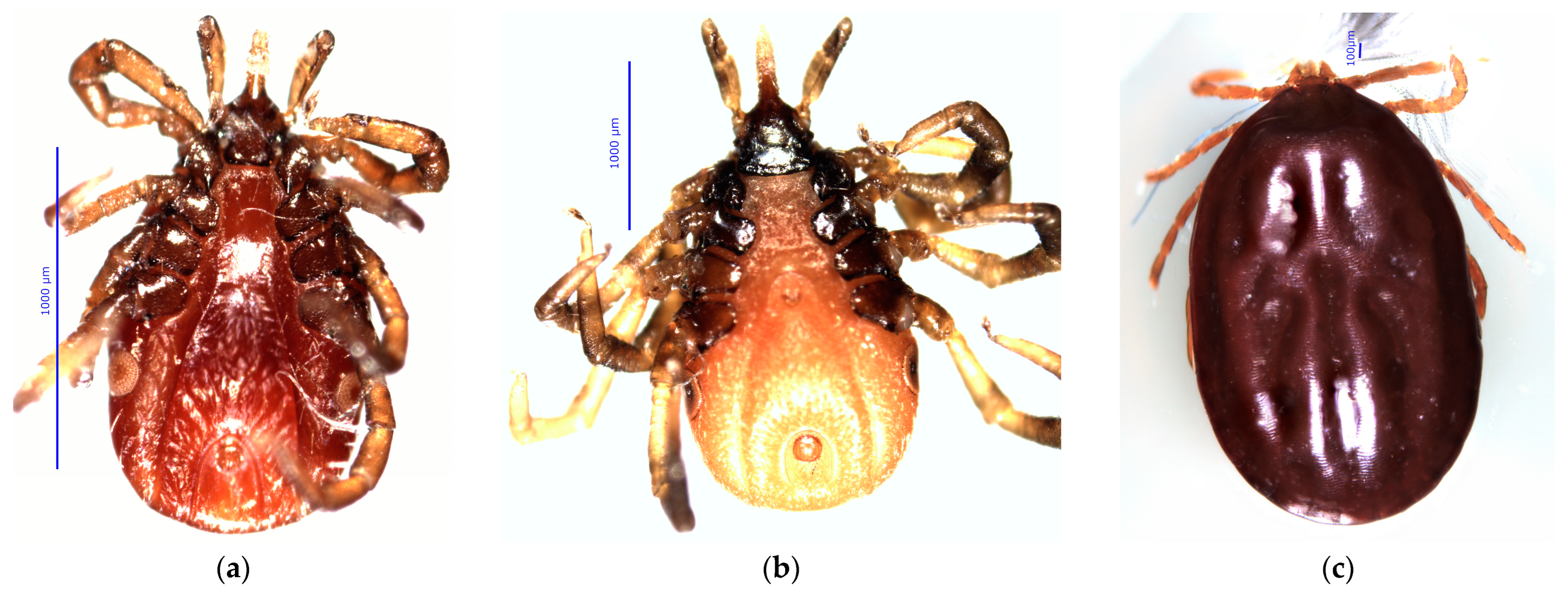
2.5. Tick Identification
2.6. DNA Extraction and PCR Amplification and Sequencing Analysis
2.7. Statistical Analyses
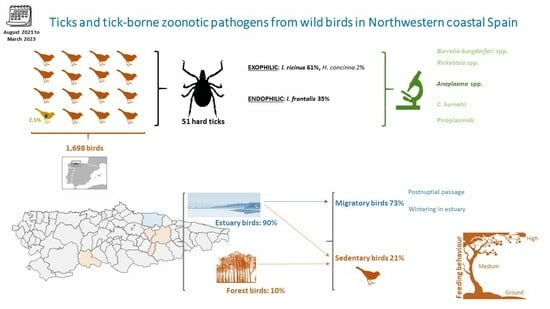
3. Results
3.1. Bird Species
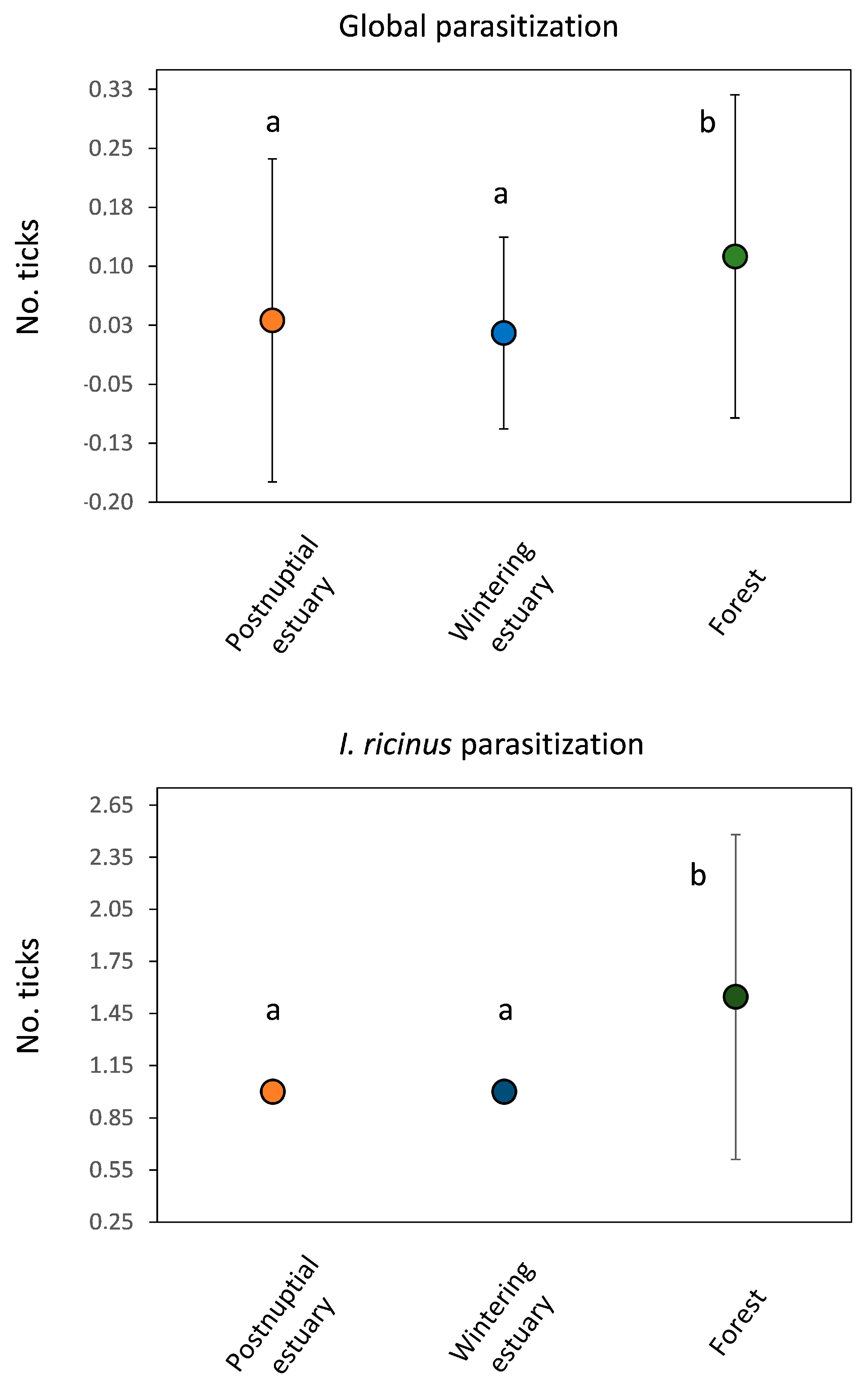
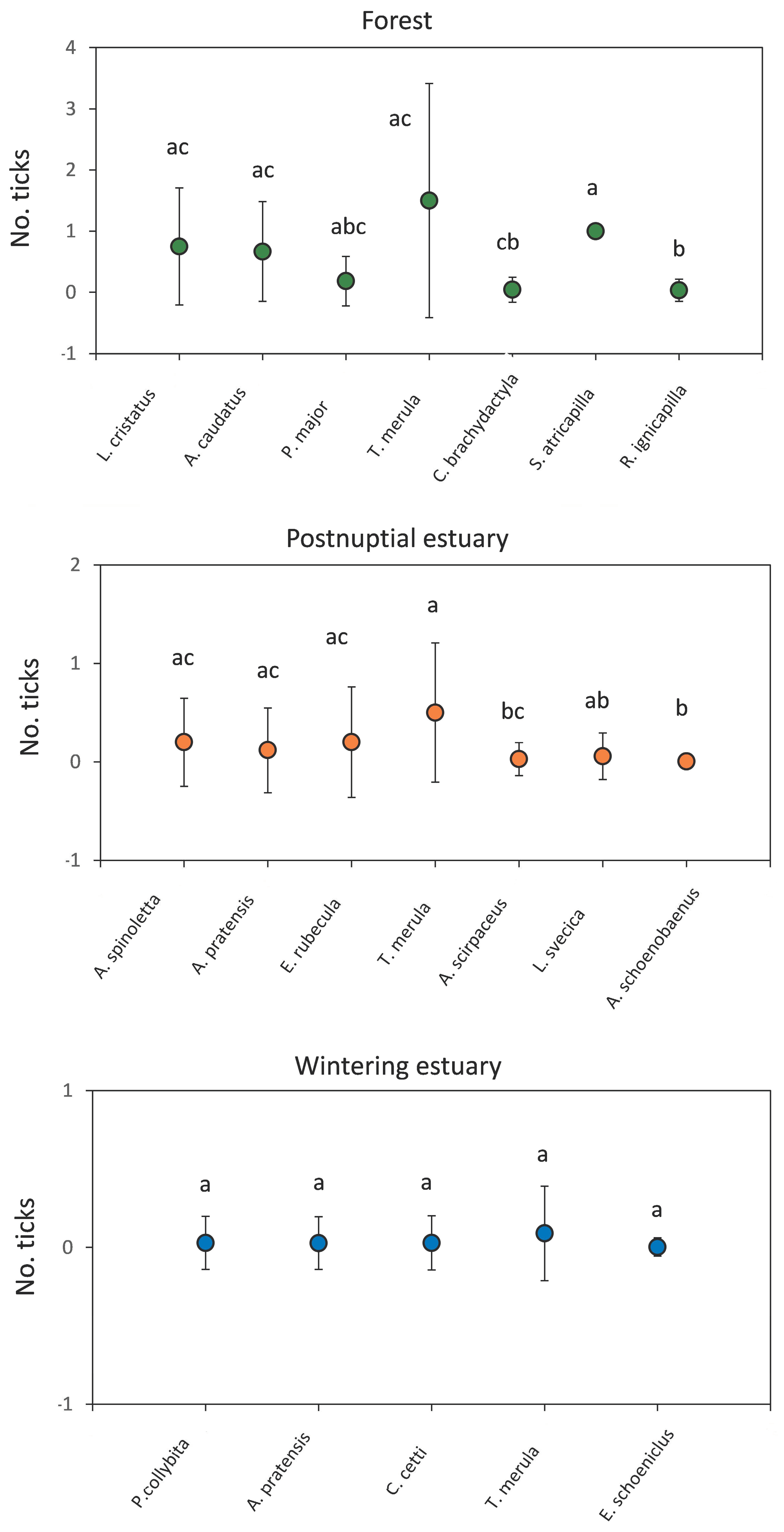
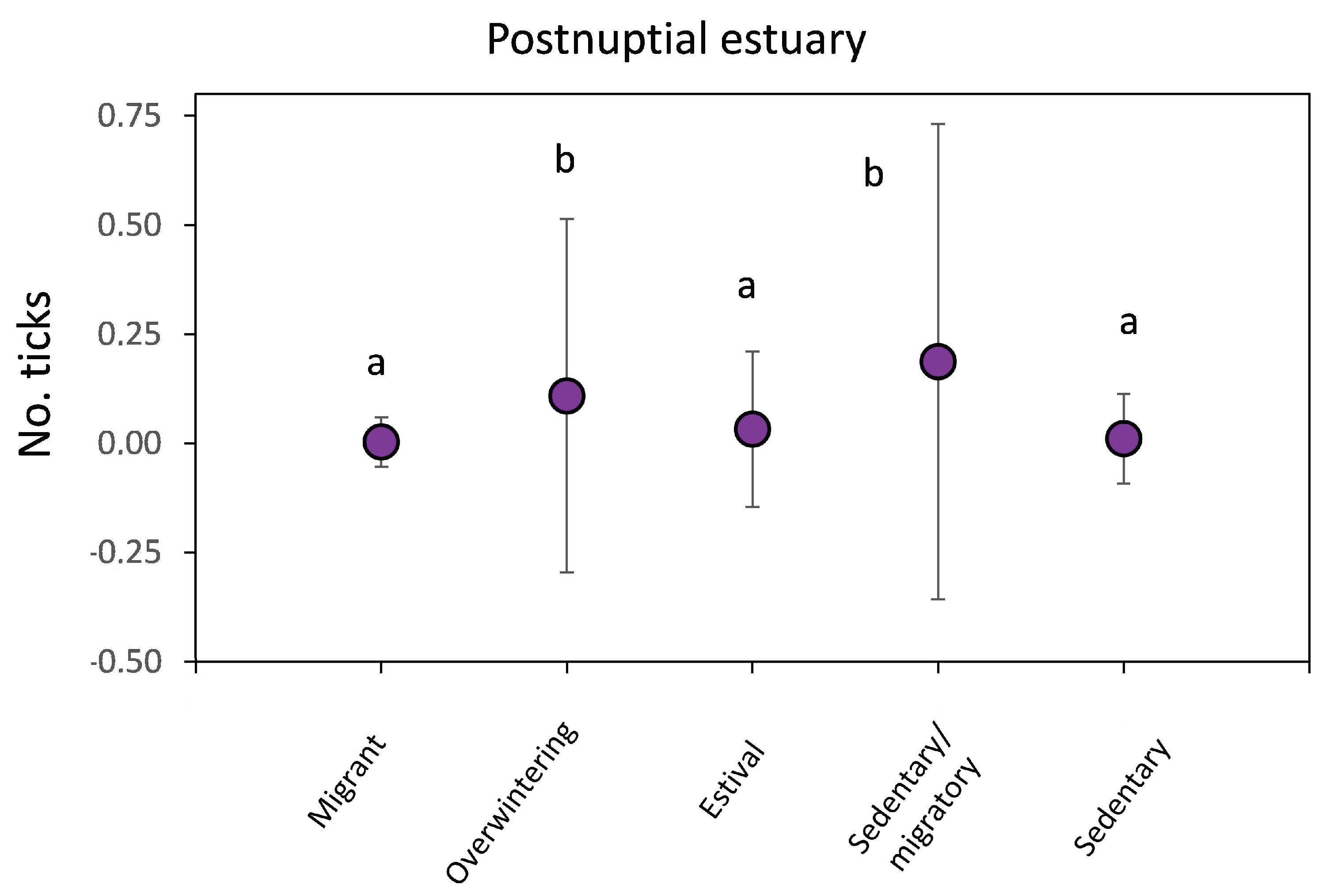
3.2. Tick Infestations
3.3. Pathogen Surveillance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brinkerhoff, R.J.; Folsom-O’Keefe, C.M.; Tsao, K.; Diuk-Wasser, M.A. Do birds affect Lyme Disease risk? Range expansion of the vector-borne pathogen Borrelia burgdorferi. Front. Ecol. Environ. 2011, 9, 103–110. [Google Scholar] [CrossRef]
- Hornok, S.; Karcza, Z.; Csörgo, T. Birds as disseminators of ixodid ticks and tick-borne pathogens: Note on the relevance to migratory routes. Ornis Hung. 2012, 20, 86–89. [Google Scholar] [CrossRef][Green Version]
- Vuong, H.B.; Canham, C.D.; Fonseca, D.M.; Brisson, D.; Morin, P.J.; Smouse, P.E.; Ostfeld, R.S. Occurrence and transmission efficiencies of Borrelia burgdorferi OspC types in avian and mammalian wildlife. Infect. Genet. Evol. 2014, 27, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Sparagano, O.; George, D.; Giangaspero, A.; Spitalska, E. Arthropods and associated arthropod-borne diseases transmitted by migrating birds. The case of ticks and tick-borne pathogens. Vet. Parasitol. 2015, 213, 61–66. [Google Scholar] [CrossRef]
- Jones, T.; Cresswell, W. The phenology mismatch hypothesis: Are declines of migrant birds linked to uneven global climate change? J. Anim. Ecol. 2010, 79, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Convention on Migratory Species (CMS). Eurasian-African Bird Migration Atlas. 2023. Available online: https://migrationatlas.org/ (accessed on 3 October 2023).
- Gordo, O.; Sanz, J.J.; Lobo, J.M. La migración de las aves sobre la península Ibérica. Quercus 2009, 280, 14–22. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=2981989 (accessed on 11 September 2023).
- Moncada-Lorén, M. España, uno de los principales corredores migratorios del mundo por su ubicación geográfica. National Geographic España. 11 May 2018. Available online: https://www.nationalgeographic.es/animales/2018/05/espana-uno-de-los-principales-corredores-migratorios-del-mundo-por-su-ubicacion-geografica (accessed on 11 September 2023).
- Palomar, A.M.; Portillo, A.; Santibáñez, P.; Mazuelas, D.; Arizaga, J.; Crespo, A.; Gutiérrez, Ó.; Cuadrado, J.F.; Oteo, J.A. Crimean-Congo hemorrhagic fever virus in ticks from migratory birds, Morocco. Emerg. Infect. Dis. 2013, 19, 260–263. [Google Scholar] [CrossRef]
- Palomar, A.M.; Santibáñez, P.; Mazuelas, D.; Roncero, L.; Santibáñez, S.; Portillo, A.; Oteo, J.A. Role of birds in dispersal of etiologic agents of tick-borne zoonoses, Spain. Emerg. Infect. Dis. 2012, 18, 1188–1191. [Google Scholar] [CrossRef]
- Alguacil, M.A. Carga hospitalaria de enfermedad de Lyme en España (2005–2019). In Boletín Epidemiológico Semanal (ISCIII); Instituto de Salud Carlos III: Madrid, Spain, 2022; Volume 30, Available online: https://revista.isciii.es/index.php/bes/article/view/1214 (accessed on 21 August 2023).
- Sutherland, W.J.; Newton, I.; Green, R. Techniques in Ecology & Conservation. In Bird Ecology and Conservation: A Handbook of Techniques; Oxford University Press: Oxford, UK, 2004. [Google Scholar] [CrossRef]
- Robinson, R.A.; Julliard, R.; Saracco, J.F. Constant effort: Studying avian population processes using standardised ringing. Ringing Migr. 2009, 24, 199–204. [Google Scholar] [CrossRef]
- Harrison, C. Guía de Campo de los Nidos, Huevos y Polluelos de las Aves de España y de Europa; Omega: Barcelona, Spain, 1991. [Google Scholar]
- Svensson, L. Identification Guide to European Passerines, 4th ed.; British Trust for Ornithology: Stockholm, Sweden, 1992. [Google Scholar]
- Svensson, L.; Mullarney, K.; Zetterstrom, D. Guía de Aves de España, Europa y Región Mediterránea; Omega: Barcelona, Spain, 2010. [Google Scholar]
- Durden, L.A.; Oliver, J.H.; Kinsey, A.A. Ticks (Acari: Ixodidae) and spirochetes (Spirochaetaceae: Spirochaetales) recovered from birds on a Georgia Barrier Island. J. Med. Entomol. 2001, 38, 231–236. [Google Scholar] [CrossRef]
- Manilla, G. Ixodida. In Fauna d’Italia: Acari; Edizioni Calderini: Bologna, Italy, 1998. [Google Scholar]
- Estrada-Peña, A.; Mihalca, A.D.; Petney, T.N. (Eds.) Ticks of Europe and North Africa. A Guide to Species Identification; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Heylen, D.; de Coninck, E.; Jansen, F.; Madder, M. Differential diagnosis of three common Ixodes spp. ticks infesting songbirds of Western Europe: Ixodes arboricola, I. frontalis and I. ricinus. Ticks Tick-Borne Dis. 2014, 5, 693–700. [Google Scholar] [CrossRef]
- Sandelin, L.L.; Tolf, C.; Larsson, S.; Wilhelmsson, P.; Salaneck, E.; Jaenson, T.G.T.; Lindgren, P.E.; Olsen, B.; Waldenström, J. Candidatus Neoehrlichia mikurensis in ticks from migrating birds in Sweden. PLoS ONE 2015, 10, e0133250. [Google Scholar] [CrossRef] [PubMed]
- Black, W.C.; Piesman, J. Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc. Natl. Acad. Sci. USA 1994, 91, 10034–10038. [Google Scholar] [CrossRef]
- Del Cerro, A.; Oleaga, A.; Somoano, A.; Barandika, J.F.; García-Pérez, A.L.; Espí, A. Molecular identification of tick-borne pathogens (Rickettsia spp., Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, Coxiella burnetii and piroplasms) in questing and feeding hard ticks from North-Western Spain. Ticks Tick-Borne Dis. 2022, 13, 101961. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.; Barandika, J.F.; Oporto, B.; Minguijon, E.; Povedano, I.; García-Perez, A.L. Risks of suffering tick-borne diseases in sheep translocated to a tick infested area: A laboratory approach for the investigation of an outbreak. Ticks Tick-Borne Dis. 2015, 6, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Massung, R.F.; Slater, K.; Owens, J.H.; Nicholson, W.L.; Mather, T.N.; Solberg, V.B.; Olson, J.G. Nested PCR assay for detection of granulocytic ehrlichiae. J. Clin. Microbiol. 1998, 36, 1090–1095. [Google Scholar] [CrossRef]
- Courtney, J.W.; Kostelnik, L.M.; Zeidner, N.S.; Massung, R.F. Multiplex real-time PCR for detection of Anaplasma phagocytophilum and Borrelia burgdorferi. J. Clin. Microbiol. 2004, 42, 3164–3168. [Google Scholar] [CrossRef]
- Schets, F.M.; de Heer, L.; de Roda Husman, A.M. Coxiella burnetii in sewage water at sewage water treatment plants in a Q fever epidemic area. Int. J. Hyg. Environ. Health 2013, 216, 698–702. [Google Scholar] [CrossRef]
- Clark, K.; Hendricks, A.; Burge, D. Molecular identification and analysis of Borrelia burgdorferi sensu lato in lizards in the southeastern United States. Appl. Environ. Microbiol. 2005, 71, 2616–2625. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.J.B.; Happ, C.M.; Mayer, L.W.; Piesman, J. Detection of Borrelia burgdorferi in ticks by species-specific amplification of the flagellin gene. Am. J. Trop. Med. Hyg. 1992, 47, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Regnery, R.L.; Spruill, C.L.; Plikaytis, B.D. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 1991, 173, 1576–1589. [Google Scholar] [CrossRef] [PubMed]
- Roux, V.; Fournier, P.E.; Raoult, D. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J. Clin. Microbiol. 1996, 34, 2058–2065. [Google Scholar] [CrossRef]
- Silaghi, C.; Hamel, D.; Thiel, C.; Pfister, K.; Passos, L.M.; Rehbein, S. Genetic variants of Anaplasma phagocytophilum in wild caprine and cervid ungulates from the Alps in Tyrol, Austria. Vector-Borne Zoonotic Dis. 2011, 11, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Overzier, E.; Pfister, K.; Herb, I.; Mahling, M.; Bock, G.; Silaghi, C. Detection of tick-borne pathogens in roe deer (Capreolus capreolus), in questing ticks (Ixodes ricinus), and in ticks infesting roe deer in southern Germany. Ticks Tick-Borne Dis. 2013, 4, 320–328. [Google Scholar] [CrossRef]
- Capligina, V.; Salmane, I.; Keišs, O.; Vilks, K.; Japina, K.; Baumanis, V.; Ranka, R. Prevalence of tick- borne pathogens in ticks collected from migratory birds in Latvia. Ticks Tick-Borne Dis. 2014, 5, 75–81. [Google Scholar] [CrossRef]
- Ciebiera, O.; Jerzak, L.; Nowak-Chmura, M.; Bocheński, M. Ticks (Acari: Ixodida) on birds (Aves) migrating through the Polish Baltic coast. Exp. Appl. Acarol. 2019, 77, 241–251. [Google Scholar] [CrossRef]
- Espí, A.; del Cerro, A.; Somoano, A.; García, V.; Prieto, J.M.; Barandika, J.F.; García-Pérez, A.L. Borrelia burgdorferi sensu lato prevalence and diversity in ticks and small mammals in a Lyme borreliosis endemic Nature Reserve in North-Western Spain. Incidence in surrounding human populations. Enferm. Infecc. Microbiol. Clin. 2017, 35, 563–568. [Google Scholar] [CrossRef]
- Diakou, A.; Norte, A.C.; Lopes de Carvalho, I.; Núncio, S.; Nováková, M.; Kautman, M.; Alivizatos, H.; Kazantzidis, S.; Sychra, O.; Literák, I. Ticks and tick-borne pathogens in wild birds in Greece. Parasitol. Res. 2016, 115, 2011–2016. [Google Scholar] [CrossRef]
- Klaus, C.; Gethmann, J.; Hoffmann, B.; Ziegler, U.; Heller, M.; Beer, M. Tick infestation in birds and prevalence of pathogens in ticks collected from different places in Germany. Parasitol. Res. 2016, 115, 2729–2740. [Google Scholar] [CrossRef]
- Zając, Z.; Kulisz, J.; Kunc-Kozioł, R.; Woźniak, A.; Filipiuk, M.; Rudolf, R.; Bartosik, K.; Cabezas-Cruz, A. Tick Infestation in Migratory Birds of the Vistula River Valley, Poland. Int. J. Environ. Res. Public Health 2022, 19, 13781. [Google Scholar] [CrossRef]
- Olsen, B.; Jaenson, T.G.T.; Bergstrom, S. Prevalence of Borrelia burgdorferi sensu lato-infected ticks on migrating birds. Appl. Environ. Microbiol. 1995, 61, 3082–3087. [Google Scholar] [CrossRef] [PubMed]
- Humair, P.F.; Turrian, N.; Aeschlimann, A.; Gern, L. Ixodes ricinus immatures on birds in a focus of Lyme borreliosis. Folia Parasitol. 1993, 40, 237–242. [Google Scholar]
- Dubska, L.; Literak, I.; Kocianova, E.; Taragelova, V.; Sychra, O. Differential role of passerine birds in distribution of Borrelia Spirochetes, based on data from ticks collected from birds during the postbreeding migration period in central Europe. Appl. Environ. Microbiol. 2009, 75, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Taragelová, V.; Koči, J.; Hanincová, K.; Kurtenbach, K.; Derdáková, M.; Ogden, N.H.; Literák, I.; Kocianová, E.; Labuda, M. Blackbirds and song thrushes constitute a key reservoir of Borrelia garinii, the causative agent of Borreliosis in central Europe. Appl. Environ. Microbiol. 2008, 74, 1289–1293. [Google Scholar] [CrossRef]
- Norte, A.C.; de Carvalho, I.L.; Ramos, J.A.; Gonçalves, M.; Gern, L.; Núncio, M.S. Diversity and seasonal patterns of ticks parasitizing wild birds in western Portugal. Exp. Appl. Acarol. 2012, 58, 327–339. [Google Scholar] [CrossRef]
- Hasle, G. Transport of ixodid ticks and tick-borne pathogens by migratory birds. Front. Cell. Infect. Microbiol. 2013, 3, 48. [Google Scholar] [CrossRef]
- Hornok, S.; Flaisz, B.; Takács, N.; Kontschán, J.; Csörgo, T.; Csipak, A.; Jaksa, B.R.; Kováts, D. Bird ticks in Hungary reflect western, southern, eastern flyway connections and two genetic lineages of Ixodes frontalis and Haemaphysalis concinna. Parasites Vectors 2016, 9, 101. [Google Scholar] [CrossRef]
- Papadopoulos, B.; Humair, P.F.; Aeschlimann, A.; Vaucher, C.; Buttiker, W. Ticks on birds in Switzerland. Acarologia 2001, 42, 3–19. [Google Scholar]
- Movila, A.; Gatewood, A.; Toderas, I.; Duca, M.; Papero, M.; Uspenskaia, I.; Conovalov, J.; Fish, D. Prevalence of Borrelia burgdorferi sensu lato in Ixodes ricinus and I. lividus ticks collected from wild birds in the Republic of Moldova. Int. J. Med. Microbiol. 2008, 298, 149–153. [Google Scholar] [CrossRef]
- James, M.C.; Furness, R.W.; Bowman, A.S.; Forbes, K.J.; Gilbert, L. The importance of passerine birds as tick hosts and in the transmission of Borrelia burgdorferi, the agent of Lyme disease: A case study from Scotland. IBIS 2011, 153, 293–302. [Google Scholar] [CrossRef]
- Movila, A.; Alekseev, A.N.; Dubinina, H.V.; Toderas, I. Detection of tick-borne pathogens in ticks from migratory birds in the Baltic region of Russia. Med. Vet. Entomol. 2013, 27, 113–117. [Google Scholar] [CrossRef]
- Poupon, M.A.; Lommano, E.; Humair, P.F.; Douet, V.; Rais, O.; Schaad, M.; Jenni, L.; Gern, L. Prevalence of Borrelia burgdorferi sensu lato in ticks collected from migratory birds in Switzerland. Appl. Environ. Microbiol. 2006, 72, 976–979. [Google Scholar] [CrossRef]
- Norte, A.C.; da Silva, L.P.; Tenreiro, P.J.Q.; Felgueiras, M.S.; Araújo, P.M.; Lopes, P.B.; Matos, C.; Rosa, A.; Ferreira, P.J.S.G.; Encarnação, P.; et al. Patterns of tick infestation and their Borrelia burgdorferi s.l. infection in wild birds in Portugal. Ticks Tick-Borne Dis. 2015, 6, 743–750. [Google Scholar] [CrossRef]
- Heylen, D.; Fonville, M.; Docters Van Leeuwen, A.; Stroo, A.; Duisterwinkel, M.; Van Wieren, S.; Diuk-Wasser, M.; De Bruin, A.; Sprong, H. Pathogen communities of songbird-derived ticks in Europe’s low countries. Parasites Vectors 2017, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, A.L.; Oporto, B.; Espí, A.; del Cerro, A.; Barral, M.; Povedano, I.; Barandika, J.F.; Hurtado, A. Anaplasmataceae in wild ungulates and carnivores in northern Spain. Ticks Tick-Borne Dis. 2016, 7, 264–269. [Google Scholar] [CrossRef]
- Remesar, S.; Díaz, P.; Prieto, A.; García-Dios, D.; Fernández, G.; López, C.M.; Panadero, R.; Díez-Baños, P.; Morrondo, P. Prevalence and molecular characterization of Anaplasma phagocytophilum in roe deer (Capreolus capreolus) from Spain. Ticks Tick-Borne Dis. 2020, 11, 101351. [Google Scholar] [CrossRef]
- Sormunen, J.J.; Klemola, T.; Vesterinen, E.J. Ticks (Acari: Ixodidae) parasitizing migrating and local breeding birds in Finland. Exp. Appl. Acarol. 2022, 86, 145–156. [Google Scholar] [CrossRef]
- Toma, L.; Mancuso, E.; d’Alessio, S.G.; Menegon, M.; Spina, F.; Pascucci, I.; Monaco, F.; Goffredo, M.; Di Luca, M. Tick species from Africa by migratory birds: A 3-year study in Italy. Exp. Appl. Acarol. 2021, 83, 147–164. [Google Scholar] [CrossRef]
- Wallménius, K.; Barboutis, C.; Fransson, T.; Jaenson, T.G.; Lindgren, P.E.; Nyström, F.; Olsen, B.; Salaneck, E.; Nilsson, K. Spotted fever Rickettsia species in Hyalomma and Ixodes ticks infesting migratory birds in the European Mediterranean area. Parasit. Vectors 2014, 7. [Google Scholar] [CrossRef] [PubMed]



| Bird Family | Bird Species | MB | FB | C. Bird No. | Tick No. | BI % | Tick Stage | Tick Species | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ad | Nf | Lv | I.r | I.f | I. | H.c | |||||||
| Acrocephalidae | Acrocephalus paludicola | m | g | 4 | 0 | 0.0 | |||||||
| Acrocephalus schoenobaenus | m | g | 233 | 1 | 0.4 | 1 | 1 | ||||||
| Acrocephalus scirpaceus | m | m | 107 | 3 | 2.8 | 3 | 1 | 2 | |||||
| Aegithalidae | Aegithalos caudatus | s | m | 7 | 3 | 42.9 | 2 | 1 | 3 | ||||
| Alaudidae | Alauda arvensis | m (s) | g | 1 | 0 | 0.0 | |||||||
| Alcedinidae | Alcedo atthis | s | 1 | 0 | 0.0 | ||||||||
| Certhiidae | Certhia brachydactyla | s | m | 25 | 1 | 4.0 | 1 | 1 | |||||
| Cettiidae | Cettia cetti | s | g | 40 | 1 | 2.5 | 1 | 1 | |||||
| Cisticolidae | Cisticola juncidis | s | m | 39 | 0 | 0.0 | |||||||
| Corvidae | Garrulus glandarius | s | g | 2 | 0 | 0.0 | |||||||
| Emberizidae | Emberiza schoeniclus | m (h) | g | 294 | 1 | 0.3 | 1 | 1 | |||||
| Emberiza pusilla | m (h) | m | 1 | 0 | 0.0 | ||||||||
| Fringillidae | Pyrrhula pyrrhula | s | m | 3 | 0 | 0.0 | |||||||
| Fringilla coelebs | s/m (h) | g | 14 | 0 | 0.0 | ||||||||
| Chloris Chloris | s/m (h) | g | 7 | 0 | 0.0 | ||||||||
| Carduelis carduelis | s | g | 6 | 0 | 0.0 | ||||||||
| Serinus serinus | s | h | 1 | 0 | 0.0 | ||||||||
| Hirundinidae | Hirundo rustica | m | h | 12 | 0 | 0,0 | |||||||
| Laniidae | Lanius collurio | m (s) | g | 4 | 0 | 0,0 | |||||||
| Locustellidae | Locustella naevia | m (s) | g | 4 | 0 | 0.0 | |||||||
| Motacillidae | Anthus spinoletta | m (h) | g | 29 | 1 | 3.4 | 1 | 1 | |||||
| Motacilla flava | m (s) | g | 4 | 1 | 25.0 | 1 | 1 | ||||||
| Anthus petrosus | m (h) | g | 1 | 0 | 0.0 | ||||||||
| Anthus pratensis | m (h) | g | 146 | 9 | 6.2 | 2 | 9 | 7 | 4 | ||||
| Anthus trivialis | m (s) | m | 1 | 0 | 0.0 | ||||||||
| Motacilla alba | s/m (h) | g | 2 | 0 | 0.0 | ||||||||
| Muscicapidae | Luscinia svecica | m (s) | g | 39 | 2 | 5.1 | 2 | 2 | |||||
| Erithacus rubecula | s/m (h) | g | 52 | 2 | 3.8 | 1 | 2 | 1 | 2 | ||||
| Saxicola rubicola | s | g | 46 | 0 | 0.0 | ||||||||
| Saxicola rubetra | m | g | 20 | 0 | 0.0 | ||||||||
| Paridae | Cyanistes caeruleus | s | h | 15 | 0 | 0.0 | |||||||
| Lophophanes cristatus | s | h | 5 | 2 | 40.0 | 1 | 2 | 3 | |||||
| Parus major | s | m | 16 | 2 | 12.5 | 2 | 2 | ||||||
| Poecile palustris | s | h | 23 | 0 | 0.0 | ||||||||
| Periparus ater | s | h | 15 | 0 | 0.0 | ||||||||
| Passeridae | Passer domesticus | s | g | 8 | 0 | 0.0 | |||||||
| Phylloscopidae | Phylloscopus collybita | m (h) | h | 283 | 8 | 2.8 | 1 | 1 | 6 | 2 | 6 | ||
| Phylloscopus ibericus | m (s) | h | 3 | 0 | 0.0 | ||||||||
| Phylloscopus trochilus | m | h | 31 | 0 | 0.0 | ||||||||
| Picidae | Dendrocopos major | s | h | 2 | 0 | 0.0 | |||||||
| Jynx torquilla | m | g | 2 | 0 | 0.0 | ||||||||
| Prunellidae | Prunella modularis | s | g | 10 | 0 | 0.0 | |||||||
| Regulidae | Regulus ignicapilla | s | h | 31 | 1 | 3.2 | 1 | 1 | |||||
| Sittidae | Sitta europaea | s | h | 3 | 0 | 0.0 | |||||||
| Sturnidae | Sturnus unicolor | s | g | 1 | 0 | 0.0 | |||||||
| Sylviidae | Sylvia communis | m | m | 12 | 0 | 0.0 | |||||||
| Sylvia atricapilla | s/m (h) | m | 27 | 1 | 3.7 | 1 | 1 | ||||||
| Sylvia melanocephala | s | m | 10 | 0 | 0.0 | ||||||||
| Troglodytidae | Troglodytes troglodytes | s | m | 16 | 0 | 0.0 | |||||||
| Turdidae | Turdus merula | s | g | 17 | 4 | 23.5 | 1 | 7 | 6 | 1 | 1 | ||
| Turdus iliacus | m (h) | g | 2 | 0 | 0.0 | ||||||||
| Turdus philomelos | s | g | 21 | 0 | 0.0 | ||||||||
| 26 families | 52 species | Total | 1698 | 43 | 2.53 | 3 | 20 | 28 | 31 | 18 | 1 | 1 | |
| Target | Target Gene | Oligo Name | Sequence (5′–3′) | References |
|---|---|---|---|---|
| Ixodidae | 16S rRNA | 16S+3 | ATACTCTAGGGATAACAGCGT | [22] |
| 16S−3 | AAATTCATAGGGTCTTCTTGTC | |||
| Anaplasma spp. | 16S rRNA | Aspp16S-F | GCTATGCCGCGTGAGTGAG | [26] |
| Aspp16S-R | AGTTTGCCGGGACTTCTTCTG | |||
| Aspp16S-Pr | FAM-CTTAGGGTTGTAAAACTC-MGB | |||
| A. phagocytophilum | 16S rRNA | ge3a | CACATGCAAGTCGAACGGATTATTC | [27] |
| ge10r | TTCCGTTAAGAAGGATCTAATCTCC | |||
| ge9f | AACGGATTATTCTTTATAGCTTGCT a | |||
| ge2 | GGCAGTATTAAAAGCAGCTCCAGG a | |||
| A. phagocytophilum | msp2 | ApMSP2-FN1 | AAGGCAGTGTTGGKTAYGGTATT | [26] |
| ApMSP2-R | TTGGTCTTGAAGCGCTCGTA | [28] | ||
| ApMSP2-Pr | FAM-TGGTGCCAGGGTTGAGCTTGAGATTG-BHQ1 | |||
| Coxiella burnetii | IS1111 | sIS1pri_f | CGGGTTAAGCGTGCTCAGTAT | [29] |
| sIS1pri_r | TCCACACGCTTCCATCACCAC | |||
| Tqpro sIS1 | FAM-AGC CCA CCT TAA GAC TGG CTA CGG TGG AT-BHQ1 | |||
| Piroplasmids | 18S rRNA | TB-F3 | TTACTTW[+G]AG[+A][+A]AAYTAGAGTG b | [26] |
| TB-R3 | CTAAGAATTTCA[+C]CTCTGACA b | |||
| TB-Pr3 | JOE-CCAA[+C]Y[+G]TT[+C][+C]TATTAA[+C][+C]ATTA-BHQ1 b | |||
| Borrelia burgdorferi s.l | flaB | Outer 1 | AARGAATTGGCAGTTCAATC | [30] |
| Outer 2 | GCATTTTCWATTTTAGCAAGTGATG | |||
| Inner 1 | ACATATTCAGAGCAGACAGAGGTTCTA | |||
| Inner 2 | GAAGGTGCTGTAGCAGGTGCTGGCTGT | [31] | ||
| Rickettsia spp. | ompA | Rp190.70p | ATGGCGAATATTTCTCCAAAA | [32] |
| Rp190.701n | GTTCCGTTAATGGCAGCATCT | [33] | ||
| Rp190.602n | AGTGCAGCATTCGCTCCCCCT | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espí, A.; del Cerro, A.; Peón-Torre, P.; González-Escudero, J.V.; Somoano, A. Ticks and Tick-Borne Zoonotic Pathogens from Wild Birds in Northwestern Coastal Spain. Zoonotic Dis. 2023, 3, 316-333. https://doi.org/10.3390/zoonoticdis3040026
Espí A, del Cerro A, Peón-Torre P, González-Escudero JV, Somoano A. Ticks and Tick-Borne Zoonotic Pathogens from Wild Birds in Northwestern Coastal Spain. Zoonotic Diseases. 2023; 3(4):316-333. https://doi.org/10.3390/zoonoticdis3040026
Chicago/Turabian StyleEspí, Alberto, Ana del Cerro, Paloma Peón-Torre, José Vicente González-Escudero, and Aitor Somoano. 2023. "Ticks and Tick-Borne Zoonotic Pathogens from Wild Birds in Northwestern Coastal Spain" Zoonotic Diseases 3, no. 4: 316-333. https://doi.org/10.3390/zoonoticdis3040026
APA StyleEspí, A., del Cerro, A., Peón-Torre, P., González-Escudero, J. V., & Somoano, A. (2023). Ticks and Tick-Borne Zoonotic Pathogens from Wild Birds in Northwestern Coastal Spain. Zoonotic Diseases, 3(4), 316-333. https://doi.org/10.3390/zoonoticdis3040026