Presence, Pathogenicity, Antibiotic Resistance, and Virulence Factors of Escherichia coli: A Review
Abstract
1. Introduction
1.1. Presence of Escherichia coli in Animal Products
1.2. Presence of Escherichia coli in Raw Fresh Produce
1.3. Presence of Escherichia coli in the Environment
2. Pathogenesis of Escherichia coli
2.1. Enteropathogenic Escherichia coli
2.2. Enterotoxigenic Escherichia coli
2.3. Enteroinvasive Escherichia coli
2.4. Enteroaggregative Escherichia coli
2.5. Enterohemorrhagic Escherichia coli
2.6. Diffusely Adherent Escherichia coli
3. Intestinal Escherichia coli Pathogens’ Virulence Factors
3.1. EPEC Virulence Factors
3.2. EHEC Virulence Factors
3.3. ETEC Virulence Factors
3.4. EIEC Virulence Factors
3.5. EAEC Virulence Factors
3.6. DAEC Virulence Factors
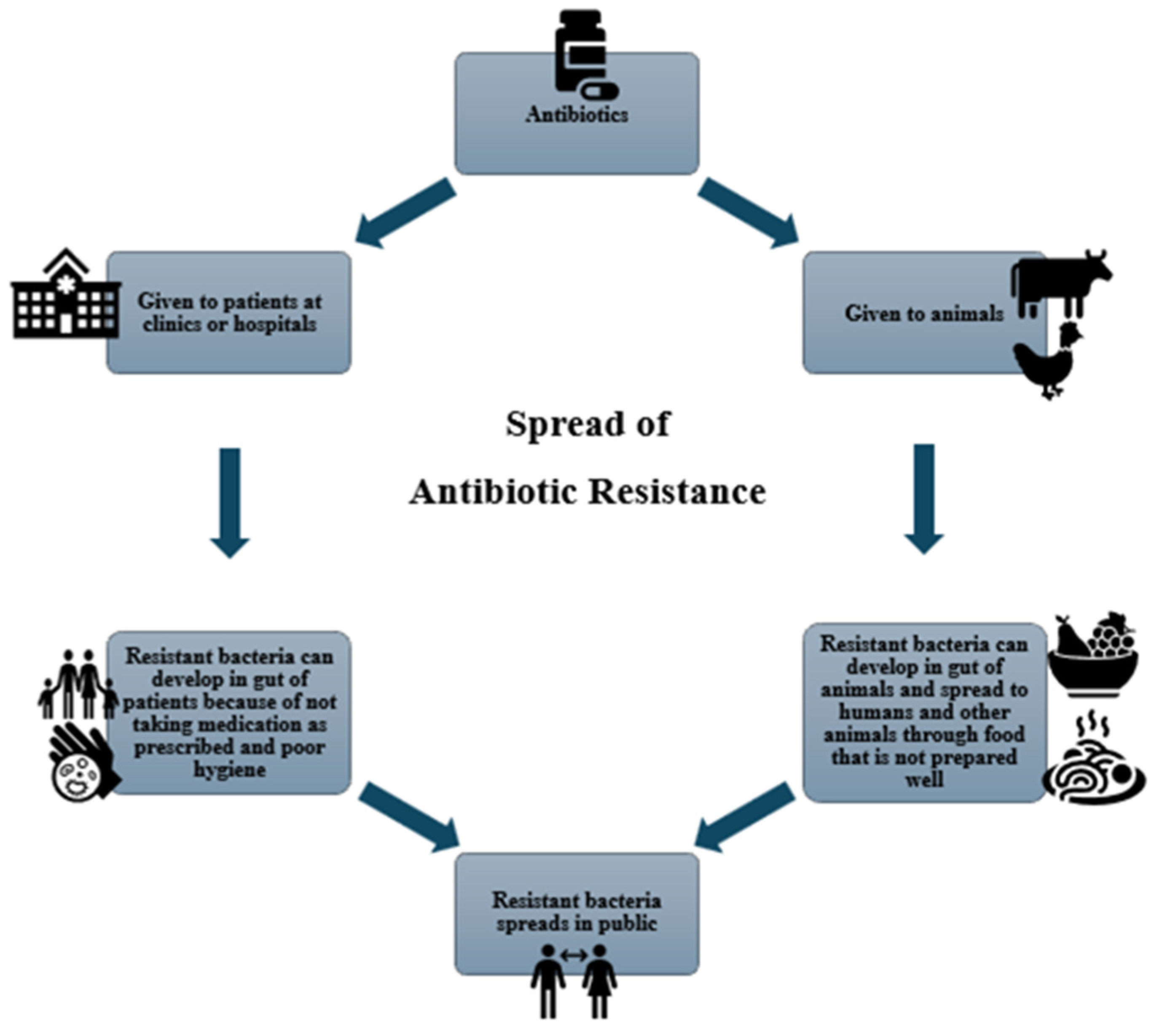
4. Antibiotics: Uses and Resistance Concerns
4.1. Genetic Basis of Antimicrobial Resistance in E. coli
4.2. Horizontal Gene Transfer
4.3. Mutations
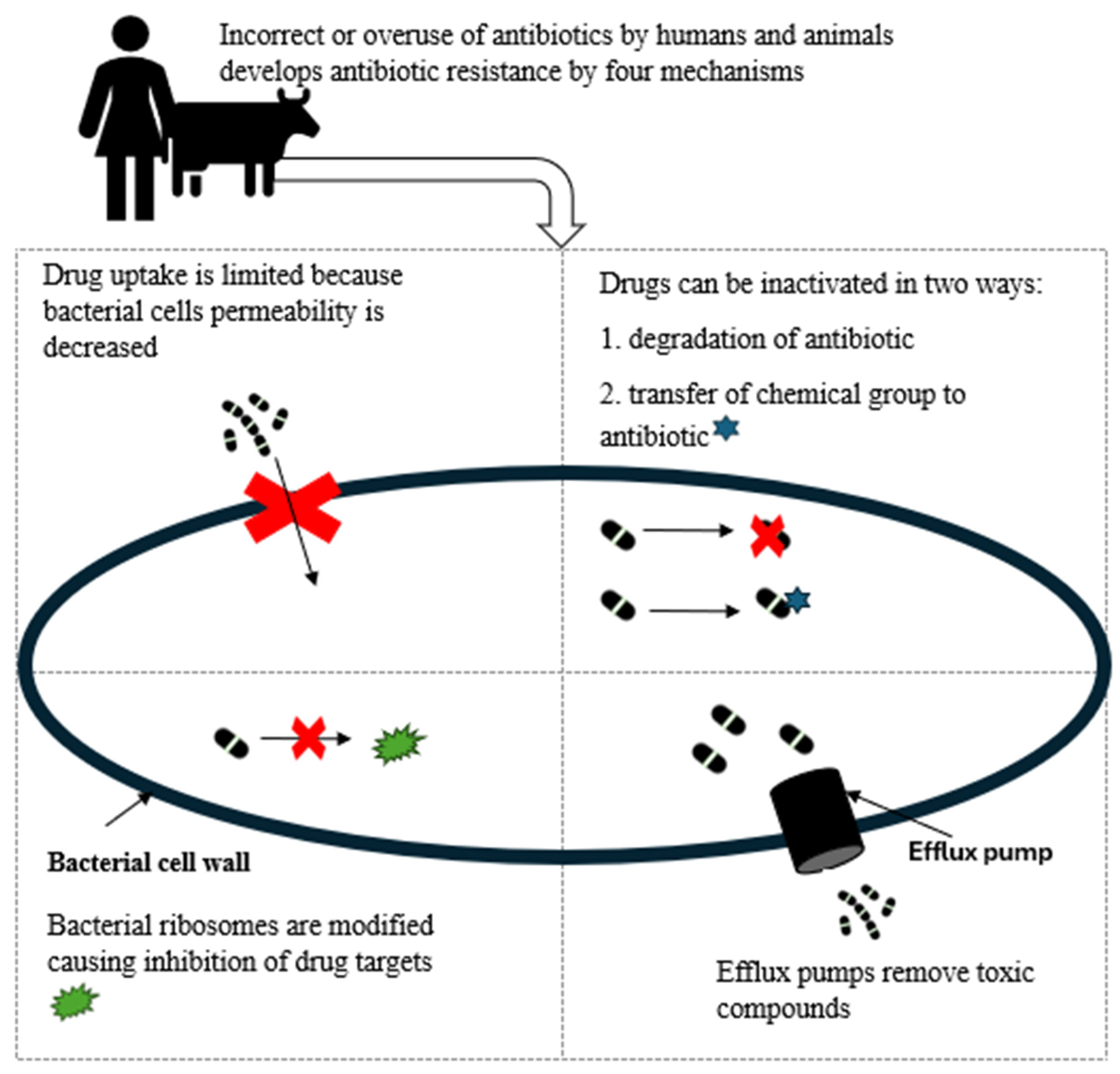
4.4. Mechanism of Antibiotic Resistance in E. coli
- (1) Limiting drug uptake
- (2) Modification of the drug target
- (3) Inactivation of the drug
- (4) Active efflux of the drug
5. Phylogenetics
6. Conclusions
Funding
Conflicts of Interest
References
- Lukjancenko, O.; Wassenaar, T.M.; Ussery, D.W. Comparison of 61 sequenced Escherichia coli genomes. Microb. Ecol. 2010, 60, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Abd El Tawab, A.A.; Ammar, A.M.; Nasef, S.A.; Reda, R.M. Prevalence of E. coli in diseased chickens with its antibiogram pattern. Benha Vet. Med. J. 2015, 28, 224–230. [Google Scholar] [CrossRef][Green Version]
- Bell, C.; Kyriakides, A. Pathogenic Escherichia coli. In Foodborne Pathogens; Elsevier: Amsterdam, The Netherlands, 2009; pp. 581–626. [Google Scholar]
- Tenaillon, O.; Skurnik, D.; Picard, B.; Denamur, E. The population genetics of commensal Escherichia coli. Nat. Rev. Microbiol. 2010, 8, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Calero, P.; Nikel, P.I. Chasing bacterial chassis for metabolic engineering: A perspective review from classical to non-traditional microorganisms. Microb. Biotechnol. 2019, 12, 98–124. [Google Scholar] [CrossRef]
- Lamprecht, C.; Romanis, M.; Huisamen, N.; Carinus, A.; Schoeman, N.; Sigge, G.O.; Britz, T.J. Escherichia coli with virulence factors and multidrug resistance in the Plankenburg River. S. Afr. J. Sci. 2014, 110, 01–06. [Google Scholar] [CrossRef]
- Jang, J.; Hur, H.G.; Sadowsky, M.J.; Byappanahalli, M.N.; Yan, T.; Ishii, S. Environmental Escherichia coli: Ecology and public health implications-a review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef]
- Ramos, S.; Silva, V.; Dapkevicius, M.L.E.; Canica, M.; Tejedor-Junco, M.T.; Igrejas, G.; Poeta, P. Escherichia coli as Commensal and Pathogenic Bacteria Among Food-Producing Animals: Health Implications of Extended Spectrum beta-lactamase (ESBL) Production. Animals 2020, 10, 2239. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Lindstedt, B.A.; Finton, M.D.; Porcellato, D.; Brandal, L.T. High frequency of hybrid Escherichia coli strains with combined Intestinal Pathogenic Escherichia coli (IPEC) and Extraintestinal Pathogenic Escherichia coli (ExPEC) virulence factors isolated from human faecal samples. BMC Infect. Dis. 2018, 18, 544. [Google Scholar] [CrossRef]
- Mafokwane, T.; Djikeng, A.; Nesengani, L.T.; Dewar, J.; Mapholi, O. Gastrointestinal Infection in South African Children under the Age of 5 years: A Mini Review. Gastroenterol. Res. Pract. 2023, 2023, 1906782. [Google Scholar] [CrossRef]
- Boroumand, M.B.; Naghmachi, M.; Ghatee, M.A. Detection of phylogenetic groups and drug resistance genes of Escherichia coli causing urinary tract infection in southwest Iran. Jundishapur J. Microbiol. 2021, 14, e112547. [Google Scholar] [CrossRef]
- Staji, H.; Khoshgoftar, J.; Vayeghan, A.J.; Bejestani, M. Phylogenetic grouping and assessment of virulence genotypes, with antibiotic resistance patterns, of Escherichia coli strains implicated in female urinary tract infections. Int. J. Enteric Pathog. 2016, 4, 4-31609. [Google Scholar] [CrossRef]
- Pakbin, B.; Brück, W.M.; Rossen, J.W. Virulence factors of enteric pathogenic Escherichia coli: A review. Int. J. Mol. Sci. 2021, 22, 9922. [Google Scholar] [CrossRef]
- Daga, A.P.; Koga, V.L.; Soncini, J.G.M.; de Matos, C.M.; Perugini, M.R.E.; Pelisson, M.; Kobayashi, R.K.T.; Vespero, E.C. Escherichia coli bloodstream infections in patients at a university hospital: Virulence factors and clinical characteristics. Front. Cell. Infect. Microbiol. 2019, 9, 191. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: A review. Food Microbiol. 2018, 73, 177–208. [Google Scholar] [CrossRef]
- Dale, A.P.; Woodford, N. Extra-intestinal pathogenic Escherichia coli (ExPEC): Disease, carriage and clones. J. Infect. 2015, 71, 615–626. [Google Scholar] [CrossRef]
- Mora-Rillo, M.; Fernández-Romero, N.; Navarro-San Francisco, C.; Díez-Sebastián, J.; Romero-Gómez, M.P.; Arnalich Fernández, F.; Arribas López, J.R.; Mingorance, J. Impact of virulence genes on sepsis severity and survival in Escherichia coli bacteremia. Virulence 2015, 6, 93–100. [Google Scholar] [CrossRef]
- Livermore, D.M. Bacterial resistance: Origins, epidemiology, and impact. Clin. Infect. Dis. 2003, 36, S11–S23. [Google Scholar] [CrossRef]
- Puvača, N.; Tankosić, J.V.; Ignjatijević, S.; Carić, M.; Prodanović, R. Antimicrobial resistance in the environment: Review of the selected resistance drivers and public health concerns. J. Agron. Technol. Eng. Manag. 2022, 5, 793–802. [Google Scholar] [CrossRef]
- Geurtsen, J.; de Been, M.; Weerdenburg, E.; Zomer, A.; McNally, A.; Poolman, J. Genomics and pathotypes of the many faces of Escherichia coli. FEMS Microbiol. Rev. 2022, 46, fuac031. [Google Scholar] [CrossRef]
- Nasrollahian, S.; Graham, J.P.; Halaji, M. A review of the mechanisms that confer antibiotic resistance in pathotypes of E. coli. Front. Cell. Infect. Microbiol. 2024, 14, 1387497. [Google Scholar] [CrossRef]
- Poirel, L.; Madec, J.-Y.; Lupo, A.; Schink, A.-K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial resistance in Escherichia coli. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Hutinel, M.; Fick, J.; Larsson, D.J.; Flach, C.-F. Investigating the effects of municipal and hospital wastewaters on horizontal gene transfer. Environ. Pollut. 2021, 276, 116733. [Google Scholar] [CrossRef] [PubMed]
- Lerminiaux, N.A.; Cameron, A.D. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019, 65, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Stoppe, N.d.C.; Silva, J.S.; Carlos, C.; Sato, M.I.; Saraiva, A.M.; Ottoboni, L.M.; Torres, T.T. Worldwide phylogenetic group patterns of Escherichia coli from commensal human and wastewater treatment plant isolates. Front. Microbiol. 2017, 8, 2512. [Google Scholar] [CrossRef]
- Montealegre, M.C.; Talavera Rodríguez, A.; Roy, S.; Hossain, M.I.; Islam, M.A.; Lanza, V.F.; Julian, T.R. High genomic diversity and heterogenous origins of pathogenic and antibiotic-resistant Escherichia coli in household settings represent a challenge to reducing transmission in low-income settings. Msphere 2020, 5, e00704-19. [Google Scholar] [CrossRef]
- Fuhrmeister, E.R.; Ercumen, A.; Pickering, A.J.; Jeanis, K.M.; Ahmed, M.; Brown, S.; Arnold, B.F.; Hubbard, A.E.; Alam, M.; Sen, D. Predictors of enteric pathogens in the domestic environment from human and animal sources in rural Bangladesh. Environ. Sci. Technol. 2019, 53, 10023–10033. [Google Scholar] [CrossRef]
- Heredia, N.; García, S. Animals as sources of food-borne pathogens: A review. Anim. Nutr. 2018, 4, 250–255. [Google Scholar] [CrossRef]
- Rani, Z.T.; Hugo, A.; Hugo, C.J.; Vimiso, P.; Muchenje, V. Effect of post-slaughter handling during distribution on microbiological quality and safety of meat in the formal and informal sectors of South Africa: A review. S. Afr. J. Anim. Sci. 2017, 47, 255–267. [Google Scholar] [CrossRef]
- Messele, Y.E.; Abdi, R.D.; Tegegne, D.T.; Bora, S.K.; Babura, M.D.; Emeru, B.A.; Werid, G.M. Analysis of milk-derived isolates of E. coli indicating drug resistance in central Ethiopia. Trop. Anim. Health Prod. 2019, 51, 661–667. [Google Scholar] [CrossRef]
- Yang, S.-C.; Lin, C.-H.; Aljuffali, I.A.; Fang, J.-Y. Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch. Microbiol. 2017, 199, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Heetun, I.; Goburdhun, D.; Neetoo, H. Comparative microbiological evaluation of raw chicken from markets and chilled outlets of Mauritius. J. Worlds Poul Res. 2015, 5, 10–18. [Google Scholar]
- Ranjbar, R.; Safarpoor Dehkordi, F.; Sakhaei Shahreza, M.H.; Rahimi, E. Prevalence, identification of virulence factors, O-serogroups and antibiotic resistance properties of Shiga-toxin producing Escherichia coli strains isolated from raw milk and traditional dairy products. Antimicrob. Resist. Infect. Control 2018, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Ludden, C.; Raven, K.E.; Jamrozy, D.; Gouliouris, T.; Blane, B.; Coll, F.; de Goffau, M.; Naydenova, P.; Horner, C.; Hernandez-Garcia, J. One health genomic surveillance of Escherichia coli demonstrates distinct lineages and mobile genetic elements in isolates from humans versus livestock. MBio 2019, 10, e02693-18. [Google Scholar] [CrossRef] [PubMed]
- Tighe-Neira, R.; Alberdi, M.; Arce-Johnson, P.; Romero-Romero, J.L.; Reyes-Díaz, M.; Inostroza-Blancheteau, C. Foods with functional properties and their potential uses in human health. In Superfood and Functional Food-An Overview of Their Processing and Utilization; Waisundara, V., Shiomi, N., Eds.; IntechOpen: London, UK, 2017. [Google Scholar]
- Hess, T.; Sutcliffe, C. The exposure of a fresh fruit and vegetable supply chain to global water-related risks. Water Int. 2018, 43, 746–761. [Google Scholar] [CrossRef]
- Luna-Guevara, J.J.; Arenas-Hernandez, M.M.; Martínez de la Peña, C.; Silva, J.L.; Luna-Guevara, M.L. The role of pathogenic E. coli in fresh vegetables: Behavior, contamination factors, and preventive measures. Int. J. Microbiol. 2019, 2019, 2894328. [Google Scholar] [CrossRef]
- Mostafidi, M.; Sanjabi, M.R.; Shirkhan, F.; Zahedi, M.T. A review of recent trends in the development of the microbial safety of fruits and vegetables. Trends Food Sci. Technol. 2020, 103, 321–332. [Google Scholar] [CrossRef]
- Nüesch-Inderbinen, M.; Stephan, R. Fresh fruit and vegetables as vehicles of bacterial foodborne disease: A review and analysis of outbreaks registered by proMED-mail associated with fresh produce. J. Food Saf. Food Qual. 2016, 67, 32–39. [Google Scholar] [CrossRef]
- Bintsis, T. Microbial pollution and food safety. AIMS Microbiol. 2018, 4, 377–396. [Google Scholar] [CrossRef]
- Feliziani, E.; Lichter, A.; Smilanick, J.L.; Ippolito, A. Disinfecting agents for controlling fruit and vegetable diseases after harvest. Postharvest Biol. Technol. 2016, 122, 53–69. [Google Scholar] [CrossRef]
- Gombas, D.; Luo, Y.; Brennan, J.; Shergill, G.; Petran, R.; Walsh, R.; Hau, H.; Khurana, K.; Zomorodi, B.; Rosen, J. Guidelines to validate control of cross-contamination during washing of fresh-cut leafy vegetables. J. Food Prot. 2017, 80, 312–330. [Google Scholar] [CrossRef] [PubMed]
- Merget, B.; Forbes, K.J.; Brennan, F.; McAteer, S.; Shepherd, T.; Strachan, N.J.; Holden, N.J. Influence of plant species, tissue type, and temperature on the capacity of Shiga-Toxigenic Escherichia coli to colonize, grow, and be internalized by plants. Appl. Environ. Microbiol. 2019, 85, e00123-19. [Google Scholar] [CrossRef] [PubMed]
- Ranjbaran, M.; Verma, M.S. Microfluidics at the interface of bacteria and fresh produce. Trends Food Sci. Technol. 2022, 128, 102–117. [Google Scholar] [CrossRef]
- Pakbin, B.; Allahyari, S.; Amani, Z.; Bruck, W.M.; Mahmoudi, R.; Peymani, A. Prevalence, Phylogroups and Antimicrobial Susceptibility of Escherichia coli Isolates from Food Products. Antibiotics 2021, 10, 1291. [Google Scholar] [CrossRef]
- Gritli, A.; Belkahla, I.; Moussa, M.B.; Abassi, M. Occurrence and characterization of Escherichia coli in raw lettuce consumed in a military hospital. J. New Sci. 2015, 11, 899–907. [Google Scholar]
- Osińska, A.; Korzeniewska, E.; Harnisz, M.; Niestępski, S. The prevalence and characterization of antibiotic-resistant and virulent Escherichia coli strains in the municipal wastewater system and their environmental fate. Sci. Total Environ. 2017, 577, 367–375. [Google Scholar] [CrossRef]
- Devane, M.L.; Moriarty, E.; Weaver, L.; Cookson, A.; Gilpin, B. Fecal indicator bacteria from environmental sources; strategies for identification to improve water quality monitoring. Water Res. 2020, 185, 116204. [Google Scholar] [CrossRef]
- Riggio, G.M.; Wang, Q.; Kniel, K.E.; Gibson, K.E. Microgreens—A review of food safety considerations along the farm to fork continuum. Int. J. Food Microbiol. 2019, 290, 76–85. [Google Scholar] [CrossRef]
- Enany, M.E.; Algammal, A.M.; Nasef, S.A.; Abo-Eillil, S.A.; Bin-Jumah, M.; Taha, A.E.; Allam, A.A. The occurrence of the multidrug resistance (MDR) and the prevalence of virulence genes and QACs resistance genes in E. coli isolated from environmental and avian sources. AMB Express 2019, 9, 192. [Google Scholar] [CrossRef]
- Nguyen, J.; Lara-Gutiérrez, J.; Stocker, R. Environmental fluctuations and their effects on microbial communities, populations and individuals. FEMS Microbiol. Rev. 2021, 45, fuaa068. [Google Scholar] [CrossRef]
- Blaak, H.; van Hoek, A.H.; Hamidjaja, R.A.; van der Plaats, R.Q.; Kerkhof-de Heer, L.; de Roda Husman, A.M.; Schets, F.M. Distribution, numbers, and diversity of ESBL-producing E. coli in the poultry farm environment. PLoS ONE 2015, 10, e0135402. [Google Scholar] [CrossRef] [PubMed]
- Bong, C.W.; Chai, S.K.; Chai, L.C.; Wang, A.J.; Lee, C.W. Prevalence and characterization of Escherichia coli in the Kelantan River and its adjacent coastal waters. Water Supply 2020, 20, 930–942. [Google Scholar] [CrossRef]
- Nguyen, J.; Fernandez, V.; Pontrelli, S.; Sauer, U.; Ackermann, M.; Stocker, R. A distinct growth physiology enhances bacterial growth under rapid nutrient fluctuations. Nat. Commun. 2021, 12, 3662. [Google Scholar] [CrossRef]
- Conway, T.; Cohen, P.S. Commensal and pathogenic Escherichia coli metabolism in the gut. In Metabolism and Bacterial Pathogenesis; Wiley: Hoboken, NJ, USA, 2015; pp. 343–362. [Google Scholar]
- Macfarlane, S.; Bahrami, B.; Macfarlane, G.T. Mucosal biofilm communities in the human intestinal tract. Adv. Appl. Microbiol. 2011, 75, 111–143. [Google Scholar] [PubMed]
- Gomes, T.A.; Elias, W.P.; Scaletsky, I.C.; Guth, B.E.; Rodrigues, J.F.; Piazza, R.M.; Ferreira, L.; Martinez, M.B. Diarrheagenic Escherichia coli. Braz. J. Microbiol. 2016, 47, 3–30. [Google Scholar] [CrossRef]
- Reygaert, W.C. Antimicrobial Mechanisms of Escherichia coli; IntechOpen: London, UK, 2017; Volume 5. [Google Scholar]
- Kolenda, R.; Burdukiewicz, M.; Schierack, P. A systematic review and meta-analysis of the epidemiology of pathogenic Escherichia coli of calves and the role of calves as reservoirs for human pathogenic E. coli. Front. Cell. Infect. Microbiol. 2015, 5, 23. [Google Scholar] [CrossRef]
- Vila, J.; Sáez-López, E.; Johnson, J.R.; Römling, U.; Dobrindt, U.; Cantón, R.; Giske, C.; Naas, T.; Carattoli, A.; Martínez-Medina, M. Escherichia coli: An old friend with new tidings. FEMS Microbiol. Rev. 2016, 40, 437–463. [Google Scholar] [CrossRef]
- Govindarajan, D.K.; Viswalingam, N.; Meganathan, Y.; Kandaswamy, K. Adherence patterns of Escherichia coli in the intestine and its role in pathogenesis. Med. Microecol. 2020, 5, 100025. [Google Scholar] [CrossRef]
- Smith, J.; Fratamico, P. Escherichia coli as a Pathogen. In Foodborne Diseases; Elsevier: Amsterdam, The Netherlands, 2017; pp. 189–208. [Google Scholar]
- Mare, A.D.; Ciurea, C.N.; Man, A.; Tudor, B.; Moldovan, V.; Decean, L.; Toma, F. Enteropathogenic Escherichia coli—A summary of the literature. Gastroenterol. Insights 2021, 12, 28–40. [Google Scholar] [CrossRef]
- Croxen, M.A.; Finlay, B.B. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 2010, 8, 26–38. [Google Scholar] [CrossRef]
- Donnenberg, M.; Kaper, J. Enteropathogenic Escherichia coli. Infect. Immun. 1992, 60, 3953–3961. [Google Scholar] [CrossRef] [PubMed]
- Baruah, S.M. The Versatile and Pathogenic Faces of Escherichia coli. Assam J. Intern. Med. 2024, 14, 1–2. [Google Scholar] [CrossRef]
- Croxen, M.A.; Law, R.J.; Scholz, R.; Keeney, K.M.; Wlodarska, M.; Finlay, B.B. Recent advances in understanding enteric pathogenic Escherichia coli. Clin. Microbiol. Rev. 2013, 26, 822–880. [Google Scholar] [CrossRef]
- Manu, D.; Lupan, I.; Popescu, O. Mechanisms of pathogenesis and antibiotics resistance in Escherichia coli. Ann. Rom. Soc. Cell Biol. 2011, 16, 7–19. [Google Scholar]
- Cepeda-Molero, M.; Berger, C.N.; Walsham, A.D.; Ellis, S.J.; Wemyss-Holden, S.; Schüller, S.; Frankel, G.; Fernández, L.Á. Attaching and effacing (A/E) lesion formation by enteropathogenic E. coli on human intestinal mucosa is dependent on non-LEE effectors. PLoS Pathog. 2017, 13, e1006706. [Google Scholar] [CrossRef]
- Martinez de la Peña, C.F.; De Masi, L.; Nisa, S.; Mulvey, G.; Tong, J.; Donnenberg, M.S.; Armstrong, G.D. BfpI, BfpJ, and BfpK minor pilins are important for the function and biogenesis of bundle-forming pili expressed by enteropathogenic Escherichia coli. J. Bacteriol. 2016, 198, 846–856. [Google Scholar] [CrossRef]
- Dubreuil, J.D.; Isaacson, R.E.; Schifferli, D.M. Animal enterotoxigenic Escherichia coli. EcoSal Plus 2016, 7. [Google Scholar] [CrossRef]
- Fleckenstein, J.M.; Kuhlmann, F.M. Enterotoxigenic Escherichia coli infections. Curr. Infect. Dis. Rep. 2019, 21, 9. [Google Scholar] [CrossRef]
- Pokharel, P.; Dhakal, S.; Dozois, C.M. The diversity of Escherichia coli pathotypes and vaccination strategies against this versatile bacterial pathogen. Microorganisms 2023, 11, 344. [Google Scholar] [CrossRef]
- von Mentzer, A.; Blackwell, G.A.; Pickard, D.; Boinett, C.J.; Joffré, E.; Page, A.J.; Svennerholm, A.-M.; Dougan, G.; Sjöling, Å. Long-read-sequenced reference genomes of the seven major lineages of enterotoxigenic Escherichia coli (ETEC) circulating in modern time. Sci. Rep. 2021, 11, 9256. [Google Scholar] [CrossRef]
- Pasqua, M.; Michelacci, V.; Di Martino, M.L.; Tozzoli, R.; Grossi, M.; Colonna, B.; Morabito, S.; Prosseda, G. The intriguing evolutionary journey of enteroinvasive E. coli (EIEC) toward pathogenicity. Front. Microbiol. 2017, 8, 2390. [Google Scholar] [CrossRef] [PubMed]
- Okhuysen, P.C.; DuPont, H.L. Enteroaggregative Escherichia coli (EAEC): A cause of acute and persistent diarrhea of worldwide importance. J. Infect. Dis. 2010, 202, 503–505. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Garcia, T.; Navarro-Garcia, F. Enteroaggregative Escherichia coli pathotype: A genetically heterogeneous emerging foodborne enteropathogen. FEMS Immunol. Med. Microbiol. 2012, 66, 281–298. [Google Scholar] [CrossRef]
- Dias, R.C.; Tanabe, R.H.; Vieira, M.A.; Cergole-Novella, M.C.; Dos Santos, L.F.; Gomes, T.A.; Elias, W.P.; Hernandes, R.T. Analysis of the virulence profile and phenotypic features of typical and atypical enteroaggregative Escherichia coli (EAEC) isolated from diarrheal patients in Brazil. Front. Cell. Infect. Microbiol. 2020, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Mancini, J.; Weckselblatt, B.; Chung, Y.K.; Durante, J.C.; Andelman, S.; Glaubman, J.; Dorff, J.D.; Bhargava, S.; Lijek, R.S.; Unger, K.P. The heat-resistant agglutinin family includes a novel adhesin from enteroaggregative Escherichia coli strain 60A. J. Bacteriol. 2011, 193, 4813–4820. [Google Scholar] [CrossRef]
- Jønsson, R.; Struve, C.; Boisen, N.; Mateiu, R.V.; Santiago, A.E.; Jenssen, H.; Nataro, J.P.; Krogfelt, K.A. Novel aggregative adherence fimbria variant of enteroaggregative Escherichia coli. Infect. Immun. 2015, 83, 1396–1405. [Google Scholar] [CrossRef]
- Jacobson, M. On the infectious causes of neonatal piglet diarrhoea—A review. Vet. Sci. 2022, 9, 422. [Google Scholar] [CrossRef]
- Harrington, S.M.; Dudley, E.G.; Nataro, J.P. Pathogenesis of enteroaggregative Escherichia coli infection. FEMS Microbiol. Lett. 2006, 254, 12–18. [Google Scholar] [CrossRef]
- Kong, H.; Hong, X.; Li, X. Current perspectivesin pathogenesis and antimicrobial resistance of enteroaggregative Escherichia coli. Microb. Pathog. 2015, 85, 44–49. [Google Scholar] [CrossRef]
- Hebbelstrup Jensen, B.; Olsen, K.E.; Struve, C.; Krogfelt, K.A.; Petersen, A.M. Epidemiology and clinical manifestations of enteroaggregative Escherichia coli. Clin. Microbiol. Rev. 2014, 27, 614–630. [Google Scholar] [CrossRef]
- Jenkins, C. Enteroaggregative Escherichia coli. In Escherichia coli, a Versatile Pathogen; Springer: Berlin/Heidelberg, Germany, 2018; pp. 27–50. [Google Scholar]
- Betz, J.; Bielaszewska, M.; Thies, A.; Humpf, H.-U.; Dreisewerd, K.; Karch, H.; Kim, K.S.; Friedrich, A.W.; Müthing, J. Shiga toxin glycosphingolipid receptors in microvascular and macrovascular endothelial cells: Differential association with membrane lipid raft microdomains. J. Lipid Res. 2011, 52, 618–634. [Google Scholar] [CrossRef] [PubMed]
- Betz, J.; Bauwens, A.; Kunsmann, L.; Bielaszewska, M.; Mormann, M.; Humpf, H.-U.; Karch, H.; Friedrich, A.W.; Müthing, J. Uncommon membrane distribution of Shiga toxin glycosphingolipid receptors in toxin-sensitive human glomerular microvascular endothelial cells. Biol. Chem. 2012, 393, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Nataro, J.P.; Kaper, J.B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998, 11, 142–201. [Google Scholar] [CrossRef]
- Schüller, S. Shiga toxin interaction with human intestinal epithelium. Toxins 2011, 3, 626–639. [Google Scholar] [CrossRef]
- Meza-Segura, M.; Zaidi, M.B.; Vera-Ponce de León, A.; Moran-Garcia, N.; Martinez-Romero, E.; Nataro, J.P.; Estrada-Garcia, T. New insights into DAEC and EAEC pathogenesis and phylogeny. Front. Cell. Infect. Microbiol. 2020, 10, 572951. [Google Scholar] [CrossRef]
- Gebisa, E.S.; Gerasu, M.A.; Leggese, D.T. A review on virulence factors of Escherichia coli. Anim. Vet. Sci. 2019, 7, 83–93. [Google Scholar] [CrossRef]
- Mainil, J. Escherichia coli virulence factors. Vet. Immunol. Immunopathol. 2013, 152, 2–12. [Google Scholar] [CrossRef]
- Clavijo, A.P.; Bai, J.; Gómez-Duarte, O.G. The Longus type IV pilus of enterotoxigenic Escherichia coli (ETEC) mediates bacterial self-aggregation and protection from antimicrobial agents. Microb. Pathog. 2010, 48, 230–238. [Google Scholar] [CrossRef]
- Bolton, D.J. Verocytotoxigenic (Shiga toxin–producing) Escherichia coli: Virulence factors and pathogenicity in the farm to fork paradigm. Foodborne Pathog. Dis. 2011, 8, 357–365. [Google Scholar] [CrossRef]
- Bergan, J.; Lingelem, A.B.D.; Simm, R.; Skotland, T.; Sandvig, K. Shiga toxins. Toxicon 2012, 60, 1085–1107. [Google Scholar] [CrossRef]
- Karpman, D.; Ståhl, A.l. Enterohemorrhagic Escherichia coli pathogenesis and the host response. In Enterohemorrhagic Escherichia coli Other Shiga Toxin-Producing E. coli; ASM Press: Washington, DC, USA, 2015; pp. 381–402. [Google Scholar]
- Nguyen, Y.; Sperandio, V. Enterohemorrhagic E. coli (EHEC) pathogenesis. Front. Cell. Infect. Microbiol. 2012, 2, 90. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; LeJeune, J.T.; Zhao, T.; Doyle, M.P. Enterohemorrhagic Escherichia coli. In Food Microbiology: Fundamentals and Frontiers; Wiley: Hoboken, NJ, USA, 2012; pp. 287–309. [Google Scholar]
- Scheutz, F.; Teel, L.D.; Beutin, L.; Piérard, D.; Buvens, G.; Karch, H.; Mellmann, A.; Caprioli, A.; Tozzoli, R.; Morabito, S. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J. Clin. Microbiol. 2012, 50, 2951–2963. [Google Scholar] [CrossRef] [PubMed]
- Gyles, C.; Fairbrother, J. Escherichia coli. Pathog. Bact. Infect. Anim. 2010, 4, 267–308. [Google Scholar]
- Nagy, B.; Fekete, P.Z. Enterotoxigenic Escherichia coli (ETEC) in farm animals. Vet. Res. 1999, 30, 259–284. [Google Scholar]
- Dhakal, R.; Wang, Q.; Howard, P.; Sintchenko, V. Genome sequences of enteroinvasive Escherichia coli sequence type 6, 99, and 311 strains acquired in Asia Pacific. Microbiol. Resour. Announc. 2019, 8, e00944-19. [Google Scholar] [CrossRef]
- Schnupf, P.; Sansonetti, P.J. Shigella pathogenesis: New insights through advanced methodologies. In Bacteria and Intracellularity; Wiley: Hoboken, NJ, USA, 2019; pp. 15–39. [Google Scholar]
- Kotloff, K.L.; Riddle, M.S.; Platts-Mills, J.A.; Pavlinac, P.; Zaidi, A.K. Shigellosis. Lancet 2018, 391, 801–812. [Google Scholar] [CrossRef]
- Lambrecht, N.J.; Bridges, D.; Wilson, M.L.; Adu, B.; Eisenberg, J.N.; Folson, G.; Baylin, A.; Jones, A.D. Associations of bacterial enteropathogens with systemic inflammation, iron deficiency, and anemia in preschool-age children in southern Ghana. PLoS ONE 2022, 17, e0271099. [Google Scholar] [CrossRef]
- Boisen, N.; Krogfelt, K.A.; Nataro, J.P. Enteroaggregative Escherichia coli. In Escherichia coli; Elsevier: Amsterdam, The Netherlands, 2013; pp. 247–273. [Google Scholar]
- Guerrieri, C.G.; Pereira, M.F.; Galdino, A.C.M.; Santos, A.L.S.d.; Elias, W.P.; Schuenck, R.P.; Spano, L.C. Typical and atypical enteroaggregative Escherichia coli are both virulent in the Galleria mellonella model. Front. Microbiol. 2019, 10, 1791. [Google Scholar] [CrossRef]
- Servin, A.L. Pathogenesis of human diffusely adhering Escherichia coli expressing Afa/Dr adhesins (Afa/Dr DAEC): Current insights and future challenges. Clin. Microbiol. Rev. 2014, 27, 823–869. [Google Scholar] [CrossRef]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Kwatra, S.; Taneja, G.; Nasa, N. Alternative routes of drug administration-transdermal, pulmonary & parenteral. Indo Glob. J. Pharm. Sci. 2012, 2, 409–426. [Google Scholar]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.K. Alternatives to antibiotics: Why and how. In NAM Perspectives; National Academy of Medicine: Washington, DC, USA, 2017. [Google Scholar]
- Meek, R.W.; Vyas, H.; Piddock, L.J.V. Nonmedical uses of antibiotics: Time to restrict their use? PLoS Biol. 2015, 13, e1002266. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Zarazaga, M.; Ben Sallem, R.; Jouini, A.; Ben Slama, K.; Torres, C. Antibiotic resistance in Escherichia coli in husbandry animals: The African perspective. Lett. Appl. Microbiol. 2017, 64, 318–334. [Google Scholar] [CrossRef]
- Ahs, J.W.; Tao, W.; Löfgren, J.; Forsberg, B.C. Diarrheal diseases in low-and middle-income countries: Incidence, prevention and management. Open Infect. Dis. J. 2010, 4, 113–124. [Google Scholar] [CrossRef]
- Hawk, D.; Tribble, D.R.; Riddle, M.S. Clinical treatment of nondysentery travelers’ diarrhea during deployment. Mil. Med. 2010, 175, 140–146. [Google Scholar] [CrossRef]
- Alzaidi, S.; Veillette, J.J.; May, S.S.; Olson, J.; Jackson, K.; Waters, C.D.; Butler, A.M.; Hutton, M.A.; Buckel, W.R.; Webb, B.J. Oral β-Lactams, Fluoroquinolones, or Trimethoprim-Sulfamethoxazole for Definitive Treatment of Uncomplicated Escherichia coli or Klebsiella Species Bacteremia From a Urinary Tract Source. In Proceedings of the Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2024; p. ofad657. [Google Scholar]
- Bielaszewska, M.; Idelevich, E.A.; Zhang, W.; Bauwens, A.; Schaumburg, F.; Mellmann, A.; Peters, G.; Karch, H. Effects of antibiotics on Shiga toxin 2 production and bacteriophage induction by epidemic Escherichia coli O104: H4 strain. Antimicrob. Agents Chemother. 2012, 56, 3277–3282. [Google Scholar] [CrossRef]
- Padmini, N.; Ajilda, A.A.K.; Sivakumar, N.; Selvakumar, G. Extended spectrum β-lactamase producing Escherichia coli and Klebsiella pneumoniae: Critical tools for antibiotic resistance pattern. J. Basic Microbiol. 2017, 57, 460–470. [Google Scholar] [CrossRef]
- Lakshminarayanan, S.; Jayalakshmy, R. Diarrheal diseases among children in India: Current scenario and future perspectives. J. Nat. Sci. Biol. Med. 2015, 6, 24. [Google Scholar] [CrossRef]
- Economou, V.; Gousia, P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist. 2015, 8, 49–61. [Google Scholar] [CrossRef]
- Gould, I.M.; Bal, A.M. New antibiotic agents in the pipeline and how they can help overcome microbial resistance. Virulence 2013, 4, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Something old, something new: Revisiting natural products in antibiotic drug discovery. Can. J. Microbiol. 2014, 60, 147–154. [Google Scholar] [CrossRef]
- Sengupta, S.; Chattopadhyay, M.K.; Grossart, H.-P. The multifaceted roles of antibiotics and antibiotic resistance in nature. Front. Microbiol. 2013, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, S.S.; Bhat, B.A.; Tariq, L.; Yaseen, S.I.; Ara, I.; Rafi, B.; Hamdani, S.N.; Hassan, T.; Rashid, O. Antibiotic resistance: The future disaster. Int. J. Res. Appl. Sci. Biotechnol. 2020, 7. [Google Scholar]
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic resistance in bacteria—A review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Huang, Z.; Pan, H.; Zhang, P.; Cao, X.; Ju, W.; Wang, C.; Zhang, J.; Meng, J.; Yuan, Z.; Xu, X. Prevalence and antimicrobial resistance patterns of diarrheagenic Escherichia coli in Shanghai, China. Pediatr. Infect. Dis. J. 2016, 35, 835–839. [Google Scholar] [CrossRef]
- Rolain, J.-M. Food and human gut as reservoirs of transferable antibiotic resistance encoding genes. Front. Microbiol. 2013, 4, 173. [Google Scholar] [CrossRef]
- Kumarasamy, K.K.; Toleman, M.A.; Walsh, T.R.; Bagaria, J.; Butt, F.; Balakrishnan, R.; Chaudhary, U.; Doumith, M.; Giske, C.G.; Irfan, S. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: A molecular, biological, and epidemiological study. Lancet Infect. Dis. 2010, 10, 597–602. [Google Scholar] [CrossRef]
- Cassir, N.; Rolain, J.-M.; Brouqui, P. A new strategy to fight antimicrobial resistance: The revival of old antibiotics. Front. Microbiol. 2014, 5, 551. [Google Scholar] [CrossRef]
- Irrgang, A.; Roschanski, N.; Tenhagen, B.-A.; Grobbel, M.; Skladnikiewicz-Ziemer, T.; Thomas, K.; Roesler, U.; Kaesbohrer, A. Prevalence of mcr-1 in E. coli from livestock and food in Germany, 2010–2015. PLoS ONE 2016, 11, e0159863. [Google Scholar] [CrossRef] [PubMed]
- Newton-Foot, M.; Snyman, Y.; Maloba, M.R.B.; Whitelaw, A.C. Plasmid-mediated mcr-1 colistin resistance in Escherichia coli and Klebsiella spp. clinical isolates from the Western Cape region of South Africa. Antimicrob. Resist. Infect. Control 2017, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Grami, R.; Mansour, W.; Mehri, W.; Bouallègue, O.; Boujaâfar, N.; Madec, J.-Y.; Haenni, M. Impact of food animal trade on the spread of mcr-1-mediated colistin resistance, Tunisia, July 2015. Eurosurveillance 2016, 21, 30144. [Google Scholar] [CrossRef] [PubMed]
- Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance. In Virulence Mechanisms of Bacterial Pathogens; Wiley: Hoboken, NJ, USA, 2016; pp. 481–511. [Google Scholar]
- Ghannad, M.S.; Mohammadi, A. Bacteriophage: Time to re-evaluate the potential of phage therapy as a promising agent to control multidrug-resistant bacteria. Iran. J. Basic Med. Sci. 2012, 15, 693. [Google Scholar]
- Kester, J.C.; Fortune, S.M. Persisters and beyond: Mechanisms of phenotypic drug resistance and drug tolerance in bacteria. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 91–101. [Google Scholar] [CrossRef]
- Schroeder, M.; Brooks, B.D.; Brooks, A.E. The complex relationship between virulence and antibiotic resistance. Genes 2017, 8, 39. [Google Scholar] [CrossRef]
- Peterson, E.; Kaur, P. Antibiotic resistance mechanisms in bacteria: Relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Front. Microbiol. 2018, 9, 2928. [Google Scholar] [CrossRef]
- Manson, J.M.; Hancock, L.E.; Gilmore, M.S. Mechanism of chromosomal transfer of Enterococcus faecalis pathogenicity island, capsule, antimicrobial resistance, and other traits. Proc. Natl. Acad. Sci. USA 2010, 107, 12269–12274. [Google Scholar] [CrossRef]
- Revitt-Mills, S.A.; Robinson, A. Antibiotic-induced mutagenesis: Under the microscope. Front. Microbiol. 2020, 11, 585175. [Google Scholar] [CrossRef]
- Arzanlou, M.; Chai, W.C.; Venter, H. Intrinsic, adaptive and acquired antimicrobial resistance in Gram-negative bacteria. Essays Biochem. 2017, 61, 49–59. [Google Scholar] [PubMed]
- Belay, W.Y.; Getachew, M.; Tegegne, B.A.; Teffera, Z.H.; Dagne, A.; Zeleke, T.K.; Abebe, R.B.; Gedif, A.A.; Fenta, A.; Yirdaw, G. Mechanism of antibacterial resistance, strategies and next-generation antimicrobials to contain antimicrobial resistance: A review. Front. Pharmacol. 2024, 15, 1444781. [Google Scholar] [CrossRef] [PubMed]
- Cag, Y.; Caskurlu, H.; Fan, Y.; Cao, B.; Vahaboglu, H. Resistance mechanisms. Ann. Transl. Med. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. Insights on the antimicrobial resistance mechanisms of bacteria. Adv. Clin. Med. Microbiol. 2016, 2. [Google Scholar]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482. [Google Scholar] [CrossRef]
- Reynolds, D.; Burnham, J.P.; Guillamet, C.V.; McCabe, M.; Yuenger, V.; Betthauser, K.; Micek, S.T.; Kollef, M.H. The threat of multidrug-resistant/extensively drug-resistant Gram-negative respiratory infections: Another pandemic. Eur. Respir. Rev. 2022, 31. [Google Scholar] [CrossRef]
- Fernández, L.; Hancock, R.E. Adaptive and mutational resistance: Role of porins and efflux pumps in drug resistance. Clin. Microbiol. Rev. 2012, 25, 661–681. [Google Scholar] [CrossRef]
- Choi, U.; Lee, C.-R. Distinct roles of outer membrane porins in antibiotic resistance and membrane integrity in Escherichia coli. Front. Microbiol. 2019, 10, 953. [Google Scholar] [CrossRef]
- Wright, G.D. Molecular mechanisms of antibiotic resistance. Chem. Commun. 2011, 47, 4055–4061. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, M.M.; Varela, M.F. Modulation of bacterial multidrug resistance efflux pumps of the major facilitator superfamily. Int. J. Bacteriol. 2013, 2013, 204141. [Google Scholar] [CrossRef]
- Schultsz, C.; Geerlings, S. Plasmid-mediated resistance in Enterobacteriaceae: Changing landscape and implications for therapy. Drugs 2012, 72, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C.; Jacoby, G.A. Mechanisms of drug resistance: Quinolone resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 12–31. [Google Scholar] [CrossRef] [PubMed]
- Nas, F. Mechanisms of Bacterial Antibiotics Resistance: A Review. J. Adv. Microbiol. 2017, 7, 1–6. [Google Scholar] [CrossRef]
- Bush, K.; Jacoby, G.A. Updated functional classification of β-lactamases. Antimicrob. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Tolmasky, M.E. Aminoglycoside modifying enzymes. Drug Resist. Updates 2010, 13, 151–171. [Google Scholar] [CrossRef]
- Ray, S.; Das, S.; Suar, M. Molecular mechanism of drug resistance. In Drug Resistance in Bacteria, Fungi, Malaria, and Cancer; Springer: Berlin/Heidelberg, Germany, 2017; pp. 47–110. [Google Scholar]
- Nikaido, H.; Pagès, J.-M. Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol. Rev. 2012, 36, 340–363. [Google Scholar] [CrossRef]
- Anes, J.; McCusker, M.P.; Fanning, S.; Martins, M. The ins and outs of RND efflux pumps in Escherichia coli. Front. Microbiol. 2015, 6, 587. [Google Scholar] [CrossRef]
- Alvarez-Ortega, C.; Olivares, J.; Martínez, J.L. RND multidrug efflux pumps: What are they good for? Front. Microbiol. 2013, 4, 7. [Google Scholar] [CrossRef]
- Abdi, S.N.; Ghotaslou, R.; Ganbarov, K.; Mobed, A.; Tanomand, A.; Yousefi, M.; Asgharzadeh, M.; Kafil, H.S. Acinetobacter baumannii efflux pumps and antibiotic resistance. Infect. Drug Resist. 2020, 13, 423–434. [Google Scholar] [CrossRef]
- Swick, M.C.; Morgan-Linnell, S.K.; Carlson, K.M.; Zechiedrich, L. Expression of multidrug efflux pump genes acrAB-tolC, mdfA, and norE in Escherichia coli clinical isolates as a function of fluoroquinolone and multidrug resistance. Antimicrob. Agents Chemother. 2011, 55, 921–924. [Google Scholar] [CrossRef]
- De Gaetano, G.V.; Lentini, G.; Famà, A.; Coppolino, F.; Beninati, C. Antimicrobial resistance: Two-component regulatory systems and multidrug efflux pumps. Antibiotics 2023, 12, 965. [Google Scholar] [CrossRef]
- Zack, K.M.; Sorenson, T.; Joshi, S.G. Types and Mechanisms of Efflux Pump Systems and the Potential of Efflux Pump Inhibitors in the Restoration of Antimicrobial Susceptibility, with a Special Reference to Acinetobacter baumannii. Pathogens 2024, 13, 197. [Google Scholar] [CrossRef] [PubMed]
- Kornelsen, V.; Kumar, A. Update on multidrug resistance efflux pumps in Acinetobacter spp. Antimicrob. Agents Chemother. 2021, 65, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Pasqua, M.; Grossi, M.; Zennaro, A.; Fanelli, G.; Micheli, G.; Barras, F.; Colonna, B.; Prosseda, G. The varied role of efflux pumps of the MFS family in the interplay of bacteria with animal and plant cells. Microorganisms 2019, 7, 285. [Google Scholar] [CrossRef]
- Gauba, A.; Rahman, K.M. Evaluation of antibiotic resistance mechanisms in gram-negative bacteria. Antibiotics 2023, 12, 1590. [Google Scholar] [CrossRef] [PubMed]
- Coura, F.M.; Diniz, A.N.; Oliveira, C.A.; Lage, A.P.; Lobato, F.C.F.; Heinemann, M.B.; Silva, R.O.S. Detection of virulence genes and the phylogenetic groups of Escherichia coli isolated from dogs in Brazil. Ciência Rural. 2018, 48, e20170478. [Google Scholar] [CrossRef]
- Ranjbar, R.; Nazari, S.; Farahani, O. Phylogenetic analysis and antimicrobial resistance profiles of Escherichia coli strains isolated from UTI-suspected patients. Iran. J. Public Health 2020, 49, 1743. [Google Scholar] [CrossRef]
- Nowrouzian, F.L.; Clermont, O.; Edin, M.; Östblom, A.; Denamur, E.; Wold, A.E.; Adlerberth, I. Escherichia coli B2 phylogenetic subgroups in the infant gut microbiota: Predominance of uropathogenic lineages in Swedish infants and enteropathogenic lineages in Pakistani infants. Appl. Environ. Microbiol. 2019, 85, e01681-19. [Google Scholar] [CrossRef]
- Chakraborty, A.; Saralaya, V.; Adhikari, P.; Shenoy, S.; Baliga, S.; Hegde, A. Characterization of Escherichia coli phylogenetic groups associated with extraintestinal infections in South Indian population. Ann. Med. Health Sci. Res. 2015, 5, 241–246. [Google Scholar]
- Baponi, S.; Taravati, A.; Dilmagani, M. Determination of Phylogenetic Groups of Escherichia coli Isolated from Human Urine in Urmia City; Crescent Journal of Medical and Biological Sciences: Encino, CA, USA, 2016. [Google Scholar]
- Halaji, M.; Fayyazi, A.; Rajabnia, M.; Zare, D.; Pournajaf, A.; Ranjbar, R. Phylogenetic group distribution of uropathogenic Escherichia coli and related antimicrobial resistance pattern: A meta-analysis and systematic review. Front. Cell. Infect. Microbiol. 2022, 12, 790184. [Google Scholar] [CrossRef]
- Hazen, T.H.; Sahl, J.W.; Fraser, C.M.; Donnenberg, M.S.; Scheutz, F.; Rasko, D.A. Refining the pathovar paradigm via phylogenomics of the attaching and effacing Escherichia coli. Proc. Natl. Acad. Sci. USA 2013, 110, 12810–12815. [Google Scholar] [CrossRef] [PubMed]
- Hazen, T.H.; Leonard, S.R.; Lampel, K.A.; Lacher, D.W.; Maurelli, A.T.; Rasko, D.A. Investigating the relatedness of enteroinvasive Escherichia coli to other E. coli and Shigella isolates by using comparative genomics. Infect. Immun. 2016, 84, 2362–2371. [Google Scholar] [CrossRef] [PubMed]
- Ingle, D.J.; Tauschek, M.; Edwards, D.J.; Hocking, D.M.; Pickard, D.J.; Azzopardi, K.I.; Amarasena, T.; Bennett-Wood, V.; Pearson, J.S.; Tamboura, B. Evolution of atypical enteropathogenic E. coli by repeated acquisition of LEE pathogenicity island variants. Nat. Microbiol. 2016, 1, 15010. [Google Scholar] [CrossRef]
- Denamur, E.; Clermont, O.; Bonacorsi, S.; Gordon, D. The population genetics of pathogenic Escherichia coli. Nat. Rev. Microbiol. 2021, 19, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Rasko, D.A.; Rosovitz, M.; Myers, G.S.; Mongodin, E.F.; Fricke, W.F.; Gajer, P.; Crabtree, J.; Sebaihia, M.; Thomson, N.R.; Chaudhuri, R. The pangenome structure of Escherichia coli: Comparative genomic analysis of E. coli commensal and pathogenic isolates. J. Bacteriol. 2008, 190, 6881–6893. [Google Scholar] [CrossRef]
- Yu, D.; Banting, G.; Neumann, N.F. A review of the taxonomy, genetics, and biology of the genus Escherichia and the type species Escherichia coli. Can. J. Microbiol. 2021, 67, 553–571. [Google Scholar] [CrossRef]
- Chaudhuri, R.R.; Henderson, I.R. The evolution of the Escherichia coli phylogeny. Infect. Genet. Evol. 2012, 12, 214–226. [Google Scholar] [CrossRef]
- Marshall, B.M.; Levy, S.B. Food animals and antimicrobials: Impacts on human health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277. [Google Scholar]
- Rather, I.A.; Kim, B.-C.; Bajpai, V.K.; Park, Y.-H. Self-medication and antibiotic resistance: Crisis, current challenges, and prevention. Saudi J. Biol. Sci. 2017, 24, 808–812. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Khan, A.U. Global economic impact of antibiotic resistance: A review. J. Glob. Antimicrob. Resist. 2019, 19, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Piddock, L.J. The crisis of no new antibiotics—What is the way forward? Lancet Infect. Dis. 2012, 12, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.G.; Gilbert, D.N.; Spellberg, B. Seven ways to preserve the miracle of antibiotics. Clin. Infect. Dis. 2013, 56, 1445–1450. [Google Scholar] [CrossRef]
- Touchon, M.; Perrin, A.; De Sousa, J.A.M.; Vangchhia, B.; Burn, S.; O’Brien, C.L.; Denamur, E.; Gordon, D.; Rocha, E.P. Phylogenetic background and habitat drive the genetic diversification of Escherichia coli. PLoS Genet. 2020, 16, e1008866. [Google Scholar] [CrossRef]
- da Silva, G.J.; Mendonça, N. Association between antimicrobial resistance and virulence in Escherichia coli. Virulence 2012, 3, 18–28. [Google Scholar] [CrossRef]
| Antimicrobial Agents | Mechanisms of Resistance | Genetic Basis |
|---|---|---|
| β-lactams | β-lactamases inactivate drugs | ampC |
| Penicillins | Active efflux | bla genes-plasmid |
| Cephalosporins | (TEM, SHV, CTX-M, NDM) | |
| Monobactams | acrAB(tolC), acrAD(tolC) | |
| Carbapenems | ||
| Aminoglycosides | Aminoglycoside-modifying enzymes | aac, ant, aph-plasmid |
| Amikacin | Modify target-16S rRNA | amrA, rmtB |
| Gentamicin | Active efflux | mdtEF(tolC) |
| Tobramycin | ||
| Tetracyclines | Limiting uptake | ompF |
| Tetracyclines | Active efflux | acrAB(tolC) |
| tetA, tetB-plasmid | ||
| Chloramphenicol | Limiting uptake | ompF |
| Active efflux | acrAB(tolC) | |
| Fluoroquinolones | Limiting uptake | ompF |
| Ciprofloxacin | Modified target-gyrase | gyrA |
| Norfloxacin | Modified target-topoisomerase IV | parC |
| Active efflux | acrAB(tolC), acrEF(tolC) | |
| mdtABC(tolC) | ||
| Metabolic pathway inhibitors | Target enzyme modification | TMP-dhfr |
| Trimethoprim/Sulfamethoxazole | SXT-dhps |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naidoo, N.; Zishiri, O.T. Presence, Pathogenicity, Antibiotic Resistance, and Virulence Factors of Escherichia coli: A Review. Bacteria 2025, 4, 16. https://doi.org/10.3390/bacteria4010016
Naidoo N, Zishiri OT. Presence, Pathogenicity, Antibiotic Resistance, and Virulence Factors of Escherichia coli: A Review. Bacteria. 2025; 4(1):16. https://doi.org/10.3390/bacteria4010016
Chicago/Turabian StyleNaidoo, Natalie, and Oliver T. Zishiri. 2025. "Presence, Pathogenicity, Antibiotic Resistance, and Virulence Factors of Escherichia coli: A Review" Bacteria 4, no. 1: 16. https://doi.org/10.3390/bacteria4010016
APA StyleNaidoo, N., & Zishiri, O. T. (2025). Presence, Pathogenicity, Antibiotic Resistance, and Virulence Factors of Escherichia coli: A Review. Bacteria, 4(1), 16. https://doi.org/10.3390/bacteria4010016






