Dietary Dill Weed (Anethum graveolens) Stimulated Disease Resistance of African Catfish (Clarias gariepinus) Against Edwardsiellosis Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Feed Preparation
2.2. Feeding Trial Condition
2.3. Sampling
2.4. Growth Performance Calculation
2.5. Hematological Profiling
2.6. Digestive Enzyme Activity Assay
2.7. Antioxidant Enzyme Activity Assay
2.8. Heat Stress Assay
2.9. Bacterial Challenge Assay
2.10. Gut Microbiota Analysis
2.10.1. DNA Preparation
2.10.2. DNA Sequencing
2.10.3. DNA Sequencing Analysis
2.11. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Hematological Profiling
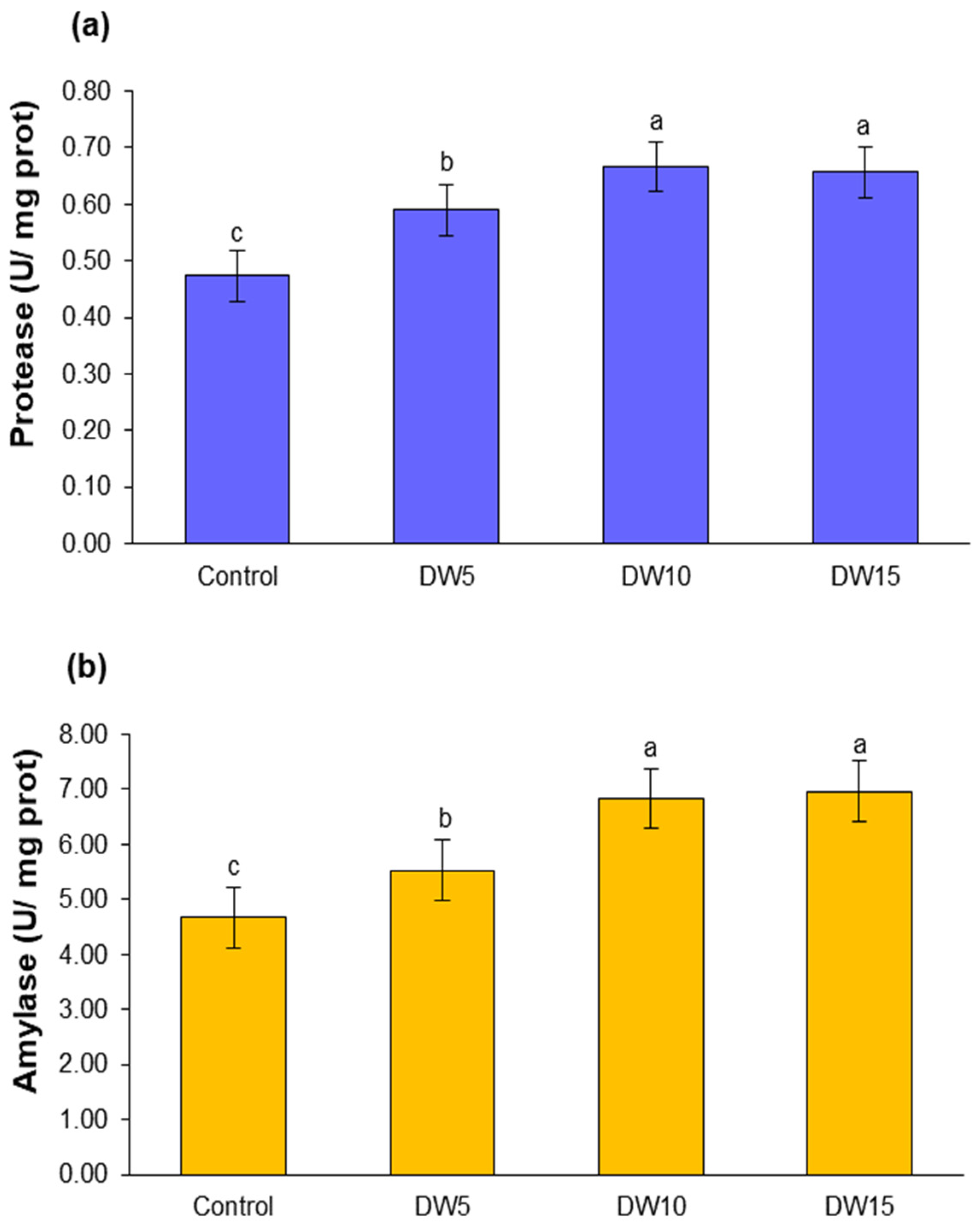
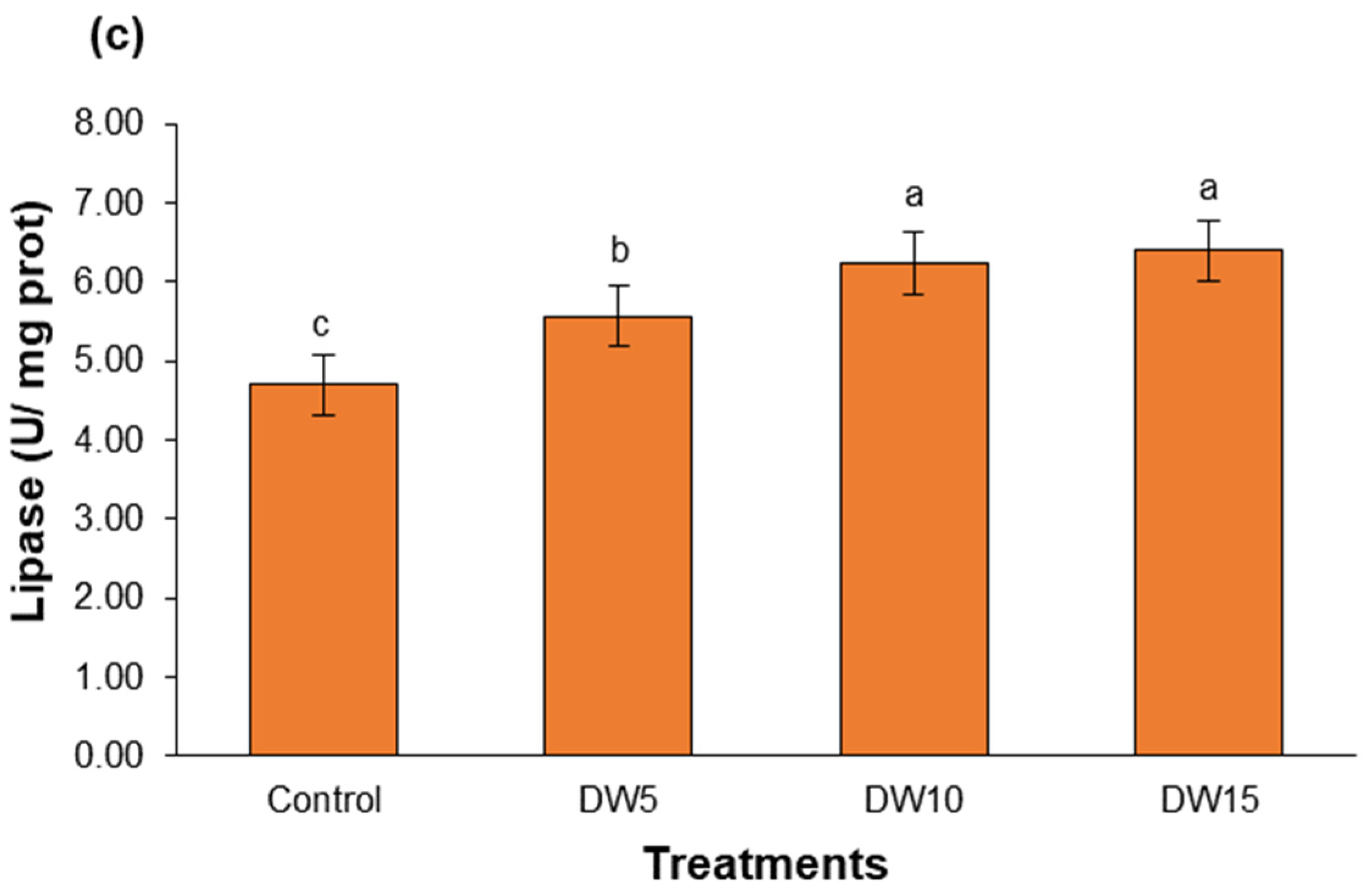
3.3. Digestive Enzyme Activity
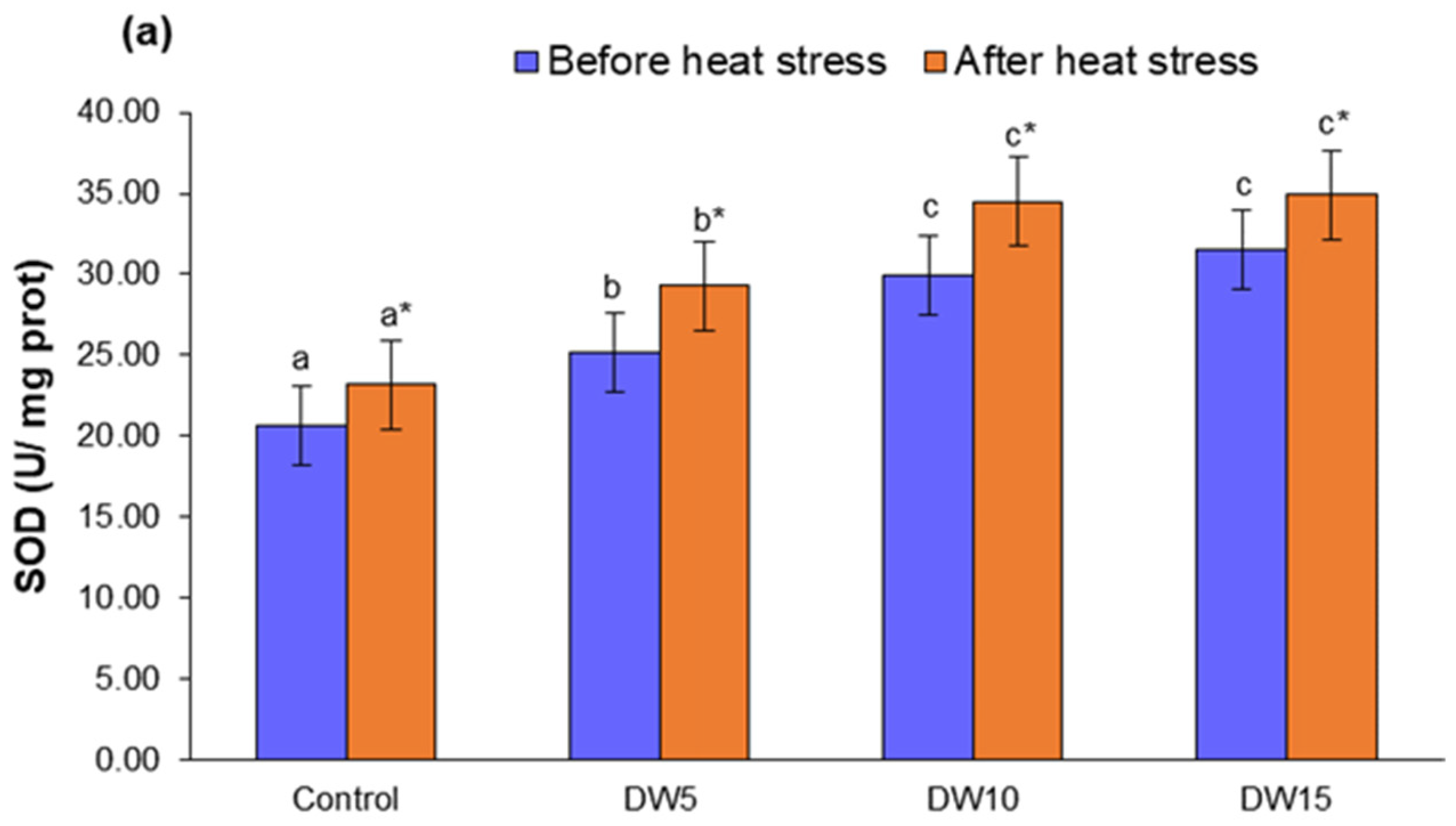
3.4. Antioxidant Enzyme Activity
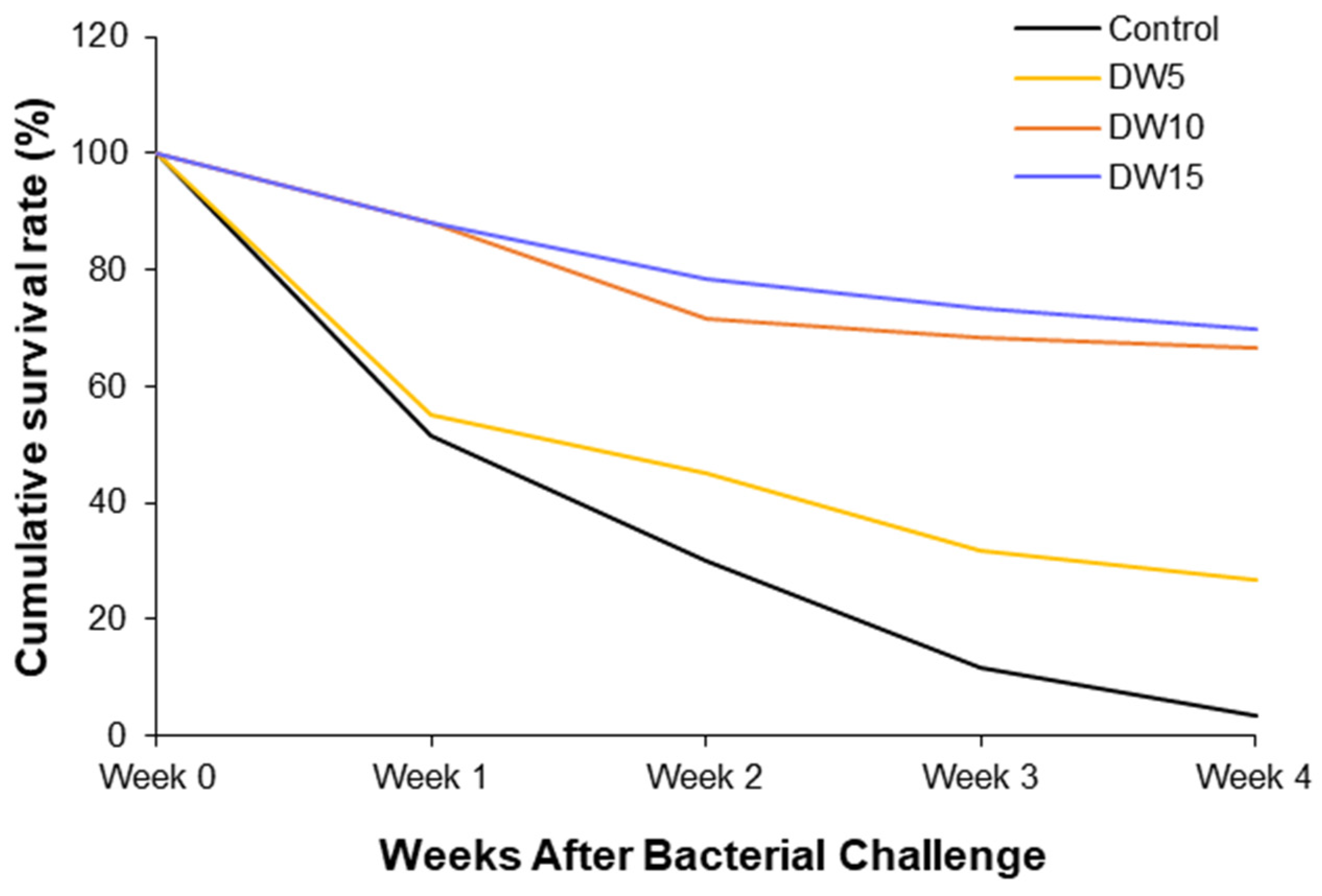
3.5. Cumulative Survival Rate of Post-Bacterial Infection
3.6. Gut Microbiota Analysis
3.7. Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, L.S.; Kon Yeu, H.; Khoo, M.I.; Mn, A.; Wee, W. Effect of dietary supplementation of turmeric, Curcuma longa leaf on growth and health status of African catfish, Clarias gariepinus. Vet. Integr. Sci. 2024, 22, 907–920. [Google Scholar] [CrossRef]
- Yada, T.; Tort, L. 10-Stress and Disease Resistance: Immune System and Immunoendocrine Interactions. In Fish Physiology; Schreck, C.B., Tort, L., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 35, pp. 365–403. [Google Scholar]
- Goh, K.W.; Abdul Kari, Z.; Wee, W.; Zakaria, N.N.A.; Rahman, M.M.; Kabir, M.A.; Abdul Hamid, N.K.; Tahiluddin, A.B.; Kamarudin, A.S.; Téllez-Isaías, G.; et al. Exploring the roles of phytobiotics in relieving the impacts of Edwardsiella tarda infection on fish: A mini-review. Front. Vet. Sci. 2023, 10, 1149514. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Duman, M. Expanding the Spectrum of Diseases and Disease Associations Caused by Edwardsiella tarda and Related Species. Microorganisms 2024, 12, 1031. [Google Scholar] [CrossRef]
- Leung, K.Y.; Wang, Q.; Zheng, X.; Zhuang, M.; Yang, Z.; Shao, S.; Achmon, Y.; Siame, B.A. Versatile lifestyles of Edwardsiella: Free-living, pathogen, and core bacterium of the aquatic resistome. Virulence 2022, 13, 5–18. [Google Scholar] [CrossRef]
- Wee, W.; Abdul Hamid, N.K.; Mat, K.; Khalif, R.I.A.R.; Rusli, N.D.; Rahman, M.M.; Kabir, M.A.; Wei, L.S. The effects of mixed prebiotics in aquaculture: A review. Aquac. Fish. 2024, 9, 28–34. [Google Scholar] [CrossRef]
- Wei, L.S.; Kari, Z.A.; Kabir, M.A.; Khoo, M.I.; Azra, M.N.; Wee, W. Promoting Growth and Health of African Catfish, Clarias gariepinus, Through Dietary Novel Supplement, Ginger, Zingiber officinale Rosc, Leaf Powder. Aquac. Stud. 2024, 24, AQUAST1719. [Google Scholar] [CrossRef]
- Ashry, A.M.; Habiba, M.M.; El-Zayat, A.M.; Badreldeen, A.H.; Younis, N.A.; Ahmed, H.A.; El-Dakroury, M.F.; Ali, M.A.M.; Dawood, M.A.O. Effects of ginger (Zingiber officinale) on the growth performance, digestive enzyme activity, antioxidative response, and antibacterial capacity of striped catfish (Pangasianodon hypophthalmus) reared in outdoor conditions. Aquac. Rep. 2023, 33, 101760. [Google Scholar] [CrossRef]
- Rahman, M.; Mamun, M.A.A.; Rathore, S.S.; Nandi, S.K.; Abdul Kari, Z.; Wei, L.S.; Tahiluddin, A.B.; Rahman, M.M.; Manjappa, N.K.; Hossain, A.; et al. Effects of dietary supplementation of natural Spirulina on growth performance, hemato-biochemical indices, gut health, and disease resistance to Aeromonas hydrophila of Stinging catfish (Heteropneustes fossilis) fingerling. Aquac. Rep. 2023, 32, 101727. [Google Scholar] [CrossRef]
- Nandi, S.K.; Suma, A.Y.; Rashid, A.; Kabir, M.A.; Goh, K.W.; Abdul Kari, Z.; Van Doan, H.; Zakaria, N.N.A.; Khoo, M.I.; Seong Wei, L. The Potential of Fermented Water Spinach Meal as a Fish Meal Replacement and the Impacts on Growth Performance, Reproduction, Blood Biochemistry and Gut Morphology of Female Stinging Catfish (Heteropneustes fossilis). Life 2023, 13, 176. [Google Scholar] [CrossRef]
- Hamid, N.K.A.; Somdare, P.O.; Md Harashid, K.A.; Othman, N.A.; Kari, Z.A.; Wei, L.S.; Dawood, M.A.O. Effect of papaya (Carica papaya) leaf extract as dietary growth promoter supplement in red hybrid tilapia (Oreochromis mossambicus × Oreochromis niloticus) diet. Saudi J. Biol. Sci. 2022, 29, 3911–3917. [Google Scholar] [CrossRef]
- Anis Mohamad Sukri, S.; Andu, Y.; Tuan Harith, Z.; Sarijan, S.; Naim Firdaus Pauzi, M.; Seong Wei, L.; Dawood, M.A.O.; Abdul Kari, Z. Effect of feeding pineapple waste on growth performance, texture quality and flesh colour of nile tilapia (Oreochromis niloticus) fingerlings. Saudi J. Biol. Sci. 2022, 29, 2514–2519. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Sim, K.Y.; Wendy, W.; Zulhisyam, A.K. Peperomia pellucida leaf extract as immunostimulator in controlling motile aeromonad septicemia due to Aeromonas hydrophila in red hybrid tilapia, Oreochromis spp. farming. Vet. World 2016, 9, 231–234. [Google Scholar] [CrossRef]
- Hammod, A.J.; Abd El-Aziz, A.H.; Areaaer, A.H.; Alfertosi, K.A. Effect of Dill Powder (Anethum graveolens) as a Dietary Supplement on Productive Performance, Mortality and Economic Figure in Broiler. IOP Conf. Ser. Earth Environ. Sci. 2020, 553, 012018. [Google Scholar] [CrossRef]
- Jangali, A.; Hedayati, M.; Khalaji, S.; Manafi, M. Oxidative stress and effects of dill (Anethum graveolens dhi) powder on the performance and health status of broilers. S. Afr. J. Anim. Sci. 2021, 51, 700–714. [Google Scholar] [CrossRef]
- Milenković, L.; Ilić, Z.S.; Stanojević, L.; Danilović, B.; Šunić, L.; Kevrešan, Ž.; Stanojević, J.; Cvetković, D. Chemical Composition and Bioactivity of Dill Seed (Anethum graveolens L.) Essential Oil from Plants Grown under Shading. Plants 2024, 13, 886. [Google Scholar] [CrossRef]
- Haidari, F.; Zakerkish, M.; Borazjani, F.; Ahmadi Angali, K.; Amoochi Foroushani, G. The effects of Anethum graveolens (dill) powder supplementation on clinical and metabolic status in patients with type 2 diabetes. Trials 2020, 21, 483. [Google Scholar] [CrossRef]
- Wei, L.S.; Adrian Susin, A.A.; Tahiluddin, A.B.; Kien, L.V.; Wee, W. Exploring the potential of black fungus, Auricularia auricula, as a feed additive in African catfish, Clarias gariepinus, farming. Heliyon 2024, 10, e33810. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Xiao, W.; Ye, J.; Xu, L.; Peng, R.; Han, Q.; Lü, Z.; Shi, H.; Jiang, X. Effects of feed transition on digestive tract digestive enzyme, morphology and intestinal community in cuttlefish (Sepia pharaonis). Front. Mar. Sci. 2022, 9, 941488. [Google Scholar] [CrossRef]
- Erlanger, B.F.; Kokowsky, N.; Cohen, W. The preparation and properties of two new chromogenic substrates of trypsin. Arch. Biochem. Biophys. 1961, 95, 271–278. [Google Scholar] [CrossRef]
- Robyt, J.; Whelan, W. The β-Amylases, Starch and Its Derivates; Academic press: London, UK, 1968; pp. 477–497. [Google Scholar]
- Winkler, U.K.; Stuckmann, M. Glycogen, hyaluronate, and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J. Bacteriol. 1979, 138, 663–670. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Noreldin, A.E.; Sewilam, H. Blood biochemical variables, antioxidative status, and histological features of intestinal, gill, and liver tissues of African catfish (Clarias gariepinus) exposed to high salinity and high-temperature stress. Environ. Sci. Pollut. Res. 2022, 29, 56357–56369. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Liew, V.K.; Kari, Z.A.; Kabir, M.A.; Azra, M.N.; Khoo, M.I.; Wee, W. Protective Effects of Dietary Etlingera elatior (Jack) Bud Flower Powder against Edwardsiella tarda Infection in African Catfish, Clarias gariepinus. Aquac. Res. 2024, 2024, 1754005. [Google Scholar] [CrossRef]
- Inglis, P.W.; Pappas, M.d.C.R.; Resende, L.V.; Grattapaglia, D. Fast and inexpensive protocols for consistent extraction of high quality DNA and RNA from challenging plant and fungal samples for high-throughput SNP genotyping and sequencing applications. PLoS ONE 2018, 13, e0206085. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2012, 41, e1. [Google Scholar] [CrossRef]
- García-López, R.; Cornejo-Granados, F.; Lopez-Zavala, A.A.; Sánchez-López, F.; Cota-Huízar, A.; Sotelo-Mundo, R.R.; Guerrero, A.; Mendoza-Vargas, A.; Gómez-Gil, B.; Ochoa-Leyva, A. Doing More with Less: A Comparison of 16S Hypervariable Regions in Search of Defining the Shrimp Microbiota. Microorganisms 2020, 8, 134. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Parks, D.H.; Chuvochina, M.; Chaumeil, P.-A.; Rinke, C.; Mussig, A.J.; Hugenholtz, P. A complete domain-to-species taxonomy for Bacteria and Archaea. Nat. Biotechnol. 2020, 38, 1079–1086. [Google Scholar] [CrossRef]
- Oni, A.I.; Adeleye, O.O.; Adebowale, T.O.; Oke, O.E. The role of phytogenic feed additives in stress mitigation in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2024, 108, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.S.; Hooi, K.Y.; Khoo, M.I.; Azra, M.N.; Wee, W. Effects of dietary kaffir lime, Citrus hystrix DC, leaf powder on the growth performance, digestive enzyme, hematology, antioxidative response, and disease resistance against Edwardsiella tarda infection in African catfish, Clarias gariepinus. Aquac. Int. 2024, 32, 7469–7485. [Google Scholar] [CrossRef]
- Mesripour, A.; Rafieian-Kopaei, M.; Bahrami, B. The effects of Anethum graveolens essence on scopolamine-induced memory impairment in mice. Res. Pharm. Sci. 2016, 11, 145–151. [Google Scholar] [PubMed]
- Bhukhai, K.; Fouquet, G.; Rittavee, Y.; Tanhuad, N.; Lakmuang, C.; Borwornpinyo, S.; Anurathapan, U.; Suksamrarn, A.; Piyachaturawat, P.; Chairoungdua, A.; et al. Enhancing Erythropoiesis by a Phytoestrogen Diarylheptanoid from Curcuma comosa. Biomedicines 2022, 10, 1427. [Google Scholar] [CrossRef]
- Bilen, S.; Özkan, O.; Alagöz, K.; Özdemir, K.Y. Effect Of dill (Anethum graveolens) and garden cress (Lepidium sativum) dietary supplementation on growth performance, digestive enzyme activities and immune responses of juvenile common carp (Cyprinus carpio). Aquaculture 2018, 495, 611–616. [Google Scholar] [CrossRef]
- Abbasi, E.; Goodarzi, M.T.; Tayebinia, H.; Saidijam, M.; Khodadadi, I. Favorable effects of Anethum graveolens on liver oxidative stress and cholesterol 7 alpha-hydroxylase levels in non-alcoholic fatty liver disease (NAFLD) rat models. Metab. Open 2021, 12, 100140. [Google Scholar] [CrossRef] [PubMed]
- Ajarem, J.S.; Maodaa, S.N.; El-Serehy, H.A.; Altoom, N.; Allam, A.A.; Hernandez-Bautista, R.; Mahmoud, A.M. Anethum graveolens Prevents Liver and Kidney Injury, Oxidative Stress and Inflammation in Mice Exposed to Nicotine Perinatally. Coatings 2021, 11, 838. [Google Scholar] [CrossRef]
- Oshaghi, E.A.; Khodadadi, I.; Tavilani, H.; Goodarzi, M.T. Effect of dill tablet (Anethum graveolens L) on antioxidant status and biochemical factors on carbon tetrachloride-induced liver damage on rat. Int. J. Appl. Basic Med. Res. 2016, 6, 111–114. [Google Scholar] [CrossRef]
- Ramírez, C.; Coronado, J.; Silva, A.; Romero, J. Cetobacterium Is a Major Component of the Microbiome of Giant Amazonian Fish (Arapaima gigas) in Ecuador. Animals 2018, 8, 189. [Google Scholar] [CrossRef]
- Tsuchiya, C.; Sakata, T.; Sugita, H. Novel ecological niche of Cetobacterium somerae, an anaerobic bacterium in the intestinal tracts of freshwater fish. Lett. Appl. Microbiol. 2008, 46, 43–48. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, X.; Zhang, Z.; Jin, Z.; Wang, G.; Ling, F. Effects of dietary Cetobacterium somerae on the intestinal health, immune parameters and resistance against Nocardia seriolae of largemouth bass, Micropterus salmoides. Fish Shellfish Immunol. 2023, 135, 108693. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, S.; Lessing, D.J.; Chu, W. The attenuating effects of synbiotic containing Cetobacterium somerae and Astragalus polysaccharide against trichlorfon-induced hepatotoxicity in crucian carp (Carassius carassius). J. Hazard. Mater. 2024, 461, 132621. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, S.; Lessing, D.J.; Guo, L.; Chu, W. Characterization of Cetobacterium somerae CPU-CS01 isolated from the intestine of healthy crucian carp (Carassius auratus) as potential probiotics against Aeromonas hydrophila infection. Microb. Pathog. 2023, 180, 106148. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zhou, W.; Xie, Y.; Li, Y.; Zhang, Z.; Yang, Y.; Olsen, R.E.; Ran, C.; Zhou, Z. Effects of Cetobacterium somerae fermentation product on gut and liver health of common carp (Cyprinus carpio) fed diet supplemented with ultra-micro ground mixed plant proteins. Aquaculture 2021, 543, 736943. [Google Scholar] [CrossRef]
- Calvo, A.; Pastor, Y.; Rosas-Val, P.; Gamazo, C. Unveiling the immunomodulatory effect of the novel probiotic Akkermansia muciniphila and its protective effect in vitro. Microbiol. Res. 2024, 283, 127677. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Zhao, D.; Yan, C.; Ren, X.; Wang, A.; Xia, X. Pasteurized Akkermansia muciniphila improves immunity and relieves other toxic effects in 5-fluorouracil treated BALB/c mice. Food Biosci. 2024, 59, 103918. [Google Scholar] [CrossRef]
- Cheng, J.; Lei, Z.; Fang, C.; Jia, W.; Xu, Y. Pasteurized Akkermansia muciniphila and its outer membrane protein Amuc_1100 alleviate alcoholic liver disease through modulating gut microbiota and host metabolism. Food Biosci. 2024, 59, 104072. [Google Scholar] [CrossRef]
- Keitel, L.; Miebach, K.; Rummel, L.; Yordanov, S.; Büchs, J. Process analysis of the anaerobe Phocaeicola vulgatus in a shake flasks and fermenter reveals pH and product inhibition. Ann. Microbiol. 2024, 74, 7. [Google Scholar] [CrossRef]
- Jin, S.; Chen, P.; Yang, J.; Li, D.; Liu, X.; Zhang, Y.; Xia, Q.; Li, Y.; Chen, G.; Li, Y.; et al. Phocaeicola vulgatus alleviates diet-induced metabolic dysfunction-associated steatotic liver disease progression by downregulating histone acetylation level via 3-HPAA. Gut Microbes 2024, 16, 2309683. [Google Scholar] [CrossRef]


| Treatments | ||||
|---|---|---|---|---|
| Parameters | Control | DW5 | DW10 | DW15 |
| Initial weight (IW) (g) | 10.5 ± 0.06 | 10.5 ± 0.06 | 10.6 ± 0.12 | 10.5 ± 0.153 |
| Final weight (FW) (g) | 143.3 ± 1.66 c | 158.0 ± 1.10 b | 171.0 ± 1.79 a | 172.9 ± 2.46 a |
| Weight gain (WG) (%) | 1260.7 ± 9.38 c | 1409.9 ± 4.88 b | 1518.8 ± 30.38 a | 1541.5 ± 4.06 a |
| Specific growth rate (SGR) (%/day) | 2.02 ± 0.005 c | 2.11 ± 0.003 b | 2.16 ± 0.015 a | 2.17 ± 0.002 a |
| Feed conversion ratio (FCR) | 1.36 ± 0.016 c | 1.22 ± 0.009 b | 1.12 ± 0.013 a | 1.11 ± 0.016 a |
| Hepatosomatic index (HSI) | 2.86 ± 0.122 d | 2.26 ± 0.088 c | 1.73 ± 0.079 a | 1.60 ± 0.083 b |
| Visceral somatic index (VSI) | 5.26 ± 0.169 c | 4.18 ± 0.301 b | 3.43 ± 0.222 a | 3.40 ± 0.232 a |
| Treatments | ||||
|---|---|---|---|---|
| Parameters | Control | DW5 | DW10 | DW15 |
| WBC/µL | 91.0 ± 1.71 c | 97.7 ± 1.96 b | 104.8 ± 3.00 a | 108.7 ± 3.40 a |
| LYM (%) | 80.8 ± 0.60 | 83.2 ± 2.53 | 80.5 ± 2.09 | 80.5 ± 3.46 |
| MON (%) | 10.4 ± 0.57 | 10.0 ± 0.21 | 10.2 ± 0.51 | 10.1 ± 0.25 |
| RBC (103/µL) | 2.3 ± 0.17 c | 3.00 ± 0.12 b | 3.5 ± 0.15 a | 3.5 ± 0.15 a |
| HGB (g/dL) | 4.7 ± 0.20 c | 5.2 ± 0.12 b | 6.1 ± 0.25 a | 5.9 ± 0.25 a |
| HCT (%) | 20.7 ± 1.10 c | 26.3 ± 2.57 b | 32.3 ± 1.93 a | 32.3 ± 1.72 a |
| MCH (pg) | 30.0 ± 0.92 | 30.6 ± 1.23 | 31.2 ± 1.45 | 30.8 ± 0.83 |
| MCHC (g/dL) | 21.9 ± 1.31 | 22.3 ± 1.27 | 22.2 ± 1.31 | 21.9 ± 1.31 |
| Parameters | Equation | R2 | Dill Weed (%) |
|---|---|---|---|
| Final weight (FW) | y = 0.0010x2 − 0.2694x + 18.2151 | 0.9174 | 0.2624 |
| Weight gain (WG) | y = 0.0000x2 − 0.0356x + 21.9694 | 0.9396 | 0.2244 |
| Specific growth rate (SGR) | y = 67.7596x2 − 274.8743x + 278.7666 | 0.9580 | 0.2290 |
| Feed conversion ratio (FCR) | y = 17.5727x2 − 48.6238x + 33.632 | 0.8994 | 0.2895 |
| Red blood cell (RBC) | y = 0.7008x2 − 3.0165x + 3.2386 | 0.9078 | 0.2772 |
| Protease activity | y = 20.3318x2 − 16.7621x + 3.3773 | 0.7749 | 0.4331 |
| Catalase (CAT) activity | y = 0.0013x2 − 0.2032x + 7.4201 | 0.8956 | 0.2950 |
| Cumulative survival rate | y = 0.0000x2 + 0.0018x − 0.0402 | 0.9883 | 0.3115 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, L.S.; Liew, V.K.; Tahiluddin, A.B.; Harikrishnan, R.; Hosain, M.E.; Azra, M.N.; Wee, W. Dietary Dill Weed (Anethum graveolens) Stimulated Disease Resistance of African Catfish (Clarias gariepinus) Against Edwardsiellosis Infection. Bacteria 2025, 4, 23. https://doi.org/10.3390/bacteria4020023
Wei LS, Liew VK, Tahiluddin AB, Harikrishnan R, Hosain ME, Azra MN, Wee W. Dietary Dill Weed (Anethum graveolens) Stimulated Disease Resistance of African Catfish (Clarias gariepinus) Against Edwardsiellosis Infection. Bacteria. 2025; 4(2):23. https://doi.org/10.3390/bacteria4020023
Chicago/Turabian StyleWei, Lee Seong, Vui Kien Liew, Albaris B. Tahiluddin, Ramasamy Harikrishnan, Md. Eilious Hosain, Mohamad Nor Azra, and Wendy Wee. 2025. "Dietary Dill Weed (Anethum graveolens) Stimulated Disease Resistance of African Catfish (Clarias gariepinus) Against Edwardsiellosis Infection" Bacteria 4, no. 2: 23. https://doi.org/10.3390/bacteria4020023
APA StyleWei, L. S., Liew, V. K., Tahiluddin, A. B., Harikrishnan, R., Hosain, M. E., Azra, M. N., & Wee, W. (2025). Dietary Dill Weed (Anethum graveolens) Stimulated Disease Resistance of African Catfish (Clarias gariepinus) Against Edwardsiellosis Infection. Bacteria, 4(2), 23. https://doi.org/10.3390/bacteria4020023








