Isolation and Identification of Pathogenic Bacteria Aeromonas veronii in Ctenopharyngodon idella (Grass Carp) and Chinese Herbal Medicine Antibacterial Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish
2.2. Chinese Herbal Medicines
2.3. Etiological Examination
2.4. Bacterial Identification
2.4.1. The Identification of the Morphological, Physiological, and Biochemical Characteristics of the Pathogenic Bacteria
2.4.2. 16S rRNA Sequence Analysis and Phylogenetic Tree Construction
2.4.3. Identification of Isolated Strains
2.5. Artificial Infection Experiment
2.6. Antibacterial Test of Chinese Herbal Medicine
2.6.1. Preparation of Herbal Extracts
2.6.2. Determination of Antibacterial Zones of Herbal Extracts
2.6.3. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.6.4. The Efficacy Determination of the Most Potent Bactericidal Herbal Extract
3. Results
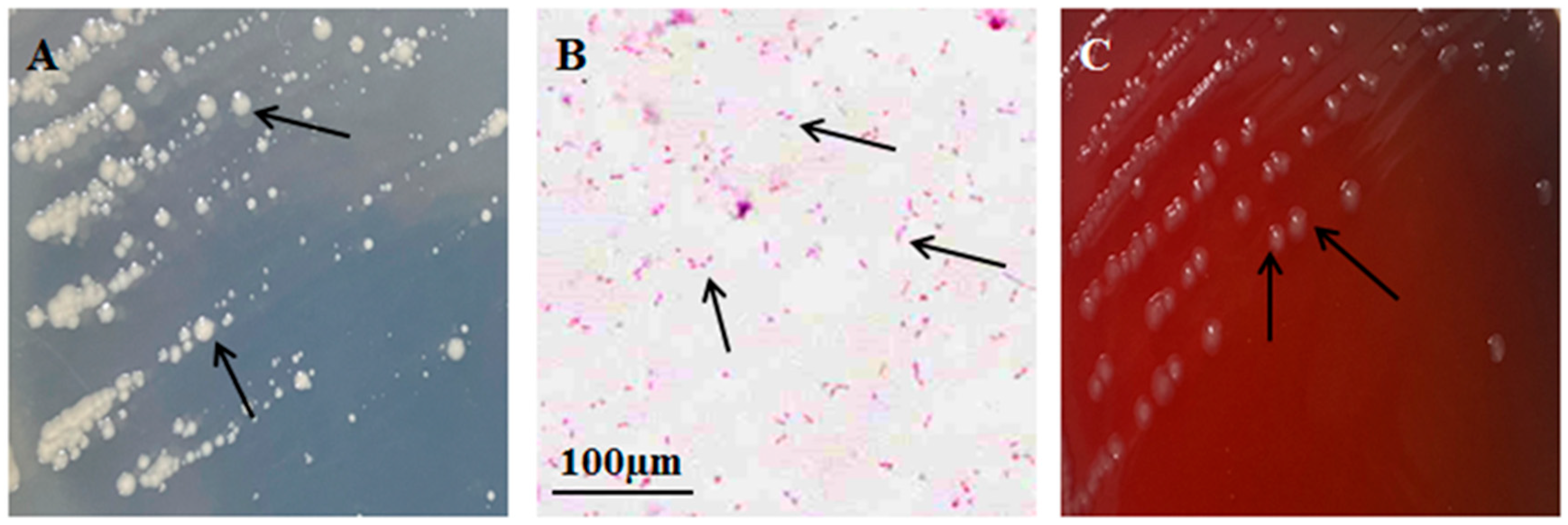
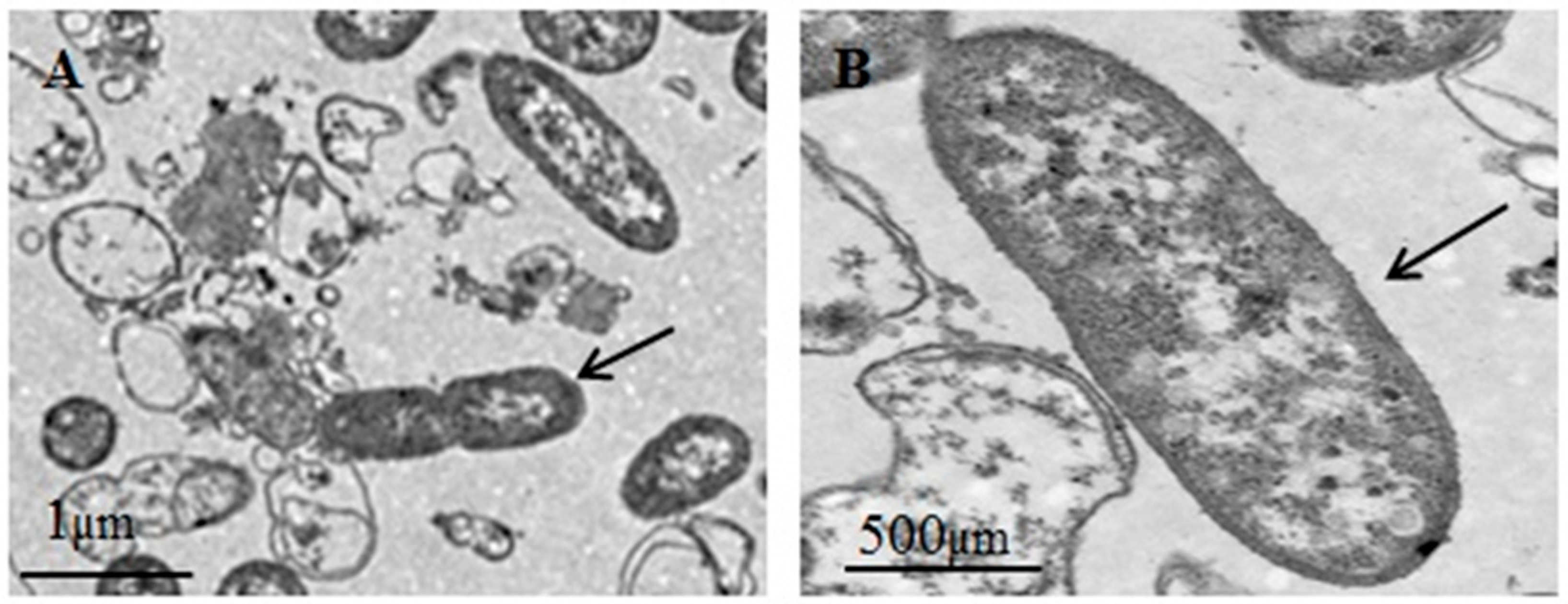
3.1. Clinical Signs, Isolation of Bacteria, and Biological Characteristics Study
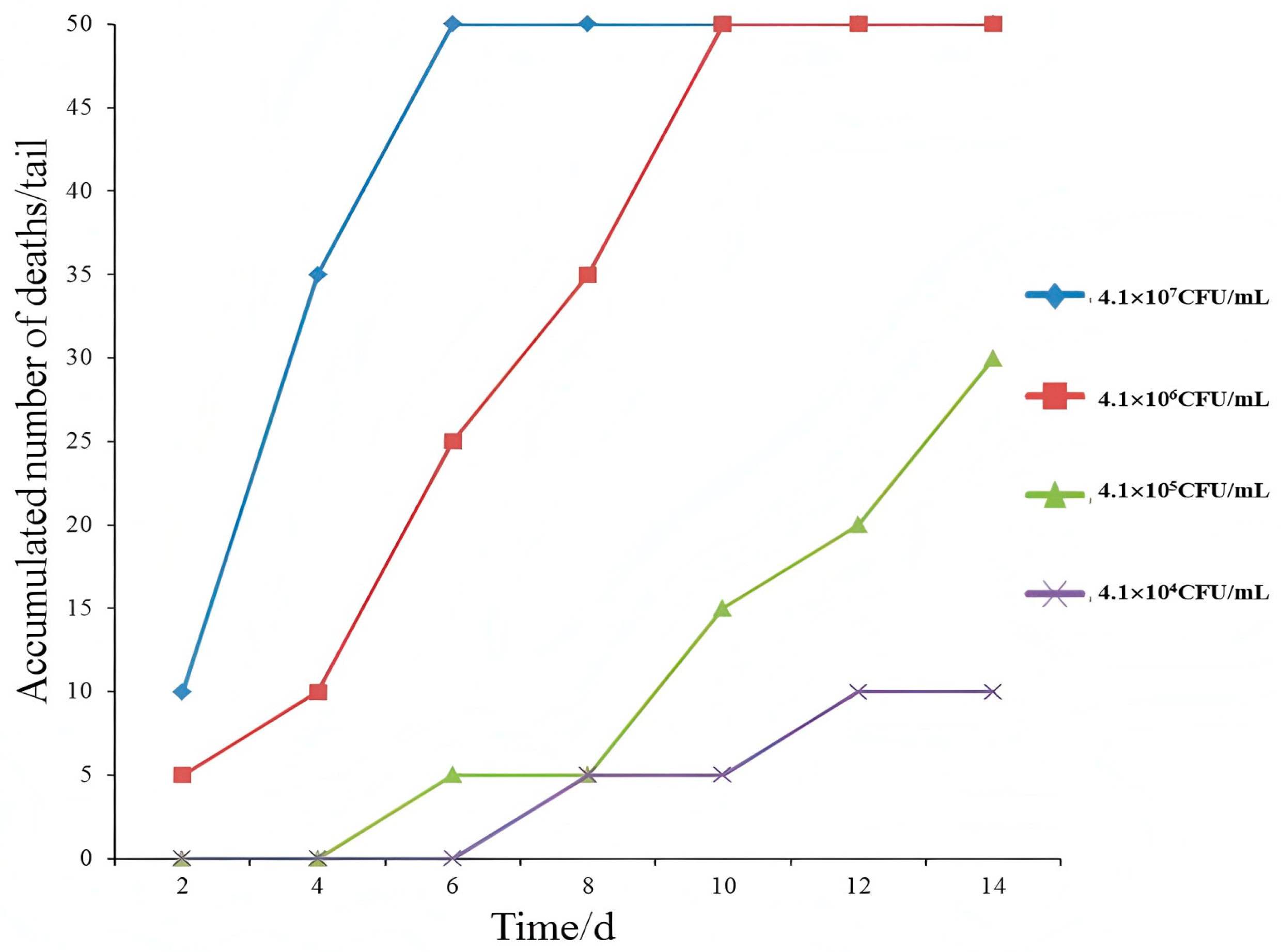
3.2. Cumulative Number of Deaths
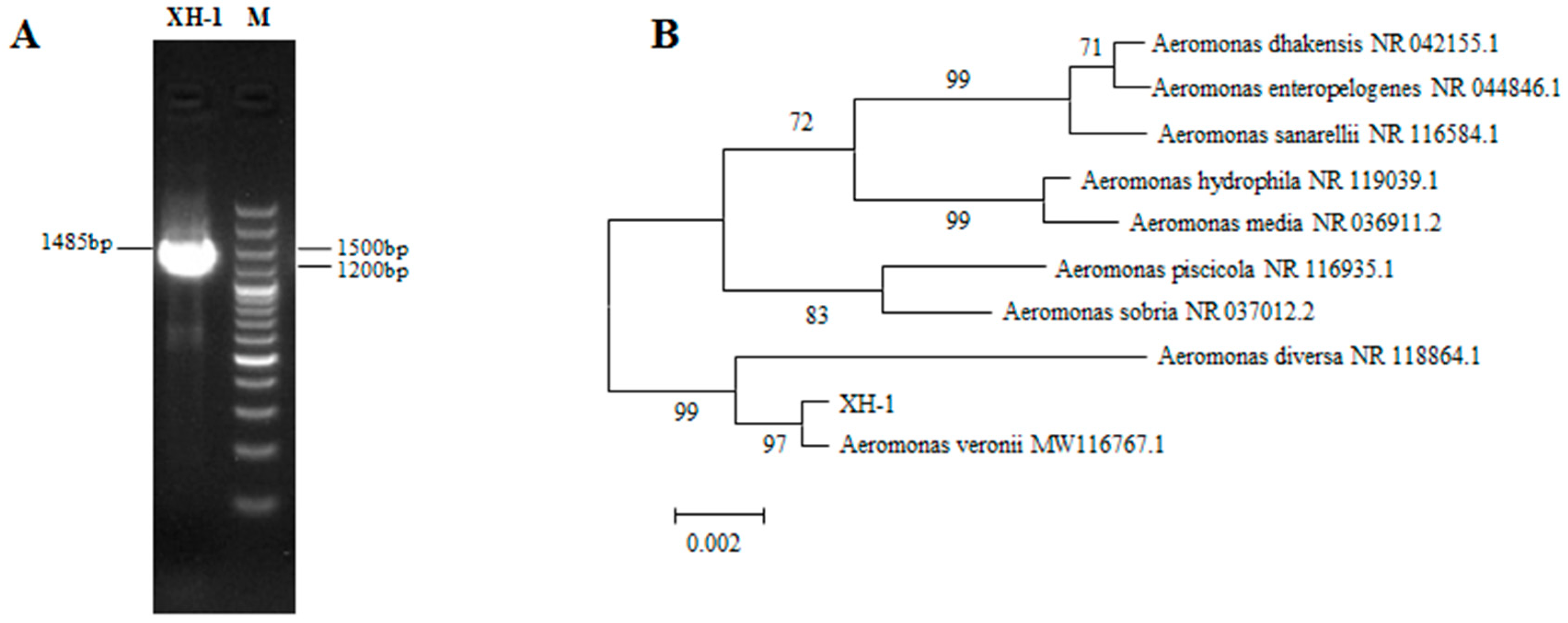
3.3. 16S rRNA Sequence Analysis, Phylogenetic Tree Construction, and the Identification of the Isolated Strain
3.4. Sensitivity of XH-1 to 14 Chinese Herbal Medicines
3.5. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
3.6. Evaluation of the Efficacy of Chinese Herbal Medicines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.L.; Lu, Z.J.; Zhan, F.F.; Li, Y.N.; Shi, F.; Zhao, L.J.; Lin, L.; Qin, Z.D. IgM expression and B lymphocyte antibacterial activity of grass carp (Ctenopharyngodon idella). J. Fish. China 2021, 45, 115–124. [Google Scholar] [CrossRef]
- Hong, Y.; Jiang, W.D.; Kuang, S.Y.; Hu, K.; Tang, L.; Liu, Y.; Jiang, J.; Zhang, Y.A.; Zhou, X.Q.; Feng, L. Growth, digestive and absorptive capacity and antioxidant status in intestine and hepatopancreas of subadult grass carp Ctenopharyngodon idellus fed graded levels of dietary threonine. J. Anim. Sci. Biotechnol. 2015, 6, 59–61. [Google Scholar] [CrossRef][Green Version]
- Kong, W.G.; Huang, C.; Tang, Y.; Zhang, D.; Wu, Z.X.; Chen, X.X. Effect of Bacillus subtilis on Aeromonas hydrophila-induced intestinal mucosal barrier function damageand inflammation in grass carp (Ctenopharyngodon idella). Sci. Rep. 2017, 7, 1588. [Google Scholar] [CrossRef]
- El-Barbary, M.I. Serum Biochemical and Histopathological Changes Associated with Aeromonas hydrophila Isolated from Oreochromis niloticus and Sparus aurata with Multiple Antibiotic Resistance Index. J. Biol. Sci. 2017, 17, 222–234. [Google Scholar] [CrossRef]
- Lehane, L.; Rawlin, G. Topically acquired bacterial zoonoses from fish: A review. Med. J. Aust. 2000, 173, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Gao, C.X.; Zeng, W.W.; Wang, Q.; Wang, Y.Y.; Li, Y.Y.; Yin, J.Y.; Chang, O.Q.; Shi, C.B. Pathogenicity and main phenotype characteristics of two Aeromonas veronii isolated from diseased grass carp. Chin. J. Prev. Vet. Med. 2019, 41, 145–150. Available online: http://yfsy.paperopen.com/#/digest?ArticleID=2897 (accessed on 8 July 2025).
- Qin, G.X.; Ai, X.H.; Xu, J.; Yang, Y.B. Dual RNA-seq of spleens extracted from channel catffsh infected with Aeromonas veronii reveals novel insights into host-pathogen interactions. Ecotoxicol. Environ. Saf. 2023, 252, 114609. [Google Scholar] [CrossRef]
- Monti, M.; Torri, A.; Amadori, E.; Rossi, A.; Bartolini, G.; Casadei, C.; Frassineti, G.L. Aeromonas veronii biovar veronii and sepsis-infrequent complication of biliary drainage placement: A case report. World J. Clin. Cases 2019, 7, 759–764. [Google Scholar] [CrossRef]
- Fernandez-Bravo, A.; Figueras, M.J. An update on the Genus Aermonas: Taxonomy, Epidemiology and pathogenicity. Microorganisms 2020, 8, 129. [Google Scholar] [CrossRef]
- Hatrongjit, R.; Kerdsin, A.; Takeuchi, D.; Wongsurawat, T.; Jenjaroenpun, P.; Chopjitt, P.; Boueroy, P.; Akeda, Y.; Hamada, S. Genomic Analysis of Aeromonas veronii C198, a Novel Mcr-3.41-Harboring isolate from a patient with septicemia in Thailand. Pathogens 2020, 9, 1031. [Google Scholar] [CrossRef]
- Li, T.; Raza, S.H.A.; Yang, B.; Sun, Y.; Wang, G.; Sun, W.; Qian, A.; Wang, C.; Kang, Y.; Shan, X. Aeromonas veronii infection in Commercial Freshwater Fish: A potential threat to Public Health. Animals 2020, 10, 608. [Google Scholar] [CrossRef] [PubMed]
- Tekedar, H.C.; Kumru, S.; Blom, J.; Perkins, A.D.; Griffin, M.J.; Abdelhamed, H.; Karsi, A.; Lawrence, M.L. Comparative genomics of Aeromonas veronii: Identification of a pathotype impacting aquaculture globally. PLoS ONE 2019, 14, e0221018. [Google Scholar] [CrossRef] [PubMed]
- Stanbaugh, C.L.; Abter, E.I.; Tompkins, A.L. Aeromonas veronii cellulitis, bacteremia, and sepsis in a patient with liver cirrhosis and end-stage renal disease following a minor abrasion with exposure to pond water: A case report and literature review. IDCases 2022, 30, e01631. [Google Scholar] [CrossRef]
- Dong, H.T.; Techatanakitarnan, C.; Jindakittikul, P.; Thaiprayoon, A.; Taengphu, S.; Charoensapsri, W.; Khunrae, P.; Rattanarojpong, T.; Senapin, S. Aeromonas jandaei and Aeromonas veronii caused disease and mortality in Nile tilapia, Oreochromis niloticus (L.). J. Fish Dis. 2017, 40, 1395–1403. [Google Scholar] [CrossRef]
- Wang, Y.E.; Xing, C.G.; Wang, G.L. Susceptibilities of Five Marine Pathogenic Vibrios to 34 Chinese Herbs. Fish. Sci. 2008, 27, 221–225. [Google Scholar] [CrossRef]
- Holt, J.G.; Kriey, N.R.; Sneath, P.H.A.; Staley, J.T. Bergey’s Manual of: Determinative Bacteriology, 9th ed.; Lippincott Williams and Wilkins: Baltimore, MA, USA, 1994. [Google Scholar]
- Krieg, N.R.; Holt, J.G. Bergey’s Manual of Systematic Bacteriology; Williams and Wilkins Baltimore: London, UK, 1984; Volume 1, pp. 545–548. [Google Scholar]
- Ke, W.J.; Qin, H.B.; Chen, Y.F.; Yang, Y.; Yu, H.; Guan, W.T.; Li, Z.Y.; Liu, F. Antibacterial effects of 25 kinds of Chinese herbal medicines on Proteus vulgaris in vitro. Fish. Sci. Technol. Inf. 2019, 46, 328–331. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Q.Z.; Li, C.T. Baeteriostasis of 50 traditional Chinese medicine herbs against Aeromonas hydrophila in vtro. Chin. J. Prev. Vet. Med. 2011, 33, 862–865. [Google Scholar] [CrossRef]
- Gao, X.H.; Xiao, Y.; Zhang, M.H.; An, W.; He, Z.K.; Shao, L.; Zhang, H.Q. In Vitro antibacterial effect of 13 Chinese herbal medicines on Pseudamonas fluoroscens. J. South. Agric. 2016, 47, 748–752. [Google Scholar] [CrossRef]
- Yang, Y.B.; Cao, H.P.; Xia, Y.T.; Yang, T.; Zhao, L.; Hu, K.; Yang, X.L. In Vitro inhibitory effect of 41 chinese herbs on three sturgeon-pathogenic bacteria. Freshw. Fish. 2013, 43, 81–84. [Google Scholar] [CrossRef]
- Wang, S. Handbook of Modern Diagnostics; Peking Union Medical College Press: Beijing, China, 1995. [Google Scholar]
- Jeney, Z.; Jeney, G. Recent achievements in studies on diseases of the common carp (Cyprinus carpio L.). Aquaculture 1995, 129, 397–420. [Google Scholar] [CrossRef]
- Kozinska, A.; Figueras, M.J.; Chacon, M.R.; Soler, L. Phenotypic characteristics and pathogenicity of Aeromonas genomospecies isolated from common carp (Cyprinus carpio L.). J. Appl. Microbiol. 2002, 93, 1034–1041. [Google Scholar] [CrossRef]
- Rahman, M.; Colque-Navarro, P.; Kühn, I.; Huys, G.; Swings, J.; Möllby, R. Identificationand characterization of pathogenic Aeromonas veronii biovar sobria associated with epizootic ulcerative syndrome in fish in Bangladesh. Appl. Environ. Microbiol. 2002, 68, 650–655. [Google Scholar] [CrossRef]
- Wahli, T.; Burr, S.E.; Pugovkin, D.; Mueller, O.; Frey, J. Aeromonas sobria, a causative agent of disease in farmed perch, Perca fluviatilis L. J. Fish Dis. 2005, 28, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M. Isolation of pathogenic bacteria from the skin ulcerous symptomatic gourami (Colisa lalia) through 16S rDNA analysis. Univ. J. Zool. 2008, 27, 21–24. [Google Scholar] [CrossRef]
- Martínez-Murcia, A.J.; Saavedra, M.J.; Mota, V.R.; Maier, T.; Stackebrandt, E.; Cousin, S. Aeromonas aquariorum sp. nov., isolated from the aquaria of ornamental fish. Int. J. Syst. Evol. Microbiol. 2008, 58, 1169–1175. [Google Scholar] [CrossRef]
- Sreedharan, K.; Philip, R.; Singh, I.S.B. Virulence potential and antibiotic susceptibility pattern of motile aeromonads associated with freshwater ornamental fish culture systems:a possible threat to public health. Braz. J. Microbiol. 2008, 43, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Kokka, R.P. The pathogenicity of Aeromonas strains relative to genospecies and pheospecies identification. FEMS Microbiol. Lett. 1991, 69, 29–33. [Google Scholar] [CrossRef][Green Version]
- Altwegg, M.; Steigerwalt, A.G.; Altwegg-Bissig, R.; Luthy-Hottenstein, J.; Brenner, D.J. Biochemical identification of Aeromonas genospecies isolated from humans. J. Clin. Microbiol. 1990, 28, 258–264. [Google Scholar] [CrossRef]
- Zaki, M.M.; Eissa, A.E.; Saeid, S. Assessment of the Immune Status in Nile Tilapia (Oreochromis niloticus) Experimentally Challenged with Toxogenic/Septicemic Bacteria During Treatment Trial with Florfenicol and Enrofloxacin. Word J. Fish. Mar. Sci. 2011, 3, 21–36. [Google Scholar]
- Zhang, T.; Li, B. Antibiotic resistance in water environment: Frontiers of fundamental research, risk assessment and control strategies. Sci. Bull. 2020, 65, 2543–2554. [Google Scholar] [CrossRef]
- Mao, Z.J.; Zhuo, H.L. Studies on the Pathogen of the Bacteria Epidemic against Mud Crab Scylla serrata. Fish. Sci. 2001, 20, 8–11. [Google Scholar] [CrossRef]
- Jin, S.; Wang, G.L.; Zhao, Q.S.; Chen, H.Q. Medical study on provenion and cure for skin ulcer disease of marine cage-cultured sea-perch. J. Oceanogr. TaiWan Strait 2000, 19, 233–236. [Google Scholar] [CrossRef]
- Song, X.H.; Cai, C.F.; Ni, J.G. Extraction and Antibacterial Activity of Active Constituents from Nine Common Chinese Herbal Medicines. Reserv. Fish. 2001, 21, 38–40. [Google Scholar] [CrossRef]
- Settharaksa, S.; Monton, C.; Charoenchai, L. Optimization of Caesalpinia sappan L. heartwood extraction procedure to obtain the highest content of brazilin and greatest antibacterial activity. J. Integr. Med. 2019, 17, 351–358. [Google Scholar] [CrossRef]
- Choi, B.M.; Kim, B.R. Upregulation of heme oxygenase-1 by brazilin via the phosphatidylinositol 3-kinase/Akt and ERK pathways and its protective effect against oxidative injury. Eur. J. Pharmacol. 2008, 580, 12–18. [Google Scholar] [CrossRef]
- Batubara, I.; Mitsunaga, T.; Ohashi, H. Brazilin from Caesalpinia sappan wood as an antiacne agent. J. Wood Sci. 2010, 56, 77–81. [Google Scholar] [CrossRef]

| Item | XH-1 | Item | XH-1 |
|---|---|---|---|
| Gram stain | − | Esculin hydrolysis | − |
| Oxidase | + | Hydrogen sulfide production | − |
| Voges–Proskauer | + | Arabinose fermentation | + |
| Indole test | + | Inositol fermentation | − |
| Glucose fermentation | + | Ornithine decarboxylase | − |
| Mannitol fermentation | + | Urea | − |
| Salicin fermentation | − | Methyl red | + |
| Homologous Strain | 16S rRNA Gene Homology (%) | Gene Bank Accession No. |
|---|---|---|
| A. veronii strain HD6454 chromosome, complete genome | 100 | CP079823.1 |
| A. veronii strain 183026 chromosome, complete genome | 100 | CP072325.1 |
| A. veronii strain AV040 chromosome, complete genome | 100 | CP095841.1 |
| A. veronii strain HD6448 chromosome, complete genome | 100 | CP087266.1 |
| A. veronii strain AEv1810 16S ribosomal RNA gene, partial sequence | 100 | ON063910.1 |
| A. veronii strain SW3814 chromosome, complete genome | 100 | CP083461.2 |
| A. veronii strain A29V chromosome, complete genome | 100 | CP080630.1 |
| A. veronii strain Colony108 chromosome | 100 | CP070210.1 |
| A. veronii strain Colony58 chromosome | 100 | CP070211.1 |
| A. veronii strain Colony121 chromosome | 100 | CP070208.1 |
| Chinese Medicine Name | Inhibition Zone Diameter (mm) | Sensitivity |
|---|---|---|
| Lignum sappa | 25.13 ± 0.24 | S |
| Pericarpium granna | 24.11 ± 0.18 | S |
| Artemisia argyi | 22.67 ± 0.12 | S |
| Scutellaria baicalensis Georgi | 19.64 ± 0.35 | S |
| Coptis chinensis | 17.33 ± 0.47 | S |
| Artemisia capillaris | 15.47 ± 0.32 | S |
| Spatholobus suberectus | 14.07 ± 0.34 | I |
| Rehmannia glutinosa | 12.53 ± 0.61 | I |
| Glycyrrhiza uralensis Fisch | 9.63 ± 0.16 | R |
| Andrographis paniculata | 0 | / |
| Dendranthema morifolium | 0 | / |
| Carthamus tinctorius | 0 | / |
| Cortex fraxini | 0 | / |
| Isatis indigotica | 0 | / |
| Chinese Medicine Name | MIC | MRC |
|---|---|---|
| Lignum sappa | 7.813 | 15.625 |
| Pericarpium granna | 7.813 | 15.625 |
| Artemisia argyi | 15.625 | 31.250 |
| Scutellaria baicalensis Georgi | 62.500 | 125.000 |
| Coptis chinensis | 125.000 | 250.000 |
| Artemisia capillaris | 125.000 | 250.000 |
| Concentration of Drug (mg/mL) | Primary Number of Bacteria (cfu/mL) | Final Number of Bacteria (cfu/mL) | |||
|---|---|---|---|---|---|
| 2 h | 8 h | 16 h | 32 h | ||
| 6.00 | 2.00 × 105 | 1.44 × 105 | 1.68 × 105 | 3.75 × 105 | 6.79 × 105 |
| 10.00 | 5.66 × 103 | 2.42 × 104 | 7.14 × 104 | 2.84 × 105 | |
| 14.00 | 0 | 0 | 7.80 × 103 | 3.27 × 104 | |
| 18.00 | 0 | 0 | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Xue, H.; Liu, G.; Sun, L.; Jiang, H. Isolation and Identification of Pathogenic Bacteria Aeromonas veronii in Ctenopharyngodon idella (Grass Carp) and Chinese Herbal Medicine Antibacterial Experiment. Bacteria 2025, 4, 34. https://doi.org/10.3390/bacteria4030034
Zhao Y, Xue H, Liu G, Sun L, Jiang H. Isolation and Identification of Pathogenic Bacteria Aeromonas veronii in Ctenopharyngodon idella (Grass Carp) and Chinese Herbal Medicine Antibacterial Experiment. Bacteria. 2025; 4(3):34. https://doi.org/10.3390/bacteria4030034
Chicago/Turabian StyleZhao, Yanhua, Hui Xue, Guoxing Liu, Li Sun, and Hucheng Jiang. 2025. "Isolation and Identification of Pathogenic Bacteria Aeromonas veronii in Ctenopharyngodon idella (Grass Carp) and Chinese Herbal Medicine Antibacterial Experiment" Bacteria 4, no. 3: 34. https://doi.org/10.3390/bacteria4030034
APA StyleZhao, Y., Xue, H., Liu, G., Sun, L., & Jiang, H. (2025). Isolation and Identification of Pathogenic Bacteria Aeromonas veronii in Ctenopharyngodon idella (Grass Carp) and Chinese Herbal Medicine Antibacterial Experiment. Bacteria, 4(3), 34. https://doi.org/10.3390/bacteria4030034







