Heartland Virus: An Evolving Story of an Emerging Zoonotic and Vector-Borne Disease
Abstract
:Simple Summary
Abstract
1. Introduction
2. Viral Etiology
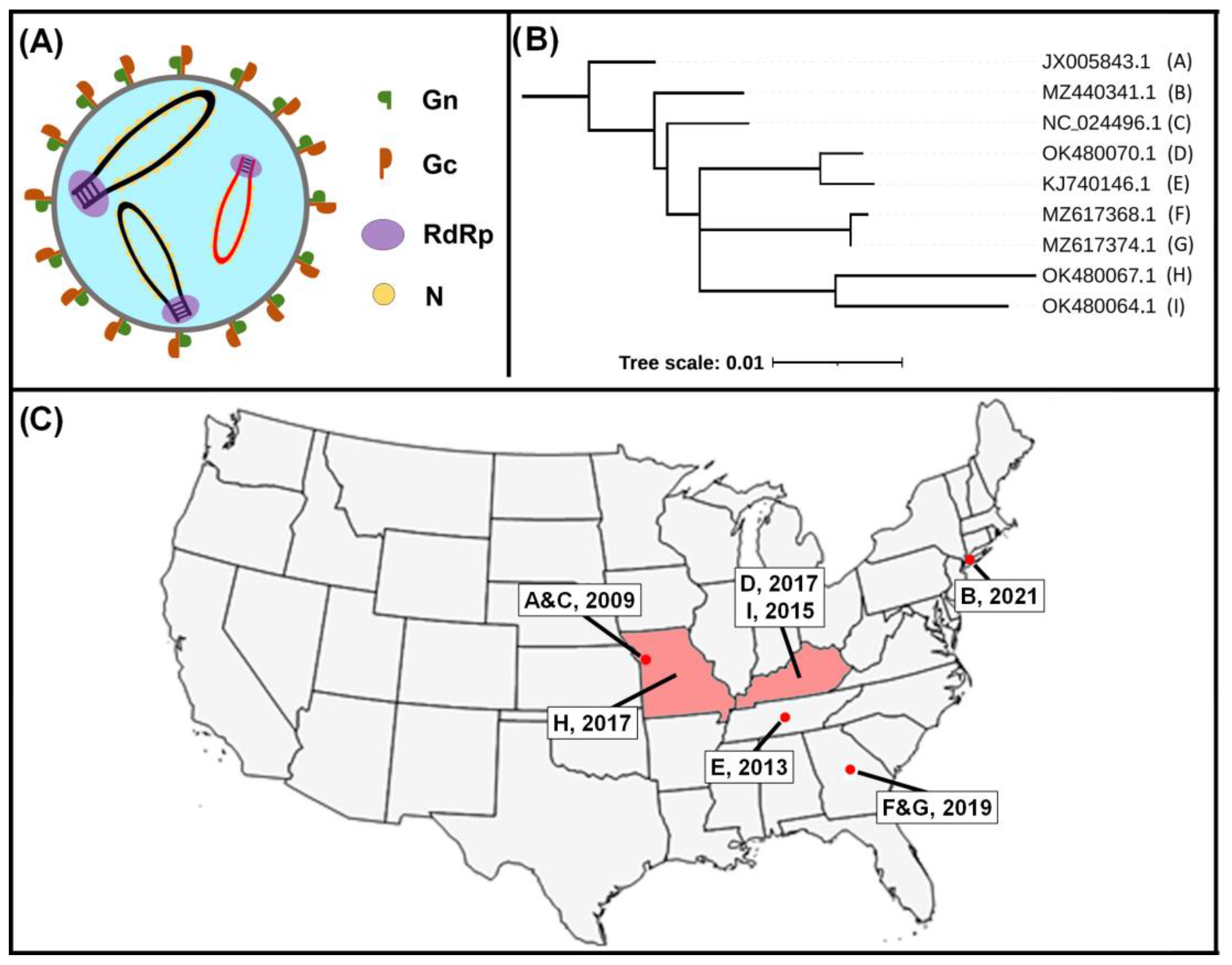
3. Epidemiology
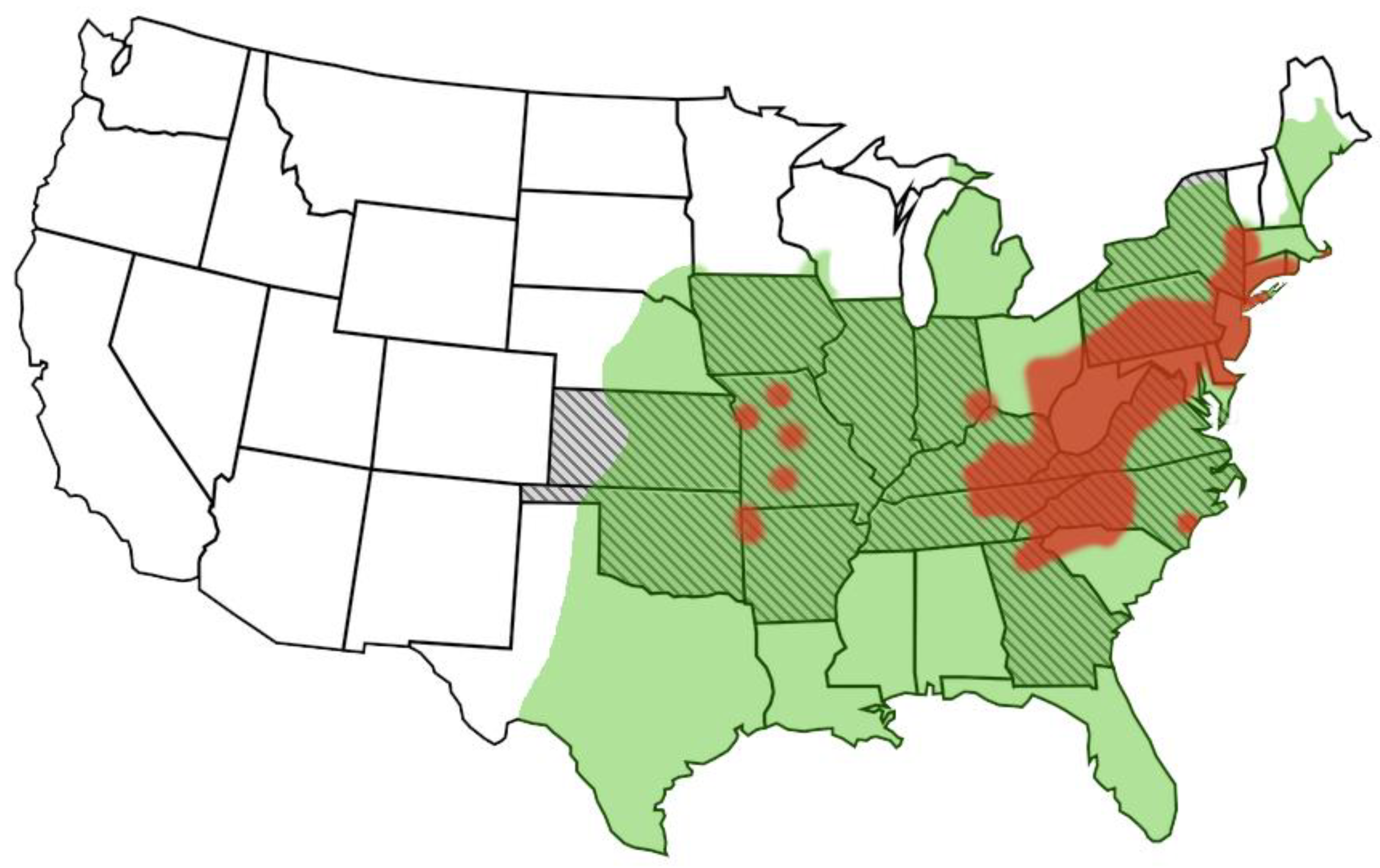
4. Host Range
5. Viral Pathogenesis
6. Transmission and Vector Ecology
7. Clinical Findings, Differential Diagnosis, Treatment, and Prognosis
8. Diagnosis and Surveillance
9. Prevention
10. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McMullan, L.K.; Folk, S.M.; Kelly, A.J.; MacNeil, A.; Goldsmith, C.S.; Metcalfe, M.G.; Batten, B.C.; Albariño, C.G.; Rollin, S.R.Z.E.; Nicholson, W.L.; et al. A new phlebovirus associated with severe febrile illness in Missouri. N. Engl. J. Med. 2012, 367, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.J.; Liang, M.F.; Zhang, S.Y.; Liu, Y.; Li, J.D.; Sun, Y.L.; Zhang, L.; Zhang, Q.F.; Popov, V.L.; Li, C.; et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 2011, 364, 1523–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, J.H.; Adkins, S.; Alkhovsky, S.V.; Avsic-Zupanc, T.; Ayllon, M.A.; Bahl, J.; Balkema-Buschmann, A.; Ballinger, M.J.; Bandte, M.; Beer, M.; et al. 2022 taxonomic update of phylum Negarnaviricota (Riboviria: Orthornavirae), including the large orders Bunyavirales and Mononegavirales. Arch. Virol. 2022, 167, 2857–2906. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Zhou, M.; Deng, F.; Wang, H.; Ning, Y.J. Combinatorial Minigenome Systems for Emerging Banyangviruses Reveal Viral Reassortment Potential and Importance of a Protruding Nucleotide in Genome "Panhandle" for Promoter Activity and Reassortment. Front. Microbiol. 2020, 11, 599. [Google Scholar] [CrossRef] [Green Version]
- Calvert, A.E.; Brault, A.C. Development and Characterization of Monoclonal Antibodies Directed Against the Nucleoprotein of Heartland Virus. Am. J. Trop. Med. Hyg. 2015, 93, 1338–1340. [Google Scholar] [CrossRef] [Green Version]
- Bosco-Lauth, A.M.; Panella, N.A.; Root, J.J.; Gidlewski, T.; Lash, R.R.; Harmon, J.R.; Burkhalter, K.L.; Godsey, M.S.; Savage, H.M.; Nicholson, W.L.; et al. Serological investigation of heartland virus (Bunyaviridae: Phlebovirus) exposure in wild and domestic animals adjacent to human case sites in Missouri 2012–2013. Am. J. Trop. Med. Hyg. 2015, 92, 1163–1167. [Google Scholar] [CrossRef] [Green Version]
- Clarke, L.L.; Ruder, M.G.; Mead, D.G.; Howerth, E.W. Heartland Virus Exposure in White-Tailed Deer in the Southeastern United States, 2001–2015. Am. J. Trop. Med. Hyg. 2018, 99, 1346–1349. [Google Scholar] [CrossRef] [Green Version]
- Lindsey, N.P.; Menitove, J.E.; Biggerstaff, B.J.; Turabelidze, G.; Parton, P.; Peck, K.; Basile, A.J.; Kosoy, O.I.; Fischer, M.; Staples, J.E. Seroprevalence of Heartland Virus Antibodies in Blood Donors, Northwestern Missouri, USA. Emerg. Infect. Dis. 2019, 25, 358–360. [Google Scholar] [CrossRef]
- Feng, K.; Deng, F.; Hu, Z.; Wang, H.; Ning, Y.J. Heartland virus antagonizes type I and III interferon antiviral signaling by inhibiting phosphorylation and nuclear translocation of STAT2 and STAT1. J. Biol. Chem. 2019, 294, 9503–9517. [Google Scholar] [CrossRef]
- Ning, Y.J.; Feng, K.; Min, Y.Q.; Deng, F.; Hu, Z.; Wang, H. Heartland virus NSs protein disrupts host defenses by blocking the TBK1 kinase-IRF3 transcription factor interaction and signaling required for interferon induction. J. Biol. Chem. 2017, 292, 16722–16733. [Google Scholar] [CrossRef]
- Rezelj, V.V.; Li, P.; Chaudhary, V.; Elliott, R.M.; Jin, D.Y.; Brennan, B. Differential Antagonism of Human Innate Immune Responses by Tick-Borne Phlebovirus Nonstructural Proteins. mSphere 2017, 2, e00234-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, B.H.; Albarino, C.G.; Hartman, A.L.; Erickson, B.R.; Ksiazek, T.G.; Nichol, S.T. Rift valley fever virus lacking the NSs and NSm genes is highly attenuated, confers protective immunity from virulent virus challenge, and allows for differential identification of infected and vaccinated animals. J. Virol. 2008, 82, 2681–2691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikegami, T.; Won, S.; Peters, C.J.; Makino, S. Rescue of infectious rift valley fever virus entirely from cDNA, analysis of virus lacking the NSs gene, and expression of a foreign gene. J. Virol. 2006, 80, 2933–2940. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, S.; Inagaki, T.; Tajima, S.; Suzuki, T.; Yoshikawa, T.; Fukushi, S.; Park, E.S.; Fujii, H.; Morikawa, S.; Tani, H.; et al. Reverse Genetics System for Heartland Bandavirus: NSs Protein Contributes to Heartland Bandavirus Virulence. J. Virol. 2022, 96, e0004922. [Google Scholar] [CrossRef]
- Lemoine, F.; Correia, D.; Lefort, V.; Doppelt-Azeroual, O.; Mareuil, F.; Cohen-Boulakia, S.; Gascuel, O. NGPhylogeny.fr: New generation phylogenetic services for non-specialists. Nucleic Acids Res. 2019, 47, W260–W265. [Google Scholar] [CrossRef] [Green Version]
- Heartland Virus Disease (Heartland) Statistics and Maps. Available online: https://www.cdc.gov/heartland-virus/statistics/index.html (accessed on 21 July 2023).
- Riemersma, K.K.; Komar, N. Heartland Virus Neutralizing Antibodies in Vertebrate Wildlife, United States, 2009-2014. Emerg. Infect. Dis. 2015, 21, 1830–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brault, A.C.; Savage, H.M.; Duggal, N.K.; Eisen, R.J.; Staples, J.E. Heartland Virus Epidemiology, Vector Association, and Disease Potential. Viruses 2018, 10, 498. [Google Scholar] [CrossRef] [Green Version]
- Staples, J.E.; Pastula, D.M.; Panella, A.J.; Rabe, I.B.; Kosoy, O.I.; Walker, W.L.; Velez, J.O.; Lambert, A.J.; Fischer, M. Investigation of Heartland Virus Disease Throughout the United States, 2013–2017. Open Forum Infect. Dis. 2020, 7, ofaa125. [Google Scholar] [CrossRef] [Green Version]
- Bopp, N.E.; Kaiser, J.A.; Strother, A.E.; Barrett, A.D.T.; Beasley, D.W.C.; Benassi, V.; Milligan, G.N.; Preziosi, M.-P.; Reece, L.M. Baseline mapping of severe fever with thrombocytopenia syndrome virology, epidemiology and vaccine research and development. npj Vaccines 2020, 5, 111. [Google Scholar] [CrossRef]
- Fang, X.; Hu, J.; Peng, Z.; Dai, Q.; Liu, W.; Liang, S.; Li, Z.; Zhang, N.; Bao, C. Epidemiological and clinical characteristics of severe fever with thrombocytopenia syndrome bunyavirus human-to-human transmission. PLoS Negl. Trop. Dis. 2021, 15, e0009037. [Google Scholar] [CrossRef]
- Chen, Y.; Jia, B.; Huang, R.; Yan, X.; Xiong, Y.; Yong, L.; Chao, W. Occupational Severe Fever With Thrombocytopenia Syndrome Following Needle-Stick Injury. Infect. Control Hosp. Epidemiol. 2017, 38, 760–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.; Wu, W.; Wang, H.; Du, Y.; Liu, L.; Kang, K.; Huang, X.; Ma, H.; Mu, F.; Zhang, S.; et al. Human-to-human transmission of severe fever with thrombocytopenia syndrome bunyavirus through contact with infectious blood. J. Infect. Dis. 2013, 207, 736–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamanaka, A.; Kirino, Y.; Fujimoto, S.; Ueda, N.; Himeji, D.; Miura, M.; Sudaryatma, P.E.; Sato, Y.; Tanaka, H.; Mekata, H.; et al. Direct Transmission of Severe Fever with Thrombocytopenia Syndrome Virus from Domestic Cat to Veterinary Personnel. Emerg. Infect. Dis. 2020, 26, 2994–2998. [Google Scholar] [CrossRef] [PubMed]
- Monzón, J.D.; Atkinson, E.G.; Henn, B.M.; Benach, J.L. Population and Evolutionary Genomics of Amblyomma americanum, an Expanding Arthropod Disease Vector. Genome Biol. Evol. 2016, 8, 1351–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Asian Longhorned Tick: What You Need to Know and What You Can Do. Available online: https://www.aphis.usda.gov/aphis/maps/animal-health/asian-longhorned-tick (accessed on 21 July 2023).
- Bosco-Lauth, A.M.; Calvert, A.E.; Root, J.J.; Gidlewski, T.; Bird, B.H.; Bowen, R.A.; Muehlenbachs, A.; Zaki, S.R.; Brault, A.C. Vertebrate Host Susceptibility to Heartland Virus. Emerg. Infect. Dis. 2016, 22, 2070–2077. [Google Scholar] [CrossRef] [Green Version]
- Clarke, L.L.; Ruder, M.G.; Mead, D.; Howerth, E.W. Experimental Infection of White-Tailed Deer (Odocoileus virginanus) with Heartland Virus. Am. J. Trop. Med. Hyg. 2018, 98, 1194–1196. [Google Scholar] [CrossRef] [Green Version]
- Maqbool, M.; Sajid, M.S.; Saqib, M.; Anjum, F.R.; Tayyab, M.H.; Rizwan, H.M.; Rashid, M.I.; Rashid, I.; Iqbal, A.; Siddique, R.M.; et al. Potential Mechanisms of Transmission of Tick-Borne Viruses at the Virus-Tick Interface. Front. Microbiol. 2022, 13, 846884. [Google Scholar] [CrossRef]
- Gargili, A.; Estrada-Peña, A.; Spengler, J.R.; Lukashev, A.; Nuttall, P.A.; Bente, D.A. The role of ticks in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus: A review of published field and laboratory studies. Antivir. Res. 2017, 144, 93–119. [Google Scholar] [CrossRef]
- Westover, J.B.; Rigas, J.D.; Van Wettere, A.J.; Li, R.; Hickerson, B.T.; Jung, K.H.; Miao, J.; Reynolds, E.S.; Conrad, B.L.; Nielson, S.; et al. Heartland virus infection in hamsters deficient in type I interferon signaling: Protracted disease course ameliorated by favipiravir. Virology 2017, 511, 175–183. [Google Scholar] [CrossRef]
- Fujii, H.; Tani, H.; Egawa, K.; Taniguchi, S.; Yoshikawa, T.; Fukushi, S.; Yamada, S.; Harada, S.; Kurosu, T.; Shimojima, M.; et al. Susceptibility of Type I Interferon Receptor Knock-Out Mice to Heartland Bandavirus (HRTV) Infection and Efficacy of Favipiravir and Ribavirin in the Treatment of the Mice Infected with HRTV. Viruses 2022, 14, 1668. [Google Scholar] [CrossRef]
- Huang, X.Y.; Du, Y.H.; Wang, H.F.; You, A.G.; Li, Y.; Su, J.; Nie, Y.F.; Ma, H.X.; Xu, B.L. Prevalence of severe fever with thrombocytopenia syndrome virus in animals in Henan Province, China. Infect. Dis. Poverty 2019, 8, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Min, Y.-Q.; Li, Y.; Sun, X.; Deng, F.; Wang, H.; Ning, Y.-J. Animal Model of Severe Fever With Thrombocytopenia Syndrome Virus Infection. Front. Microbiol. 2022, 12, 797189. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Nabeshima, T.; Inoue, S.; Tun, M.M.N.; Obata, M.; Hu, W.; Shimoda, H.; Kurihara, S.; Izumikawa, K.; Morita, K.; et al. Severe Fever with Thrombocytopenia Syndrome in Cats and Its Prevalence among Veterinarian Staff Members in Nagasaki, Japan. Viruses 2021, 13, 1142. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, K.; Nonoue, N.; Noda, A.; Kasajima, N.; Noguchi, K.; Takano, A.; Shimoda, H.; Orba, Y.; Muramatsu, M.; Sakoda, Y.; et al. Fatal Tickborne Phlebovirus Infection in Captive Cheetahs, Japan. Emerg. Infect. Dis. 2018, 24, 1726–1729. [Google Scholar] [CrossRef] [Green Version]
- Ishijima, K.; Tatemoto, K.; Park, E.; Kimura, M.; Fujita, O.; Taira, M.; Kuroda, Y.; Mendoza, M.V.; Inoue, Y.; Harada, M.; et al. Lethal Disease in Dogs Naturally Infected with Severe Fever with Thrombocytopenia Syndrome Virus. Viruses 2022, 14, 1963. [Google Scholar] [CrossRef]
- Park, S.-J.; Kim, Y.-I.; Casel, M.A.; Kim, E.-H.; Kim, S.-M.; Yu, K.-M.; Rollon, R.; Jang, S.-G.; Jeong, H.W.; Choi, Y.K. Infection Route Impacts the Pathogenesis of Severe Fever with Thrombocytopenia Syndrome Virus in Ferrets. Viruses 2022, 14, 1184. [Google Scholar] [CrossRef]
- Park, E.S.; Shimojima, M.; Nagata, N.; Ami, Y.; Yoshikawa, T.; Iwata-Yoshikawa, N.; Fukushi, S.; Watanabe, S.; Kurosu, T.; Kataoka, M.; et al. Severe Fever with Thrombocytopenia Syndrome Phlebovirus causes lethal viral hemorrhagic fever in cats. Sci. Rep. 2019, 9, 11990. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.; Jiang, H.; Liang, M.; Han, Y.; Gu, W.; Zhang, F.; Zhu, H.; Wu, W.; Chen, T.; Li, C.; et al. SFTS virus infection in nonhuman primates. J. Infect. Dis. 2015, 211, 915–925. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.H.; Huang, W.W.; He, W.Q.; He, X.Y.; Wang, X.H.; Lin, Y.L.; Zhao, Z.J.; Zheng, Y.T.; Pang, W. Longitudinal analysis of immunocyte responses and inflammatory cytokine profiles in SFTSV-infected rhesus macaques. Front. Immunol. 2023, 14, 1143796. [Google Scholar] [CrossRef]
- Jin, C.; Liang, M.; Han, Y.; Jiang, H.; Li, C.; Zhu, H.; Wei, Q.; Qin, C.; Li, D. Pathogenesis of emerging severe fever with thrombocytopenia syndrome virus in a non-human primate model (P3021). J. Immunol. 2013, 190, 55.11. [Google Scholar] [CrossRef]
- McClung, K.L.; Little, S.E. Amblyomma americanum (Lone star tick). Trends Parasitol. 2023, 39, 70–71. [Google Scholar] [CrossRef]
- Tuten, H.C.; Burkhalter, K.L.; Noel, K.R.; Hernandez, E.J.; Yates, S.; Wojnowski, K.; Hartleb, J.; Debosik, S.; Holmes, A.; Stone, C.M. Heartland Virus in Humans and Ticks, Illinois, USA, 2018–2019. Emerg. Infect. Dis. 2020, 26, 1548–1552. [Google Scholar] [CrossRef] [PubMed]
- Aziati, I.D.; Jnr, D.M.; Antia, A.; Joshi, A.; Aviles-Gamboa, A.; Lee, P.; Harastani, H.; Wang, D.; Adalsteinsson, S.A.; Boon, A.C.M. Prevalence of Bourbon and Heartland viruses in field collected ticks at an environmental field station in St. Louis County, Missouri, USA. Ticks Tick Borne Dis. 2023, 14, 102080. [Google Scholar] [CrossRef] [PubMed]
- Newman, B.C.; Sutton, W.B.; Moncayo, A.C.; Hughes, H.R.; Taheri, A.; Moore, T.C.; Schweitzer, C.J.; Wang, Y. Heartland Virus in Lone Star Ticks, Alabama, USA. Emerg. Infect. Dis. 2020, 26, 1954–1956. [Google Scholar] [CrossRef] [PubMed]
- Romer, Y.; Adcock, K.; Wei, Z.; Mead, D.G.; Kirstein, O.; Bellman, S.; Piantadosi, A.; Kitron, U.; Vazquez-Prokopec, G.M. Isolation of Heartland Virus from Lone Star Ticks, Georgia, USA, 2019. Emerg. Infect. Dis. 2022, 28, 786–792. [Google Scholar] [CrossRef]
- Savage, H.M.; Godsey, M.S., Jr.; Tatman, J.; Burkhalter, K.L.; Hamm, A.; Panella, N.A.; Ghosh, A.; Raghavan, R.K. Surveillance for Heartland and Bourbon Viruses in Eastern Kansas, June 2016. J. Med. Entomol. 2018, 55, 1613–1616. [Google Scholar] [CrossRef] [Green Version]
- Savage, H.M.; Godsey, M.S.; Lambert, A.; Panella, N.A.; Burkhalter, K.L.; Harmon, J.R.; Lash, R.R.; Ashley, D.C.; Nicholson, W.L. First detection of heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am. J. Trop. Med. Hyg. 2013, 89, 445–452. [Google Scholar] [CrossRef]
- Godsey, M.S.; Savage, H.M.; Burkhalter, K.L.; Bosco-Lauth, A.M.; Delorey, M.J. Transmission of Heartland Virus (Bunyaviridae: Phlebovirus) by Experimentally Infected Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 2016, 53, 1226–1233. [Google Scholar] [CrossRef] [Green Version]
- Raghavan, R.K.; Peterson, A.T.; Cobos, M.E.; Ganta, R.; Foley, D. Current and Future Distribution of the Lone Star Tick, Amblyomma americanum (L.) (Acari: Ixodidae) in North America. PLoS ONE 2019, 14, e0209082. [Google Scholar] [CrossRef]
- Gregory, N.; Fernandez, M.P.; Diuk-Wasser, M. Risk of tick-borne pathogen spillover into urban yards in New York City. Parasites Vectors 2022, 15, 288. [Google Scholar] [CrossRef]
- Noden, B.H.; Roselli, M.A.; Loss, S.R. Effect of Urbanization on Presence, Abundance, and Coinfection of Bacteria and Protozoa in Ticks in the US Great Plains. J. Med. Entomol. 2022, 59, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, C.; Cheng, C.; Zhang, G.; Yu, T.; Lawrence, K.; Li, H.; Sun, J.; Yang, Z.; Ye, L.; et al. Rapid Spread of Severe Fever with Thrombocytopenia Syndrome Virus by Parthenogenetic Asian Longhorned Ticks. Emerg. Infect. Dis. 2022, 28, 363–372. [Google Scholar] [CrossRef]
- Cumbie, A.N.; Trimble, R.N.; Eastwood, G. Pathogen Spillover to an Invasive Tick Species: First Detection of Bourbon Virus in Haemaphysalis longicornis in the United States. Pathogens 2022, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Raney, W.R.; Perry, J.B.; Hermance, M.E. Transovarial Transmission of Heartland Virus by Invasive Asian Longhorned Ticks under Laboratory Conditions. Emerg. Infect. Dis. 2022, 28, 726–729. [Google Scholar] [CrossRef]
- Whitlow, A.M.; Schurch, R.; Mullins, D.; Eastwood, G. The Influence of Southwestern Virginia Environmental Conditions on the Potential Ability of Haemaphysalis longicornis, Amblyomma americanum, and Amblyomma maculatum to Overwinter in the Region. Insects 2021, 12, 1000. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-N.; Li, T.-Q.; Liu, Q.-M.; Wu, Y.-Y.; Luo, M.-Y.; Gong, Z.-Y. Vectors, Hosts, and the Possible Risk Factors Associated with Severe Fever with Thrombocytopenia Syndrome. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 8518189. [Google Scholar] [CrossRef]
- Zhuang, L.; Sun, Y.; Cui, X.M.; Tang, F.; Hu, J.G.; Wang, L.Y.; Cui, N.; Yang, Z.D.; Huang, D.D.; Zhang, X.A.; et al. Transmission of Severe Fever with Thrombocytopenia Syndrome Virus by Haemaphysalis longicornis Ticks, China. Emerg. Infect. Dis. 2018, 24, 868–871. [Google Scholar] [CrossRef] [Green Version]
- Muehlenbachs, A.; Fata, C.R.; Lambert, A.J.; Paddock, C.D.; Velez, J.O.; Blau, D.M.; Staples, J.E.; Karlekar, M.B.; Bhatnagar, J.; Nasci, R.S.; et al. Heartland virus-associated death in tennessee. Clin. Infect. Dis. 2014, 59, 845–850. [Google Scholar] [CrossRef] [Green Version]
- Pastula, D.M.; Turabelidze, G.; Yates, K.F.; Jones, T.F.; Lambert, A.J.; Panella, A.J.; Kosoy, O.I.; Velez, J.O.; Fisher, M.; Staples, E.; et al. Notes from the field: Heartland virus disease—United States, 2012–2013. MMWR 2014, 63, 270–271. [Google Scholar]
- Fill, M.A.; Compton, M.L.; McDonald, E.C.; Moncayo, A.C.; Dunn, J.R.; Schaffner, W.; Bhatnagar, J.; Zaki, S.R.; Jones, T.F.; Shieh, W.J. Novel Clinical and Pathologic Findings in a Heartland Virus-Associated Death. Clin. Infect. Dis. 2017, 64, 510–512. [Google Scholar] [CrossRef]
- Hevey, M.A.; O’Halloran, J.A.; Jagger, B.W.; Staples, J.E.; Lambert, A.J.; Panella, A.J.; Kosoy, O.I.; Turabelidze, G.; Raymer, D.S.; Ewald, G.A.; et al. Heartland virus infection in a heart transplant recipient from the Heartland. Transpl. Infect. Dis. 2019, 21, e13098. [Google Scholar] [CrossRef] [PubMed]
- Decker, M.D.; Morton, C.T.; Moncayo, A.C. One Confirmed and 2 Suspected Cases of Heartland Virus Disease. Clin. Infect. Dis. 2020, 71, 3237–3240. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, A.P., 2nd; Prusinski, M.A.; O’Connor, C.; Maffei, J.G.; Ngo, K.A.; Koetzner, C.A.; Santoriello, M.P.; Romano, C.L.; Xu, G.; Ribbe, F.; et al. Heartland Virus Transmission, Suffolk County, New York, USA. Emerg. Infect. Dis. 2021, 27, 3128–3132. [Google Scholar] [CrossRef] [PubMed]
- Ahlers, C.G.; Matthews, H.; Perez, R.; Naggie, S. Secondary hemophagocytic lymphohistiocytosis due to Heartland virus. BMJ Case Rep. 2022, 15, e253082. [Google Scholar] [CrossRef]
- Liu, S.; Kannan, S.; Meeks, M.; Sanchez, S.; Girone, K.W.; Broyhill, J.C.; Martines, R.B.; Bernick, J.; Flammia, L.; Murphy, J.; et al. Fatal Case of Heartland Virus Disease Acquired in the Mid-Atlantic Region, United States. Emerg. Infect. Dis. 2023, 29, 992–996. [Google Scholar] [CrossRef]
- Basile, A.J.; Horiuchi, K.; Goodman, C.H.; Kosoy, O.; Panella, A.J.; Velez, J.O.; Pastula, D.M.; Brault, A.C.; Staples, J.E.; Calvert, A.E. Development of diagnostic microsphere-based immunoassays for Heartland virus. J. Clin. Virol. 2021, 134, 104693. [Google Scholar] [CrossRef]
- Biggs, H.M.; Behravesh, C.B.; Bradley, K.K.; Dahlgren, F.S.; Drexler, N.A.; Dumler, J.S.; Folk, S.M.; Kato, C.Y.; Lash, R.R.; Levin, M.L.; et al. Diagnosis and Management of Tickborne Rickettsial Diseases: Rocky Mountain Spotted Fever and Other Spotted Fever Group Rickettsioses, Ehrlichioses, and Anaplasmosis—United States. MMWR Recomm. Rep. 2016, 65, 1–44. [Google Scholar] [CrossRef] [Green Version]
- Song, R.; Chen, Z.; Li, W. Severe fever with thrombocytopenia syndrome (SFTS) treated with a novel antiviral medication, favipiravir (T-705). Infection 2020, 48, 295–298. [Google Scholar] [CrossRef]
- Suemori, K.; Saijo, M.; Yamanaka, A.; Himeji, D.; Kawamura, M.; Haku, T.; Hidaka, M.; Kamikokuryo, C.; Kakihana, Y.; Azuma, T.; et al. A multicenter non-randomized, uncontrolled single arm trial for evaluation of the efficacy and the safety of the treatment with favipiravir for patients with severe fever with thrombocytopenia syndrome. PLoS Negl. Trop. Dis. 2021, 15, e0009103. [Google Scholar] [CrossRef]
- Simpson, K.; Chapman, P.; Klag, A. Long-term outcome of primary immune-mediated thrombocytopenia in dogs. J. Small Anim. Pr. 2018, 59, 674–680. [Google Scholar] [CrossRef]
- Lewis, D.C.; Meyers, K.M. Canine idiopathic thrombocytopenic purpura. J. Vet. Intern. Med. 1996, 10, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Best, M.P.; Fry, D.R. Primary immune-mediated thrombocytopenia and immune-mediated neutropenia suspected in a 21-week-old Maine Coon cat. Aust. Vet. J. 2014, 92, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Garon, C.L.; Scott, M.A.; Selting, K.A.; Cohn, L.A. Idiopathic thrombocytopenic purpura in a cat. J. Am. Anim. Hosp. Assoc. 1999, 35, 464–470. [Google Scholar] [CrossRef]
- Yasuda, J.; Okada, K.; Sato, J.; Sato, R.; Tachibana, Y.; Takashima, K.; Naito, Y. Idiopathic thrombocytopenia in Japanese black cattle. J. Vet. Med. Sci./Jpn. Soc. Vet. Sci. 2002, 64, 87–89. [Google Scholar] [CrossRef] [Green Version]
- Larson, V.L.; Perman, V.; Stevens, J.B. Idiopathic thrombocytopenic purpura in two horses. J. Am. Vet. Med. Assoc. 1983, 183, 328–330. [Google Scholar] [PubMed]
- Seo, J.-W.; Kim, D.; Yun, N.; Kim, D.-M. Clinical Update of Severe Fever with Thrombocytopenia Syndrome. Viruses 2021, 13, 1213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Y.; Xu, Y. Antiviral Treatment Options for Severe Fever with Thrombocytopenia Syndrome Infections. Infect. Dis. Ther. 2022, 11, 1805–1819. [Google Scholar] [CrossRef] [PubMed]
- Dualis, H.; Zefong, A.C.; Joo, L.K.; Dadar Singh, N.K.; Syed Abdul Rahim, S.S.; Avoi, R.; Jeffree, M.S.; Hassan, M.R.; Ibrahim, M.Y.; Omar, A. Factors and outcomes in Severe Fever with Thrombocytopenia Syndrome (SFTS): A systematic review. Ann. Med. Surg. 2021, 67, 102501. [Google Scholar] [CrossRef]
- Xia, T.; Wu, X.; Hong, E.; Jung, K.; Lai, C.-J.; Kwak, M.-J.; Seo, H.; Kim, S.; Jiang, Z.; Cha, I.; et al. Glucosylceramide is essential for Heartland and Dabie bandavirus glycoprotein-induced membrane fusion. PLoS Pathog. 2023, 19, e1011232. [Google Scholar] [CrossRef]
- Savage, H.M.; Godsey, M.S., Jr.; Panella, N.A.; Burkhalter, K.L.; Ashley, D.C.; Lash, R.R.; Ramsay, B.; Patterson, T.; Nicholson, W.L. Surveillance for Heartland Virus (Bunyaviridae: Phlebovirus) in Missouri During 2013: First Detection of Virus in Adults of Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 2016, 53, 607–612. [Google Scholar] [CrossRef]
- Savage, H.M.; Godsey, M.S., Jr.; Panella, N.A.; Burkhalter, K.L.; Manford, J.; Trevino-Garrison, I.C.; Straily, A.; Wilson, S.; Bowen, J.; Raghavan, R.K. Surveillance for Tick-Borne Viruses Near the Location of a Fatal Human Case of Bourbon Virus (Family Orthomyxoviridae: Genus Thogotovirus) in Eastern Kansas, 2015. J. Med. Entomol. 2018, 55, 701–705. [Google Scholar] [CrossRef]
- Warang, A.; Zhang, M.; Zhang, S.; Shen, Z. A panel of real-time PCR assays for the detection of Bourbon virus, Heartland virus, West Nile virus, and Trypanosoma cruzi in major disease-transmitting vectors. J. Vet. Diagn. Investig. 2021, 33, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Shelite, T.R.; Bopp, N.E.; Moncayo, A.; Reynolds, E.S.; Thangamani, S.; Melby, P.C.; Bloch, K.; Aguilar, P.V.; Travi, B.L. Isothermal Recombinase Polymerase Amplification-Lateral Flow Point-of-Care Diagnostic Test for Heartland Virus. Vector Borne Zoonotic Dis. 2021, 21, 110–115. [Google Scholar] [CrossRef]
- Preventing Tick Bites. Available online: https://www.cdc.gov/ticks/avoid/on_people.html (accessed on 21 July 2023).
- Preventing Ticks on Your Pets. Available online: https://www.cdc.gov/ticks/avoid/on_pets.html (accessed on 21 July 2023).
- Wang, Y.; Griffiths, A.; Brackney, D.E.; Verardi, P.H. Generation of Multiple Arbovirus-like Particles Using a Rapid Recombinant Vaccinia Virus Expression Platform. Pathogens 2022, 11, 1505. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T. Vaccine Development for Severe Fever with Thrombocytopenia Syndrome. Viruses 2021, 13, 627. [Google Scholar] [CrossRef]
- Dong, F.; Li, D.; Wen, D.; Li, S.; Zhao, C.; Qi, Y.; Jangra, R.K.; Wu, C.; Xia, D.; Zhang, X.; et al. Single dose of a rVSV-based vaccine elicits complete protection against severe fever with thrombocytopenia syndrome virus. NPJ Vaccines 2019, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Kollars, T.M., Jr.; Oliver, J.H., Jr.; Durden, L.A.; Kollars, P.G. Host association and seasonal activity of Amblyomma americanum (Acari: Ixodidae) in Missouri. J. Parasitol. 2000, 86, 1156–1159. [Google Scholar] [CrossRef] [PubMed]
- Childs, J.E.; Paddock, C.D. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu. Rev. Entomol. 2003, 48, 307–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rand, M.L.; Wright, J.F. Virus-associated idiopathic thrombocytopenic purpura. Transfus. Sci. 1998, 19, 253–259. [Google Scholar] [CrossRef]
- Stasi, R.; Willis, F.; Shannon, M.S.; Gordon-Smith, E.C. Infectious causes of chronic immune thrombocytopenia. Hematol. Oncol. Clin. North. Am. 2009, 23, 1275–1297. [Google Scholar] [CrossRef]
- Jones, R.; Kulkarni, M.A.; Davidson, T.M.V.; Talbot, B. Arbovirus vectors of epidemiological concern in the Americas: A scoping review of entomological studies on Zika, dengue and chikungunya virus vectors. PLoS ONE 2020, 15, e0220753. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Hu, S.; Liu, X.; Yang, J.; Liu, D.; Wu, L.; Wang, H.; Hu, Z.; Deng, F.; Shen, S. Migration, recombination, and reassortment are involved in the evolution of severe fever with thrombocytopenia syndrome bunyavirus. Infect. Genet. Evol. 2017, 47, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Sall, A.A.; Zanotto, P.M.; Sene, O.K.; Zeller, H.G.; Digoutte, J.P.; Thiongane, Y.; Bouloy, M. Genetic reassortment of Rift Valley fever virus in nature. J. Virol. 1999, 73, 8196–8200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calzolari, M.; Chiapponi, C.; Bellini, R.; Bonilauri, P.; Lelli, D.; Moreno, A.; Barbieri, I.; Pongolini, S.; Lavazza, A.; Dottori, M. Isolation of three novel reassortant phleboviruses, Ponticelli I, II, III, and of Toscana virus from field-collected sand flies in Italy. Parasit. Vectors 2018, 11, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantlo, E.K.; Haley, N.J. Heartland Virus: An Evolving Story of an Emerging Zoonotic and Vector-Borne Disease. Zoonotic Dis. 2023, 3, 188-202. https://doi.org/10.3390/zoonoticdis3030016
Mantlo EK, Haley NJ. Heartland Virus: An Evolving Story of an Emerging Zoonotic and Vector-Borne Disease. Zoonotic Diseases. 2023; 3(3):188-202. https://doi.org/10.3390/zoonoticdis3030016
Chicago/Turabian StyleMantlo, Emily K., and Nicholas J. Haley. 2023. "Heartland Virus: An Evolving Story of an Emerging Zoonotic and Vector-Borne Disease" Zoonotic Diseases 3, no. 3: 188-202. https://doi.org/10.3390/zoonoticdis3030016
APA StyleMantlo, E. K., & Haley, N. J. (2023). Heartland Virus: An Evolving Story of an Emerging Zoonotic and Vector-Borne Disease. Zoonotic Diseases, 3(3), 188-202. https://doi.org/10.3390/zoonoticdis3030016






