Abstract
Antimicrobial resistance (AMR) is a growing global health emergency that threatens the effectiveness of modern medicine, exacerbating healthcare costs, morbidity, and mortality, particularly in low- and middle-income countries (LMICs). Traditional approaches to antimicrobial development and stewardship have proven inadequate in curbing the rapid emergence and spread of resistant pathogens. This review explores cutting-edge biotechnological innovations as sustainable, precision-based solutions to combat AMR and promote global health equity. A comprehensive narrative review was conducted using literature published between 2018 and 2023 from PubMed, ScienceDirect, and Web of Science. Peer-reviewed studies focusing on novel antimicrobial strategies were thematically analyzed, with attention to efficacy, feasibility, and translational readiness. Key innovations identified include nanotechnology-enhanced antimicrobial delivery, bacteriophage therapy, CRISPR-Cas gene editing, immunotherapy, and personalized medicine. These strategies demonstrated substantial in vitro and in vivo efficacy, such as >90% MRSA biofilm reduction via silver nanoparticles and 95% carbapenem susceptibility restoration in E. coli using CRISPR-Cas9. When integrated with machine learning and rapid diagnostics, these approaches enable precision-targeted therapies and data-informed stewardship, offering scalable solutions adaptable to diverse healthcare systems. Antimicrobial resistance demands urgent, equitable innovation. Integrating biotechnologies like CRISPR, phage therapy, and nanomedicine with data-driven tools offers promising solutions. To ensure real-world impact, we recommend establishing regionally tailored translational research platforms and public–private partnerships as the most effective strategy to scale innovations and strengthen AMR response in low-resource settings.
1. Introduction
Antimicrobial resistance (AMR) presents an existential threat to modern medicine, undermining decades of progress in infectious disease treatment and jeopardizing routine surgical and medical procedures [1,2]. The World Health Organization (WHO) projects that, if unaddressed, AMR could cause up to 10 million deaths annually by 2050—surpassing cancer as the leading cause of mortality [3]. This growing crisis not only endangers individual health but also threatens global public health systems, economic security, and food supply chains [4,5].
The rapid evolution of resistance mechanisms among pathogens has far outpaced the development of new antimicrobials. Current drug pipelines remain limited, and traditional containment strategies are proving inadequate to curb the accelerating spread of resistant organisms [6]. Vulnerable populations—such as neonates, the elderly, and patients with chronic illnesses—are especially affected, facing prolonged hospitalizations, increased treatment costs, and elevated mortality rates from infections once easily treatable [7].
Compounding the problem, the widespread use of antibiotics in agriculture continues to contribute to resistance selection and transmission across ecosystems [8,9]. Furthermore, global surveillance reports indicate alarming trends in resistance patterns in conflict-affected and post-COVID-19 regions. In Eastern Ukraine and Syria, disrupted healthcare systems and unregulated antibiotic use have been linked to increased rates of carbapenem-resistant Klebsiella pneumoniae [10]. WHO’s 2022 AMR brief also highlighted a surge in resistance during the COVID-19 pandemic, particularly in low- and middle-income countries (LMICs), where empirical treatments were often used in the absence of diagnostics.
Given these challenges, there is a critical need for transformative strategies that go beyond conventional antimicrobials. Emerging technologies—such as nanotechnology for targeted drug delivery [10], bacteriophage therapy for pathogen-specific eradication [11], CRISPR-based gene editing for resistance gene silencing [12], immunotherapy to boost host defenses [13], and precision medicine to tailor interventions to individual and microbial profiles [14], offer promising solutions. These innovations, when integrated into health systems alongside surveillance and stewardship programs, have the potential to redefine AMR control in both high-income and resource-limited settings.
This narrative review synthesizes the current landscape of innovative biotechnological interventions for AMR and evaluates their feasibility, readiness, and applicability for global scale-up, with a special focus on equity and sustainability.
2. Methodology
A comprehensive, integrative narrative review was conducted to evaluate the current landscape of innovative strategies addressing antimicrobial resistance (AMR). The methodology included a systematic literature search, application of predefined inclusion and exclusion criteria, structured data extraction, and thematic synthesis aligned with PRISMA 2020 guidelines. The protocol to conduct this review was registered in the international prospective register for systematic review PROSPERO CRD420250608857.
2.1. Literature Search
A structured search was performed in December 2023 across three major databases: PubMed, Web of Science, and ScienceDirect, to identify relevant peer-reviewed studies. The search was limited to publications from January 2018 to December 2023 to ensure contemporary relevance. Search terms included combinations of keywords such as “antimicrobial resistance,” “infection control,” “bacteriophage therapy,” “CRISPR-Cas,” “nanotechnology,” “immunotherapy,” and “precision medicine”, using Boolean operators (AND, OR) to optimize results.
2.2. Inclusion and Exclusion Criteria
Inclusion criteria focused on peer-reviewed empirical studies published in English, emphasizing innovative strategies, technologies, or methodologies directly targeting AMR. Eligible study designs included experimental, quasi-experimental, and observational studies with clinical or implementation data. Articles were excluded if they were not peer-reviewed (e.g., opinion pieces, editorials), lacked empirical data, or addressed only traditional antimicrobials without discussing novel or alternative interventions.
3. Study Selection and Data Extraction
From an initial yield of 1023 articles, duplicates and irrelevant titles were removed, and titles and abstracts were screened for eligibility. Full-text reviews led to the final inclusion of 145 articles. The PRISMA flow diagram (Figure 1) details the selection process. A standardized data extraction matrix was developed to collect information on strategy type (e.g., bacteriophage, CRISPR), study design, outcomes measured (e.g., efficacy, safety), and contextual factors such as feasibility and scalability.
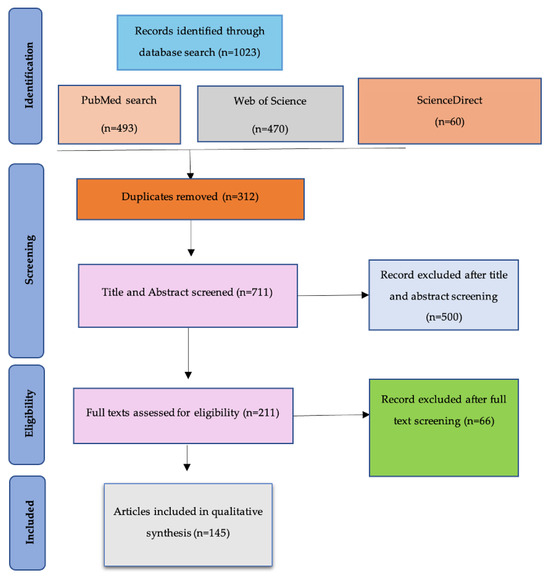
Figure 1.
PRISMA flow diagram illustrating the literature screening and selection process for studies included in the review.
Thematic Analysis
Thematic synthesis was employed to organize the findings into five primary thematic domains: nanotechnology, bacteriophage therapy, CRISPR-Cas systems, immunotherapy, and personalized medicine. This process involved iterative reading of the selected studies, open coding of relevant findings, and inductive categorization into thematic clusters. The resulting themes were refined through consensus discussions to ensure internal consistency and analytical coherence. This allowed for integration of heterogeneous evidence, including studies with varying designs and outcome measures, within a coherent narrative framework. Figure 1 presents the PRISMA 2020 flow diagram, which outlines the process of literature identification, screening, eligibility assessment, and final inclusion.
4. Results
4.1. Nanotechnology in Antimicrobial Delivery and Targeting
Nanotechnology has emerged as a transformative tool in infectious disease management, particularly in overcoming limitations of conventional antimicrobials. Among the 145 studies included, 32 focused on nanotechnology-based interventions for AMR. Engineered nanoparticles—including silver (AgNPs), gold nanoparticles, and liposomes—demonstrated enhanced drug delivery, biofilm disruption, and targeted activity against multidrug-resistant pathogens.
Silver nanoparticles (AgNPs) disrupted bacterial membranes, induced oxidative stress via reactive oxygen species (ROS), and interfered with DNA replication, exhibiting broad efficacy against MRSA and Escherichia coli [15,16]. Liposomes encapsulated antibiotics effectively, enhancing drug penetration into biofilm-embedded bacteria, a common challenge in device-associated infections [16]. Gold nanoparticles showed efficacy against Pseudomonas aeruginosa by triggering ROS-mediated cell death and enabled pathogen-specific targeting when functionalized with bacterial ligands (Table 1) [17].

Table 1.
A summary of key nanoparticles.
Topical formulations using hydrogels and nanocomposites offered localized and sustained antimicrobial delivery, minimizing systemic toxicity [18,19]. Nine studies investigated combination nanotherapies that co-delivered antibiotics and adjuvants, showing synergistic efficacy in bypassing resistance mechanisms [20].
Peptides nanomaterials have emerged as promising solutions for targeted antimicrobial delivery due to their inherent chemical structures and bioactivities. Wang et al. (2024) review highlights advances in designing peptide- and peptoid-based nanocarriers that enhance stability and specificity by enabling selective binding to bacterial membranes and controlled release of antimicrobial agents [21]. These nanomaterials employ self-assembly and chemical modifications to optimize pharmacokinetics, reduce toxicity and improves therapeutic efficacy. Similarly, Magana et al. (2020) reveals that antimicrobial peptides (AMPs) are promising therapeutic agents that exhibit antimicrobial activities by interfering with bacterial cell membranes, blocking essential intercellular processes and modulate immune responses to strengthen host defenses [22]. Generally, these nanomaterials represent a significant step in antimicrobial therapy strategies by enabling precision targeting of pathogens and addressing the challenge of antimicrobial resistant infections.
Additionally, Kumar et al. (2019) reported antibacterial activities of carbon-based graphene nanoparticles against bacterial pathogens including E. coli [23]. The results of this study showed that graphene particles induce both physical damage of bacterial cell membranes through sharp edges and oxidative stressing through charge transfers. These biotechnological innovations underscore nanotechnology’s utility in both systemic and localized infection control.
4.2. Bacteriophage Therapy
Bacteriophage therapy has re-emerged as a promising strategy in the fight against antimicrobial resistance (AMR), particularly in the treatment of infections caused by multidrug-resistant (MDR) bacteria. Bacteriophages viruses that selectively infect and lyse bacterial cells offer a targeted approach that preserves the host microbiome and minimizes the collateral damage often associated with broad-spectrum antibiotics [20].
Mechanistically, bacteriophages attach to specific bacterial receptors, inject their genetic material, and hijack the bacterial machinery to replicate. This replication culminates in cell lysis, releasing progeny phages capable of infecting neighboring pathogenic cells [24]. This self-amplifying nature allows for sustained antibacterial activity and offers a scalable therapeutic model adaptable to various infection contexts.
Clinical and preclinical evidence demonstrates the efficacy of phage therapy in several critical use cases. In controlled clinical trials, phage cocktails targeting Pseudomonas aeruginosa significantly reduced bacterial loads, especially in immunocompromised patients and individuals with cystic fibrosis. In one notable trial published in Nature Biotechnology, engineered phages were administered intravenously, resulting in improved clinical outcomes and bacterial clearance superior to that achieved with antibiotics alone [24].
Case-based studies have further illustrated phage therapy’s capacity to resolve recalcitrant infections. A landmark report in Clinical Infectious Diseases documented the successful eradication of a persistent methicillin-resistant Staphylococcus aureus (MRSA) infection using a custom phage preparation. This outcome followed repeated antibiotic failures and underscores the potential of phages in personalized medicine [25].
Additionally, experimental models have demonstrated phage efficacy against Salmonella enterica, a common foodborne pathogen. In vivo studies showed that targeted phage administration in animal models significantly decreased bacterial colonization and improved clinical outcomes, suggesting future application in both human and veterinary medicine [26].
Beyond their direct bactericidal action, phages exhibit synergistic effects when co-administered with antibiotics. This combination therapy can enhance bacterial killing, reduce required antibiotic dosages, and slow the emergence of resistance [27]. Furthermore, due to their host specificity, phages spare beneficial commensals preserving the host’s microbiota and supporting immune homeostasis.
Despite the success, bacteria have adapted several resistance mechanisms against phages that limit the performance of the therapy [28]. Bacteria can modify their surface receptors and block the adsorption of phages on their surfaces, secrete small molecules with anti-phage properties [29] and secret toxins to block the progress of phage replication and assembly [28].
Nevertheless, public access and usage of phage therapy in various countries face some regulatory challenges. In Eastern Europe, phage treatment can only be administered to patients in Phage Therapy Unit [30], while in Western Europe and United States, the application of individual phage therapy is under specific regulations that require the availability of clinical trials and approved protocols for Good Manufacturing practices (GMPs). In these countries, the phage therapy is still considered as experimental treatment [31].
4.3. CRISPR-Cas Systems for Precision Antimicrobial Therapy
CRISPR-Cas technology has rapidly gained momentum as a transformative platform in combating antimicrobial resistance (AMR), offering unmatched specificity in targeting resistance-conferring genes. Of the 145 studies reviewed, 19 focused on the development and application of CRISPR-based antimicrobial interventions predominantly utilizing CRISPR-Cas9 and CRISPR-Cas12 systems. These technologies enable precise gene editing, allowing for the targeted elimination of resistance determinants while preserving the host microbiota’s integrity [32].
CRISPR-Cas systems operate through guide RNAs that direct Cas proteins to specific genomic loci within bacterial DNA. Upon binding, Cas enzymes induce double-stranded breaks or nucleotide modifications, disrupting resistance genes and restoring bacterial susceptibility to conventional antimicrobials. This targeted approach avoids the broad-spectrum collateral damage often caused by traditional antibiotics [33].
Several studies report substantial success in restoring antimicrobial sensitivity in drug-resistant pathogens. For instance, CRISPR-Cas9 targeting the blaNDM-1 gene—which encodes the New Delhi metallo-beta-lactamase responsible for carbapenem resistance in Escherichia coli and other Enterobacteriaceae—achieved 95% restoration of drug susceptibility [34]. Similarly, CRISPR-Cas12 has been employed to disable the mecA gene in Staphylococcus aureus, the driver of methicillin resistance in MRSA, yielding 89% eradication efficacy (Table 2) [35]. These findings were validated in vivo. Wu et al. (2021) [36] demonstrated that CRISPR-Cas9 therapy effectively reduced gastrointestinal colonization by E. coli harboring blaNDM-1 in murine models, reinforcing the translational potential of this strategy [33,35]. Such precision-based interventions offer a compelling therapeutic option for multidrug-resistant infections that fail standard treatment protocols.

Table 2.
CRISPR-Cas Applications in Antimicrobial Resistance Management.
Beyond therapeutics, CRISPR also plays a pivotal role in diagnostics. Molecular platforms such as SHERLOCK and DETECTR integrate CRISPR-based detection to rapidly identify resistance genes from clinical specimens, facilitating point-of-care AMR surveillance and enabling timely, tailored interventions [32].
Another emerging innovation involves the engineering of bacteriophages to serve as CRISPR delivery vectors. These phages concurrently infect resistant bacteria and deliver CRISPR-Cas elements, thereby achieving dual-action bacterial lysis and resistance gene editing [37]. This approach holds promise in high-mutation environments where resistance evolution is rapid.
CRISPR-Cas antimicrobials exhibit the critical advantage of selectively targeting resistant strains while sparing susceptible and commensal microbes reducing the risk of dysbiosis and secondary infections. This ecological precision is especially valuable in immunocompromised patients, where microbiome stability is integral to recovery.
4.4. Immunotherapy for Enhanced Host Resistance
Immunotherapy has gained significant traction as a promising adjunct or alternative to conventional antimicrobials, offering a host-directed strategy to combat antimicrobial resistance (AMR). Among the 145 studies reviewed, 21 focused on immunotherapeutic approaches—including monoclonal antibodies (mAbs), immune-modulatory therapies, and vaccine-enhanced strategies—aimed at strengthening host immunity and mitigating pathogen virulence.
4.4.1. Monoclonal Antibodies
At the forefront of this advancement are monoclonal antibodies, which target specific bacterial toxins or virulence factors without directly killing the pathogens. By neutralizing these pathogenic elements, mAbs exert their protective effects without placing selective pressure on bacterial populations, thus minimizing the risk of resistance emergence [38].
A notable clinical success involves mAbs directed against Clostridium difficile toxins. These antibodies bind and neutralize TcdA and TcdB, significantly reducing both the recurrence and severity of C. difficile infections [39]. Their efficacy has been particularly demonstrated in cases of recurrent or refractory disease, where conventional antibiotics often fail, underscoring their value in treatment-resistant scenarios.
In Staphylococcus aureus especially methicillin-resistant S. aureus (MRSA)—monoclonal antibodies targeting α-toxin have shown considerable promise. Preclinical studies demonstrated that anti-α-toxin mAbs not only reduced bacterial load but also improved survival in systemic infection models, offering a potential path for targeted immunoprophylaxis in high-risk patients [38].
Recent investigations have also explored synergistic strategies combining mAbs with vaccines. This dual approach enhances immunogenicity and immunologic memory, particularly in populations with compromised immune systems such as the elderly who may mount inadequate responses to vaccination alone. Such combination therapies improve both the breadth and durability of protection against bacterial pathogens [40].
4.4.2. Immune Checkpoint Inhibitors
The checkpoint inhibitors have revolutionized cancer therapy and are now being evaluated for infectious disease contexts. By blocking suppressive pathways such as PD-1/PD-L1, these agents reinvigorate T-cell responses previously dampened during chronic infections. Early trials suggest potential in controlling persistent bacterial infections; however, safety concerns and unintended immune activation remain areas for cautious advancement [41].
Despite their promise, immunotherapies face important challenges. One such issue is the emergence of resistance through antigenic variation where bacteria alter or mask the antigens targeted by mAbs. This highlights the need for next-generation antibodies with broader epitope recognition, higher affinity, and enhanced neutralization capabilities [38,39]. A summary of immunotherapeutic modalities, bacterial targets, and clinical outcomes is presented in Table 3.

Table 3.
Immunotherapy Targets and Outcomes.
4.4.3. Host-Directed Therapies
Intracellular pathogens rely on host’s cellular machinery for survival by reprogramming host gene expression to disrupt innate defense mechanisms such as apoptosis [42]. Through co-evolution, these pathogens have developed abilities to manipulate or mimic host factors and secrete effector molecules that suppress immune responses [43]. Host-directed therapy (HDT) is an emerging nanomaterial-based approach that target host factors that pathogens exploit, thereby blocking their replication and persistence [44]. By acting on host immune pathways essential for pathogen’s life cycle, HDT can be effective biotechnological approach for eliminating both drug-sensitive and resistant pathogens in the body [45].
4.5. Precision Medicine and Personalized Treatment Plans
Personalized medicine represents a transformative shift in antimicrobial stewardship, offering individualized therapeutic strategies that leverage diagnostic and genomic innovations to optimize treatment outcomes and mitigate antimicrobial resistance (AMR). Among the studies reviewed, 17 focused on precision medicine applications, emphasizing the integration of rapid diagnostics, genomic profiling, and patient-specific data to guide targeted antimicrobial therapy.
Rapid diagnostic technologies—such as polymerase chain reaction (PCR) and next-generation sequencing (NGS)—form the cornerstone of personalized medicine in infectious disease management. These tools enable real-time identification of pathogens and resistance determinants directly from clinical specimens [46]. By shortening the diagnostic window, clinicians can avoid empirical use of broad-spectrum antibiotics and initiate pathogen-specific therapy. For instance, NGS allows whole-genome analysis of microbial isolates within hours, accurately identifying resistance genes such as blaNDM-1, mecA, or vanA—critical for tailoring effective treatment regimens [47,48].
The clinical feasibility of these technologies continues to improve. Rapid PCR platforms now deliver actionable results within 30–60 min, enabling timely treatment decisions in emergency and intensive care settings. These rapid interventions have been associated with improved clinical outcomes and reduced reliance on unnecessary antimicrobials that drive resistance development [47].
Beyond diagnostics, the integration of electronic health records (EHRs) is pivotal in developing personalized treatment strategies. EHRs allow clinicians to access comprehensive patient-specific data—including prior antimicrobial use, allergy history, microbiome status, and comorbidities—to guide informed therapeutic decisions. This individualized approach not only enhances efficacy but also reduces risks associated with antibiotic overuse and microbiome disruption [35].
Personalized medicine also fosters multidisciplinary collaboration. Microbiologists, infectious disease specialists, geneticists, and clinical pharmacists work in tandem to develop and monitor tailored treatment protocols. This collaborative framework supports real-time AMR surveillance, optimizes antimicrobial use, and reinforces adherence to stewardship guidelines.
An overview of the bacteriophage infection process and convergence of diagnostics, genomics, and clinical data analytics in personalized medicine is summarized in Figure 2a and Figure 2b, respectively.
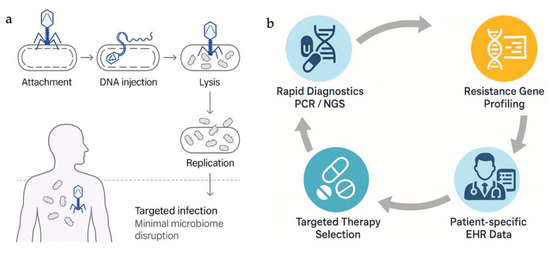
Figure 2.
(a). Mechanism of action and clinical application of bacteriophage therapy. (The illustration depicts phage attachment, DNA injection, replication, bacterial lysis, and reinfection of nearby pathogens—highlighting high specificity and minimal impact on the human microbiome) and (b). Personalized Medicine in AMR Management (The diagram depicts the integration of PCR/NGS diagnostics, resistance gene profiling, patient-specific EHR data, and targeted therapy selection in a closed-loop system supporting antimicrobial stewardship).
4.6. Machine Learning Approaches
Machine learning (ML) approaches are increasingly pivotal in strengthening global efforts to combat antimicrobial resistance (AMR). A growing body of evidence supports the application of ML algorithms in predicting antimicrobial susceptibility and optimizing therapy—especially in critical care environments such as intensive care units (ICUs). Feretzakis et al. (2020) [15] demonstrated that ML-enhanced models improved empirical antibiotic selection in Greek ICUs, reducing treatment delays and improving patient outcomes [13]. Advanced ML techniques, such as association rule mining, have further supported AMR surveillance by identifying co-resistance patterns. For example, Sakagianni et al. (2022) used this method in ICU settings to uncover clusters of antibiotic co-resistance, which were then used to inform local empirical treatment guidelines [46]. Despite these promising applications, several ethical considerations must be addressed. A 2025 review of biomedical big data highlighted persistent concerns related to data privacy, algorithmic bias, and the lack of transparent governance frameworks. These concerns underscore the need for equity-driven and ethically grounded deployment of ML technologies in clinical settings [14].
4.7. Translational Research
Parallel to technological innovation, translational research pathways—such as phased clinical trials and public–private partnerships (PPPs)—are essential for moving AMR innovations from bench to bedside. Early-phase clinical evaluations have tested novel interventions like bacteriophage therapy and CRISPR-Cas-based antimicrobials. For instance, Plumet et al. (2022) reviewed Phase I/II trials of phage therapies and highlighted key regulatory hurdles, including dose standardization and manufacturing protocols [39]. Similarly, Rabaan et al. (2023) analyzed CRISPR-Cas trials and emphasized the importance of minimizing off-target effects to align with evolving regulatory standards [40].
Public–private partnerships have also played a crucial role in accelerating innovation and expanding access in low- and middle-income countries (LMICs). One such initiative, the Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X), supports the development of regionally adaptable solutions, including phage banks and mobile diagnostic labs. A 2023 report published in npj Antimicrobial Resistance credited CARB-X with significantly enhancing diagnostic and therapeutic capacity in resource-constrained settings [49].
When integrated with global frameworks such as the World Health Organization’s Global Antimicrobial Resistance and Use Surveillance System (GLASS), these ML-driven and translational initiatives form a comprehensive approach to ensuring AMR interventions are not only scientifically robust but also accessible, equitable, and sustainable across diverse health systems. The synergy of ML and translational research approaches to combat AMR is shown in Figure 3.
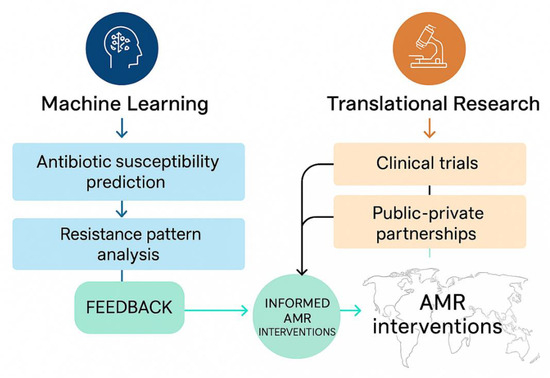
Figure 3.
Machine learning and translational research pathways in AMR intervention. (The diagram illustrates the synergy between ML-driven prediction/analysis and translational research components—including clinical trials and public–private partnerships—feeding into informed AMR interventions through iterative feedback loops).
5. Discussion
Antimicrobial resistance (AMR) continues to threaten global health security, prompting a demand for transformative and equitable approaches that transcend conventional antimicrobial therapies. The innovative interventions discussed—ranging from nanotechnology-based drug delivery systems to bacteriophage therapy, CRISPR-Cas genome editing, immunotherapy, and personalized medicine—offer hope for reshaping infection control. Yet, translating these laboratory advances into real-world impact is far from straightforward. Regulatory, ethical, and logistical barriers, especially in resource-limited settings, continue to hinder broad implementation. The transition of these technologies from proof-of-concept to clinical practice requires structured, evidence-informed pathways.
Gene-editing systems such as CRISPR-Cas9 and CRISPR-Cas12, while highly effective in targeting resistance genes, raise ethical concerns about unintended genome modifications and ecological consequences. These issues demand rigorous oversight and the development of context-specific regulatory frameworks that balance innovation with safety and ethical integrity [33]. Without addressing these foundational gaps, even the most promising interventions may remain inaccessible to those who need them most.
In many low- and middle-income countries (LMICs), implementing technologies such as next-generation sequencing (NGS) and nanomedicine is challenged by high costs, limited infrastructure, and a shortage of skilled personnel. However, these challenges are not insurmountable. Adaptations like local phage banks, open-source diagnostic platforms, and mobile molecular testing units provide scalable alternatives. Global initiatives such as the WHO’s Global Antimicrobial Resistance and Use Surveillance System (GLASS) exemplify how data sharing and coordinated surveillance can support countries with limited resources while contributing to a broader international knowledge base [49]. These strategies can ensure that innovation is not confined to high-income settings but equitably benefits populations worldwide.
Structured translational pathways, such as phased clinical trials and public–private partnerships (PPPs), are pivotal in bridging the gap between laboratory innovation and clinical application for AMR technologies. Phased clinical trials provide a systematic approach to evaluate safety, efficacy, and scalability, progressing through Phase I to IV to generate robust evidence for regulatory approval. For example, bacteriophage therapy trials can standardize dosing protocols [50], while CRISPR-Cas trials can assess off-target risks, addressing ethical concerns [33]. PPPs, like the Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X), combine private sector innovation with public sector oversight to fund development, support technology transfer, and build local capacity in LMICs [49]. By integrating with global initiatives like GLASS and local solutions such as mobile testing units, these pathways ensure equitable access, enabling technologies to address AMR effectively across diverse settings.
In parallel with biomedical advances, the integration of data science—particularly machine learning (ML)—is reshaping how clinicians predict, diagnose, and respond to antimicrobial resistance. ML has demonstrated potential in predicting antibiotic susceptibility using routine clinical and microbiological data, especially in intensive care settings where time-critical decisions are paramount [13]. Studies have shown that algorithms using basic demographic and culture data can improve empirical antibiotic selection, reduce treatment delays, and improve outcomes [46].
Recent studies showcase how ML, when leveraged in LMIC contexts, can improve AMR surveillance and decision-making. For instance, ML models trained on United Kingdom E. coli genomic data achieved up to 94% accuracy when validated with Ugandan, Nigerian, and Tanzanian datasets in predicting ampicillin resistance [51]. In Uganda, ML methods like logistic regression and gradient boosting performed well in predicting resistance to TB drugs using WGS and clinical data, although transferability to other regional datasets highlights generalizability challenges [52]. Moreover, antimicro.ai, developed in Kenya, exemplifies a local AI tool designed to predict antimicrobial susceptibility using open datasets—demonstrating the potential for LMIC-led innovation in AMR management [53]. Overall, these real-world examples demonstrate how ML can transform AMR surveillance from reactive monitoring to predictive, intervention-focused systems integrating ML and precision medicine into AMR strategies within resource-constrained settings.
These capabilities have the potential to shift AMR management toward earlier, evidence-driven interventions that slow resistance spread. However, their deployment must be underpinned by strong ethical frameworks that address data privacy, algorithmic bias, and patient consent [14]. The rise of generative AI in healthcare underscores the urgent need for secure, transparent, and accountable data governance.
To ensure successful implementation and scale-up of these innovations, interdisciplinary collaboration is essential. An inclusive, global model for integrating these technologies into health systems could be achieved through a multi-stakeholder consortium that unites researchers, regulators, industry partners, and policymakers. Such a collaborative framework would facilitate knowledge transfer, accelerate technology adoption, and harmonize global efforts. By focusing on shared goals such as equitable access, infrastructure development, and workforce training, this consortium could bridge the gap between innovation and implementation [36,54].
Finally, equity must remain at the center of all AMR-related interventions. Technological innovation alone cannot solve the AMR crisis if it remains inaccessible to the populations most at risk. Contextualizing these solutions within national AMR action plans, embedding them in health systems strengthening agendas, and supporting implementation research will be crucial to understanding what works, where, and why. Personalized medicine, for example, holds great promise in aligning treatment to pathogen and patient profiles, but its benefits will only be realized through cost-effective diagnostics, electronic health records integration, and clinician training in diverse health systems [55].
To enhance the feasibility and impact of these strategies in low- and middle-income countries (LMICs), a range of coordinated policy actions is required. Establishing regional bacteriophage banks offers a localized and cost-effective approach to managing multidrug-resistant pathogens such as Klebsiella pneumoniae and MRSA, particularly when governments collaborate with academic and healthcare institutions to develop production facilities and train microbiologists. Making rapid molecular testing accessible through subsidies for open-source diagnostics, including CRISPR-based SHERLOCK kits, can further strengthen early detection and response capacities. Mobile diagnostic units—piloted in partnership with global initiatives like CARB-X—could significantly expand testing coverage in rural and underserved areas. In parallel, healthcare workforce development should prioritize hands-on training in nanotechnology, CRISPR-Cas, and machine learning through collaborations with regional universities, ensuring clinicians and laboratory professionals are equipped to implement these technologies effectively [52]. National health policies should explicitly integrate AMR control strategies, such as nanomedicine and phage therapy, supported by earmarked funding aligned with WHO GLASS guidelines. Public–private partnerships (PPPs) can facilitate innovation scale-up by investing in local nanoparticle production, expanding electronic health record systems, and promoting equitable technology transfer. In addition, engaging community health workers in AMR surveillance through low-cost diagnostic tools can generate locally relevant data for real-time trend monitoring and targeted interventions. A robust ethical and regulatory framework will also be critical, requiring the establishment of regional ethics boards and alignment of regulatory standards with WHO recommendations to ensure timely, safe, and equitable access to novel therapies. These combined efforts can drive the successful implementation of biotechnological innovations and ensure their benefits are equitably distributed within LMIC health systems [56].
While this narrative review highlights transformative AMR interventions, certain limitations must be acknowledged. The analysis is based solely on peer-reviewed, English-language literature, excluding grey literature and regional studies that may offer additional insights. Moreover, the heterogeneity in trial designs and technology maturity levels limited direct comparisons across interventions. Future reviews should consider systematic methodologies and include stakeholder consultations to validate feasibility in diverse health systems.
6. Future Directions
Integrating environmental reservoirs of antimicrobial resistance (AMR) into biotechnological approaches will enhance monitoring of antibiotic-resistant genes (ARGs) in the environment. Advanced approaches such as CRISPR-enriched metagenomics improve the detection of low-abundance ARGs in wastewater [57]. Recent genome-based studies show that mobile genetic elements play a key role in ARG transmission within wastewater treatment systems [58]. Additionally, synthetic biology provides promising avenues for AMR control, with AI-designed antimicrobial peptides offering customizable and less immunogenic options. These advances highlight how biotechnological tools can identify AMR hotspots and inform targeted interventions.
7. Conclusions
Antimicrobial resistance (AMR) represents a dynamic and escalating global health challenge that demands innovative, scalable, and equitable solutions. This narrative review outlines a strategic blueprint for addressing AMR through the integration of advanced biotechnologies—such as nanotechnology-based drug delivery, bacteriophage therapy, CRISPR-Cas systems, immunotherapy, and personalized medicine alongside robust public health infrastructures and data-driven interventions. These emerging therapies, particularly when supported by machine learning (ML) analytics, hold transformative potential for optimizing clinical outcomes, improving diagnostic precision, and reducing reliance on conventional antibiotics.
However, realizing this potential requires more than scientific innovation; it demands the resolution of regulatory and ethical challenges, especially concerning gene-editing and AI-driven technologies, as well as the development of equitable access strategies for resource-limited settings where the AMR burden is greatest. Low-cost adaptations, inclusive data-sharing frameworks, and region-specific implementation pathways must be prioritized.
Furthermore, building a resilient and sustainable global response calls for interdisciplinary collaboration among researchers, policymakers, healthcare leaders, and communities, supported by strong capacity-building and funding mechanisms. Ultimately, a future-proof strategy against AMR hinges on merging innovation with ethical governance, equity-driven policies, and global solidarity, ensuring that antimicrobial efficacy is preserved and public health systems are strengthened for generations to come.
Author Contributions
All authors equally contributed to the conceptualization, literature search, and writing of the manuscript. Both authors participated in drafting and revising the manuscript and have approved the final version for submission. All authors have read and agreed to the published version of the manuscript.
Funding
This work did not receive any specific funding from public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were generated or analyzed in this study.
Acknowledgments
During the preparation of this manuscript/study, the author(s) used AI for the purposes of generating some of the figures through guidance. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
All authors declare no competing interests related to this work.
References
- Sakagianni, A.; Koufopoulou, C.; Koufopoulos, P.; Kalantzi, S.; Theodorakis, N.; Nikolaou, M.; Paxinou, E.; Kalles, D.; Verykios, V.S.; Myrianthefs, P.; et al. Data-driven approaches in antimicrobial resistance: Machine learning solutions. Antibiotics 2024, 13, 1052. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, P.; Bennis, N.X.; Daran, J.-M.; Daran-Lapujade, P. gEL DNA: A Cloning- and Polymerase Chain Reaction–Free Method for CRISPR-Based Multiplexed Genome Editing. CRISPR J. 2021, 4, 896–913. [Google Scholar] [CrossRef]
- Au, A.; Lee, H.; Ye, T.; Dave, U.; Rahman, A. Bacteriophages: Combating antimicrobial resistance in food-borne bacteria prevalent in agriculture. Microorganisms 2021, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in nanomaterial-based targeted drug delivery systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef]
- Liu, C.S.C.; Pandey, R. Integrative genomics would strengthen AMR understanding through ONE health approach. Heliyon 2024, 10, e34719. Available online: https://www.cell.com/heliyon/fulltext/S2405-8440(24)10750-5 (accessed on 9 August 2025). [CrossRef]
- Dighe, S.; Jog, S.; Momin, M.; Sawarkar, S.; Omri, A. Intranasal drug delivery by nanotechnology: Advances in and challenges for Alzheimer’s disease management. Pharmaceutics 2023, 16, 58. [Google Scholar] [CrossRef]
- ECDC. Carbapenem-Resistant Klebsiella Pneumoniae in Eastern Ukraine and Syria; ECDC: Stockholm, Sweden; WHO Regional Office for Europe: Copenhagen, Denmark, 2023; Available online: https://www.ecdc.europa.eu (accessed on 4 August 2025).
- Feretzakis, G.; Sakagianni, A.; Loupelis, E.; Kalles, D.; Skarmoutsou, N.; Martsoukou, M.; Christopoulos, C.; Lada, M.; Petropoulou, S.; Velentza, A.; et al. Machine learning for antibiotic resistance prediction: A prototype using off-the-shelf techniques and entry-level data to guide empiric antimicrobial therapy. Healthc. Inform. Res. 2021, 27, 214–221. [Google Scholar] [CrossRef]
- Ferreira, M.; Ogren, M.; Dias, J.N.R.; Silva, M.; Gil, S.; Tavares, L.; Aires-Da-Silva, F.; Gaspar, M.M.; Aguiar, S.I. Liposomes as antibiotic delivery systems: A promising nanotechnological strategy against antimicrobial resistance. Molecules 2021, 26, 2047. [Google Scholar] [CrossRef]
- Zalewska-Piątek, B. Phage Therapy—Challenges, Opportunities and Future Prospects. Pharmaceuticals 2023, 16, 1638. [Google Scholar] [CrossRef]
- Fortaleza, J.A.G.; Ong, C.J.N.; De Jesus, R. Efficacy and clinical potential of phage therapy in treating methicillin-resistant Staphylococcus aureus (MRSA) infections: A review. Eur. J. Microbiol. Immunol. 2024, 14, 13–25. [Google Scholar] [CrossRef]
- Gupta, S.L.; Basu, S.; Soni, V.; Jaiswal, R.K. Immunotherapy: An alternative promising therapeutic approach against cancers. Mol. Biol. Rep. 2022, 49, 9903–9913. [Google Scholar] [CrossRef] [PubMed]
- Gwenzi, W.; Chaukura, N.; Muisa-Zikali, N.; Teta, C.; Musvuugwa, T.; Rzymski, P.; Abia, A.L.K. Insects, rodents, and pets as reservoirs, vectors, and sentinels of antimicrobial resistance. Antibiotics 2021, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Hetta, H.F.; Ramadan, Y.N.; Al-Harbi, A.I.; Ahmed, E.A.; Battah, B.; Ellah, N.H.A.; Zanetti, S.; Donadu, M.G. Nanotechnology as a promising approach to combat multidrug resistant bacteria: A comprehensive review and future perspectives. Biomedicines 2023, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Feretzakis, G.; Loupelis, E.; Sakagianni, A.; Kalles, D.; Martsoukou, M.; Lada, M.; Skarmoutsou, N.; Christopoulos, C.; Valakis, K.; Velentza, A.; et al. Using Machine Learning Techniques to Aid Empirical Antibiotic Therapy Decisions in the Intensive Care Unit of a General Hospital in Greece. Antibiotics 2020, 9, 50. [Google Scholar] [CrossRef]
- Jo, S.J.; Kwon, J.; Kim, S.G.; Lee, S.-J. The biotechnological application of bacteriophages: What to do and where to go in the middle of the post-antibiotic era. Microorganisms 2023, 11, 2311. [Google Scholar] [CrossRef]
- Kaprou, G.D.; Bergšpica, I.; Alexa, E.A.; Alvarez-Ordóñez, A.; Prieto, M. Rapid methods for antimicrobial resistance diagnostics. Antibiotics 2021, 10, 209. [Google Scholar] [CrossRef]
- Kaur, K.; Singh, S.; Kaur, R. Impact of antibiotic usage in food-producing animals on food safety and possible antibiotic alternatives. Microbe 2024, 4, 100097. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Goda, D.A.; Khalil, M.I.; Al-Zaban, M.I. Novel biogenic silver nanoparticle-induced reactive oxygen species inhibit the biofilm formation and virulence activities of methicillin-resistant Staphylococcus aureus (MRSA) strain. Front. Bioeng. Biotechnol. 2020, 8, 433. [Google Scholar] [CrossRef]
- Khan, F.; Kang, M.-G.; Jo, D.-M.; Chandika, P.; Jung, W.-K.; Kang, H.W.; Kim, Y.-M. Phloroglucinol-gold and-zinc oxide nanoparticles: Antibiofilm and antivirulence activities towards Pseudomonas aeruginosa PAO1. Mar. Drugs 2021, 19, 601. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, B.; Fan, J.; Wang, F.; Liu, Q. A Study of the Dengue Epidemic and Meteorological Factors in Guangzhou, China, by Using a Zero-Inflated Poisson Regression Model. Asia Pac. J. Public Health 2014, 26, 48–57. [Google Scholar] [CrossRef]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial Properties of Graphene-Based Nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef] [PubMed]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Xu, S.; Tang, Z.; Li, Z.; Zhang, L. Use of antimicrobials in food animals and impact of transmission of antimicrobial resistance on humans. Biosaf. Health 2021, 03, 32–38. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; De Filippis, A.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver nanoparticles: Bactericidal and mechanistic approach against drug resistant pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef]
- Bleriot, I.; Pacios, O.; Blasco, L.; Fernández-García, L.; López, M.; Ortiz-Cartagena, C.; Barrio-Pujante, A.; García-Contreras, R.; Pirnay, J.-P.; Wood, T.K.; et al. Improving phage therapy by evasion of phage resistance mechanisms. JAC-Antimicrob. Resist. 2024, 6, dlae017. [Google Scholar] [CrossRef]
- Hardy, A.; Kever, L.; Frunzke, J. Antiphage small molecules produced by bacteria–beyond protein-mediated defenses. Trends Microbiol. 2023, 31, 92–106. [Google Scholar] [CrossRef]
- Żaczek, M.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Łusiak-Szelachowska, M.; Górski, A. Phage therapy in Poland—A centennial journey to the first ethically approved treatment facility in Europe. Front. Microbiol. 2020, 11, 1056. [Google Scholar] [CrossRef]
- Yang, Q.; Le, S.; Zhu, T.; Wu, N. Regulations of phage therapy across the world. Front. Microbiol. 2023, 14, 1250848. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, T.; Shahwan, M.; Aatif, M. Origin of antibiotics and antibiotic resistance, and their impacts on drug development: A narrative review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Li, P.; Wan, P.; Zhao, R.; Chen, J.; Li, X.; Li, J.; Xiong, W.; Zeng, Z. Targeted Elimination of blaNDM-5 Gene in Escherichia coli by Conjugative CRISPR-Cas9 System. Infect. Drug Resist. 2022, 15, 1707–1716. [Google Scholar] [CrossRef]
- O’neill, J.I.M. Antimicrobial resistance: Tackling a crisis for the health and wealth of nations. Rev. Antimicrob. Resist. 2014. Available online: https://cir.nii.ac.jp/crid/1370857593729357568 (accessed on 9 August 2025).
- Olson, E.G.; Micciche, A.C.; Rothrock, M.J., Jr.; Yang, Y.; Ricke, S.C. Application of bacteriophages to limit Campylobacter in poultry production. Front. Microbiol. 2022, 12, 458721. [Google Scholar] [CrossRef]
- Wu, Y.; Battalapalli, D.; Hakeem, M.J.; Selamneni, V.; Zhang, P.; Draz, M.S.; Ruan, Z. Engineered CRISPR-Cas systems for the detection and control of antibiotic-resistant infections. J. Nanobiotechnol. 2021, 19, 401. [Google Scholar] [CrossRef]
- Fage, C.; Lemire, N.; Moineau, S. Delivery of CRISPR-Cas systems using phage-based vectors. Curr. Opin. Biotechnol. 2021, 68, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Oon, Y.-L.; Ayaz, M.; Deng, M.; Li, L.; Song, K. Waterborne pathogens detection technologies: Advances, challenges, and future perspectives. Front. Microbiol. 2023, 14, 1286923. [Google Scholar] [CrossRef] [PubMed]
- Plumet, L.; Ahmad-Mansour, N.; Dunyach-Remy, C.; Kissa, K.; Sotto, A.; Lavigne, J.-P.; Costechareyre, D.; Molle, V. Bacteriophage therapy for Staphylococcus aureus infections: A review of animal models, treatments, and clinical trials. Front. Cell. Infect. Microbiol. 2022, 12, 907314. [Google Scholar] [CrossRef] [PubMed]
- Rabaan, A.A.; Al Fares, M.A.; Almaghaslah, M.; Alpakistany, T.; Al Kaabi, N.A.; Alshamrani, S.A.; Alshehri, A.A.; Almazni, I.A.; Saif, A.; Hakami, A.R.; et al. Application of CRISPR-Cas system to mitigate superbug infections. Microorganisms 2023, 11, 2404. [Google Scholar] [CrossRef]
- Yi, M.; Zheng, X.; Niu, M.; Zhu, S.; Ge, H.; Wu, K. Combination strategies with PD-1/PD-L1 blockade: Current advances and future directions. Mol. Cancer 2022, 21, 28. [Google Scholar] [CrossRef]
- Wallis, R.S.; O’gArra, A.; Sher, A.; Wack, A. Host-directed immunotherapy of viral and bacterial infections: Past, present and future. Nat. Rev. Immunol. 2023, 23, 121–133. [Google Scholar] [CrossRef]
- Bergman, P.; Raqib, R.; Rekha, R.S.; Agerberth, B.; Gudmundsson, G.H. Host Directed Therapy Against Infection by Boosting Innate Immunity. Front. Immunol. 2020, 11, 1209. [Google Scholar] [CrossRef] [PubMed]
- Gholap, A.D.; Khuspe, P.R.; Pardeshi, S.R.; Uddin, J.; Das, U.; Hatvate, N.T.; Rojekar, S.; Giram, P.; Khalid, M.; Choonara, Y.E.; et al. Achieving Optimal Health with Host-Directed Therapies (HDTs) in Infectious Diseases—A New Horizon. Adv. Ther. 2025, 8, 2400169. [Google Scholar] [CrossRef]
- Rastogi, S.; Chandra, P. Host-Directed Omics Approaches to Tackle Antimicrobial Resistance. In Antimicrobial Resistance: Factors to Findings; Soni, V., Akhade, A.S., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 327–357. [Google Scholar]
- Sakagianni, A.; Feretzakis, G.; Kalles, D.; Loupelis, E.; Rakopoulou, Z.; Dalainas, I.; Fildisis, G. Discovering Association Rules in Antimicrobial Resistance in Intensive Care Unit. In Studies in Health Technology and Informatics; Mantas, J., Gallos, P., Zoulias, E., Hasman, A., Househ, M.S., Diomidous, M., Liaskos, J., Charalampidou, M., Eds.; IOS Press: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Salam, A.; Al-Amin, Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial resistance: A growing serious threat for global public health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Chen, H.; Li, N.; Liang, W. The Application of the CRISPR-Cas System in Antibiotic Resistance. Infect. Drug Resist. 2022, 15, 4155–4168. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as drug delivery systems: A review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Khatami, A.; Foley, D.A.; Warner, M.S.; Barnes, E.H.; Peleg, A.Y.; Li, J.; Stick, S.; Burke, N.; Lin, R.C.Y.; Warning, J.; et al. Standardised treatment and monitoring protocol to assess safety and tolerability of bacteriophage therapy for adult and paediatric patients (STAMP study): Protocol for an open-label, single-arm trial. BMJ Open 2022, 12, e065401. [Google Scholar] [CrossRef]
- Nsubuga, M.; Galiwango, R.; Jjingo, D.; Mboowa, G. Generalizability of machine learning in predicting antimicrobial resistance in E. coli: A multi-country case study in Africa. BMC Genom. 2024, 25, 287. [Google Scholar] [CrossRef]
- Babirye, S.R.; Nsubuga, M.; Mboowa, G.; Batte, C.; Galiwango, R.; Kateete, D.P. Machine learning-based prediction of antibiotic resistance in Mycobacterium tuberculosis clinical isolates from Uganda. BMC Infect. Dis. 2024, 24, 1391. [Google Scholar] [CrossRef]
- Darzi, A.; Koivuniemi, A.; Acharya, A.; Dryden, S.; Kohli, P.; Mason, J.; Papa, E.; Singh, A.P.; Soni, L. Harnessing Artificial Intelligence to Tackle Antimicrobial Resistance. Imperial College London. 2025. Available online: https://www.imperial.ac.uk/Stories/harnessing-artificial-intelligence-tackle-antimicrobial-resistance/ (accessed on 8 August 2025).
- WHO. Global Antimicrobial Resistance and Use Surveillance System; WHO: Geneva, Switzerland, 2022; Available online: https://www.who.int/initiatives/glass (accessed on 5 August 2023).
- Yamin, D.; Uskoković, V.; Wakil, A.M.; Goni, M.D.; Shamsuddin, S.H.; Mustafa, F.H.; Alfouzan, W.A.; Alissa, M.; Alshengeti, A.; Almaghrabi, R.H.; et al. Current and future technologies for the detection of antibiotic-resistant bacteria. Diagnostics 2023, 13, 3246. [Google Scholar] [CrossRef]
- Sammut, S.M. The role of the biotechnology industry in addressing health inequities in Africa: Strengthening the entire health care value chain. J. Commer. Biotechnol. 2021, 26, 57–68. Available online: https://commercialbiotechnology.com/menuscript/index.php/jcb/article/view/1008 (accessed on 9 August 2025). [CrossRef]
- Mao, Y.; Shisler, J.L.; Nguyen, T.H. Enhanced detection for antibiotic resistance genes in wastewater samples using a CRISPR-enriched metagenomic method. Water Res. 2025, 274, 123056. [Google Scholar] [CrossRef]
- Abdulkadir, N.; Saraiva, J.P.; Zhang, J.; Stolte, S.; Gillor, O.; Harms, H.; Rocha, U.; Rosato, A.E. Genome-centric analyses of 165 metagenomes show that mobile genetic elements are crucial for the transmission of antimicrobial resistance genes to pathogens in activated sludge and wastewater. Microbiol. Spectr. 2024, 12, e02918–e02923. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).