PVDF Hybrid Nanocomposites with Graphene and Carbon Nanotubes and Their Thermoresistive and Joule Heating Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanocomposites
2.3. Preparation of Test Samples
2.4. Experimental Methods
2.4.1. X-ray Diffraction Analysis
2.4.2. Differential Scanning Calorimetry
2.4.3. Scanning Electron Microscopy
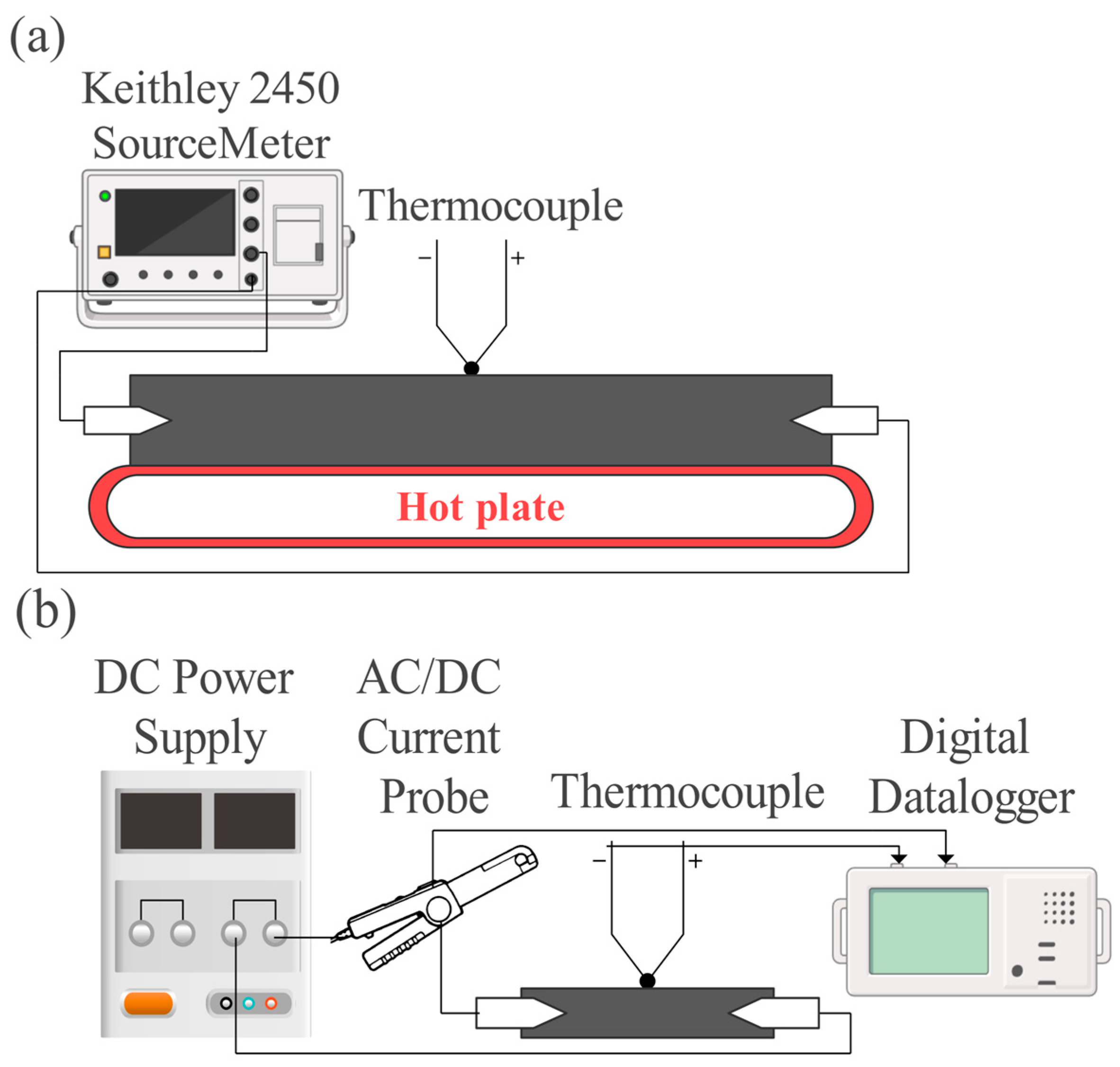
2.4.4. Thermoresistive Characterization
3. Results and Discussion
3.1. Structural and Morphological Characterization
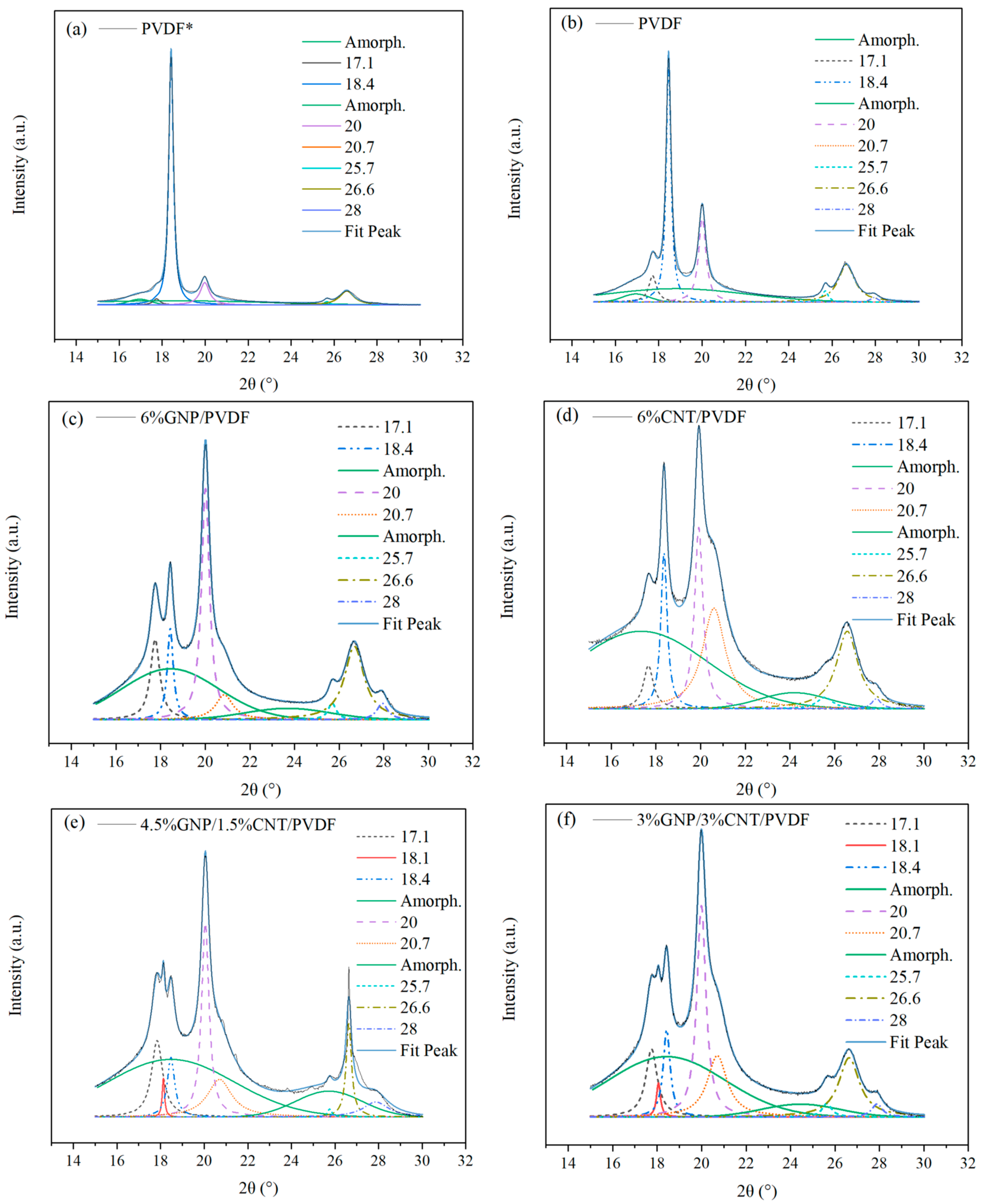
3.1.1. X-ray Diffraction Analysis
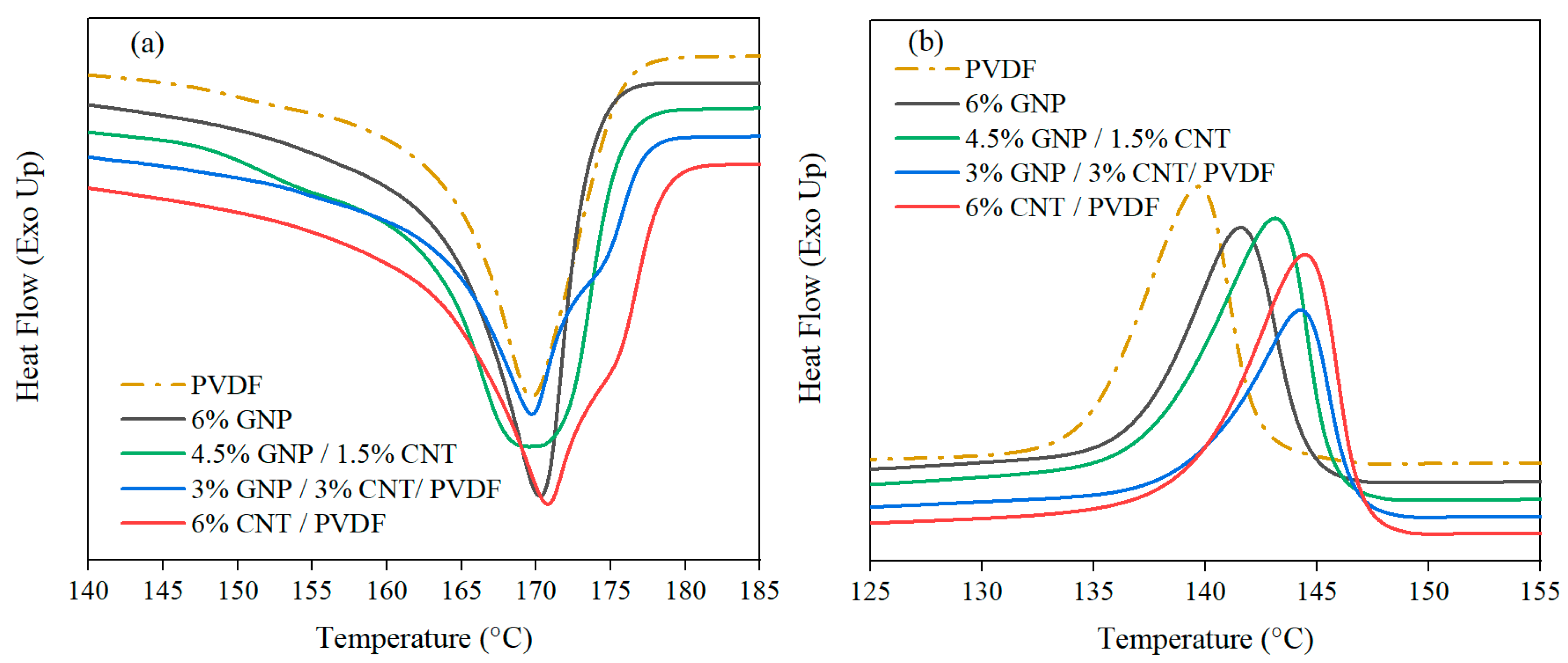
3.1.2. Differential Scanning Calorimetry
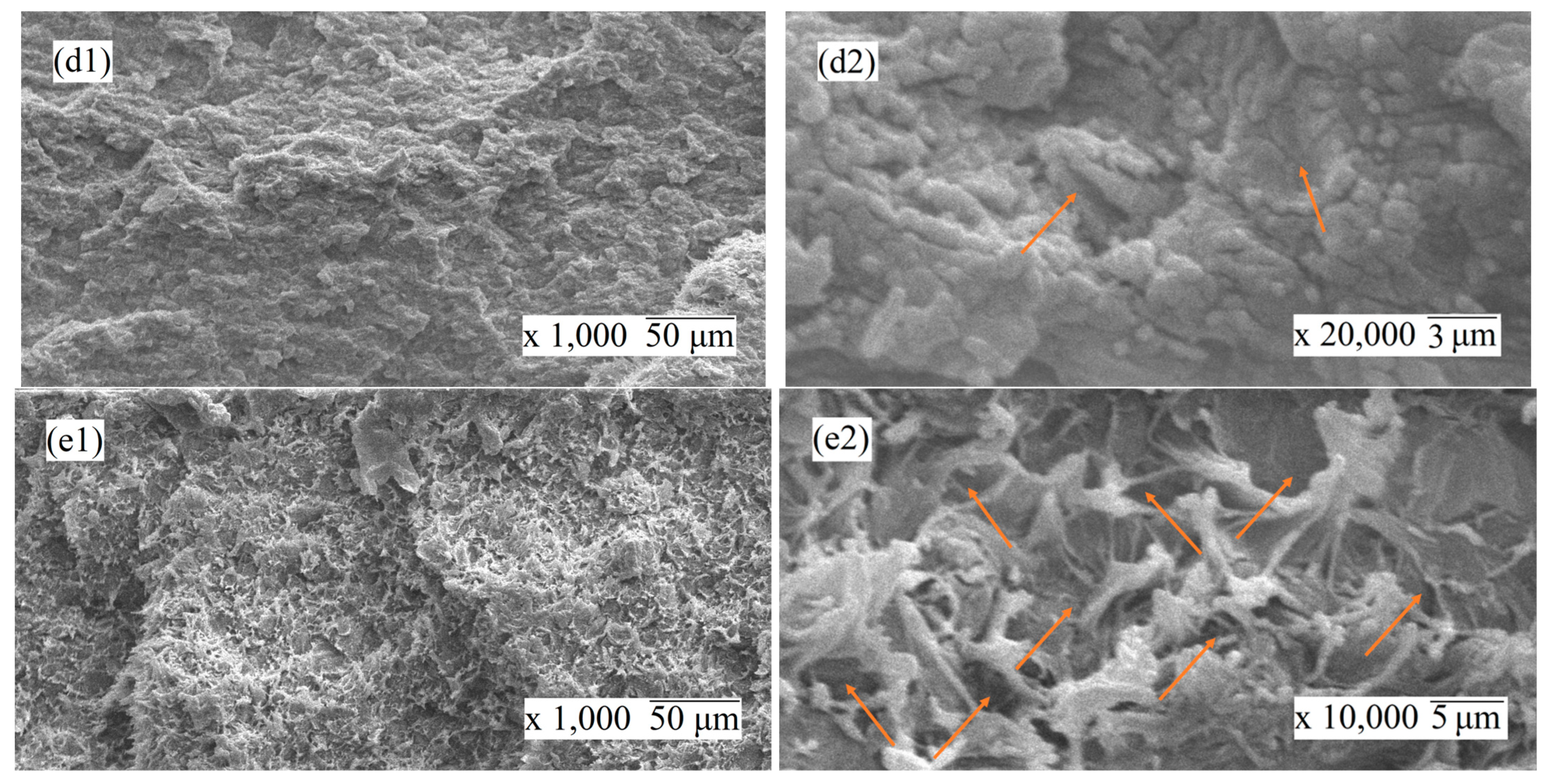
3.1.3. Scanning Electron Microscopy
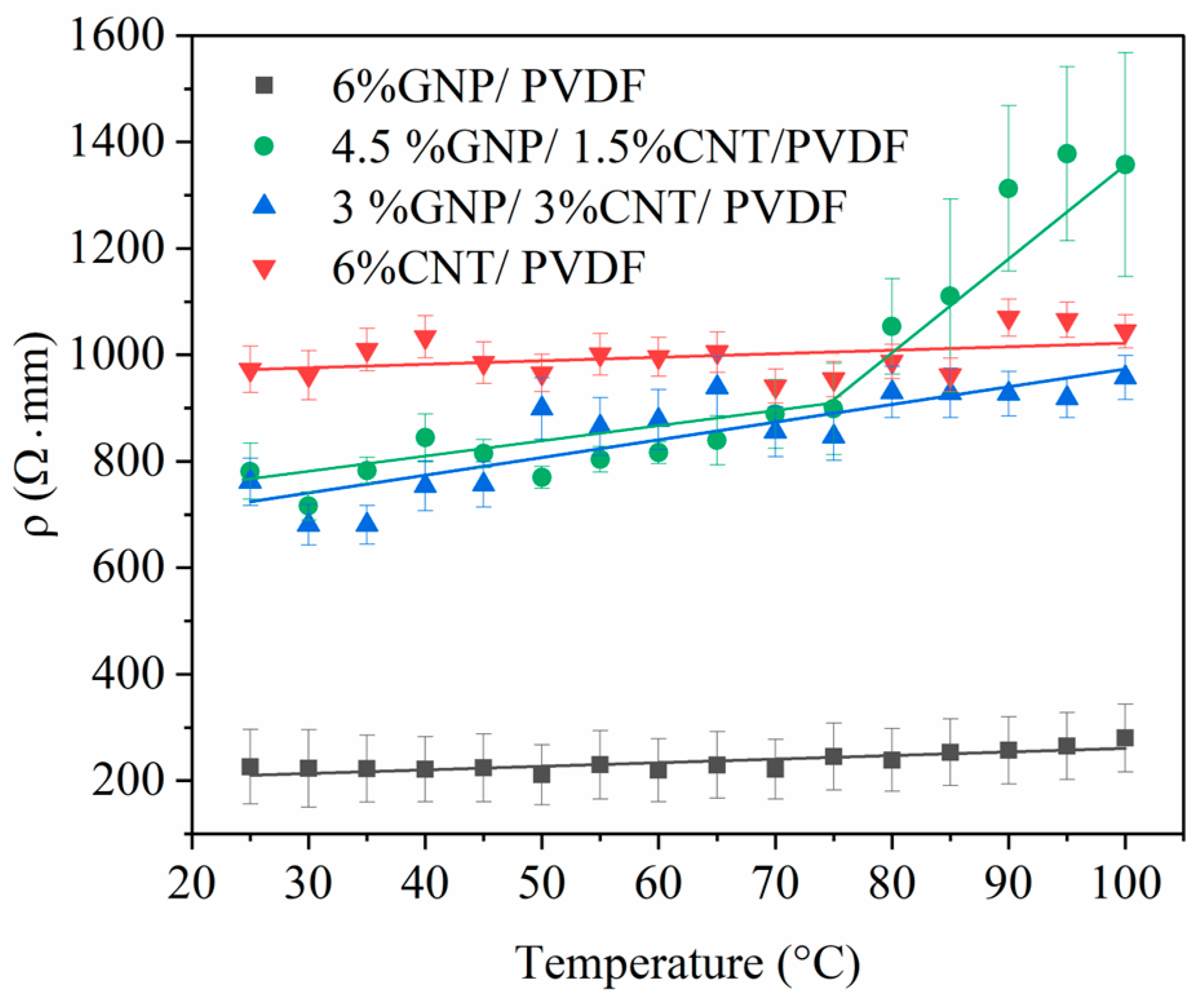
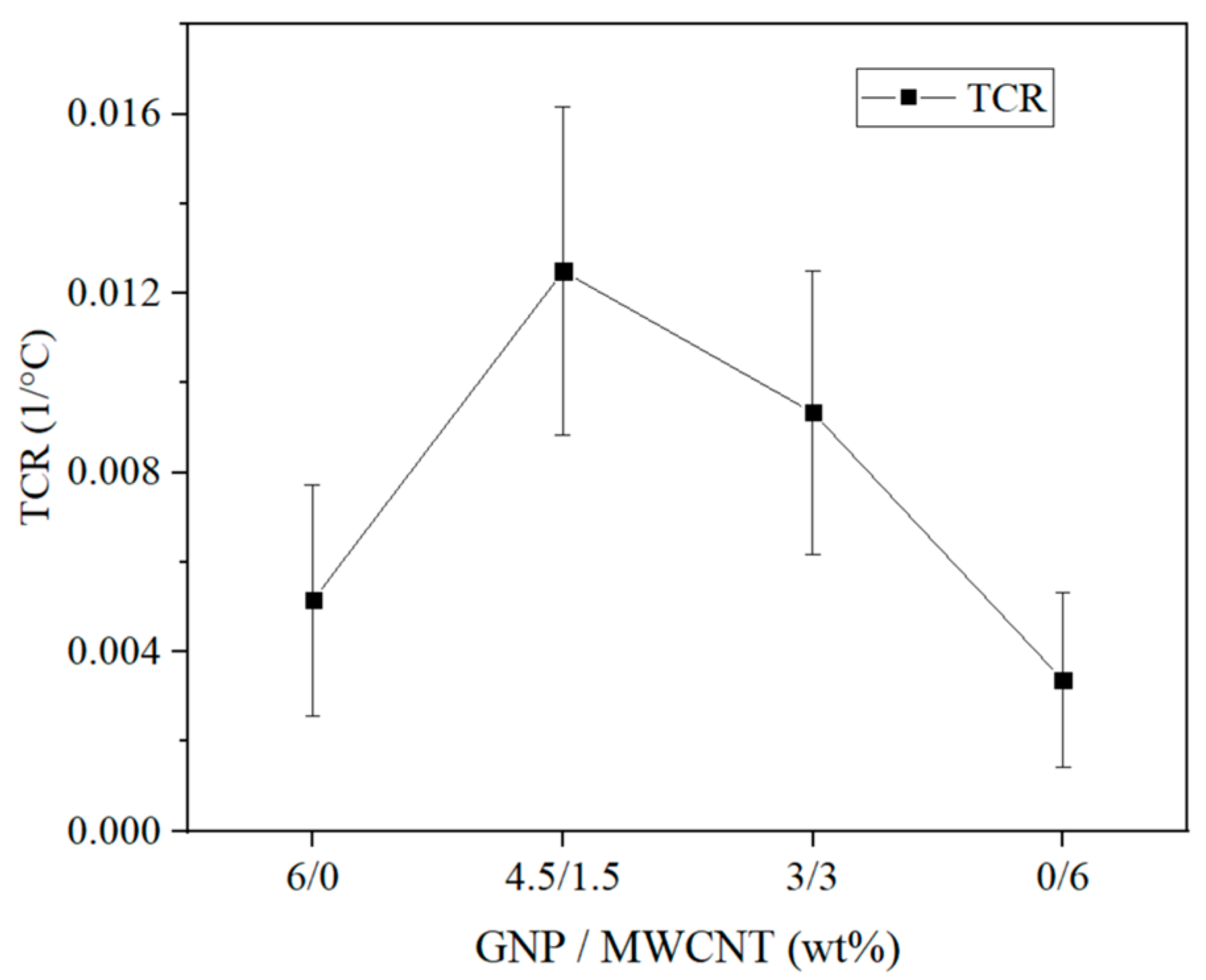
3.2. Thermoresistive Characterization
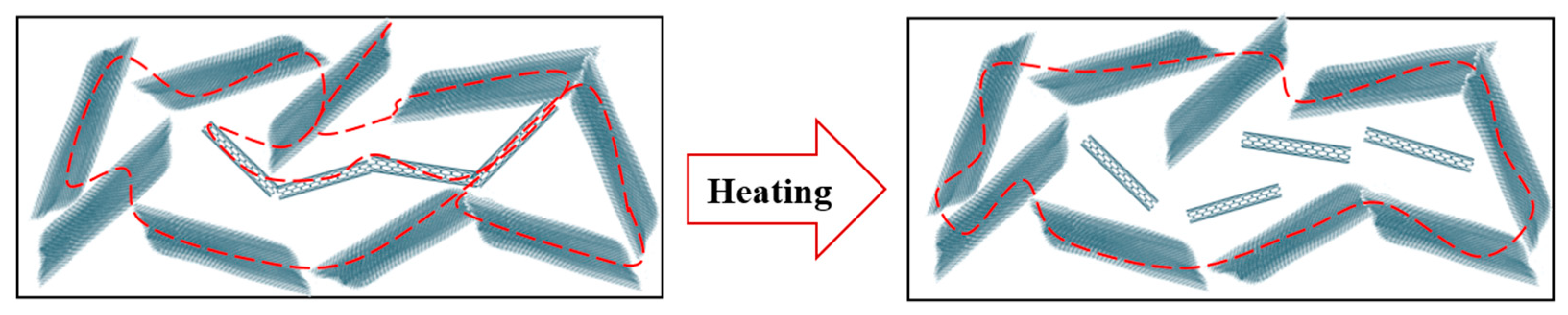
3.2.1. Thermoresistive Behavior
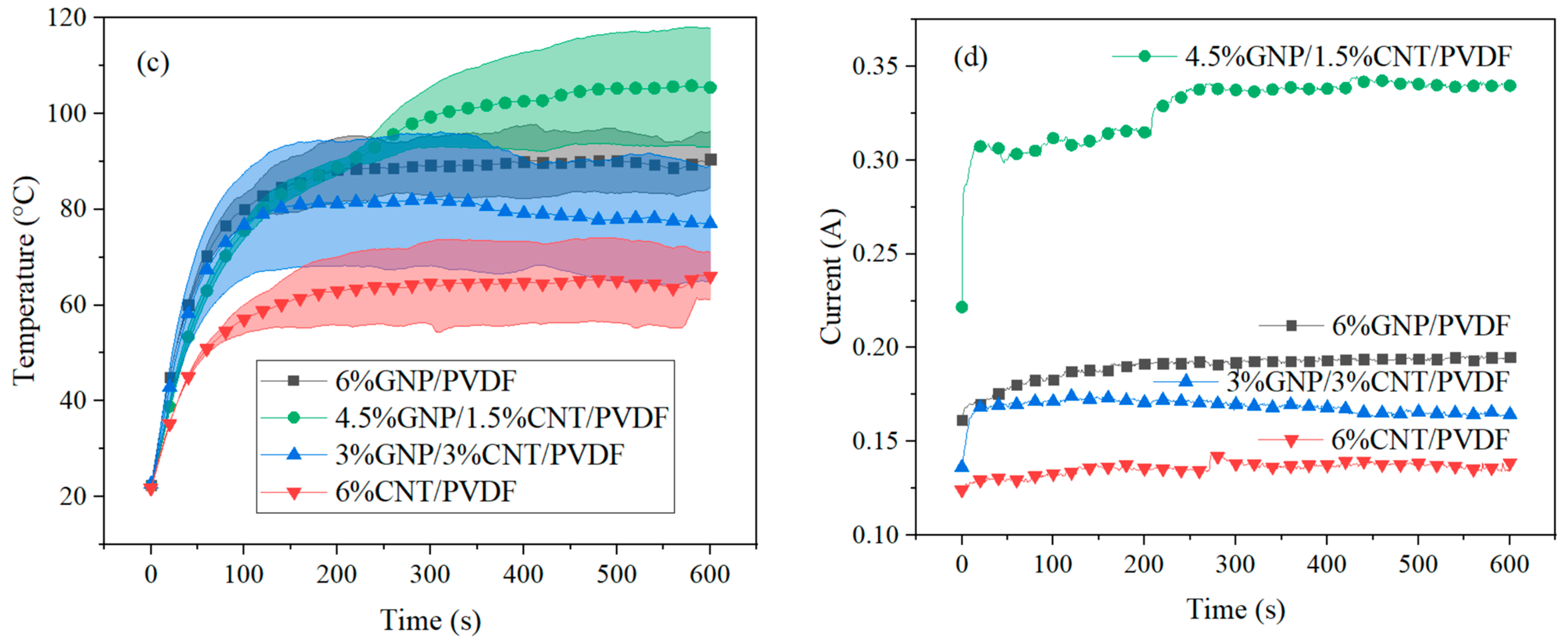
3.2.2. Heating Elements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batakliev, T.; Petrova-Doycheva, I.; Angelov, V.; Georgiev, V.; Ivanov, E.; Kotsilkova, R.; Casa, M.; Cirillo, C.; Adami, R.; Sarno, M.; et al. Effects of Graphene Nanoplatelets and Multiwall Carbon Nanotubes on the Structure and Mechanical Properties of Poly (lactic acid) Composites: A Comparative Study. Appl. Sci. 2019, 9, 469. [Google Scholar] [CrossRef]
- Spinelli, G.; Lamberti, P.; Tucci, V.; Kotsilkova, R.; Ivanov, E.; Menseidov, D.; Naddeo, C.; Romano, V.; Guadagno, L.; Adami, R.; et al. Nanocarbon/Poly (Lactic) Acid for 3D Printing: Effect of Fillers Content on Electromagnetic and Thermal Properties. Materials 2019, 12, 2369. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, E.; Kotsilkova, R.; Xia, H.; Chen, Y.; Donato, R.K.; Donato, K.; Godoy, A.P.; Di Maio, R.; Silvestre, C.; Cimmino, S.; et al. PLA/Graphene/MWCNT Composites with Improved Electrical and Thermal Properties Suitable for FDM 3D Printing Applications. Appl. Sci. 2019, 9, 1209. [Google Scholar] [CrossRef]
- Avilés, F. Thermoresistivity of Carbon Nanostructures and Their Polymeric Nanocomposites. Adv. Mater. Interfaces 2023, 10, 2300218. [Google Scholar] [CrossRef]
- Dinh, T.; Phan, H.-P.; Dao, D.V.; Woodfield, P.; Qamar, A.; Nguyen, N.-T. Graphite on Paper as Material for Sensitive Thermoresistive Sensors. J. Mater. Chem. C 2015, 3, 8776–8779. [Google Scholar] [CrossRef]
- Balam, A.; Cen-Puc, M.; May-Pat, A.; Abot, J.L.; Avilés, F. Influence of Polymer Matrix on the Sensing Capabilities of Carbon Nanotube Polymeric Thermistors. Smart Mater. Struct. 2020, 29, 015012. [Google Scholar] [CrossRef]
- Ji, S.; Wu, X.; Jiang, Y.; Wang, T.; Liu, Z.; Cao, C.; Ji, B.; Chi, L.; Li, D.; Chen, X. Self-Reporting Joule Heating Modulated Stiffness of Polymeric Nanocomposites for Shape Reconfiguration. ACS Nano 2022, 16, 16833–16842. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, G.; Guarini, R.; Kotsilkova, R.; Batakliev, T.; Ivanov, E.; Romano, V. Experimental and Simulation Studies of Temperature Effect on Thermophysical Properties of Graphene-Based Polylactic Acid. Materials 2022, 15, 986. [Google Scholar] [CrossRef]
- Al-Harthi, M.A.; Hussain, M. Effect of the Surface Functionalization of Graphene and MWCNT on the Thermodynamic, Mechanical and Electrical Properties of the Graphene/MWCNT-PVDF Nanocomposites. Polymers 2022, 14, 2976. [Google Scholar] [CrossRef]
- Cerrada, M.L.; Arranz-Andrés, J.; Caballero-González, A.; Blázquez-Blázquez, E.; Pérez, E. The β Form in PVDF Nanocomposites with Carbon Nanotubes: Structural Features and Properties. Polymers 2023, 15, 1491. [Google Scholar] [CrossRef]
- Fan, B.; Bedoui, F.; Weigand, S.; Bai, J. Conductive Network and β Polymorph Content Evolution Caused by Thermal Treatment in Carbon Nanotubes-BaTiO 3 Hybrids Reinforced Polyvinylidene Fluoride Composites. J. Phys. Chem. C 2016, 120, 9511–9519. [Google Scholar] [CrossRef]
- Loura, N.; Gkartzou, E.; Trompeta, A.-F.; Konstantopoulos, G.; Klonos, P.A.; Kyritsis, A.; Charitidis, C.A. Development of CNT-Based Nanocomposites with Ohmic Heating Capability towards Self-Healing Applications in Extrusion-Based 3D Printing Technologies. C 2023, 9, 111. [Google Scholar] [CrossRef]
- Spinelli, G.; Guarini, R.; Kotsilkova, R.; Ivanov, E.; Vertuccio, L.; Romano, V.; Guadagno, L. Joule Heating Effect in Carbon-Based Epoxy Resin: An Experimental and Numerical Study. Bulg. Chem. Commun. 2023, 55, 335–343. [Google Scholar]
- Zeng, Y.; Lu, G.; Wang, H.; Du, J.; Ying, Z.; Liu, C. Positive Temperature Coefficient Thermistors Based on Carbon Nanotube/Polymer Composites. Sci. Rep. 2014, 4, 6684. [Google Scholar] [CrossRef] [PubMed]
- Cen-Puc, M.; Pool, G.; Oliva-Avilés, A.I.; May-Pat, A.; Avilés, F. Experimental Investigation of the Thermoresistive Response of Multiwall Carbon Nanotube/Polysulfone Composites under Heating-Cooling Cycles. Compos. Sci. Technol. 2017, 151, 34–43. [Google Scholar] [CrossRef]
- Lamberti, P.; De Vivo, B.; Spinelli, G.; Tucci, V.; Guadagno, L.; Raimondo, M.; Vertuccio, L. Analysis of the Effects of Hydrotalcite Inclusion on the Temperature-Sensing Properties of CNT-Epoxy Nanocomposites. IEEE Sens. J. 2016, 16, 7977–7985. [Google Scholar] [CrossRef]
- Dai, H.; Thostenson, E.T.; Schumacher, T. Comparative Study of the Thermoresistive Behavior of Carbon Nanotube-Based Nanocomposites and Multiscale Hybrid Composites. Compos. B. Eng. 2021, 222, 109068. [Google Scholar] [CrossRef]
- Verma, P.; Schiffer, A.; Kumar, S. Thermo-Resistive and Thermo-Piezoresistive Sensitivity of Carbon Nanostructure Engineered Thermoplastic Composites Processed via Additive Manufacturing. Polym. Test. 2021, 93, 106961. [Google Scholar] [CrossRef]
- Kumar, A.; Hsieh, P.-Y.; Shaikh, M.O.; Kumar, R.K.R.; Chuang, C.-H. Flexible Temperature Sensor Utilizing MWCNT Doped PEG-PU Copolymer Nanocomposites. Micromachines 2022, 13, 197. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.; Cen, F.; Yu, X.; Zhou, W.; Jiang, S.; Yu, Y. Polymer Positive Temperature Coefficient Composites with Room-Temperature Curie Point and Superior Flexibility for Self-Regulating Heating Devices. Polymer 2023, 265, 125587. [Google Scholar] [CrossRef]
- Kumar, P.; Yu, S.; Shahzad, F.; Hong, S.M.; Kim, Y.-H.; Koo, C.M. Ultrahigh Electrically and Thermally Conductive Self-Aligned Graphene/Polymer Composites Using Large-Area Reduced Graphene Oxides. Carbon 2016, 101, 120–128. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, G.; Zhang, B.; Zhang, Z. The Influence of Crystalline and Aggregate Structure on PTC Characteristic of Conductive Polyethylene/Carbon Black Composite. Eur. Polym. J. 1998, 34, 1221–1227. [Google Scholar] [CrossRef]
- Chen, L.; Hou, J.; Chen, Y.; Wang, H.; Duan, Y.; Zhang, J. Synergistic Effect of Conductive Carbon Black and Silica Particles for Improving the Pyroresistive Properties of High Density Polyethylene Composites. Compos. B. Eng. 2019, 178, 107465. [Google Scholar] [CrossRef]
- Pech-Pisté, R.; Cen-Puc, M.; Balam, A.; May-Pat, A.; Avilés, F. Multifunctional Sensing Properties of Polymer Nanocomposites Based on Hybrid Carbon Nanostructures. Mater. Today Commun. 2020, 25, 101472. [Google Scholar] [CrossRef]
- Kono, A.; Shimizu, K.; Nakano, H.; Goto, Y.; Kobayashi, Y.; Ougizawa, T.; Horibe, H. Positive-Temperature-Coefficient Effect of Electrical Resistivity below Melting Point of Poly (Vinylidene Fluoride) (PVDF) in Ni Particle-Dispersed PVDF Composites. Polymer 2012, 53, 1760–1764. [Google Scholar] [CrossRef]
- Fernandes, L.C.; Correia, D.M.; García-Astrain, C.; Pereira, N.; Tariq, M.; Esperança, J.M.S.S.; Lanceros-Méndez, S. Ionic-Liquid-Based Printable Materials for Thermochromic and Thermoresistive Applications. ACS Appl. Mater. Interfaces 2019, 11, 20316–20324. [Google Scholar] [CrossRef] [PubMed]
- Maria Joseph Raj, N.P.; Alluri, N.R.; Chandrasekhar, A.; Khandelwal, G.; Kim, S.-J. Self-Powered Ferroelectric NTC Thermistor Based on Bismuth Titanate. Nano Energy 2019, 62, 329–337. [Google Scholar] [CrossRef]
- Kotsilkova, R.; Ivanov, E.; Georgiev, V.; Ivanova, R.; Menseidov, D.; Batakliev, T.; Angelov, V.; Xia, H.; Chen, Y.; Bychanok, D.; et al. Essential Nanostructure Parameters to Govern Reinforcement and Functionality of Poly(lactic) Acid Nanocomposites with Graphene and Carbon Nanotubes for 3D Printing Application. Polymers 2020, 12, 1208. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive Phases of Poly (Vinylidene Fluoride): Determination, Processing and Applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Georgopoulou, A.; Diethelm, P.; Wagner, M.; Spolenak, R.; Clemens, F. Soft self-regulating heating elements for thermoplastic elastomer-based electronic skin applications. 3D Print Addit. Manuf. 2023, 11, e828–e838. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, W.; Zhou, Y.; Chen, Y.; Cao, X.; Yang, Y.; Xu, J.; Jiang, Y. Effect of Crystalline Phase on the Dielectric and Energy Storage Properties of Poly (Vinylidene Fluoride). J. Mater. Sci. Mater. Electron. 2016, 27, 7280–7286. [Google Scholar] [CrossRef]
- Jurczuk, K.; Galeski, A.; Mackey, M.; Hiltner, A.; Baer, E. Orientation of PVDF α and γ Crystals in Nanolayered Films. Colloid Polym. Sci. 2015, 293, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Navarro Oliva, F.S.; Sahihi, M.; Lenglet, L.; Ospina, A.; Guenin, E.; Jaramillo-Botero, A.; Goddard, W.A.; Bedoui, F. Nanoparticle Size and Surface Chemistry Effects on Mechanical and Physical Properties of Nano-Reinforced Polymers: The Case of PVDF-Fe3O4 Nano-Composites. Polym. Test. 2023, 117, 107851. [Google Scholar] [CrossRef]
- Jen, Y.-M.; Huang, J.-C.; Zheng, K.-Y. Synergistic Effect of Multi-Walled Carbon Nanotubes and Graphene Nanoplatelets on the Monotonic and Fatigue Properties of Uncracked and Cracked Epoxy Composites. Polymers 2020, 12, 1895. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, X.; Wu, C. Effect of Preparation Methods on Crystallization Behavior and Tensile Strength of Poly(vinylidene fluoride) Membranes. Membranes 2013, 3, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-X.; Liu, X.; Wu, J.; Wong, S.-C.; Chase, G.G.; Qu, J.-P. Electrospun Poly (Vinylidene Fluoride) Membranes Functioning as Static Charge Storage Device with Controlled Crystalline Phase by Inclusions of Nanoscale Graphite Platelets. J. Mater. Sci. 2018, 53, 3038–3048. [Google Scholar] [CrossRef]
- Shuai, C.; Zeng, Z.; Yang, Y.; Qi, F.; Peng, S.; Yang, W.; He, C.; Wang, G.; Qian, G. Graphene Oxide Assists Polyvinylidene Fluoride Scaffold to Reconstruct Electrical Microenvironment of Bone Tissue. Mater. Des. 2020, 190, 108564. [Google Scholar] [CrossRef]
- Tsonos, C.; Soin, N.; Tomara, G.; Yang, B.; Psarras, G.C.; Kanapitsas, A.; Siores, E. Electromagnetic Wave Absorption Properties of Ternary Poly (Vinylidene Fluoride)/Magnetite Nanocomposites with Carbon Nanotubes and Graphene. RSC Adv. 2016, 6, 1919–1924. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, H.; Pu, L.; Li, L. Fabrication of Poly(Vinylidene Fluoride)/Multiwalled Carbon Nanotube Nanocomposite Foam via Supercritical Fluid Carbon Dioxide: Synergistic Enhancement of Piezoelectric and Mechanical Properties. Compos. Sci. Technol. 2020, 192, 108108. [Google Scholar] [CrossRef]
- Typical Properties of Kynar® PVDF. Available online: https://www.ipolymer.com/pdf/PVDF.pdf (accessed on 12 March 2024).
- Palacios-Aguilar, E.; Bonilla-Rios, J.; Sanchez-Fernandez, J.A.; Vargas-Martinez, A.; Lozoya-Santos, J.D.J.; Ramırez-Mendoza, R. Comparing the Elasticity of the Melt and Electrical Conductivity of the Solid of PP-HDPE Copolymer CNT Composites Obtained by Direct Compounding versus Dilution of a PP Masterbatch. J. Intell. Mater. Syst. Struct. 2021, 32, 1105–1115. [Google Scholar] [CrossRef]
- Kynar® PVDF Performance Characteristics & Data Brochure. Arkema High Performance Polymers. Available online: https://hpp.arkema.com/en/product-families/kynar-fluoropolymer-family/download-performance-characteris/ (accessed on 5 April 2024).
- Ramakers-van Dorp, E.; Möginger, B.; Hausnerova, B. Thermal expansion of semi-crystalline polymers: Anisotropic thermal strain and crystallite orientation. Polymer 2020, 191, 122249. [Google Scholar] [CrossRef]
- Dellinger, J.H. The Temperature Coefficient of Resistance of Copper, Issue 147; U.S. Department of Commerce and Labor, Bureau of Standards: Washington, DC, USA, 1911. [Google Scholar]



| Filler | GNP | MWCNT |
|---|---|---|
| Trade Name | SE1233 | NC7000 |
| Purity, C wt.% | >97 | 90 |
| Particle size, D50, μm | <50 | - |
| Outer diameter, nm | - | 9.5 |
| Inner diameter, nm | - | 5 |
| Length, μm | - | 1.5 |
| SSA, m2/g | 400–600 | 250–300 |
| Shape | 2D | 1D |
| Volume Resistivity, Ω.cm | - | 10−4 |
| Sample | PVDF wt.% | GNP wt.% | MWCNT wt.% | GNP/MWCNT wt.% |
|---|---|---|---|---|
| PVDF* | 100 | - | - | - |
| PVDF | 100 | - | - | - |
| Monofiller composites | ||||
| 6% GNP/PVDF | 94.0 | 6.0 | - | 6:0 |
| 6% CNT/PVDF | 94.0 | - | 6.0 | 0:6 |
| Hybrid filler composites | ||||
| 4.5% GNP/1.5% CNT/PVDF | 94.0 | 4.5 | 1.5 | 4.5:1.5 |
| 3% GNP/3% CNT/PVDF | 94.0 | 3.0 | 3.0 | 3.0:3.0 |
| Composition (Hot-Pressed Samples) | Aα + γ | Aα | Aβ | Aamorph. | Aα,γ,β | Xc % | α + γ % | α % | β % |
|---|---|---|---|---|---|---|---|---|---|
| PVDF* | 22,704 | 275 | 139 | 10,037 | 23,118 | 70.6 ± 2.3 | 69.3 ± 2.2 | 0.9 ± 0.10 | 0.4 ± 0.01 |
| PVDF | 20,419 | 517 | 71 | 11,460 | 21,008 | 64.7 ± 0.8 | 62.9 ± 0.8 | 1.6 ± 0.02 | 0.2 ± 0.01 |
| 6% GNP/PVDF | 17,009 | 474 | 1037 | 14,917 | 18,521 | 55.4 ± 0.7 | 50.9 ± 0.6 | 1.4 ± 0.03 | 3.1 ± 0.08 |
| 4.5% GNP/1.5% CNT/PVDF | 10,836 | 90 | 2617 | 19,710 | 13,543 | 40.6 ± 0.5 | 32.5 ±0.4 | 0.3 ± 0.01 | 7.8 ± 0.23 |
| 3% GNP/3% CNT/PVDF | 13,088 | 461 | 3919 | 15,333 | 17,468 | 53.2 ± 1.0 | 39.9 ± 0.7 | 1.4 ± 0.10 | 11.9 ± 0.51 |
| 6% CNT/PVDF | 12,575 | 278 | 4064 | 19,394 | 16,917 | 46.5 ± 4.5 | 34.6 ± 3.6 | 0.7 ± 0.46 | 11.2 ± 0.39 |
| Composition | ΔHm [J/g] | Tm1, °C | Tm2, °C | Tc, °C | Xc, % |
|---|---|---|---|---|---|
| First Heating Cycle | |||||
| PVDF* | 53.1 | 169.9 | - | 134.5 | 50.7 ± 3.6 |
| PVDF | 53.2 | 170.1 | - | 139.7 | 50.8 ± 0.5 |
| 6% GNP–PVDF | 53.4 | 170.4 | - | 141.6 | 54.3 ± 0.7 |
| 4.5% GNP–1.5% CNT–PVDF | 46.0 | 170.3 | - | 143.1 | 46.7 ± 0.3 |
| 3% GNP–3% CNT–PVDF | 52.6 | 169.7 | 174.8 | 144.2 | 53.4 ± 1.3 |
| 6% CNT–PVDF | 53.5 | 170.9 | 175.7 | 144.5 | 53.5 ± 0.4 |
| Second Heating Cycle | |||||
| PVDF* | 51.9 | 169.5 | - | 135.7 | 49.6 ± 0.6 |
| PVDF | 51.7 | 168.0 | - | 139.9 | 49.4 ± 0.4 |
| 6% GNP–PVDF | 52.0 | 169.1 | - | 141.6 | 52.8 ± 1.1 |
| 4.5% GNP–1.5% CNT–PVDF | 48.3 | 169.6 | - | 143.2 | 49.1 ± 0.5 |
| 3% GNP–3% CNT–PVDF | 50.9 | 169.1 | 172.9 | 144.2 | 51.7 ± 1.4 |
| 6% CNT–PVDF | 50.2 | 169.5 | 173.8 | 144.5 | 51.0 ± 0.8 |
| Sample | Constant Voltage of 8 V (1) | Heat 1 (J) | Constant Voltage of 10 V (2) | Heat 2 (J) | ||||
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Current (A) | Time (s) | Temperature (°C) | Current (A) | Time (s) | |||
| 6% GNP–PVDF | 50.6 | 0.12 | 600 | 556.6 | 90.3 | 0.20 | 600 | 1170.0 |
| 4.5% GNP–1.5% CNT–PVDF | 44.3 | 0.13 | 600 | 643.8 | 105.5 | 0.34 | 600 | 2039.0 |
| 3% GNP–3% CNT–PVDF | 46.9 | 0.10 | 600 | 459.7 | 77.0 | 0.16 | 600 | 986.0 |
| 6% CNT–PVDF | 42.5 | 0.08 | 600 | 368.8 | 66.2 | 0.14 | 600 | 892.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoyanova, S.; Ivanov, E.; Hegde, L.R.; Georgopoulou, A.; Clemens, F.; Bedoui, F.; Kotsilkova, R. PVDF Hybrid Nanocomposites with Graphene and Carbon Nanotubes and Their Thermoresistive and Joule Heating Properties. Nanomaterials 2024, 14, 901. https://doi.org/10.3390/nano14110901
Stoyanova S, Ivanov E, Hegde LR, Georgopoulou A, Clemens F, Bedoui F, Kotsilkova R. PVDF Hybrid Nanocomposites with Graphene and Carbon Nanotubes and Their Thermoresistive and Joule Heating Properties. Nanomaterials. 2024; 14(11):901. https://doi.org/10.3390/nano14110901
Chicago/Turabian StyleStoyanova, Stiliyana, Evgeni Ivanov, Lohitha R. Hegde, Antonia Georgopoulou, Frank Clemens, Fahmi Bedoui, and Rumiana Kotsilkova. 2024. "PVDF Hybrid Nanocomposites with Graphene and Carbon Nanotubes and Their Thermoresistive and Joule Heating Properties" Nanomaterials 14, no. 11: 901. https://doi.org/10.3390/nano14110901






