Taxonomic and Functional Profiling of Bacterial Communities in Leather Biodegradation: Insights into Metabolic Pathways and Diversity
Abstract
1. Introduction
2. Materials and Methods
2.1. Assay Determining Microorganisms’ Ability to Degrade Leather (ISO:20136:2020)
2.2. Sample Collection and Preparation
2.3. RNA Extraction and Quality Control
2.4. 16S and Transcriptomic Library Preparation
2.5. 16S and Transcriptomic Library Sequencing
2.6. 16S Analysis
2.7. Metatranscriptomic Analysis
3. Results
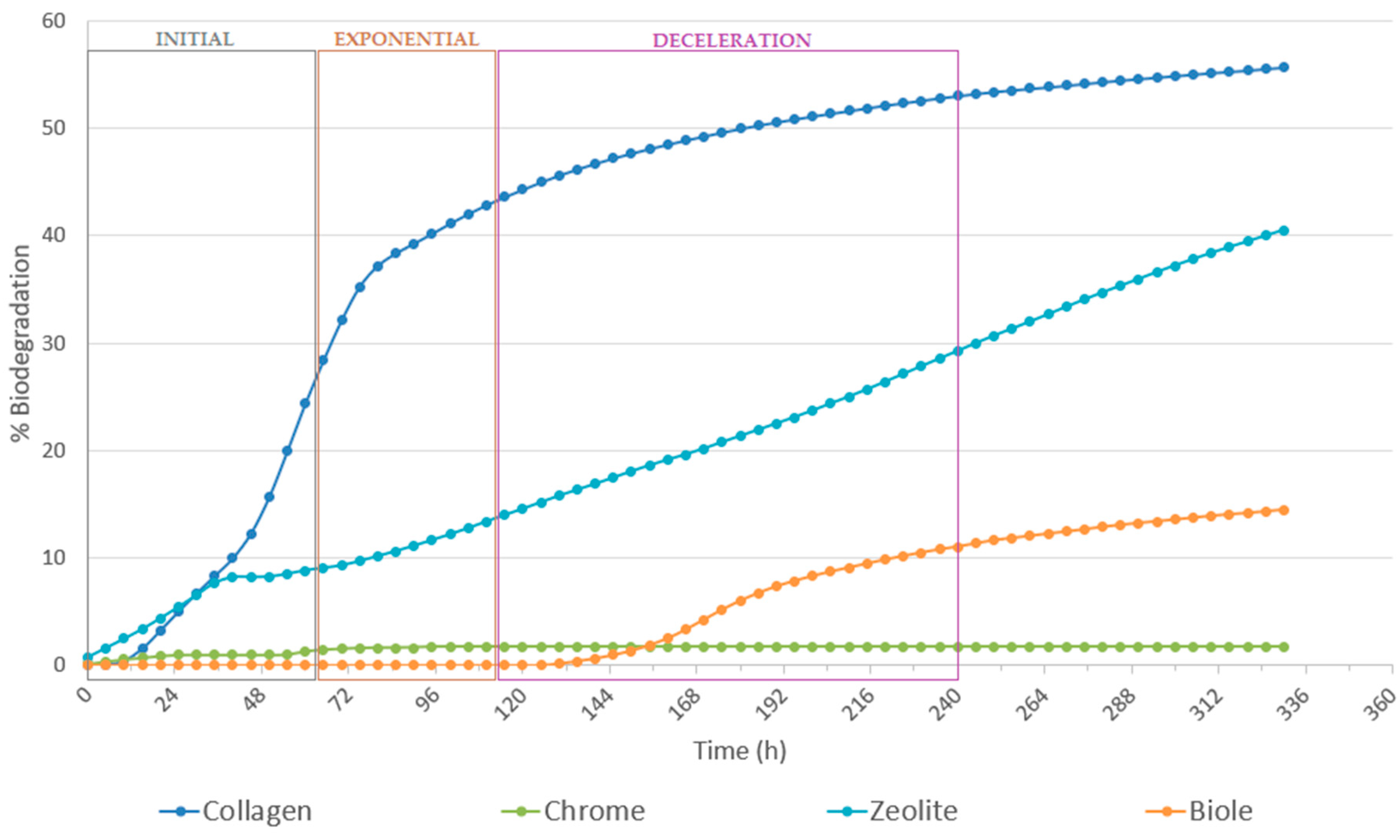
3.1. Assay Determining Microorganisms’ Ability to Degrade Leather (ISO:20136:2020)
3.2. 16S and Transcriptomic Library Sequencing
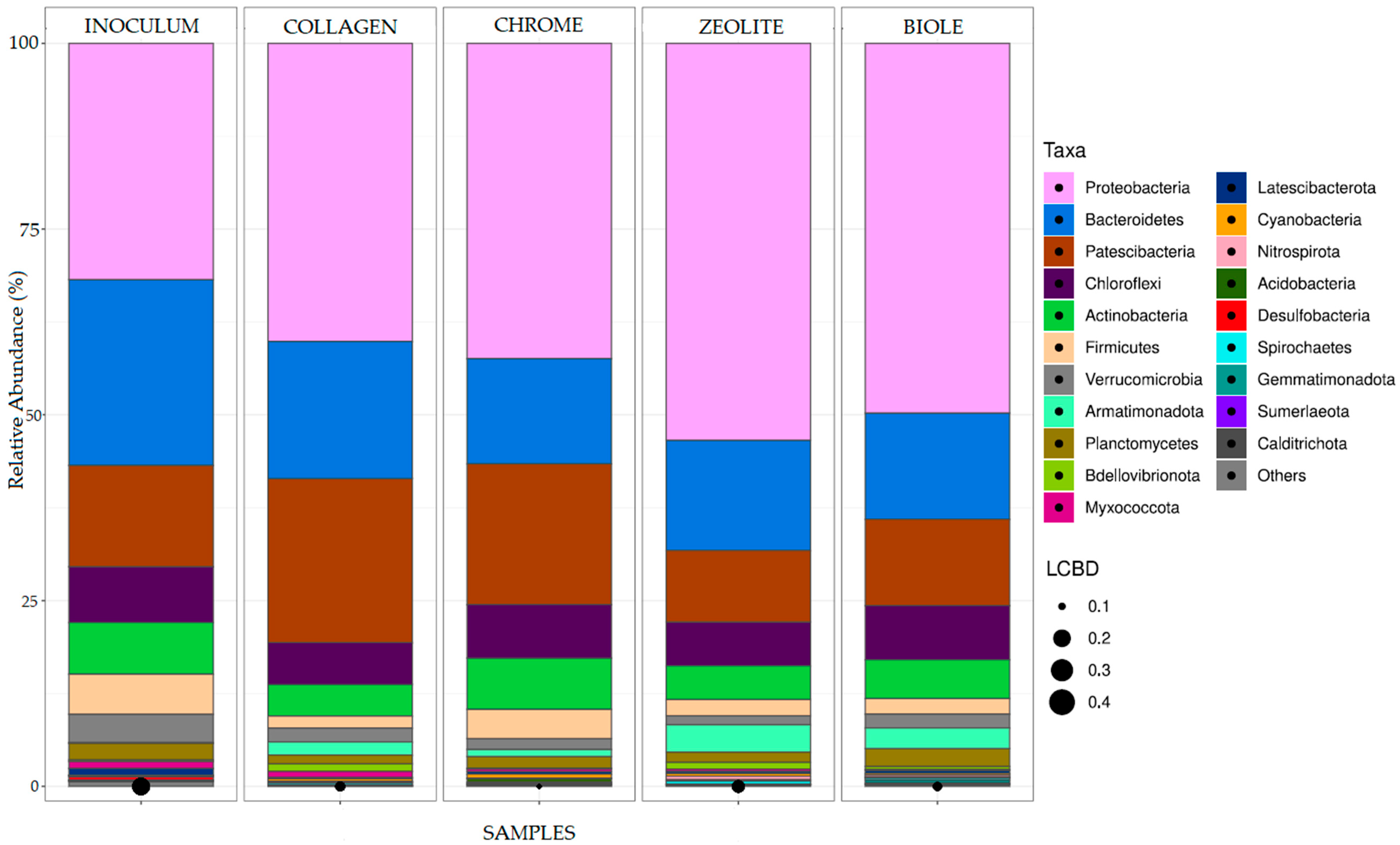
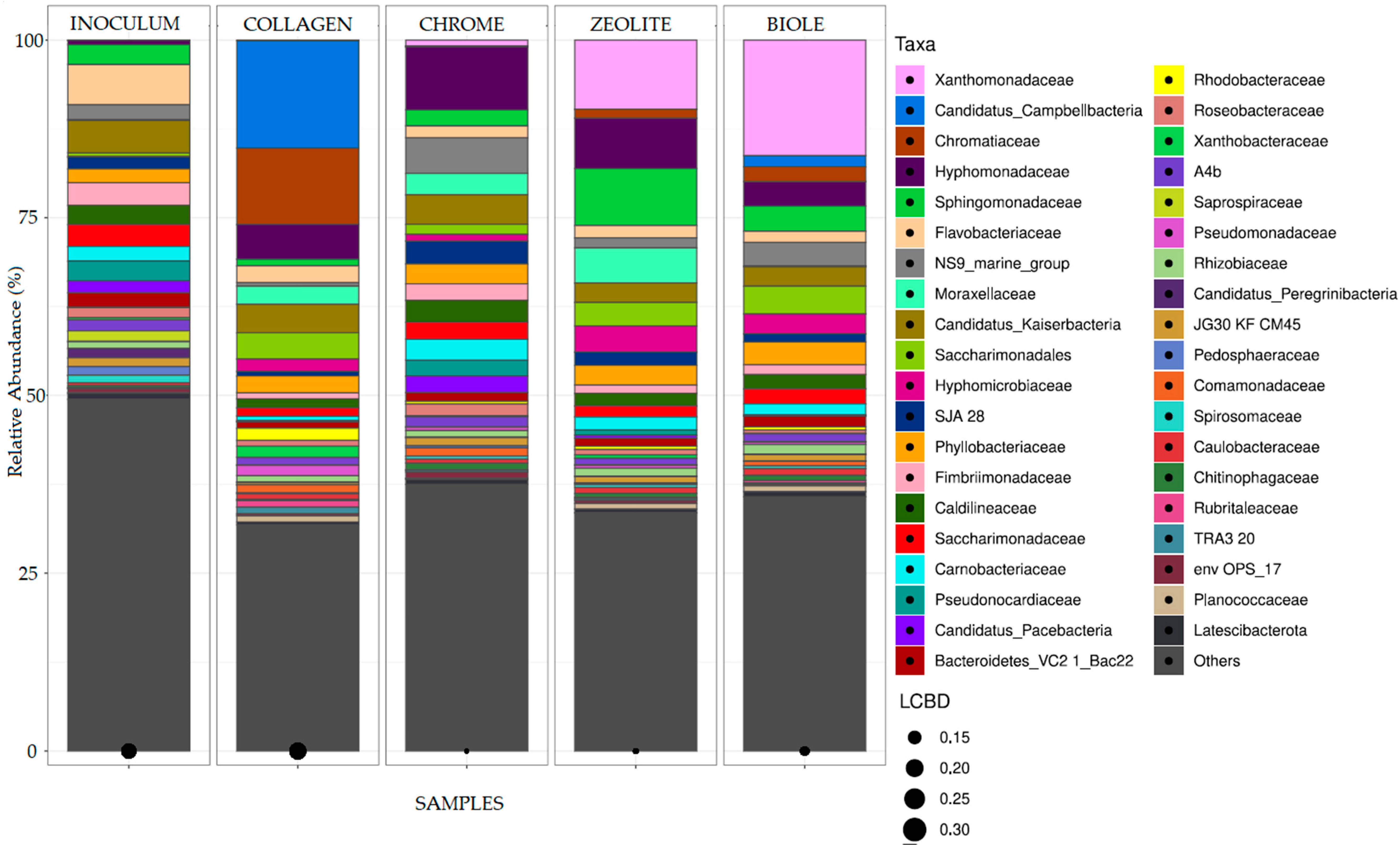
3.3. 16S rRNA Gene Sequence Analysis of Bacterial Communities
3.4. Alpha Diversity
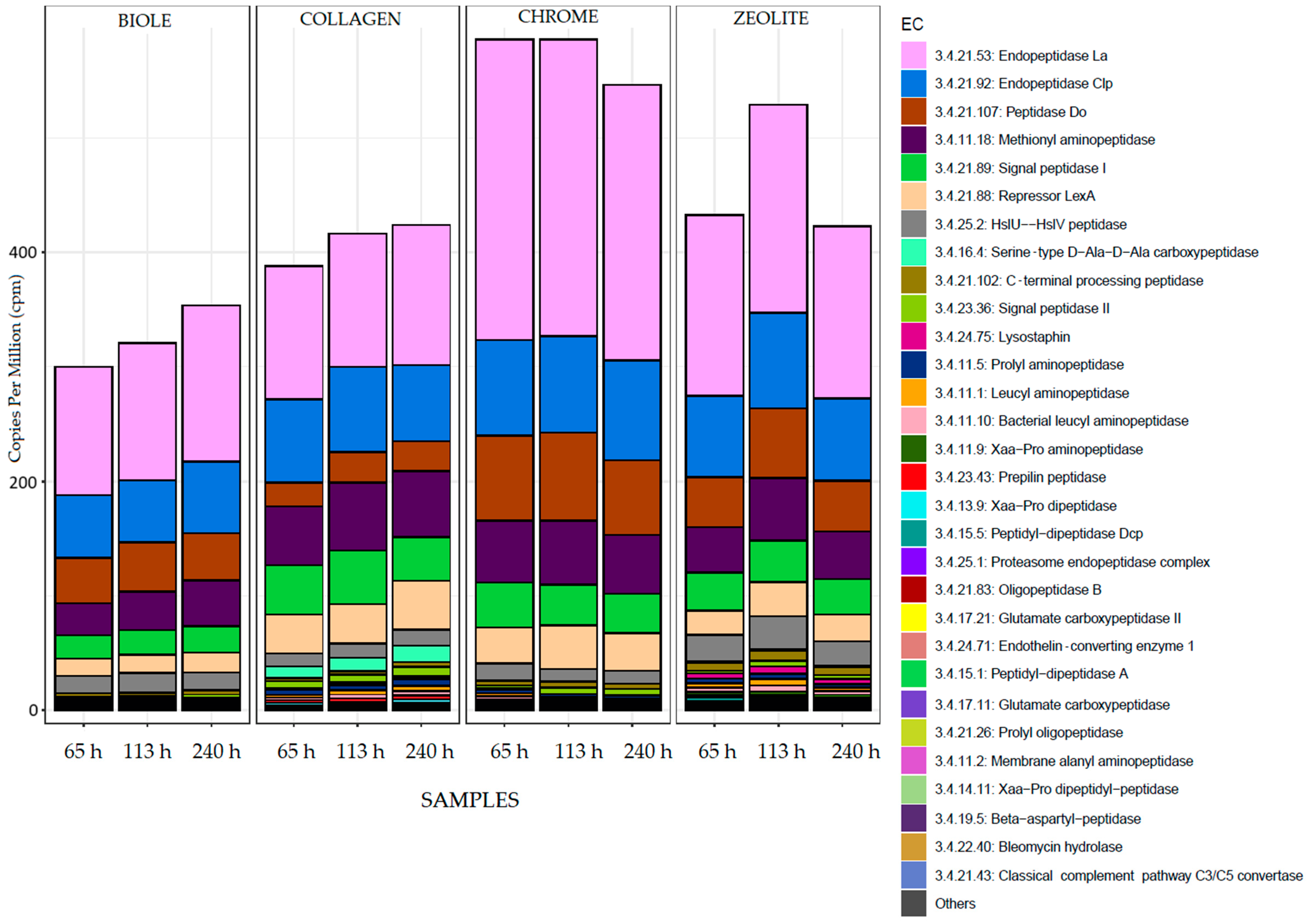
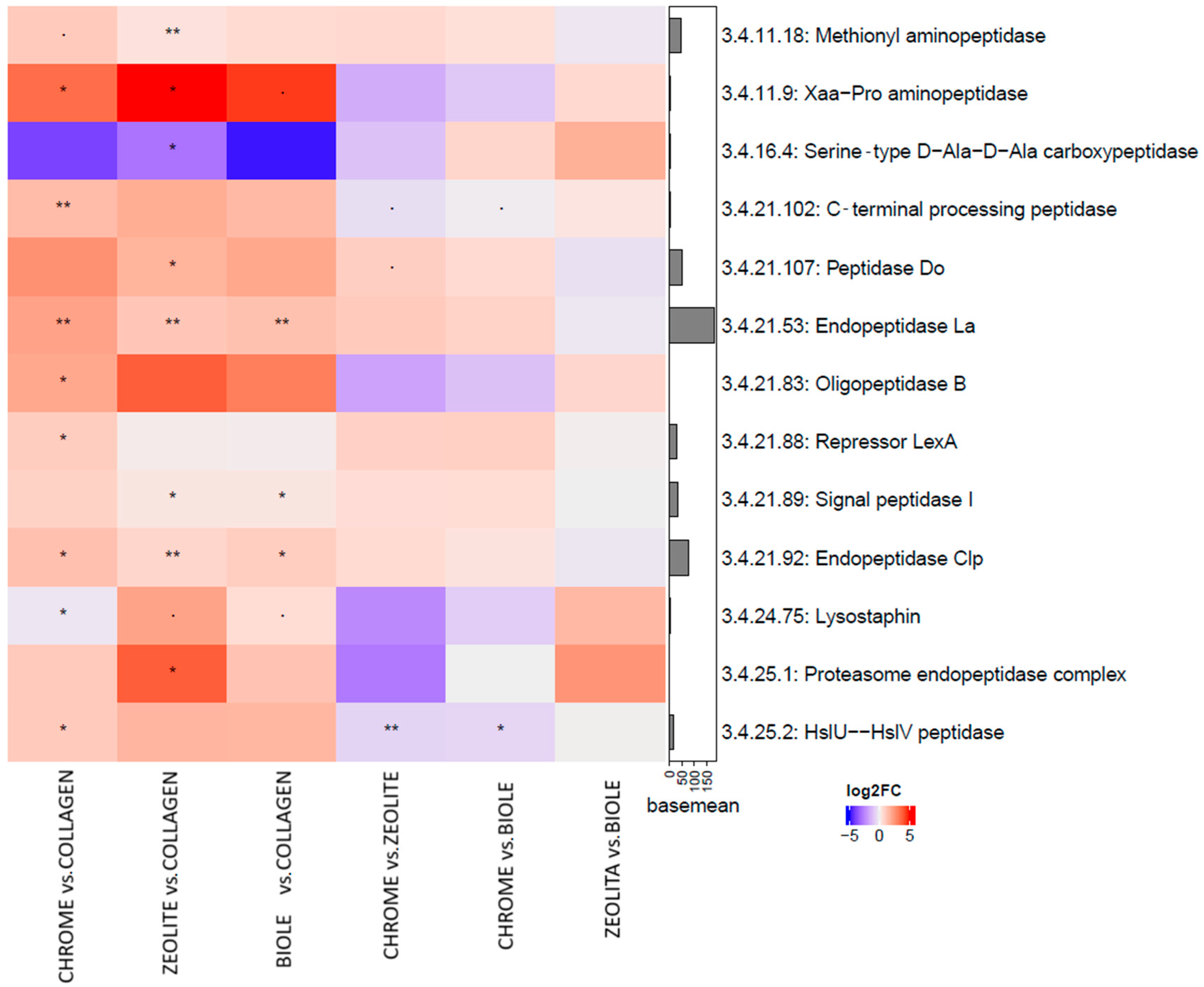
3.5. Metatranscriptomic Analysis
3.5.1. Alpha Diversity
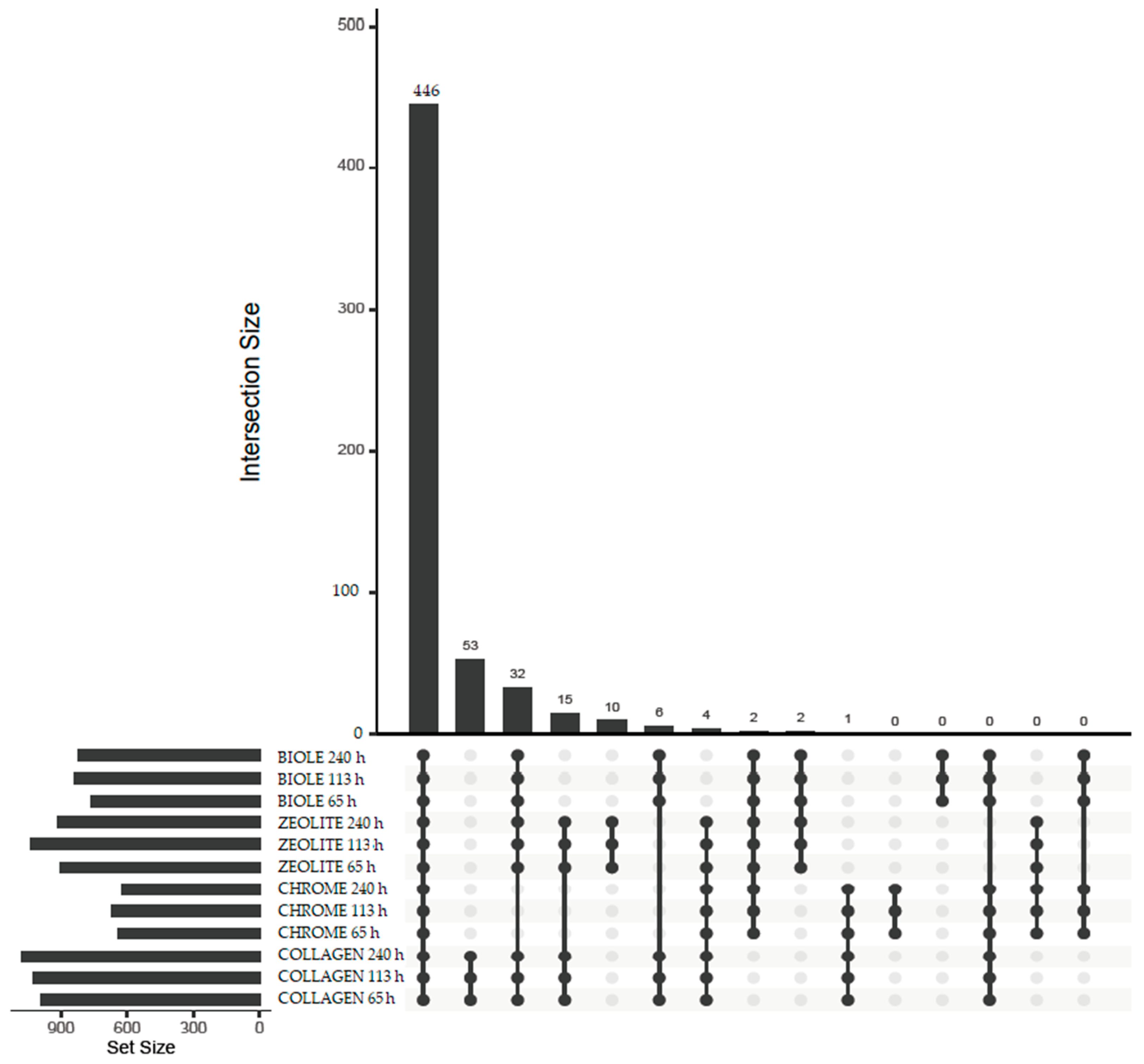
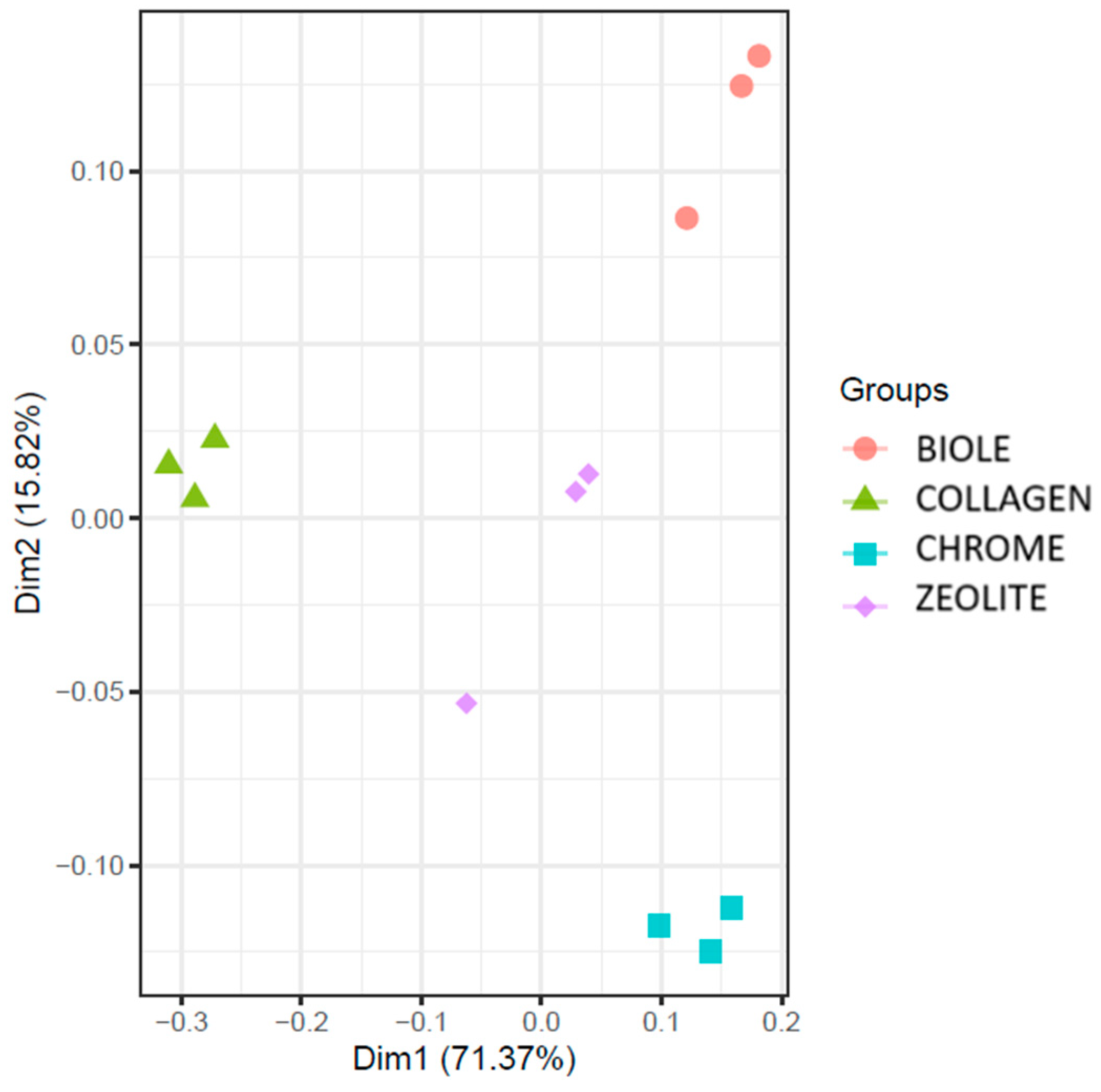
3.5.2. Beta Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Narayanan, P.; Janardhanan, S.K. An Approach Towards Identification of Leather from Leather-like Polymeric Material Using FTIR-ATR Technique. Collagen Leather 2024, 6, 1. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, X.; Zheng, M.; Yue, O.; Xie, L.; Zha, S.; Dong, S.; Li, T.; Song, Y.; Huang, M.; et al. Leather for Flexible Multifunctional Bio-Based Materials: A Review. J. Leather Sci. Eng. 2022, 4, 16. [Google Scholar] [CrossRef]
- Onem, E.; Yorgancioglu, A.; Karavana, H.A.; Yilmaz, O. Comparison of Different Tanning Agents on the Stabilization of Collagen via Differential Scanning Calorimetry. J. Therm. Anal. Calorim. 2017, 129, 615–622. [Google Scholar] [CrossRef]
- Meyer, M.; Dietrich, S.; Schulz, H.; Mondschein, A. Comparison of the Technical Performance of Leather, Artificial Leather, and Trendy Alternatives. Coatings 2021, 11, 226. [Google Scholar] [CrossRef]
- Covington, A.D. Tanning Chemistry: The Science of Leather; RSC Publishing: Cambridge, UK, 2009; ISBN 978-0-85404-170-1. [Google Scholar]
- China, C.R.; Maguta, M.M.; Nyandoro, S.S.; Hilonga, A.; Kanth, S.V.; Njau, K.N. Alternative Tanning Technologies and Their Suitability in Curbing Environmental Pollution from the Leather Industry: A Comprehensive Review. Chemosphere 2020, 254, 126804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ingham, B.; Cheong, S.; Ariotti, N.; Tilley, R.D.; Naffa, R.; Holmes, G.; Clarke, D.J.; Prabakar, S. Real-Time Synchrotron Small-Angle X-ray Scattering Studies of Collagen Structure During Leather Processing. Ind. Eng. Chem. Res. 2018, 57, 63–69. [Google Scholar] [CrossRef]
- Vico, A.; Maestre-Lopez, M.I.; Arán-Ais, F.; Orgilés-Calpena, E.; Bertazzo, M.; Marhuenda-Egea, F.C. Assessment of the Biodegradability and Compostability of Finished Leathers: Analysis Using Spectroscopy and Thermal Methods. Polymers 2024, 16, 1908. [Google Scholar] [CrossRef]
- Alugoju, P.; Rao, C.S.V.; Babu, R.; Thankappan, R. Assessment of Biodegradability of Synthetic Tanning Agents Used in Leather Tanning Process. Int. J. Eng. Technol. 2011, 3, 302–308. [Google Scholar]
- Stefan, D.; Dima, R.; Pantazi-Bajenaru, M.; Ferdes, M.; Meghea, A. Identifying Microorganisms Able to Perform Biodegradation of Leather Industry Waste. Mol. Cryst. Liq. Cryst. 2012, 556, 301–308. [Google Scholar] [CrossRef]
- Masi, C.; Gemechu, G.; Tafesse, M. Isolation, Screening, Characterization, and Identification of Alkaline Protease-Producing Bacteria from Leather Industry Effluent. Ann. Microbiol. 2021, 71, 24. [Google Scholar] [CrossRef]
- Pandey, K.; Saharan, B.S.; Kumar, R.; Jabborova, D.; Duhan, J.S. Modern-Day Green Strategies for the Removal of Chromium from Wastewater. J. Xenobiotics 2024, 14, 1670–1696. [Google Scholar] [CrossRef]
- Abdulmalik, A.F.; Yakasai, H.M.; Usman, S.; Muhammad, J.B.; Jagaba, A.H.; Ibrahim, S.; Babandi, A.; Shukor, M.Y. Characterization and Invitro Toxicity Assay of Bio-Reduced Hexavalent Chromium by Acinetobacter sp. Isolated from Tannery Effluent. Case Stud. Chem. Environ. Eng. 2023, 8, 100459. [Google Scholar] [CrossRef]
- Kanagaraj, G.; Elango, L. Chromium and Fluoride Contamination in Groundwater Around Leather Tanning Industries in Southern India: Implications from Stable Isotopic Ratio δ53Cr/δ52Cr, Geochemical and Geostatistical Modelling. Chemosphere 2019, 220, 943–953. [Google Scholar] [CrossRef]
- Bisht, H.; Kumar, N. Identification and Characterization of Aluminium Tolerant Bacteria Isolated from Soil Contaminated by Electroplating and Automobile Waste. Nat. Environ. Pollut. Technol. 2023, 22, 411–416. [Google Scholar] [CrossRef]
- Farh, M.E.-A.; Kim, Y.-J.; Sukweenadhi, J.; Singh, P.; Yang, D.-C. Aluminium Resistant, Plant Growth Promoting Bacteria Induce Overexpression of Aluminium Stress Related Genes in Arabidopsis thaliana and Increase the Ginseng Tolerance Against Aluminium Stress. Microbiol. Res. 2017, 200, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Arti; Mehra, R. Analysis of Heavy Metals and Toxicity Level in the Tannery Effluent and the Environs. Environ. Monit. Assess. 2023, 195, 554. [Google Scholar] [CrossRef]
- Gillard, B.; Chatzievangelou, D.; Thomsen, L.; Ullrich, M.S. Heavy-Metal-Resistant Microorganisms in Deep-Sea Sediments Disturbed by Mining Activity: An Application Toward the Development of Experimental In Vitro Systems. Front. Mar. Sci. 2019, 6, 462. [Google Scholar] [CrossRef]
- Ashraf, S.; Naveed, M.; Afzal, M.; Ashraf, S.; Rehman, K.; Hussain, A.; Zahir, Z.A. Bioremediation of Tannery Effluent by Cr- and Salt-Tolerant Bacterial Strains. Environ. Monit. Assess. 2018, 190, 716. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, A.; Panigrahi, A.K. Chromium Contamination in Soil and Its Bioremediation: An Overview. In Advances in Bioremediation and Phytoremediation for Sustainable Soil Management: Principles, Monitoring and Remediation; Malik, J.A., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 229–248. ISBN 978-3-030-89984-4. [Google Scholar]
- Fatima, Z.; Azam, A.; Iqbal, M.Z.; Badar, R.; Muhammad, G. A Comprehensive Review on Effective Removal of Toxic Heavy Metals from Water Using Genetically Modified Microorganisms. Desalination Water Treat. 2024, 319, 100553. [Google Scholar] [CrossRef]
- Kulakovskaya, T. Inorganic Polyphosphates and Heavy Metal Resistance in Microorganisms. World J. Microbiol. Biotechnol. 2018, 34, 139. [Google Scholar] [CrossRef]
- Fardami, A.; Balarabe, U.; Sabitu, M.; Lawal, A.; Adamu, A.; Aliyu, A.; Lawal, I.; Abdullahi Dalhatu, I.; Zainab, M.; Farouq, A. Mechanisms of Bacterial Resistance to Heavy Metals: A Mini Review. UMYU Sci. 2023, 2, 76–87. [Google Scholar] [CrossRef]
- Pande, V.; Pandey, S.C.; Sati, D.; Bhatt, P.; Samant, M. Microbial Interventions in Bioremediation of Heavy Metal Contaminants in Agroecosystem. Front. Microbiol. 2022, 13, 824084. [Google Scholar] [CrossRef]
- Hu, J.; Xiao, Z.; Zhou, R.; Deng, W.; Wang, M.; Ma, S. Ecological Utilization of Leather Tannery Waste with Circular Economy Model. J. Clean. Prod. 2011, 19, 221–228. [Google Scholar] [CrossRef]
- Rosu, L.; Varganici, C.; Crudu, A.; Rosu, D. Influence of Different Tanning Agents on Bovine Leather Thermal Degradation. J. Therm. Anal. Calorim. 2018, 134, 583–594. [Google Scholar] [CrossRef]
- Moktadir, M.A.; Ahmadi, H.B.; Sultana, R.; Zohra, F.-T.; Liou, J.J.H.; Rezaei, J. Circular Economy Practices in the Leather Industry: A Practical Step Towards Sustainable Development. J. Clean. Prod. 2020, 251, 119737. [Google Scholar] [CrossRef]
- Alam, M.A.; Mondal, A.K.; Uddin, M.T.; Razzaq, M.A.; Chowdhury, M.J.; Saha, M.S. Chemical Investigation and Separation of Chromium from Chrome Cake of BSCIC Tannery Industrial Estate at Hemayetpur, Dhaka, Bangladesh. J. Environ. Public Health 2023, 2023, 6685856. [Google Scholar] [CrossRef]
- Hashem, M.A.; Sahen, M.S.; Hasan, M.; Payel, S.; Nur-A-Tomal, M.S. Tannery Liming Sludge in Compost Production: Sustainable Waste Management. Biomass Convers. Biorefinery 2023, 13, 9305–9314. [Google Scholar] [CrossRef]
- Cervantes, C.; Campos-García, J.; Devars, S.; Gutiérrez-Corona, F.; Loza-Tavera, H.; Torres-Guzmán, J.C.; Moreno-Sánchez, R. Interactions of Chromium with Microorganisms and Plants. FEMS Microbiol. Rev. 2001, 25, 335–347. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Kumar, S.; Singh, D. Tannery Effluent Treatment and Its Environmental Impact: A Review of Current Practices and Emerging Technologies. Water Qual. Res. J. 2023, 58, 128–152. [Google Scholar] [CrossRef]
- Mohd, S.; Sangale, M.K.; Ade, A.B. (Eds.) Plastic Waste Disposal and Reuse of Plastic Waste. In Bioremediation Technology for Plastic Waste; Springer: Singapore, 2019; pp. 21–30. ISBN 978-981-13-7492-0. [Google Scholar]
- Verma, S.K.; Sharma, P.C. Current Trends in Solid Tannery Waste Management. Crit. Rev. Biotechnol. 2023, 43, 805–822. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, Z. Biodegradability of Leather: A Crucial Indicator to Evaluate Sustainability of Leather. Collagen Leather 2024, 6, 12. [Google Scholar] [CrossRef]
- Bonilla-Espadas, M.; Zafrilla, B.; Lifante-Martínez, I.; Camacho, M.; Orgilés-Calpena, E.; Arán-Aís, F.; Bertazzo, M.; Bonete, M.-J. Selective Isolation and Identification of Microorganisms with Dual Capabilities: Leather Biodegradation and Heavy Metal Resistance for Industrial Applications. Microorganisms 2024, 12, 1029. [Google Scholar] [CrossRef]
- Sahoo, S.; Sahoo, R.K.; Gaur, M.; Behera, D.U.; Sahu, A.; Das, A.; Dey, S.; Dixit, S.; Subudhi, E. Environmental Carbapenem-resistant Acinetobacter baumannii in Wastewater Receiving Urban River System of Eastern India: A Public Health Threat. Int. J. Environ. Sci. Technol. 2023, 20, 9901–9910. [Google Scholar] [CrossRef]
- Jeong, J.; Ahn, S.; Truong, T.C.; Kim, J.-H.; Weerawongwiwat, V.; Lee, J.-S.; Yoon, J.-H.; Sukhoom, A.; Kim, W. Description of Mycolicibacterium arenosum sp. nov. Isolated from Coastal Sand on the Yellow Sea Coast. Curr. Microbiol. 2024, 81, 73. [Google Scholar] [CrossRef]
- Ali, A.; Li, M.; Su, J.; Li, Y.; Wang, Z.; Bai, Y.; Ali, E.F.; Shaheen, S.M. Brevundimonas diminuta Isolated from Mines Polluted Soil Immobilized Cadmium (Cd2+) and zinc (Zn2+) Through Calcium Carbonate Precipitation: Microscopic and Spectroscopic Investigations. Sci. Total Environ. 2022, 813, 152668. [Google Scholar] [CrossRef]
- Jia, X.; Tan, R.; Peng, B. Preparation and Application of Polyethylene Glycol Triazine Derivatives as a Chrome-Free Tanning Agent for Wet-White Leather Manufacturing. Environ. Sci. Pollut. Res. 2022, 29, 7732–7742. [Google Scholar] [CrossRef]
- Rivela, B.; Moreira, M.T.; Bornhardt, C.; Méndez, R.; Feijoo, G. Life Cycle Assessment as a Tool for the Environmental Improvement of the Tannery Industry in Developing Countries. Environ. Sci. Technol. 2004, 38, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.A.; Sattar, S.; Nawaz, R.; Al-Hussain, S.A.; Rizwan, M.; Bukhari, A.; Waseem, M.; Irfan, A.; Inam, A.; Zaki, M.E.A. Enhancing Chromium Removal and Recovery from Industrial Wastewater Using Sustainable and Efficient Nanomaterial: A Review. Ecotoxicol. Environ. Saf. 2023, 263, 115231. [Google Scholar] [CrossRef]
- Mukherjee, A.; Reddy, M.S. Metatranscriptomics: An Approach for Retrieving Novel Eukaryotic Genes from Polluted and Related Environments. 3 Biotech 2020, 10, 71. [Google Scholar] [CrossRef]
- Ojala, T.; Kankainen, M.; Kankuri, E. Understanding Human Health Through Metatranscriptomics. Trends Mol. Med. 2023, 29, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Vyskočilová, G.; Carşote, C.; Ševčík, R.; Badea, E. Burial-Induced Deterioration in Leather: A FTIR-ATR, DSC, TG/DTG, MHT and SEM Study. Herit. Sci. 2022, 10, 7. [Google Scholar] [CrossRef]
- ISO. ISO 20136:2020. Available online: https://www.iso.org/standard/75892.html (accessed on 20 November 2023).
- Español—Curtidos Segorbe S.L. Available online: http://www.curtidosegorbe.com/curtidos-segorbe-s-l/espanol/ (accessed on 22 February 2024).
- Collagen from Bovine Achilles Tendon Powder, Suitable for Substrate for Collagenase|9007-34-5. Available online: https://www.sigmaaldrich.com/ES/es/search/9007-34-5?focus=products&page=1&perpage=30&sort=relevance&term=9007-34-5&type=cas_number (accessed on 20 November 2023).
- RNAprotect® Handbook 2019. Available online: https://www.qiagen.com/us/resources/download.aspx?id=38566945-e96b-46a4-a287-ec026849da4b&lang=en (accessed on 20 November 2023).
- QIAsymphony RNA Kit. Available online: https://www.qiagen.com/us/products/discovery-and-translational-research/dna-rna-purification/rna-purification/total-rna/qiasymphony-rna-kit (accessed on 4 January 2025).
- RNeasy MinElute Cleanup Kit|RNA Concentration|QIAGEN. Available online: https://www.qiagen.com/us/products/discovery-and-translational-research/dna-rna-purification/rna-purification/rna-clean-up/rneasy-minelute-cleanup-kit (accessed on 4 January 2025).
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Nextera XT DNA Library Prep Kit|Sequence Small Genomes, Plasmids, cDNA. Available online: https://emea.illumina.com/products/by-type/sequencing-kits/library-prep-kits/nextera-xt-dna.html (accessed on 20 November 2023).
- Quant-iTTM PicoGreenTM dsDNA Assay Kits and dsDNA Reagents. Available online: https://www.thermofisher.com/order/catalog/product/es/en/P7589 (accessed on 20 November 2023).
- Illumina Ribo-Zero Plus rRNA Depletion Kit|Standalone rRNA Depletion. Available online: https://emea.illumina.com/products/by-type/molecular-biology-reagents/ribo-zero-plus-rrna-depletion.html (accessed on 5 January 2025).
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2’s q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Silva—High Quality Ribosomal RNA Database. Available online: https://www.arb-silva.de/ (accessed on 10 February 2025).
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A Fast and Accurate Illumina Paired-End reAd Merger. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Preprint 2022, 3-1. Available online: http://CRAN.Rproject.org/package=vegan (accessed on 20 November 2023).
- HUMAnN3—The Huttenhower Lab. Available online: https://huttenhower.sph.harvard.edu/humann/ (accessed on 20 November 2023).
- UniRef|UniProt. Available online: https://www.uniprot.org/uniref/ (accessed on 8 February 2025).
- Ijaz, U.Z.; Sivaloganathan, L.; McKenna, A.; Richmond, A.; Kelly, C.; Linton, M.; Stratakos, A.C.; Lavery, U.; Elmi, A.; Wren, B.W. Comprehensive Longitudinal Microbiome Analysis of the Chicken Cecum Reveals a Shift from Competitive to Environmental Drivers and a Window of Opportunity for Campylobacter. Front. Microbiol. 2018, 9, 2452. [Google Scholar] [CrossRef]
- Butt, M.Q.; Zeeshan, N.; Ashraf, N.M.; Akhtar, M.A.; Ashraf, H.; Afroz, A.; Shaheen, A.; Naz, S. Environmental Impact and Diversity of Protease-Producing Bacteria in Areas of Leather Tannery Effluents of Sialkot, Pakistan. Environ. Sci. Pollut. Res. 2021, 28, 54842–54851. [Google Scholar] [CrossRef]
- Tian, R.; Ning, D.; He, Z.; Zhang, P.; Spencer, S.J.; Gao, S.; Shi, W.; Wu, L.; Zhang, Y.; Yang, Y.; et al. Small and Mighty: Adaptation of Superphylum Patescibacteria to Groundwater Environment Drives Their Genome Simplicity. Microbiome 2020, 8, 51. [Google Scholar] [CrossRef]
- Chaudhary, D.K.; Dahal, R.H.; Kim, J. Flavobacterium silvisoli sp. nov., Isolated from Forest Soil. Int. J. Syst. Evol. Microbiol. 2019, 69, 2762–2766. [Google Scholar] [CrossRef]
- Sharma, M.; Khurana, H.; Singh, D.N.; Negi, R.K. The Genus Sphingopyxis: Systematics, Ecology, and Bioremediation Potential—A Review. J. Environ. Manag. 2021, 280, 111744. [Google Scholar] [CrossRef]
- Graciano-Ávila, G.; Aguirre-Calderón, Ó.A.; Alanís-Rodríguez, E.; Lujan-Soto, J.E. Composición, Estructura y Diversidad de Especies Arbóreas en un Bosque Templado del Noroeste de México. Ecosistemas Recur. Agropecu. 2017, 4, 535–542. [Google Scholar] [CrossRef]
- Moreno, C.E. Métodos para Medir la Biodiversidad; SEA: Singapore, 2001; ISBN 978-84-922495-2-7. [Google Scholar]
- Weiss, S.; Xu, Z.Z.; Peddada, S.; Amir, A.; Bittinger, K.; Gonzalez, A.; Lozupone, C.; Zaneveld, J.R.; Vázquez-Baeza, Y.; Birmingham, A.; et al. Normalization and Microbial Differential Abundance Strategies Depend upon Data Characteristics. Microbiome 2017, 5, 27. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- ENZYME—2.7.7.6 DNA-Directed RNA Polymerase. Available online: https://enzyme.expasy.org/EC/2.7.7.6 (accessed on 18 February 2025).
- Lauranzano, E.; Pozzi, S.; Pasetto, L.; Stucchi, R.; Massignan, T.; Paolella, K.; Mombrini, M.; Nardo, G.; Lunetta, C.; Corbo, M.; et al. Peptidylprolyl Isomerase A Governs TARDBP Function and Assembly in Heterogeneous Nuclear Ribonucleoprotein Complexes. Brain 2015, 138, 974–991. [Google Scholar] [CrossRef]
- Osaki, S.; Walaas, O. Kinetic Studies of Ferrous Ion Oxidation with Crystalline Human Ferroxidase: II. Rate constants at various steps and formation of a possible enzyme-substrate complex. J. Biol. Chem. 1967, 242, 2653–2657. [Google Scholar] [CrossRef]
- Valeika, V.; Beleska, K.; Valeikiene, V.; Sirvaityte, J. Common Tormentil Tannins as Tanning Material for Leather Processing. Вісник Київськoгo Націoнальнoгo Університету Технoлoгій Та Дизайну 2012, 3, 88–94. [Google Scholar]
- Bhaskar, N.; Sakhare, P.Z.; Suresh, P.V.; Gowda, L.; Mahendrakar, N. Biostabilization and Preparation of Protein Hydrolysates from Delimed Leather Fleshings. J. Sci. Ind. Res. 2007, 66, 1054–1063. [Google Scholar]
- Chang, A.; Jeske, L.; Ulbrich, S.; Hofmann, J.; Koblitz, J.; Schomburg, I.; Neumann-Schaal, M.; Jahn, D.; Schomburg, D. BRENDA, the ELIXIR Core Data Resource in 2021: New Developments and Updates. Nucleic Acids Res. 2020, 49, D498–D508. [Google Scholar] [CrossRef]
- Tschesche, H.; Kupfer, S. C-Terminal-Sequence Determination by Carboxypeptidase C from Orange Leaves. Eur. J. Biochem. 1972, 26, 33–36. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, C.; Ren, Y.; Wang, R.; Zhang, C.; Han, S.; Ju, Z.; Zhao, Z.; Sun, C.; Wu, M. Pseudomonas mangrovi sp. nov., Isolated from Mangrove soil. Int. J. Syst. Evol. Microbiol. 2019, 69, 377–383. [Google Scholar] [CrossRef]
- Wang, J.; Song, J.J.; Franklin, M.C.; Kamtekar, S.; Im, Y.J.; Rho, S.H.; Seong, I.S.; Lee, C.S.; Chung, C.H.; Eom, S.H. Crystal Structures of the HslVU Peptidase–ATPase Complex Reveal an ATP-Dependent Proteolysis Mechanism. Structure 2001, 9, 177–184. [Google Scholar] [CrossRef]
- Urakami, T.; Araki, H.; Oyanagi, H.; Suzuki, K.-I.; Komagata, K. Transfer of Pseudomonas aminovorans (Den Dooren de Jong 1926) to Aminobacter gen. nov. as Aminobacter aminovorans comb. nov. and Description of Aminobacter aganoensis sp. nov. and Aminobacter niigataensis sp. nov. Int. J. Syst. Evol. Microbiol. 1992, 42, 84–92. [Google Scholar] [CrossRef]
- Li, J.-M.; Ou, J.-H.; Verpoort, F.; Surmpalli, R.Y.; Huang, W.-Y.; Kao, C.-M. Toxicity Evaluation of a Heavy-Metal-Polluted River: Pollution Identification and Bacterial Community Assessment. Water Environ. Res. 2023, 95, e10904. [Google Scholar] [CrossRef]
- Gupta, R.S.; Lo, B.; Son, J. Phylogenomics and Comparative Genomic Studies Robustly Support Division of the Genus Mycobacterium into an Emended Genus Mycobacterium and Four Novel Genera. Front. Microbiol. 2018, 9, 67. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Mishra, S.K.; Misra, S.; Dixit, V.K.; Pandey, S.; Khare, P.; Khan, M.H.; Dwivedi, S.; Lehri, A. Evaluation of Fertility Indicators Associated with Arsenic-Contaminated Paddy Fields Soil. Int. J. Environ. Sci. Technol. 2018, 15, 2447–2458. [Google Scholar] [CrossRef]
- Dou, X.; Zhang, J.; Zhang, C.; Ma, D.; Chen, L.; Zhou, G.; Li, J.; Duan, Y. Calcium Carbonate Regulates Soil Organic Carbon Accumulation by Mediating Microbial Communities in Northern China. CATENA 2023, 231, 107327. [Google Scholar] [CrossRef]
- Martínez-Martínez, J.G.; Rosales-Loredo, S.; Hernández-Morales, A.; Arvizu-Gómez, J.L.; Carranza-Álvarez, C.; Macías-Pérez, J.R.; Rolón-Cárdenas, G.A.; Pacheco-Aguilar, J.R. Bacterial Communities Associated with the Roots of Typha spp. and Its Relationship in Phytoremediation Processes. Microorganisms 2023, 11, 1587. [Google Scholar] [CrossRef]
- Panda, J.; Sarkar, P. Bioremediation of Chromium by Novel Strains Enterobacter aerogenes T2 and Acinetobacter sp. PD 12 S2. Environ. Sci. Pollut. Res. 2012, 19, 1809–1817. [Google Scholar] [CrossRef]
- Ilias, M.; Rafiqullah, I.M.; Debnath, B.C.; Mannan, K.S.B.; Mozammel Hoq, M. Isolation and Characterization of Chromium(VI)-Reducing Bacteria from Tannery Effluents. Indian J. Microbiol. 2011, 51, 76–81. [Google Scholar] [CrossRef]
- Gong, D.; Ye, F.; Pang, C.; Lu, Z.; Shang, C. Isolation and Characterization of Pseudomonas sp. Cr13 and Its Application in Removal of Heavy Metal Chromium. Curr. Microbiol. 2020, 77, 3661–3670. [Google Scholar] [CrossRef]
- Izrael-Živković, L.; Rikalović, M.; Gojgić-Cvijović, G.; Kazazić, S.; Vrvić, M.; Brčeski, I.; Beškoski, V.; Lončarević, B.; Gopčević, K.; Karadžić, I. Cadmium Specific Proteomic Responses of a Highly Resistant Pseudomonas aeruginosa san ai. RSC Adv. 2018, 8, 10549–10560. [Google Scholar] [CrossRef]
- Biswas, J.K.; Mondal, M.; Rinklebe, J.; Sarkar, S.K.; Chaudhuri, P.; Rai, M.; Shaheen, S.M.; Song, H.; Rizwan, M. Multi-Metal Resistance and Plant Growth Promotion Potential of a Wastewater Bacterium Pseudomonas aeruginosa and Its Synergistic Benefits. Environ. Geochem. Health 2017, 39, 1583–1593. [Google Scholar] [CrossRef]
- Biernacka, D.; Gorzelak, P.; Klein, G.; Raina, S. Regulation of the First Committed Step in Lipopolysaccharide Biosynthesis Catalyzed by LpxC Requires the Essential Protein LapC (YejM) and HslVU Protease. Int. J. Mol. Sci. 2020, 21, 9088. [Google Scholar] [CrossRef]
- Hoppe, I.J.; Brandstetter, H.; Schönauer, E. Biochemical Characterisation of a Collagenase from Bacillus cereus Strain Q1. Sci. Rep. 2021, 11, 4187. [Google Scholar] [CrossRef]
- Ng, H.W.; Zhang, Y.; Naffa, R.; Prabakar, S. Monitoring the Degradation of Collagen Hydrogels by Collagenase Clostridium Histolyticum. Gels 2020, 6, 46. [Google Scholar] [CrossRef]
- Ruan, Z.; Xu, M.; Xing, Y.; Jiang, Q.; Yang, B.; Jiang, J.; Xu, X. Interspecies Metabolic Interactions in a Synergistic Consortium Drive Efficient Degradation of the Herbicide Bromoxynil Octanoate. J. Agric. Food Chem. 2022, 70, 11613–11622. [Google Scholar] [CrossRef]

| Sample | Tanning Type | % 12C | % N | % H | % S |
|---|---|---|---|---|---|
| Collagen | None | 51.0 | 10.3 | 7.0 | 0.9 |
| M3 chrome AV-1821 | Chrome-tanned (heavy metal-based) | 43.2 | 7.8 | 2.4 | 0.0 |
| M6 Zeolite | Zeolite-tanned (mineral-based) | 42.2 | 6.2 | 2.3 | 0.0 |
| M7 Biole | Syntan-tanned (organic phenol-based) | 45.2 | 7.5 | 2.5 | 0.0 |
| Erlenmeyer Position | Sample | Sample Number | Time (h) |
|---|---|---|---|
| - | 50:50 inoculum ratio | 220645 | 0 |
| 10 | Collagen—16S rRNA | 220649 | 65 |
| 11 | Chrome—16S rRNA | 220653 | 65 |
| 12 | Zeolite—16S rRNA | 220657 | 65 |
| 13 | Biole—16S rRNA | 220661 | 65 |
| 10 | Collagen—65 h | 220646C | 65 |
| 11 | Chrome—65 h | 220650C | 65 |
| 12 | Zeolite—65 h | 220654C | 65 |
| 13 | Biole—65 h | 220658C | 65 |
| 10 | Collagen—113 h | 220647C | 113 |
| 11 | Chrome—113 h | 220651C | 113 |
| 12 | Zeolite—113 h | 220655C | 113 |
| 13 | Biole—113 h | 220659C | 113 |
| 10 | Collagen—240 h | 220648C | 240 |
| 11 | Chrome—240 h | 220652C | 240 |
| 12 | Zeolite—240 h | 220656C | 240 |
| 13 | Biole—240 h | 220660C | 240 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonilla-Espadas, M.; Bertazzo, M.; Lifante-Martinez, I.; Camacho, M.; Orgilés-Calpena, E.; Arán-Aís, F.; Bonete, M.-J. Taxonomic and Functional Profiling of Bacterial Communities in Leather Biodegradation: Insights into Metabolic Pathways and Diversity. Bacteria 2025, 4, 37. https://doi.org/10.3390/bacteria4030037
Bonilla-Espadas M, Bertazzo M, Lifante-Martinez I, Camacho M, Orgilés-Calpena E, Arán-Aís F, Bonete M-J. Taxonomic and Functional Profiling of Bacterial Communities in Leather Biodegradation: Insights into Metabolic Pathways and Diversity. Bacteria. 2025; 4(3):37. https://doi.org/10.3390/bacteria4030037
Chicago/Turabian StyleBonilla-Espadas, Manuela, Marcelo Bertazzo, Irene Lifante-Martinez, Mónica Camacho, Elena Orgilés-Calpena, Francisca Arán-Aís, and María-José Bonete. 2025. "Taxonomic and Functional Profiling of Bacterial Communities in Leather Biodegradation: Insights into Metabolic Pathways and Diversity" Bacteria 4, no. 3: 37. https://doi.org/10.3390/bacteria4030037
APA StyleBonilla-Espadas, M., Bertazzo, M., Lifante-Martinez, I., Camacho, M., Orgilés-Calpena, E., Arán-Aís, F., & Bonete, M.-J. (2025). Taxonomic and Functional Profiling of Bacterial Communities in Leather Biodegradation: Insights into Metabolic Pathways and Diversity. Bacteria, 4(3), 37. https://doi.org/10.3390/bacteria4030037








