Potential Risks Associated with the Growth of Nitrifying Bacteria in Drinking Water Distribution Lines and Storage Tanks: A Systematic Literature Review
Abstract
1. Introduction
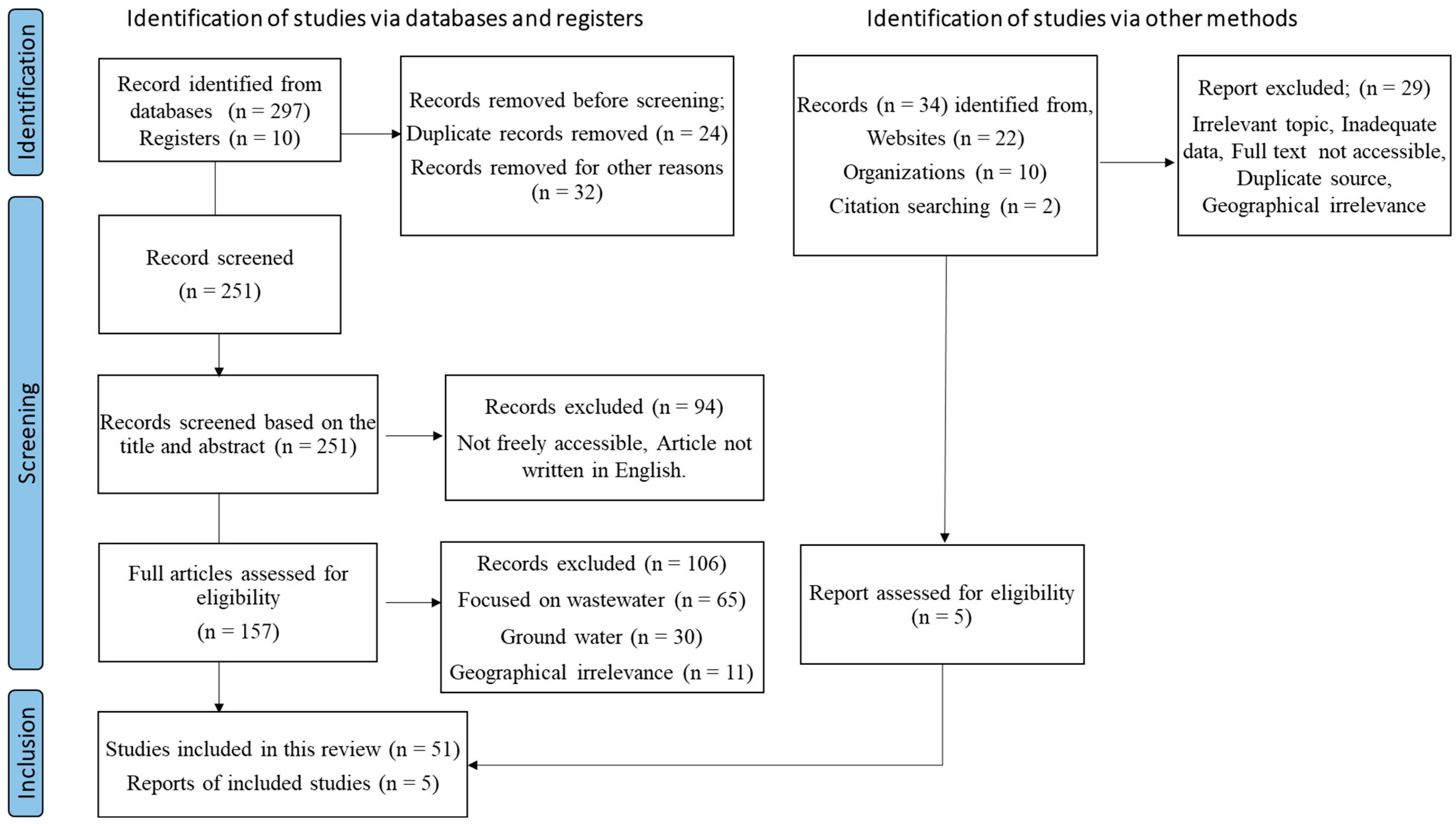
2. Research Methodology
2.1. Literature Search Strategy
2.2. Literature Selection Criteria
2.3. Data Extraction
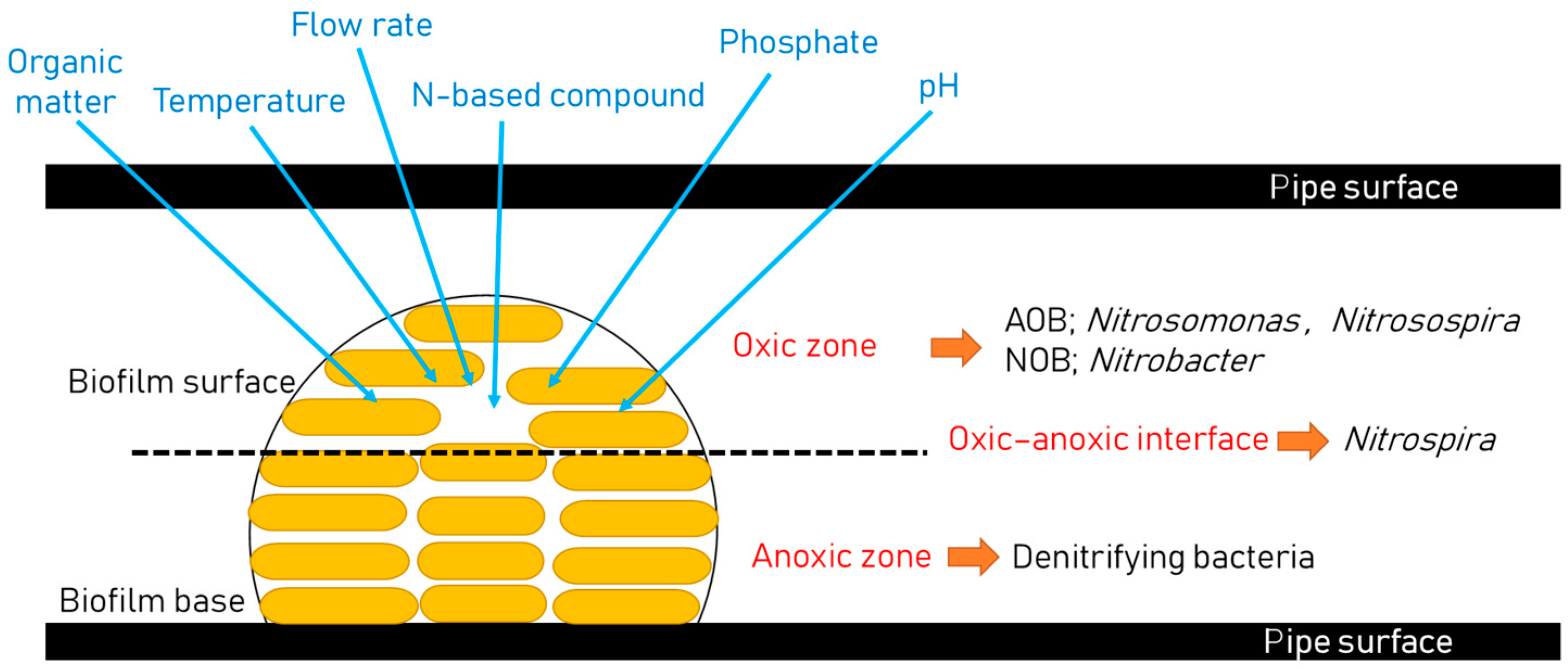
3. Diversity of Nitrifying Bacteria and Their Growth Conditions
3.1. Growth of Nitrifiers and Their Diversity
| Types of Bacteria Found | Sources Where Bacteria Are Found | Characteristics | References | |
|---|---|---|---|---|
| AOB (ammonia-oxidizing bacteria) | Nitrosomonas oligotropha Nitrosomonas europaea Nitrosomonas ureae | Drinking water distribution system | The genus Nitrosomonas dominates in chloraminated systems, thrives in oxygen-limited, low ammonia environments, and is monochloramine (MCA)-tolerant. | [2,8] |
| Nitrosococcus mobilis Nitrosococcus oceani Nitrosococcus halophilus | [8] | |||
| Nitrosomonas Nitrosospira | Chloraminated drinking distribution system | Nitrosospira is dominant in chloraminated systems. | [8,9] | |
| Nitrosomonas oligotropha | Efficient ammonia oxidizer in low-substrate environments; associated with biofilms | |||
| NOB (nitrite-oxidizing bacteria) | Nitrospira spp. | Distribution systems and storage tanks | High affinity for ammonia | [2] |
| Nitrobacter spp. | Full-scale chloraminated drinking water systems | Less abundant compared to Nitrospira | [12] | |
| Nitrospira spp. | Thrives in low-ammonia environments. | [12] | ||
| Nitrobacter spp., Nitrospira spp. | Distribution system | Nitrobacter is less dominant than Nitrospira. | [9] | |
3.2. Biofilm Formation
4. Factors Influencing Nitrification in Distribution Systems
4.1. Temperature Effect
4.2. Effect of Disinfectant Residual Levels
4.3. Effect of the Chlorine-to-Ammonia Ratio
4.4. pH Effect
4.5. Effect of Water Storage Tanks
5. Potential Health Impacts of Nitrifying Bacteria
5.1. Effect of the Formation of DBPs
| Disinfection by Product Group | Important Precursors | Disinfectant | Types of N-DBP | References |
|---|---|---|---|---|
| Haloacetonitriles (HANs) R3CCN | Aspartic Acid, Tryptophan, Kynurenic Acid, Algal Organic Matter (AOM) | Chlorine, Chloramine | Dichloroacetonitrile (DCAN), Bromochloroacetonitrile (BCAN), Dibromoacetonitrile (DBAN), Trichloroacetonitrile (TCAN), Chloroacetonitrile (MCAN), Bromobutanenitrile (MBAN) | [20,38] |
| Haloacetamides (HAcAms) R3CCONH2 | Aspartic Acid, Tyrosine | Chlorine, Chloramine | Chloroacetamide (CAAm), Bromoacetamide (BAAm), Dichloroacetamide (DCAcAm), Dibromoacetamide (DBAcAm), Trichloroacetamide (TCAcAm) | [20,38] |
| Halonitromethanes (HNMs) R3CH | Nitromethane, Nitrophenols, Glycine, Lysine | Chlorine, Ozone-Chlorination | Trichloromethane (Chloroform), Tribromomethane (Bromoform), Bromodichloromethane, Dibromochloromethane, Dichloronitromethane (DCNM), Dibromonitromethane (DBNM), Bromochloronitromethane (BCNM), Bromodichloronitromethane (BDCNM), Dibromochloronitromethane (DBCNM) | [20,38,40] |
| Nitrosamines | Dimethylamine (DMA), Trimethylamine (TMA), Ranitidine, Nizatidine | N-Nitrosodimethylamine (NDMA), N-Nitrosopyrrolidine (NPYR), N-Nitrosomorpholine (NMOR), N-Nitrosodiethylamine (NDEA), N-Nitrosoethylmethylamine (NEMA), N-Nitrosodi-n-propylamine (NDPA), N-Nitrosodi-n-butylamine (NDBA), N-Nitrosopiperidine (NPIP) | [20,38,40] | |
| Cyanogen Halides (CNXs) | Glycine, Formaldehyde, Amino Acids (Serine, Threonine) | Chloramine, Chlorination | Cyanogen Chloride (CNCl), Cyanogen Bromide (CNBr) | [20] |
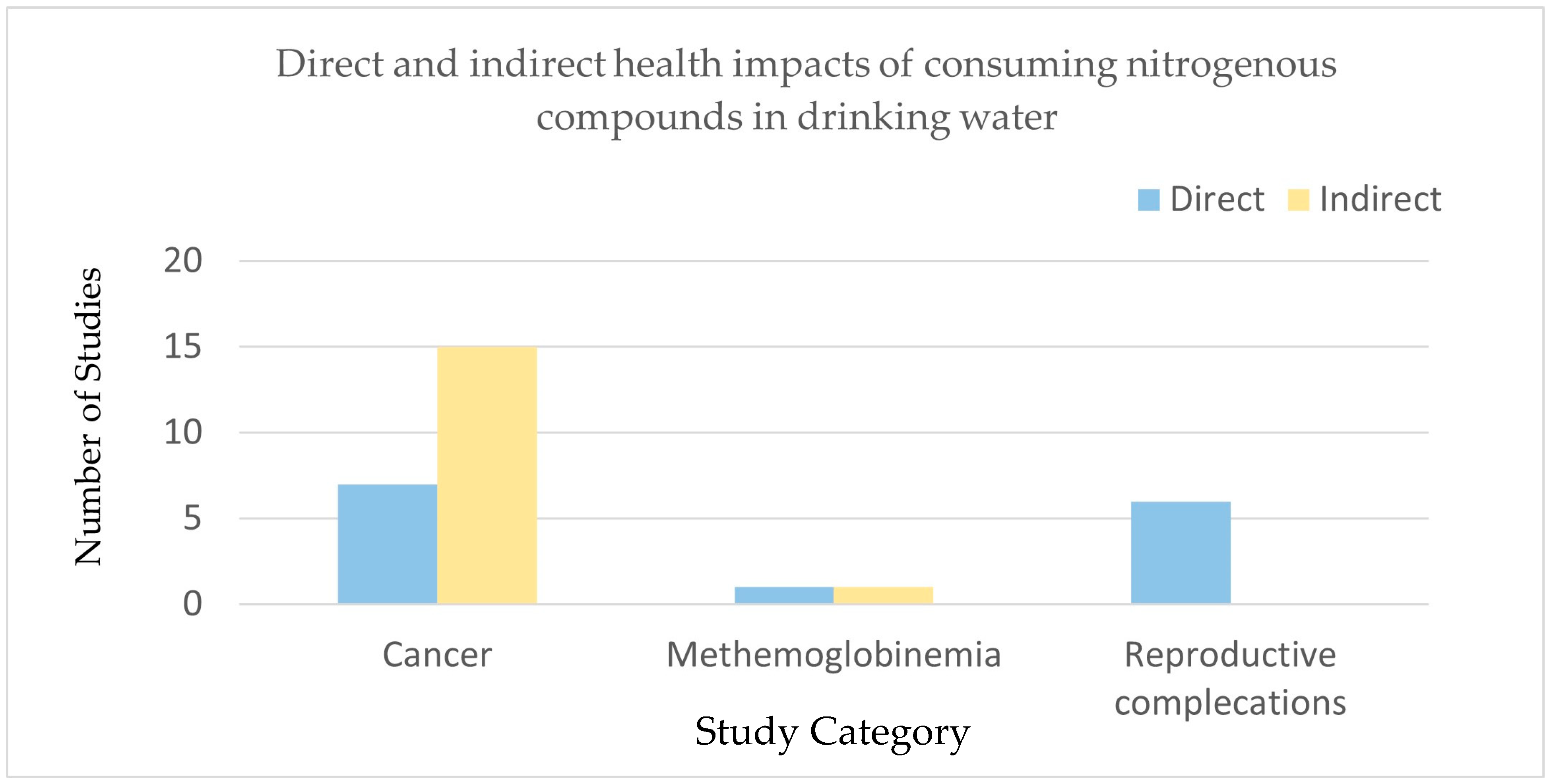
5.2. Health Effects of Nitrogenous Substances Produced by Nitrifying Bacteria
| Diseases Reported | Exposure Details | Year of Reporting | Study Area | Summary of the Findings | References | |
|---|---|---|---|---|---|---|
| Cancer | Colorectal cancer | Nitrate exposure through drinking water and diet | 2008–2013 | Nine locations from Spain and two from Italy | A significant association between nitrate exposure (below 50 mg/L of nitrate) and the colorectal cancer risk. | [47] |
| Cancer | Bladder cancer | Nitrate exposure through drinking water and diet | 1986–2010 | Iowa, USA | The study identified 258 bladder cancer cases and found a significant association for women exposed to drinking water with >5 mg/L NO3−N for ≥4 years. | [26,32] |
| Cancer | Breast cancer | Waterborne ingested nitrate and dietary ingested nitrate | 2008–2013 | Eight Spanish regions | No overall association with waterborne ingested nitrate and breast cancer. | [16] |
| Cancer | Breast cancer | Nitrate intake from drinking water and folate intake | 1986 | Iowa, USA | The results indicated that neither dietary nor water nitrate intake was significantly associated with the breast cancer risk when considering all participants. Among women with a high folate intake (≥400 µg/day), those exposed to elevated nitrate levels in public water had an increased risk of breast cancer (hazard ratio [HR] = 1.40), as did users of private well water (HR = 1.38). | [18] |
| Cancer | Kidney cancer | Nitrate intake from drinking water and DBPs | 1986–2010 | Iowa, USA | The study examined the association of nitrate and DBP exposure with the kidney cancer risk among older women. Women in the highest 95th percentile of average nitrate–nitrogen exposure had a significantly increased risk of kidney cancer. The study did not find any independent associations between total trihalomethanes (THMs), individual THMs (like chloroform and bromodichloromethane), or haloacetic acids (HAAs) and the kidney cancer risk. | [24] |
| Cancer | Thyroid cancer, hyperthyroidism, and hypothyroidism | Nitrate in public water and dietary nitrate intake | 1986–2004 | Iowa, USA | Higher nitrate levels in public water (>5 mg/L nitrate–nitrogen for ≤5 years) are associated with an increased thyroid cancer risk (RR = 2.6; 95% confidence interval (CI: 1.1–6.2). Higher dietary nitrate intake (highest vs. lowest quartile) is also linked to an increased thyroid cancer risk (RR = 2.9; 95% CI: 1.0–8.1; P-trend = 0.046). | [13] |
| Increased dietary nitrate intake is associated with a higher prevalence of hypothyroidism (OR = 1.2; 95% CI: 1.1–1.4). No significant association was found between nitrate exposure through public water or dietary intake and the prevalence of hyperthyroidism and hypothyroidism. | ||||||
| Cancer | Bladder cancer | Long-term nitrate exposure in public water supplies | 1986–1989 | Iowa, USA | No significant association between bladder cancer and increasing nitrate levels in drinking water at the levels studied (up to 5.5 mg/L nitrate–nitrogen) (highest quartile OR: 0.8 for women, 0.5 for men). | [32] |
| Cancer | Colon Cancer | Nitrate in public drinking water (Avg. >5 mg/L for >10 years) | 1986–1989 | Lowa, USA | No significant association in the general population (odds ratio (OR) = 1.2 (95%), confidence interval [CI] = 0.9–1.6). An increased risk was associated with low vitamin C intake (OR = 2.0, CI: 1.2–3.3) and high meat intake (OR = 2.2, CI: 1.4–3.6). | [48] |
| Rectal cancer | Nitrate in public drinking water (Avg. >5 mg/L for >10 years) | No increased risk of rectal cancer with elevated nitrate exposure (OR = 1.1, CI: 0.7–1.5). | ||||
| Cancer | Non-Hodgkin lymphoma (NHL) | Nitrate exposure in drinking water (public supplies) | 1980–1984 | Minnesota, USA | The study did not find a statistically significant association between nitrate levels in drinking water and the risk of NHL (median of highest exposure category = 2.4 mg nitrate/L (range = 0.1–7.2 mg/L)) | [22] |
| Cancer | Non-Hodgkin lymphoma (NHL) | Nitrate levels in drinking water (public supplies) | 1983–1986 | Nebraska | An increase in the risk of non-Hodgkin lymphoma among individuals exposed to nitrate levels in the highest quartile (4.0 mg/L nitrate-N) over 40 years. | [34] |
| Cancer | Gastric cancer | Nitrate and nitrite intake from food and water | 1986–1992 | The Netherlands | No significant association was found between dietary nitrate intake and the gastric cancer risk (rate ratio (RR) highest/lowest quintile = 0.80, 95% CI 0.47–1.37). The study did not find a risk of gastric cancer among people with a higher nitrate intake from drinking water (RR highest/lowest quintile = 0.88, 95% Cl 0.59–1.32, trend-P = 0.39) or a higher intake of nitrite (RR highest/lowest quintile = 1.44, 95% Cl 0.95–2.18, trend-P = 0.24). | [30] |
| Cancer | Brain tumors | Nitrate levels in drinking water (public supplies) | 1987–1988 | Germany | The study found no statistically significant association between nitrate levels in drinking water and the risk of primary brain tumors. | [29] |
| Cancer | Brain cancer | 1988–1993 | USA 66 counties in eastern Nebraska | No association between long-term average nitrate levels in public water supplies (PWSs) and adult brain cancer | [31] | |
| Cancer | Ovarian cancer | There were positive associations with ovarian cancer (RR = 1, 1.52, 1.81, and 1.84) | [36] | |||
| Uterine cancer | Nitrate in drinking water | 1986–1998 | USA | There were inverse associations with uterine cancer (RR = 1, 0.86, 0.86 and 0.55). | ||
| Rectal cancer | There were inverse associations with rectal cancer (RR = 1, 0.72, 0.95, and 0.471). | |||||
| Non-Hodgkin lymphoma, leukemia, melanoma, colon cancer, breast cancer, lung cancer, rectal cancer, pancreatic cancer, kidney cancer | There were no associations with increasing nitrate levels in drinking water. | |||||
| Cancer | Bladder cancer | Nitrate exposure from food, drinking water, and total estimated nitrate exposure | After 9 years of follow-up from 1986 | The Netherlands | No significant association between nitrate intake and the bladder cancer risk. Multivariate RRs for the highest vs. lowest quintiles were 1.06 (95% CI, 0.81–1.31) for food, 1.06 (95% CI, 0.82–1.37) for drinking water, and 1.09 (95% CI, 0.84–1.42) for total nitrate exposure. | [37] |
| Cancer | Childhood brain tumors (CBTs) | Household water source (well water vs. public water) and nitrate/nitrite levels in residential water | 836 CBT cases and 1485 controls from five countries. | The CBT risk did not increase with increasing nitrate levels. The astrocytoma risk was associated with higher nitrite levels (odds ratio [OR] = 4.3, 95% CI: 1.4, 12.6 for nitrite levels of 1–<5 mg/L nitrite ion; OR = 5.7, 95% CI: 1.2, 27.2 for nitrite > or =5 mg/L). | [27] | |
| Cancer | Renal cell carcinoma | Nitrate in public water supplies (levels of 5 mg/L and 10 mg/L or higher) and dietary nitrate and nitrite intake | 1986–1989 | Iowa, USA | No overall association with renal cell carcinoma for average nitrate levels or years of exposure to public water supplies >5 and >10 mg/L nitrate–nitrogen (10+ years >5 mg/L odds ratio (OR) = 1.03, 95% confidence interval (CI) 0.66, 1.60). | [35] |
| Methemoglobinemia | Oxygen transport impairment | Nitrate/nitrite in drinking water (>10 mg/L NO3-N) | Since 1941 | High nitrate intake (>10 mg/L NO3-N) can cause methemoglobinemia (blue baby syndrome) in infants, impairing oxygen transport. Infants under 6 months are most vulnerable due to immature enzyme systems. | [21] | |
| Reproductive toxicity | Fetal growth restriction, lower birth weights | Nitrate/nitrite exposure in drinking water | Animal studies indicate potential reproductive toxicity, with nitrate/nitrite linked to fetal malformations and reduced fertility. However, human data are limited and inconclusive. The evidence from animal models suggests developmental toxicity, including birth defects and growth retardation, at high doses. Human epidemiological studies show inconsistent results. | |||
| Pregnancy related issues | Preterm delivery and low birth weight | Nitrate and atrazine exposure through drinking water | 2004–2008 | 46 counties in four Midwestern states (Ohio, Indiana, Iowa, and Missouri) | Neither atrazine nor nitrate exposure was associated with an increased risk of term low birth weight (LBW). Nitrate exposure was significantly associated with increased rates of VPTD (very preterm delivery) (RR1ppm = 1.08, 95% CI: 1.02, 1.15) and very low birth weight (VLBW) (RR1ppm = 1.17, 95% CI: 1.08, 1.25). | [28] |
| Pregnancy complications | Neural tube defects (NTDs) | Prenatal nitrate exposure through drinking water | 1997–2005 | Iowa and Texas | A significant association was found between high prenatal nitrate intake and neural tube defects. | [49] |
| Spina bifida | Prenatal nitrate intake (≥5.42 mg/day from drinking water) | Mothers of babies with spina bifida were 2.0 times more likely (95% CI: 1.3–3.2) to consume ≥5 mg nitrate daily compared to <0.91 mg. | ||||
| Limb deficiency | Mothers were 1.8 times more likely (95% CI: 1.1–3.1) to consume ≥5.42 mg nitrate daily during the critical period (1 month preconception to the first trimester) compared to <1.0 mg. | |||||
| Cleft palate | The risk of cleft palate was 1.9 times higher (95% CI: 1.2–3.1) for mothers consuming ≥5.42 mg nitrate daily compared to <1.0 mg. | |||||
| Cleft lip | Mothers were 1.8 times more likely (95% CI: 1.1–3.1) to consume ≥5.42 mg nitrate daily compared to <1.0 mg. | |||||
| Methemoglobinemia | Infant methemoglobinemia | Breastfeeding mothers ingesting water with a nitrate content up to 100 ppm | Not specified | United States | No significant increase in the nitrate concentration in breast milk, even with a high maternal intake of nitrate. Breastfeeding infants are not at risk of methemoglobinemia. | [23] |
6. Control Measures
6.1. Breakpoint Chlorination
6.2. Nitrification Monitoring and Control Plan
6.3. American Water Works Association (AWWA) Manual M56
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOB | Ammonia-oxidizing bacteria |
| NOB | Nitrite-oxidizing bacteria |
| DWDN | Drinking water distribution networks |
| NDBPs | Nitrogenous disinfection by-products |
| DBP | Disinfection by-products |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| TTHMs | Total trihalomethanes |
| TP | Total phosphorus |
| NMCP | Nitrification Monitoring and Control Plan |
| AWWA | American Water Works Association |
| NMCP | Monitoring and Control Plan on Nitrification |
| TRC | Total residual chlorine |
| NDMA | N-nitrosodimethylamine |
| HANs | Haloacetonitriles |
| HAcAms | Haloacetamides |
| CNX | Cyanogen halides |
| HNMs | Halonitromethanes |
| WHO | World Health Organization |
| BPC | Periodic breakpoint chlorination |
References
- AWWA. Nitrification; U.S. Environmental Protection Agency (EPA). 2002. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/nitrification_1.pdf (accessed on 15 August 2002).
- Cruz, M.C.; Woo, Y.; Flemming, H.C.; Wuertz, S. Nitrifying Niche Differentiation in Biofilms from Full-Scale Chloraminated Drinking Water Distribution System. Water Res. 2020, 176, 115738. [Google Scholar] [CrossRef] [PubMed]
- Cunliffe, D.A. Bacterial Nitrification in Chloraminated Water Supplies. Appl. Environ. Microbiol. 1991, 57, 3399–3402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lipponen, M.T.; Suutari, M.H.; Martikainen, P.J. Occurrence of Nitrifying Bacteria and Nitrification in Finnish Drinking Water Distribution Systems. Water Res. 2002, 36, 4319–4329. [Google Scholar] [CrossRef]
- Hossain, S.; Chow, C.W.; Hewa, G.A. Review of Nitrification Monitoring and Control Strategies in Drinking Water System. Int. J. Environ. Res. Public Health 2022, 19, 4003. [Google Scholar] [CrossRef]
- Scott, D.B.; Van Dyke, M.I.; Anderson, W.B.; Huck, P.M. Influence of Water Quality on Nitrifier Regrowth in Two Full-Scale Drinking Water Distribution Systems. Can. J. Microbiol. 2015, 61, 965–976. [Google Scholar] [CrossRef]
- Erdei-Tombor, P.; Kiskó, G.; Taczman-Brückner, A. Biofilm Formation in Water Distribution Systems. Processes 2024, 12, 280. [Google Scholar] [CrossRef]
- Lipponen, M.T.; Martikainen, P.J.; Vasara, R.E.; Servomaa, K.; Zacheus, O.; Kontro, M.H. Occurrence of Nitrifiers and Diversity of Ammonia-Oxidizing Bacteria in Developing Drinking Water Biofilms. Water Res. 2004, 38, 4424–4434. [Google Scholar] [CrossRef]
- Regan, J.M.; Harrington, G.W.; Noguera, D.R. Ammonia- and Nitrite-Oxidizing Bacterial Communities in a Pilot-Scale Chloraminated Drinking Water Distribution System. Appl. Environ. Microbiol. 2002, 68, 73–81. [Google Scholar] [CrossRef]
- Bal Krishna, K.C.; Sathasivan, A. Effect of Silver in Severely Nitrified Chloraminated Bulk Waters. Water Sci. Technol. Water Supply 2012, 12, 415–421. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Ding, N. Chloramine Disinfection-Induced Nitrification Activities and Their Potential Public Health Risk Indications within Deposits of a Drinking Water Supply System. Int. J. Environ. Res. Public Health 2020, 17, 772. [Google Scholar] [CrossRef]
- Regan, J.M.; Harrington, G.W.; Noguera, D.R. Diversity of Nitrifying Bacteria in Full-Scale Chloraminated Distribution Systems. Water Res. 2003, 37, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.H.; Kilfoy, B.A.; Weyer, P.J.; Anderson, K.E.; Folsom, A.R.; Cerhan, J.R. Nitrate Intake and the Risk of Thyroid Cancer and Thyroid Disease. Epidemiology 2010, 21, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Rungvetvuthivitaya, M.; Song, R.; Campbell, M.; Ray, C. A Kinetic Study of Nitrification Inhibition in Water Distribution Systems Using Low Levels of Chlorite. J. Water Supply Res. Technol. AQUA 2014, 63, 497–506. [Google Scholar] [CrossRef]
- Ward, M.H.; Jones, R.R.; van Breda, S.G. Drinking Water Nitrate and Human Health: An Updated Review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef]
- Espejo-Herrera, N.; Gracia-Lavedan, E.; Pollan, M.; Aragonés, N.; Boldo, E.; Perez-Gomez, B.; Altzibar, J.M.; Amiano, P.; Zabala, A.J.; Ardanaz, E.; et al. Ingested Nitrate and Breast Cancer in the Spanish Multicase-Control Study on Cancer (MCC-Spain). Environ. Health Perspect. 2016, 124, 1042–1049. [Google Scholar] [CrossRef]
- Harrington, G.W.; Noguera, D.R.; Vanhoven, D.J. Pilot-Scale Evaluation of Nitrification Control Strategies. J. Am. Water Works Assoc. 2002, 94, 78–89. [Google Scholar] [CrossRef]
- Inoue-Choi, M.; Ward, M.H.; Robien, K. Interaction of Nitrate and Folate on the Risk of Breast Cancer among Postmenopausal Women. Nutr. Cancer 2012, 64, 685–694. [Google Scholar] [CrossRef]
- Wolfe, R.L.; Lieu, N.I.; Means, E.G. Ammonia-Oxidizing Bacteria in a Chloraminated Distribution System: Seasonal Occurrence, Distribution, and Disinfection Resistance. Appl. Environ. Microbiol. 1990, 56, 451–462. [Google Scholar] [CrossRef]
- Bond, T.; Templeton, M.R.; Graham, N. Precursors of Nitrogenous Disinfection By-Products in Drinking Water—A Critical Review and Analysis. J. Hazard. Mater. 2012, 235–236, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.M.; Steinberg, V.E. Health Implications of Nitrate and Nitrite in Drinking Water: An Update on Methemoglobinemia Occurrence and Reproductive and Developmental Toxicity. Regul. Toxicol. Pharmacol. 1996, 23, 35–43. [Google Scholar] [CrossRef]
- Freedman, D.M.; Cantor, K.P.; Helzlsouer, K.J. A Case-Control Study of Nitrate in Drinking Water and Non-Hodgkin’s Lymphoma in Minnesota. Arch. Environ. Health 2000, 55, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Greer, F.R.; Shannon, M. Infant Methemoglobinemia: The Role of Dietary Nitrate in Food and Water. Pediatrics 2005, 116, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.R.; Weyer, P.J.; Ward, M.H. Ingested Nitrate, Disinfection By-Products, and Kidney Cancer Risk in Older Women. Epidemiology 2017, 28, 703–711. [Google Scholar] [CrossRef]
- Jones, R.R.; Weyer, P.J.; Ward, M.H. Nitrate from Drinking Water and Diet and Bladder Cancer among Postmenopausal Women in Iowa. Environ. Health Perspect. 2016, 124, 1751–1758. [Google Scholar] [CrossRef]
- Krasner, S.W. The Formation and Control of Emerging Disinfection By-Products of Health Concern. Philos. Trans. R. Soc. A 2009, 367, 4077–4095. [Google Scholar] [CrossRef]
- Mueller, B.A.; Nielsen, S.S.; Preston-Martin, S.; Holly, E.A.; Cordier, S.; Filippini, G.; Peris-Bonet, R.; Choi, N.W. Household Water Source and the Risk of Childhood Brain Tumours: Results of the SEARCH International Brain Tumor Study. Int. J. Epidemiol. 2004, 33, 1209–1216. [Google Scholar] [CrossRef]
- Stayner, L.T.; Almberg, K.; Turyk, M. Atrazine and Nitrate in Drinking Water and the Risk of Preterm Delivery and Low Birth Weight in Four Midwestern States. Environ. Res. 2017, 152, 294–303. [Google Scholar] [CrossRef]
- Steindorf, K.; Schlehofer, B.; Wahrendorf, J. Nitrate in Drinking Water: A Case-Control Study on Primary Brain Tumors with an Embedded Drinking Water Survey in Germany. Int. J. Epidemiol. 1994, 23, 451–457. [Google Scholar] [CrossRef]
- Van Loon, A.J.M.; Botterweck, A.A.M.; Goldbohm, R.A.; Brants, H.A.M.; Van Klaveren, J.D.; Van den Brandt, P.A. Intake of Nitrate and Nitrite and the Risk of Gastric Cancer: A Prospective Cohort Study. Br. J. Cancer 1998, 78, 129–135. [Google Scholar] [CrossRef]
- Ward, M.H.; Cantor, K.P.; Cerhan, J.; Lynch, C.F.; Hartge, P. Nitrate in Public Water Supplies and Risk of Cancer: Results from Recent Studies in the Midwestern United States. Epidemiology 2004, 15, S214. [Google Scholar] [CrossRef]
- Ward, M.H.; Cantor, K.P.; Riley, D.; Merkle, S.; Lynch, C.F. Nitrate in Public Water Supplies and Risk of Bladder Cancer. Epidemiology 2003, 14, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.H.; deKok, T.M.; VanDerslice, J. Workgroup Report: Drinking-Water Nitrate and Health—Recent Findings and Research Needs. Environ. Health Perspect. 2005, 113, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.H.; Mark, S.D.; Cantor, K.P.; Weisenburger, D.D.; Correa-Villasenor, A.; Zahm, S.H. Drinking Water Nitrate and the Risk of Non-Hodgkin’s Lymphoma. Epidemiology 1996, 7, 465–471. Available online: https://pubmed.ncbi.nlm.nih.gov/8862975/ (accessed on 1 September 1996). [CrossRef] [PubMed]
- Ward, M.H.; Rusiecki, J.A.; Lynch, C.F.; Cantor, K.P. Nitrate in Public Water Supplies and the Risk of Renal Cell Carcinoma. Cancer Causes Control 2007, 18, 1141–1151. [Google Scholar] [CrossRef]
- Weyer, P.J.; Cerhan, J.R.; Kross, B.C.; Hallberg, G.R.; Kantamneni, J.; Breuer, G.; Jones, M.P.; Zheng, W.; Lynch, C.F. Municipal Drinking Water Nitrate Level and Cancer Risk in Older Women: The Iowa Women’s Health Study. Epidemiology 2001, 12, 327–338. [Google Scholar] [CrossRef]
- Zeegers, M.P.; Selen, R.F.; van den Brandt, P.A. Nitrate Intake Does Not Influence Bladder Cancer Risk: The Netherlands Cohort Study. Environ. Health Perspect. 2006, 114, 1527–1531. [Google Scholar] [CrossRef]
- Liew, D.; Linge, K.L.; Joll, C.A. Formation of Nitrogenous Disinfection By-Products in 10 Chlorinated and Chloraminated Drinking Water Supply Systems. Environ. Monit. Assess. 2016, 188, 518. [Google Scholar] [CrossRef]
- Shen, Y. Formation of Nitrogenous Disinfection By-Products (N-DBPs) in Drinking Water: Emerging Concerns and Current Issues. IOP Conf. Ser. Earth Environ. Sci. 2021, 801, 012015. [Google Scholar] [CrossRef]
- Zeng, T.; Mitch, W.A. Impact of Nitrification on the Formation of N-Nitrosamines and Halogenated Disinfection Byproducts within Distribution System Storage Facilities. Environ. Sci. Technol. 2016, 50, 2964–2973. [Google Scholar] [CrossRef]
- Kimbrough, D.E. Impact of Local Climate Change on Drinking Water Quality in a Distribution System. Water Qual. Res. J. 2019, 54, 179–192. [Google Scholar] [CrossRef]
- Odell, L.H.; Kirmeyer, G.J.; Wilczak, A.; Jacangelo, J.G.; Marcinko, J.P.; Wolfe, R.L. Controlling Nitrification in Chloraminated Systems. J. Am. Water Works Assoc. 1996, 88, 86–98. [Google Scholar] [CrossRef]
- Sarker, D.C.; Patel, C.M.; Anwar, A.H. Evaluation of Zinc and Copper for Co-Inhibition of Nitrification in Mild Nitrified Drinking Water. J. Environ. Chem. Eng. 2018, 6, 2939–2943. [Google Scholar] [CrossRef]
- OSD, H.B.; Prasad, G.V.K.S.V.; Al-Fatlawy, R. Effect of Water Storage Tank Material on Quality of Water with Storage Period. In E3S Web of Conferences; EDP Sciences: Paris, France, 2024; Volume 529, p. 03010. [Google Scholar] [CrossRef]
- Manga, M.; Ngobi, T.G.; Okeny, L.; Acheng, P.; Namakula, H.; Kyaterekera, E.; Nansubuga, I.; Kibwami, N. The Effect of Household Storage Tanks/Vessels and User Practices on the Quality of Water: A Systematic Review of Literature. Environ. Syst. Res. 2021, 10, 18. [Google Scholar] [CrossRef]
- Coss, A.; Cantor, K.P.; Ward, M.H. Pancreatic Cancer and Drinking Water and Dietary Sources of Nitrate and Nitrite. Am. J. Epidemiol. 2004, 159, 693–701. [Google Scholar] [CrossRef]
- Espejo-Herrera, N.; Gràcia-Lavedan, E.; Villanueva, C.M. Colorectal Cancer Risk and Nitrate Exposure through Drinking Water and Diet. Int. J. Cancer 2016, 139, 334–346. [Google Scholar] [CrossRef]
- De Roos, A.J.; Ward, M.H.; Lynch, C.F.; Cantor, K.P. Nitrate in Public Water Supplies and the Risk of Colon and Rectum Cancers. Epidemiology 2003, 14, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Brender, J.D.; Weyer, P.J.; Canfield, M.A. Prenatal Nitrate Intake from Drinking Water and Selected Birth Defects in Offspring of Participants in the National Birth Defects Prevention Study. Environ. Health Perspect. 2013, 121, 1083–1089. [Google Scholar] [CrossRef]
- New Hampshire Department of Environmental Services (NHDES) of Drinking Water and Groundwater Bureau (DWGB). Controlling Nitrification in Chloraminated Drinking Water Supplies: Guidance Document and Nitrification Monitoring and Control Plan Template; Drinking Water and Groundwater Bureau (DWGB): Concord, New Hampshire, USA, 2021. Available online: https://www.des.nh.gov/sites/g/files/ehbemt341/files/documents/wd-21-02.pdf (accessed on 21 January 2021).
- Fleming, K.; Harrington, G.W.; Noguera, D.R. Nitrification Potential Curves: A New Strategy for Nitrification Prevention. J. Am. Water Works Assoc. 2005, 97, 6. [Google Scholar] [CrossRef]

| Reactions Involved | Responsible Bacteria | Reaction Description |
|---|---|---|
| NH3 + O2 → NO2− + 3H+ + 2e− | AOB | Ammonia oxidation process (the first step of nitrification that converts ammonia into nitrite) |
| NO2− + H2O → NO3− + 2H+ +2e− | NOB | Nitrite oxidation process (second step of nitrification) |
| NH4+ + NO2− → N2 + 2H2O | Anammox bacteria | Anaerobic ammonium oxidation (anammox process) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekanayake, A.N.; Gunawardana, W.; Weerasooriya, R. Potential Risks Associated with the Growth of Nitrifying Bacteria in Drinking Water Distribution Lines and Storage Tanks: A Systematic Literature Review. Bacteria 2025, 4, 33. https://doi.org/10.3390/bacteria4030033
Ekanayake AN, Gunawardana W, Weerasooriya R. Potential Risks Associated with the Growth of Nitrifying Bacteria in Drinking Water Distribution Lines and Storage Tanks: A Systematic Literature Review. Bacteria. 2025; 4(3):33. https://doi.org/10.3390/bacteria4030033
Chicago/Turabian StyleEkanayake, Amandhi N., Wasana Gunawardana, and Rohan Weerasooriya. 2025. "Potential Risks Associated with the Growth of Nitrifying Bacteria in Drinking Water Distribution Lines and Storage Tanks: A Systematic Literature Review" Bacteria 4, no. 3: 33. https://doi.org/10.3390/bacteria4030033
APA StyleEkanayake, A. N., Gunawardana, W., & Weerasooriya, R. (2025). Potential Risks Associated with the Growth of Nitrifying Bacteria in Drinking Water Distribution Lines and Storage Tanks: A Systematic Literature Review. Bacteria, 4(3), 33. https://doi.org/10.3390/bacteria4030033





