Highlights
- Microbes degrade dyes through enzymatic reactions, breaking down complex dye molecules into simpler, non-toxic substances.
- Degradation of dyes through exposure to light is often enhanced by catalysts.
- Nanoparticles enhance dye degradation through catalytic activity, often involving advanced oxidation processes (AOPs).
Abstract
Textile dyes pose a major environmental threat due to their toxicity, persistence in water bodies, and resistance to conventional wastewater treatment. To address this, researchers have explored biological and physicochemical degradation methods, focusing on microbial, photolytic, and nanoparticle-mediated approaches, among others. Microbial degradation depends on fungi, bacteria, yeasts, and algae, utilizing enzymatic pathways involving oxidoreductases like laccases, peroxidases, and azoreductases to breakdown or modify complex dye molecules. Photolytic degradation employs hydroxyl radical generation and electron-hole pair formation, while nanoparticle-mediated degradation utilizes titanium dioxide (TiO2), zinc oxide (ZnO), and silver (Ag) nanoparticles to enhance dye removal. To improve efficiency, microbial consortia have been developed to enhance decolorization and mineralization, offering a cost-effective and eco-friendly alternative to physicochemical methods. Photocatalytic degradation, particularly using TiO2, harnesses light energy for dye breakdown. Research advancements focus on shifting TiO2 activation from UV to visible light through doping and composite materials, while optimizing surface area and mesoporosity for better adsorption. Nanoparticle-mediated approaches benefit from a high surface area and rapid adsorption, with ongoing improvements in synthesis, functionalization, and reusability, particularly through magnetic nanoparticle integration. These emerging technologies provide sustainable solutions for dye degradation. The primary aim of this review is to comprehensively evaluate and synthesize current research and advancements in the degradation of azo dyes through microbial methods, photolytic processes, and nanotechnology-based approaches. The review also provides detailed information on salient mechanistic aspects of these methods, efficiencies, advantages, challenges, and potential applications in industrial and environmental contexts.
1. Introduction
Dyes are substances primarily used to impart color to various materials, including fabrics, plastics, paper, leather, and more. They have played a pivotal role in human history, dating back thousands of years when natural sources such as plants, minerals, and animal-derived substances were used to create vibrant hues [1,2]. Throughout civilizations, dyes have been crucial in art, trade, fashion, and cultural expression. Dyes can be classified into various categories based on their source, chemical structure, application method, and color-fastness. Natural dyes, extracted from plants, animals, or minerals, were the earliest forms of colorants used by ancient civilizations [3,4,5]. However, synthetic dyes, derived from chemical compounds, dominate modern dyeing processes due to their consistency, versatility, and cost-effectiveness [6]. The development of synthetic dyes in the 19th century marked a significant turning point, revolutionizing industries by providing a wider range of colors, increased stability, and ease of production compared to natural dyes [7]. William Henry Perkin made the unintentional discovery of the first synthetic dye to be successfully marketed in 1856. These dyes are colored compounds that, when added to fibers, impart a permanent color that will not fade when exposed to water, oxidizing agents, or microbiological attack [5].
Globally, over 0.7 million tons of synthetic dye are manufactured each year, and approximately 80% of all organic dyes comprise azo dyes. The expansion of the global textile sector in recent years has led to a proportional increase in the utilization of synthetic dyes [8]. This surge in dye usage has been linked to a rise in environmental pollution caused by wastewater contaminated with dye substances [9]. The amount of water used and wastewater produced by the textile industry, including dry processing mills and woven fabric finishing mills, varies based on the specific processing methods employed in transforming fibers into textile materials [10]. The textile industry is one of the biggest producers of pollutants in liquid effluent, because dyeing processes require large amounts of water. Majority of textile industries produce enormous amounts of wastewater that contain spent dyes that are very challenging to remove [11,12]. These dyes cause wastewaters to become colored, which lowers the amount of dissolved oxygen and causes oxygen sag in the receiving water bodies. Furthermore, the presence of some of these dyes in wastewaters has been linked to the development of tumors, cancers, and different allergies [13,14]. Thus, it is essential to develop low-cost and efficient methods of degrading these dyes. Various physicochemical approaches have been used to remove dye from wastewater discharge. However, these physical or chemical methods have inherent drawbacks that make them economically impractical [15,16]. Higher energy and chemical requirements, incomplete elimination of organic metabolites and resistant azo dyes, significant sludge generation leading to potential secondary pollution, and the need for labor-intensive procedures are among the drawbacks [17,18]. Microbial or enzymatic decolorization and degradation, on the other hand, provide an environmentally friendly and economically viable alternative to chemical breakdown processes. These methods have the potential to save water and provide a more sustainable solution [19]. Bioremediation, a crucial area of study in environmental sciences, involves utilizing microbial methods to address pollution [20,21]. In this process, microbes adapt to toxic waste, leading to the development of resilient strains that convert harmful chemicals into less harmful forms [22]. The breakdown of stubborn compounds within the microbial system relies on biotransformation enzymes. Enzymatic mechanisms like laccase, lignin peroxidase, hexane oxidase, NADH-DCIP reductase, aminopyrine N-demethylase, and tyrosinase have been reported to degrade complex organic substances [23].
A range of biotechnological methods, primarily employing bacteria and sometimes combined with physicochemical processes, has been explored to efficiently address azo dye pollution. Azo dyes, being resistant to biodegradation, can be effectively decolorized and degraded from textile effluent using microbial or enzymatic treatments. These methods offer numerous advantages, including being environmentally friendly and cost-competitive, generating less sludge, producing non-toxic or completely mineralized end products, and requiring lower water consumption compared to physicochemical methods [24]. The effectiveness of microbial decolorization depends on the adaptability and activity of selected microorganisms, leading to the testing of numerous species for dye decolorization and mineralization in recent studies [25]. Isolating potent species for degradation is an intriguing aspect of effluent treatment. Various microorganisms, such as bacteria, fungi, yeasts, actinomycetes, algae, and plants (utilized in phytoremediation), exhibit the ability to decolorize a wide array of dyes. Furthermore, under specific environmental conditions, these organisms can even completely mineralize numerous azo dyes [26]. Given the extensive use of synthetic dyes and their adverse environmental impacts, there is a critical need for sustainable and effective wastewater treatment solutions. While physicochemical methods remain widely used, their limitations necessitate the exploration of alternative strategies. Microbial and enzymatic bioremediation emerge as promising approaches that offer an eco-friendly and cost-effective solution for dye degradation. Further research into optimizing microbial strains and enhancing biotechnological applications will be essential in advancing sustainable wastewater management and mitigating the environmental hazards posed by dye effluents.
2. Microorganism-Mediated Dye
Utilizing microorganisms instead of traditional physicochemical methods for dye degradation offers significant environmental benefits, providing clean, green, cost-effective, and sustainable solutions for treating contaminated effluents without generating toxic byproducts or substantial sludge [27]. Microbial dye degradation can be categorized based on the types of microorganisms involved, including fungi, bacteria, algae, and microbial consortia. Each group exhibits distinct mechanisms and efficiency in dye degradation, offering potential solutions for bioremediation.
2.1. Dye Degradation by Fungi
Fungi play a crucial role in dye degradation, either as biosorbents or biodegraders, benefiting from their high biomass availability, cost-effectiveness, and strong mechanical stability across different pH conditions [28]. The rigid structure of macrofungi enhances biosorption properties, with adsorption capacity increasing at higher temperatures, though excessive temperatures may inhibit decolorization by deactivating adsorbent surfaces [29]. Species such as Cunninghamella elegans and Trametes versicolor have exhibited superior dye removal efficiency compared to activated carbon, while others like Aspergillus ochraceus, Phlebia, Pleurotus, and Bjerkandera adusta demonstrate significant biodegradation capabilities [30]. The enzymatic systems of fungi, particularly lignin peroxidase, manganese peroxidase, and laccase, play a critical role in breaking down complex dye structures. However, challenges such as long hydraulic retention times and the need to sustain fungal viability in bioreactors for complete degradation need to be addressed to improve their large-scale applicability [31].
Saccharomyces cerevisiae is capable of absorbing high dye concentrations, and a study showed that the dyes like basic green 4 and basic yellow 2 are discolored by the Saccharomyces cerevisiae up to 96% for the basic green and 93% for the basic yellow [32]. In another example, Candida tropicalis adsorbed 94% of Remazol blue and 44% of Reactive red, while Trichosporon akiyoshidainum achieved 63% adsorption of Reactive blue and 90% of Reactive red [33]. Yeast-mediated dye degradation is linked to their metabolism, requiring glucose as a carbon source, and triggering the production of oxidases, reductases, and NADH-DCIP reductase for azo bond reduction [34].
2.2. Dye Degradation by Algae
Algae are increasingly recognized as cost-effective and scalable solutions for dye effluent treatment. As photosynthetic organisms, they thrive in contaminated environments without inhibition from dyes, making them promising for bioremediation [35]. Both live and dead algal cells exhibit dye removal properties through biosorption and enzymatic degradation, with azoreductase facilitating the breakdown of azo dyes into less toxic aromatic amines. Different algal species degrade dyes at varying efficiencies based on dye structure and environmental conditions [36]. Given their renewable biomass potential and self-sustaining nature, algae present an eco-friendly alternative to conventional dye removal techniques.
2.3. Dye Degradation by Bacteria
Bacteria are widely employed in industrial wastewater treatment due to their rapid degradation rates and adaptability to diverse environmental conditions. Under anaerobic conditions, bacteria can transform azo dyes into aromatic amines, while certain aerobic bacteria utilize azoreductase enzymes to break azo bonds efficiently [37]. For example, Micrococcus spp. demonstrates the rapid decolorization of reactive dyes under anaerobic conditions, while aerobic conditions further enhance degradation rates. Other bacteria, including Pseudomonas and Geobacillus stearothermophilus, exhibit high mineralization efficiency, leading to the complete breakdown of dyes into non-toxic metabolites [38]. Additionally, lignolytic bacteria, known for their ability to degrade lignin, have emerged as potent agents for synthetic dye degradation. Species such as Pseudomonas, Bacillus, and Rhodococcus possess lignin peroxidase, manganese peroxidase, and laccase enzymes, enabling them to oxidize and fragment complex dye molecules. Their ability to operate under diverse environmental conditions and utilize low-cost substrates, such as agricultural and industrial waste, makes them attractive for large-scale wastewater treatment [31]. However, challenges such as heavy metal interference and optimization of conditions for maximum degradation efficiency remain significant hurdles [39].
2.4. Microbial Consortia and Genetic Engineering for Enhanced Dye Degradation
While single microbial strains often struggle to completely degrade complex dye molecules, microbial consortia exhibit superior performance due to their combined enzymatic activities. Mixed microbial populations can decolorize dyes more efficiently than pure cultures by targeting different dye structures simultaneously or utilizing metabolites produced by other strains [40]. For example, microbial consortia containing Yarrowia sp. SSA1642, Barnettozyma californica SSA1518, and Sterigmatomyces halophilus SSA1511 decolorized 99% of Red HE3B within 12 h, compared to over 60 h for single strains [41]. Fungal–bacterial cocultures further enhance dye degradation by exploiting oxidoreductive enzymes from both microbial types [37], For example, the coculturing of Dichotomomyces cejpii MRCH 1-2 plus Phoma tropica MRCH 1-3 exhibited the maximum decolorization of the dyes like methyl red and Reactive blue up to the concentration of 50 mg/L [42]. Genetic modification of microorganisms offers another promising approach to enhance dye degradation stability and efficiency. Engineered strains with enhanced azoreductase activity have demonstrated improved degradation rates, making them highly effective for bioremediation applications [43]. For example, recombinant Escherichia coli expressing azoreductase genes from Klebsiella pneumoniae has successfully degraded methyl orange dye. Similarly, genetically modified E. coli strains have shown enhanced efficiency in breaking down various synthetic dyes [44]. The potential of genetic engineering to enhance microbial resilience against inhibitory substances and optimize dye degradation pathways holds immense promise for the future of sustainable wastewater treatment.
Microorganism-mediated dye degradation presents a sustainable and efficient alternative to conventional physicochemical methods. Fungi, yeasts, algae, and bacteria each offer unique advantages in dye degradation (Table 1), while microbial consortia enhance decolorization efficiency through synergistic metabolic interactions [31]. The integration of genetic engineering techniques further strengthens microbial capabilities, enabling higher degradation rates and stability under industrial conditions. However, challenges such as optimizing microbial survival in bioreactors, overcoming inhibitory substances in wastewater, and scaling up these technologies require further research and innovation. Moving forward, interdisciplinary approaches combining microbiology, genetic engineering, and environmental engineering will be critical in advancing microbial bioremediation strategies for effective and sustainable dye pollution management [45].

Table 1.
Microbial decolorization and degradation of dyes.
3. Mechanism of Microbial Degradation
In an in vivo process, microorganisms typically produce enzymes that degrade azo dyes. However, for higher concentrations of azo dye degradation, isolated enzymes are preferred. Commercially available enzymes like azoreductase, laccase, peroxidase, and oxidase are used in industrial applications for this purpose, functioning both intracellularly and extracellularly [60]. These enzymes can be substrate-specific and can be immobilized on substrates to enhance degradation efficiency. Because enzymes are self-degradable, they do not pose environmental risks, and the byproducts of enzyme-mediated degradation can serve as media supplements for microbial culture growth [29]. Enzymes follow different degradation mechanisms, with some being substrate-specific while others not (Figure 1). The process often involves cofactors and redox-active coenzyme intermediates like FADH, NADPH, and NADH, which transfer electrons from the enzyme to the azo dye, weakening and breaking the azo bonds to produce colorless, odorless, and sometimes toxic intermediates like aromatic amines [61]. These intermediates can further degrade into smaller compounds such as water, carbon dioxide, and ammonia in aerobic conditions. Generally, azo dye degradation starts with the reduction of double bonds under anaerobic conditions, followed by aerobic processes for complete mineralization. Despite the formation of some auto-oxidized byproducts, enzymes like azoreductase and laccase are commonly used for the efficient degradation of azo dyes [62].
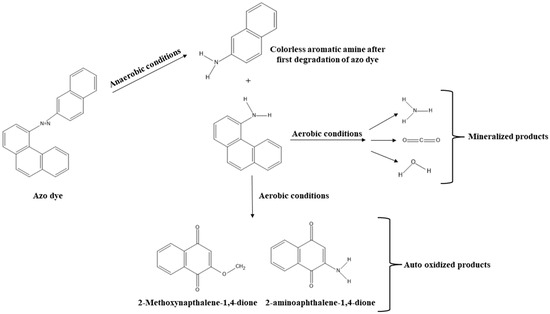
Figure 1.
The mechanisms of azo dye degradation into less toxic metabolites involve enzymatic or chemical processes that breakdown the dye molecules, reducing their toxicity.
Azoreductase enzymes, found in both the cytoplasm and membrane of microbial cells, play a crucial role in the reductive cleavage of azo dyes. These enzymes degrade dyes through redox-active intermediate-dependent pathways, requiring low-molecular-weight reducing equivalents such as flavin adenine dinucleotide (FADH) or nicotinamide adenine dinucleotide (NADH) as electron donors [63]. The electron transfer process leads to the cleavage of the azo bond (-N=N-), forming colorless but potentially toxic aromatic amines. Cytoplasmic azoreductases exhibit variable efficiency depending on the dye structure; they are less effective for sulfonated azo dyes due to their complex composition and poor diffusion properties but efficiently degrade water-soluble, non-sulfonated azo dyes under anaerobic conditions [61]. Conversely, membrane-bound azoreductases catalyze electron transfer directly to azo dyes using redox-active coenzymes, resulting in decolorization. The resulting aromatic amines, which are often cytotoxic and mutagenic, require further aerobic or microaerophilic oxidation to achieve complete mineralization into non-toxic end products [27].
Laccases, extracellular copper-containing oxidoreductases primarily secreted by fungi and certain plants, follow distinct oxidative degradation pathways. These enzymes catalyze the oxidation of phenolic and non-phenolic aromatic compounds through a nonspecific free radical mechanism, generating phenoxy radicals that undergo secondary oxidative transformations [64]. Unlike azoreductases, which rely on reductive cleavage, laccase-driven degradation occurs through oxidative polymerization, leading to the breakdown of complex dye structures into less toxic derivatives. While anthraquinone dyes can be directly oxidized by laccases, azo and indigo dyes require the presence of redox mediators such as ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) and anthraquinone dyes, which facilitate electron transfer between the enzyme and dye molecules [65]. The oxidation of azo dyes by laccases leads to the formation of phenolic and quinonoid intermediates rather than toxic aromatic amines, which subsequently undergo hydrolysis and further oxidation into simpler, environmentally benign compounds such as nitrophenols and nitrobenzene derivatives [66].
In addition to azoreductases and laccases, other key enzymatic systems involved in dye degradation include peroxidases, such as lignin peroxidase (LiP) and manganese peroxidase (MnP), which are predominantly secreted by white-rot fungi. These enzymes catalyze hydrogen peroxide-mediated oxidative cleavage of dye molecules, breaking down complex aromatic structures into smaller, biodegradable compounds [67]. LiP can cleave non-phenolic aromatic rings via one-electron oxidation, while MnP oxidizes phenolic dyes through manganese-dependent radical formation. These oxidative reactions often lead to the formation of carboxylated or hydroxylated intermediates, which can be further mineralized through microbial metabolic pathways [68]. The role of oxidoreductases such as NADH-DCIP reductase has gained increasing attention in microbial dye degradation. This enzyme facilitates the electron transfer necessary for azo bond cleavage under anaerobic conditions, allowing the efficient degradation of azo dyes in wastewater treatment systems [69]. The synergistic action of multiple enzymes, often within microbial consortia, enhances the degradation process by providing complementary pathways for dye breakdown, ultimately ensuring the complete mineralization of complex dye molecules into environmentally safe end products [70].
4. Photocatalytic Dye Degradation
Most studies on photocatalytic dye degradation (Table 2) have focused on using titanium dioxide (TiO2) as a photocatalyst. However, a major drawback of TiO2 is its absorption limited to the UV region due to its band gap of about 3.2 eV. Among the different phases of TiO2, the anatase form is the most commonly used because of its superior photon absorption characteristics [71]. The phase composition of TiO2 plays a crucial role in dye degradation. The anatase and rutile phases are the most studied, with anatase being preferred due to the triangular arrangement of oxygen ions on its surface, which allows the significant absorption of organic molecules [16]. This arrangement creates favorable reaction conditions for degrading absorbed organic pollutants, unlike the rutile phase. Pure anatase with a small proportion of the rutile phase is conducive to mesoporosity, enhancing dye adsorption [72].
In the context of photolytic degradation, most studies primarily focus on the anatase and rutile phases of TiO2 due to their established photocatalytic efficiency. However, recent advancements illustrated that faceted TiO2 exhibits superior photocatalytic activity compared to conventional Degussa P-25. The enhanced performance of faceted TiO2 can be attributed to its well-defined crystalline facets, which influence charge separation, surface reactivity, and adsorption properties, all of which are critical for efficient dye degradation [73]. Specific crystal facets of anatase TiO2, such as {001}, {101}, and {010}, demonstrate varied photocatalytic behaviors. The {001} facets, for instance, possess higher surface energy, leading to increased reactivity and superior degradation efficiency. These facets can generate a higher concentration of reactive oxygen species (ROS), such as hydroxyl radicals (•OH) and superoxide anions (O2•−), which actively participate in breaking down organic dye molecules [74]. Moreover, the interplay between different facets can further optimize charge carrier dynamics, reducing electron-hole recombination and enhancing overall photocatalytic performance. Studies suggest that tailored facet engineering of TiO2 can significantly improve light absorption and extend its photocatalytic response beyond the UV region. This can be particularly advantageous for wastewater treatment applications, where visible light-active photocatalysts are highly desirable [75]. Additionally, integrating faceted TiO2 with other semiconductors or noble metals can further enhance its activity by promoting charge transfer processes and increasing surface interactions with pollutants. Given these advantages, including faceted TiO2 in discussions of photocatalytic degradation would provide a more comprehensive understanding of the potential advancements in dye degradation technologies [73,75,76]. Exploring its structural modifications, facet-dependent reactivity, and synergistic coupling with other materials could lead to the development of more efficient and sustainable photocatalysts for environmental remediation.
4.1. Role of Total Organic Carbon (TOC) in Photodegradation
In most photocatalytic degradation studies, chemical oxygen demand (COD) is commonly used as a measure of organic pollutant degradation. COD provides an estimate of the total oxidizable organic and inorganic substances in a sample. However, it does not differentiate between partial degradation and complete mineralization of organic compounds. To obtain a more comprehensive evaluation of photodegradation efficiency, Total Organic Carbon (TOC) is increasingly being considered a more reliable parameter [77]. TOC directly quantifies the amount of carbon present in organic compounds, making it a better indicator of mineralization, as it tracks the conversion of organic pollutants into CO2 and H2O. Unlike COD, which can be influenced by the presence of inorganic reducing agents, TOC ensures that the degradation process is monitored based on the complete breakdown of organic structures. This distinction is crucial in assessing whether photodegradation leads to non-toxic end products, which is a key factor in environmental safety and wastewater treatment applications [13]. Additionally, TOC measurement allows researchers to differentiate between intermediate degradation products and complete mineralization. Many photocatalytic processes result in the formation of intermediate organic compounds, which may still pose environmental risks. By incorporating TOC analysis, studies can determine whether the degradation process effectively removes organic pollutants or merely transforms them into other potentially harmful byproducts [78]. Given its significance, future photocatalytic studies should integrate TOC analysis alongside COD measurements to provide a more accurate assessment of photodegradation efficiency. This approach would ensure a better understanding of degradation pathways, promote the development of more effective photocatalytic systems, and support sustainable wastewater treatment strategies [79].
4.2. Role of Semiconductors and Noble Metals in Photodegradation
While TiO2 remains one of the most widely studied photocatalysts for dye degradation, its high band gap (~3.2 eV for anatase) restricts its absorption primarily to the UV region, thereby limiting its overall efficiency under visible light [77]. To overcome this limitation and enhance photocatalytic activity, various modifications have been explored, including coupling TiO2 with other semiconductors and noble metals. These modifications improve charge carrier dynamics, increase visible light absorption, and enhance the overall degradation efficiency of organic dyes [80]. Pairing TiO2 with other semiconductors or metal oxides, sulfides or nitrides such as zinc oxide (ZnO), cadmium sulfide (CdS), tungsten trioxide (WO3), and graphitic carbon nitride (g-C3N4) extends its absorption range into the visible spectrum while simultaneously improving charge separation efficiency. The formation of heterojunctions between TiO2 and these semiconductors facilitates electron transfer, reducing electron-hole recombination and increasing the generation of reactive oxygen species (ROS), which play a crucial role in dye degradation [81]. For example, TiO2-ZnO nanocomposites exhibit improved charge transfer kinetics, whereas TiO2-CdS heterojunctions enhance visible light-driven degradation due to the lower band gap of CdS. Similarly, TiO2-g-C3N4 composites have gained attention for their ability to utilize a broader light spectrum, thereby improving overall photocatalytic performance [82].
Another effective strategy to enhance the photocatalytic properties of TiO2 involves the deposition of noble metals such as silver (Ag), platinum (Pt), and gold (Au) onto its surface. Noble metals act as electron sinks, preventing rapid electron-hole recombination and thereby extending the lifetime of charge carriers. This increased charge carrier availability facilitates more oxidation–reduction reactions, improving dye degradation efficiency [83]. Additionally, noble metal nanoparticles exhibit localized surface plasmon resonance (LSPR), which significantly enhances light absorption in the visible region. For example, Au- and Ag-modified TiO2 photocatalysts demonstrate superior photocatalytic performance under visible light due to plasmonic excitation effects, while platinum (Pt), with its excellent electron-trapping properties, further enhances charge separation, promoting efficient dye degradation even under low-intensity light sources [84]. By integrating TiO2 with both semiconductors and noble metals, researchers can achieve a synergistic effect that optimizes charge transfer, broadens light absorption, and enhances photocatalytic efficiency. These modifications not only accelerate degradation rates but also improve selectivity toward specific dye pollutants, making TiO2-based photocatalysts highly effective for wastewater treatment applications [85]. Future research should continue exploring these hybrid materials, focusing on optimizing compositions, improving stability, and enhancing the reusability of modified TiO2 photocatalysts. The integration of semiconductor heterojunctions and noble metal nanoparticles presents a promising direction for the development of advanced photocatalytic systems capable of achieving efficient and sustainable dye degradation [75].
4.3. Role of High-Intensity Irradiation in Photocatalytic Dye Degradation
A common assumption in photocatalytic dye degradation is that increasing light intensity leads to improved degradation efficiency. However, this effect is highly dependent on the photocatalytic material rather than just the intensity of irradiation [86]. While an optimal level of irradiation is necessary to generate sufficient electron-hole pairs in TiO2, excessively high-intensity irradiation can lead to undesirable effects such as rapid electron-hole recombination, saturation of active sites, and potential photo corrosion, which can negatively impact degradation efficiency [81]. The relationship between light intensity and photocatalytic performance follows a non-linear trend. At low intensities, the degradation rate increases as more electron-hole pairs are generated. However, beyond a threshold, the system reaches a saturation point where additional photon energy does not contribute significantly to the reaction. Additionally, in some cases, excessive irradiation may lead to the recombination of charge carriers, reducing the availability of free radicals for degradation. The impact of light intensity should therefore be considered in relation to the photocatalyst’s absorption properties, surface area, and structural modifications, rather than assuming a direct correlation with efficiency. A more comprehensive review of this topic would help clarify the role of irradiation intensity and guide the optimization of photocatalytic systems for practical applications [87].

Table 2.
Photocatalytic degradation of various dyes and their efficiency rates.
Table 2.
Photocatalytic degradation of various dyes and their efficiency rates.
| Dye | Degradation Efficiency (%) | Time | Reference |
|---|---|---|---|
| Methyl Blue | 92 | 1 h 20 min | [88] |
| Rhodamine B | 98.5 | 15 min | [89] |
| Crystal violet | 85 | 90 min | [90] |
| Basic fuchsin | 80 | 1 h 20 min | [91] |
| Reactive Black 5 | 75 | 90 min | [92] |
| Methyl orange | 85 | 30–90 min | [93] |
| Cango Red | 92.7 | 50 min | [94] |
| Methylene blue | 38 | 50 min | [94] |
| Reactive Black 5 | 66.7 | - | [95] |
| Acid Orange 7 | 94.36 | 120 min | [96] |
| Remazol blue | 93 | 90 min | [97] |
5. Mechanism of Photocatalytic Degradation of Dyes
In the dye sensitization mechanism, the process begins with the absorption of photons possessing energy equal to or greater than the material’s band gap. This causes the excitation of an electron from the valence band to the conduction band of a semiconductor metal oxide, resulting in the formation of an electron and a positive hole pair; for example, in the case of TiO2, the process initiates with the absorption of photons possessing energy equal to or greater than the band gap of TiO2, which excites an electron (e−) from the valence band (VB) to the conduction band (CB), creating a hole (h+) in the VB [98]. These charge carriers actively participate in oxidation and reduction reactions, leading to the formation of multiple radical species that drive the degradation process. Holes in the valence band oxidize water (H2O) or hydroxide ions (OH−), generating hydroxyl radicals (•OH), which are highly reactive and play a significant role in breaking down organic dye molecules. Concurrently, electrons in the conduction band reduce molecular oxygen (O2) to form superoxide anions (O2•−), which further participate in degradation reactions. These superoxide anions can interact with protons to form hydroperoxyl radicals (HO2•), adding another oxidative pathway to the degradation process [73,76,85]. Additionally, further interactions between radical species can lead to the formation of singlet oxygen (O2), which enhances the overall breakdown of organic contaminants. The synergistic action of these reactive oxygen species (ROS) enables the effective degradation of dye molecules, breaking them down into smaller, less harmful compounds and ultimately leading to the complete mineralization into CO2 and H2O (Figure 2). A comprehensive understanding of these mechanisms provides deeper insight into the photocatalytic process beyond just hydroxyl radicals, highlighting the roles of different radical species in TiO2-based dye degradation. This knowledge can refine experimental approaches and optimize the efficiency of photocatalytic systems, paving the way for more effective and sustainable applications in wastewater treatment [76,87].
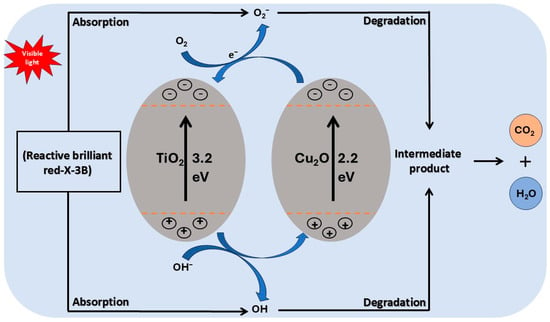
Figure 2.
The mechanisms of dye degradation by photocatalysts (Cu2O/TiO2) involve the absorption of light by the catalyst, which generates reactive species that breakdown dye molecules into less harmful substances.
6. Dye Degradation by Nanoparticles and Their Mechanism
Nanoparticles are highly effective in the reductive degradation of harmful and toxic dyes due to their unique physical and chemical properties, unlike bulk materials. These versatile nanoparticles find applications in wastewater treatment, medicine, energy generation, and bioremediation, as illustrated in Figure 3. They act as efficient catalysts in reductive reactions. Various nanoparticles such as silver (Ag), gold (Au), zinc (Zn), and silica have been synthesized from biological sources for use in multiple fields [99,100]. Silver nanoparticles (AgNPs) are widely used for their excellent antimicrobial and catalytic properties. AgNPs synthesized by Actinidia deliciosa fruit extract can completely degrade methylene blue dye in 33 min in the presence of a catalyst [101]. Similarly, AgNPs synthesized using Parkia roxburghii leaf extract achieve the complete degradation of methylene blue and rhodamine B in just 12 min [102]. Gold nanoparticles (AuNPs) are utilized in drug delivery, cancer treatment, and gene delivery. AuNPs synthesized by Paderia foetida Linn effectively degrade toxic dyes like methylene blue and rhodamine B within 12 min. Additionally, AuNPs mediated by Sterculia acuminata can degrade organic dyes such as methyl orange, methylene blue, and direct blue in just 4 min [103]. Zinc oxide nanoparticles (ZnO NPs) are extensively used due to their high surface area, non-toxicity, stable structure, photosensitivity, higher reactivity, and cost-effectiveness. ZnO NPs are effective photocatalysts for dye degradation [104]. For example, ZnO NPs synthesized using Artocarpus heterophyllus leaf extract completely degrade rose Bengal dye in 1 h. Similarly, biologically synthesized ZnO NPs from Aspergillus sp. effectively degrade methylene blue dye. Other metal nanoparticles such as nickel (Ni), silica (Si), molybdenum (Mb), titanium (Ti), and copper (Cu) are emerging due to their unique properties [105]. For instance, palladium (Pd) nanoparticles synthesized from Catharanthus roseus leaf extract efficiently degrade phenol red. Moreover, zirconium oxide nanoparticles synthesized using Lagerstroemia speciosa leaves can degrade methyl orange dye up to 94.5% [106]. In recent years, biological sources for dye degradation have become a viable alternative for removing dyes from polluted water and soil. Researchers have utilized engineered metal nanoparticles from various sources to achieve this as in Figure 3 [107]. The mechanism of dye degradation by different metal nanoparticles in the presence of light is depicted in Figure 3. Post-degradation, the presence of degraded products is confirmed using high-performance liquid chromatography–mass spectrometry (HPLC-MS) [108,109]. For instance, Fe–Ni bimetallic nanoparticles degrade Orange G into products such as naphthol amine and aniline derivatives. Additionally, HPLC-MS has detected unknown intermediate byproducts after degrading methyl red dye using immobilized TiO2 and ZnO nanoparticles. TiO2 nanoparticles have been found to degrade acid red 88, resulting in byproducts like 4-amino naphthalene sulfonic acid, naphthalene-2-ol, and others [110].
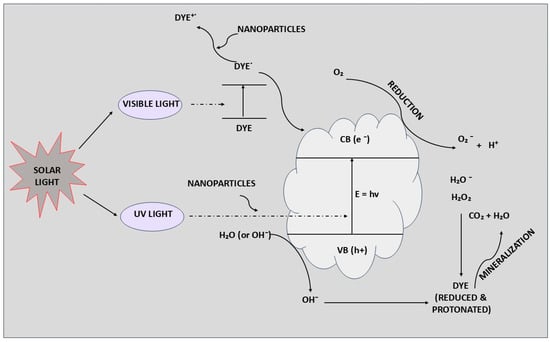
Figure 3.
The degradation mechanisms of dyes by metal nanoparticles involve catalytic processes where the nanoparticles facilitate the breakdown of dye molecules into less harmful substances through advanced oxidation reactions and other chemical interactions.
7. Advantages and Limitations of Microbial, Photolytic, and Nanotechnology-Based Dye Degradation
The increasing release of synthetic dyes into aquatic ecosystems presents with serious environmental threats due to their toxicity and potential carcinogenic and teratogenic effects. Various dye degradation methods, including microbial, photolytic, and nanotechnology-based approaches, have been developed, each offering distinct advantages and limitations (Table 3) [111]. Microbial degradation is an eco-friendly and cost-effective method that utilizes bacteria, fungi, and algae to breakdown complex dye molecules into non-toxic metabolites, with species like Pseudomonas aeruginosa and Aspergillus niger demonstrating high degradation efficiency. However, microbial degradation is often slow, highly dependent on environmental conditions, and may lead to incomplete mineralization or accumulation of toxic intermediates, limiting its large-scale application [112]. Photolytic degradation, particularly using catalysts like titanium dioxide (TiO2), is highly efficient, non-selective, and does not produce secondary pollutants, making it suitable for various dye structures. However, its dependency on UV light, high energy consumption, and catalyst deactivation pose significant challenges, requiring optimization for real-world applications [113]. Recent research has shown that visible light-driven photocatalysts such as BiVO4/Ag can improve efficiency, achieving over 90% degradation of Rhodamine B within 40 min. Nanotechnology-based dye degradation, utilizing nanomaterials like Fe3O4, silver nanoparticles, and carbon nanotubes, offers enhanced catalytic activity, rapid adsorption, and easy separation, making it a promising alternative. However, high production costs, environmental concerns related to nanoparticle toxicity, and aggregation issues hinder its large-scale adoption [78]. Studies have demonstrated that Fe3O4 nanoparticles functionalized with chitosan can achieve 99% removal of Reactive Black 5 dye, while Ag/ZnO nanocomposites have shown 94% degradation of Malachite Green. Given the limitations of individual methods, hybrid approaches integrating microbial, photolytic, and nanotechnology-based techniques present a promising solution for efficient dye removal. For example, microbial consortia can initiate dye breakdown, enhancing subsequent photocatalytic degradation, while nanoparticles improve adsorption and catalytic efficiency [95]. Recent advancements suggest that integrated bioreactor systems combining these methods can optimize dye degradation by ensuring sequential and synergistic interactions. Moreover, economic and environmental considerations must be addressed to scale up these technologies, with research focusing on cost-effective catalyst production, sustainability, and regulatory frameworks for safe nanoparticle use. Governments and environmental agencies can incentivize sustainable dye degradation through subsidies, strict discharge regulations, and collaboration with industries generating dye-laden wastewater, such as textiles and pharmaceuticals [63]. Furthermore, interdisciplinary research, education, and public awareness initiatives are crucial for promoting eco-friendly wastewater treatment technologies. The path to effective dye degradation lies in leveraging microbial, photolytic, and nanotechnology-based methods in an integrated manner, fostering sustainability, and optimizing technological advancements to mitigate dye pollution and protect aquatic ecosystems.

Table 3.
Comparative analysis of microbial, photolytic, and nanotechnology-based dye degradation: advantages, limitations, and emerging insights.
8. Impact of Azo Dyes on Health and Safety
A growing body of evidence highlights the grave urgency to prioritize health and safety measures concerning azo dye exposure, particularly in occupational and consumer settings. Beyond industrial hazards, the infiltration of azo dyes into food and water sources exacerbates public health risks (Table 4). The consumption of non-food-grade azo dyes in adulterated food products has been linked to gastrointestinal disorders, liver toxicity, and endocrine disruptions, disproportionately affecting vulnerable populations such as children and pregnant women [13,111,114]. Moreover, ingestion of azo dye-contaminated drinking water has been associated with oxidative stress, DNA damage, and an increased prevalence of neurological disorders, metabolic imbalances, and immune suppression [115]. Given these far-reaching health implications, global enforcement of stringent safety standards, occupational hazard reduction strategies, and interdisciplinary research collaborations is imperative. The transition towards non-toxic, biodegradable dyes, alongside advancements in remediation technologies, can significantly mitigate health hazards while fostering environmental sustainability [75]. Additionally, raising public awareness about the risks of azo dye exposure and encouraging safer manufacturing practices can further contribute to minimizing health and ecological threats [13]. A concerted effort involving policymakers, researchers, industries, and consumers will be crucial in addressing the long-term consequences of azo dye contamination and ensuring a safer, more sustainable future.

Table 4.
Health risks and safety considerations of different dye classes and their metabolic effects.
9. Emerging Technologies in Dye Degradation
The accumulation of dyes and dye-laden wastewater not only causes environmental pollution but also poses significant health and esthetic concerns. Studies have linked exposure to certain textile dyes with various health disorders. For example, benzidine-based dyes have been associated with bladder cancer due to their carcinogenic nature and metabolic conversion into harmful aromatic amines [131]. Similarly, Remazol Brilliant Blue and Disperse Blue 3 dyes have been shown to cause skin irritation and allergic dermatitis upon prolonged exposure [119,132]. Azo dyes, which constitute the largest class of synthetic dyes, are of particular concern because of their potential to form toxic aromatic amines upon degradation, leading to mutagenic and genotoxic effects [112]. For example, the dye Congo Red has been reported to be converted into benzidine, which is linked with cancer [133]. The presence of textile dyes in aquatic environments is due to their complex molecular structures, which make them resistant to conventional wastewater treatment methods. Many researchers have highlighted the inefficiency of single treatment approaches, particularly in meeting increasingly stringent environmental regulations [134]. For example, ref. [135] reported that, after a single treatment of Reactive Black 5 using coagulation–flocculation, nearly 96.2% of the dye remained undegraded, raising unaddressed concerns about its residual toxicity. Similarly, an investigation reported previously [136,137] demonstrated that traditional activated sludge processes were only able to remove 55% of methylene blue dye, necessitating additional treatment steps for complete degradation. Industrial wastewater often contains a complex mixture of dyes, heavy metals, and organic pollutants, making it impossible for a single treatment process to efficiently remove all contaminants. Research teams [138] have analyzed dye-laden water samples from textile effluent and found the presence of Reactive Blue 19, lead, and cadmium. The mixture of these contaminants was successfully removed (up to 95%) using a combinatorial approach involving adsorption, advanced oxidation processes (AOPs), and membrane filtration. Similarly, a study by [139] demonstrated that a combination of Fenton oxidation and microbial degradation effectively removed 93% of Congo Red dye along with associated heavy metals such as chromium and mercury. Recognizing the limitations of single-treatment strategies, researchers have shown considerable interest in integrated and hybrid processes for dye removal. It is essential to differentiate between these approaches; an integrated system sequentially applies multiple treatment methods, while a hybrid system combines two or more methods into a single treatment step.
Hybrid techniques are gaining traction because of enhanced efficiency and reduced costs and resource consumption [140]. For example, a hybrid system combining electrocoagulation with photocatalysis achieved a 92% reduction in dye concentration, significantly outperforming either method alone [141]. Several integrated approaches have also been successfully applied for textile wastewater treatment. For example, a study explored the combination of ozonation followed by biological treatment, achieving 97% decolorization of Reactive Orange 16 dye while reducing chemical oxygen demand (COD) by 85% [142]. In another study, researchers investigated a two-stage process involving adsorption on activated carbon followed by enzymatic degradation using laccase-producing bacteria, leading to a 98% removal of Malachite Green and crystal violet [143]. These findings highlight the effectiveness of integrating biological and physicochemical methods to enhance dye degradation. Hybrid approaches have also been extended to nanoparticle-based treatment systems. For example, TiO2-photocatalysis has been enhanced by doping with silver (Ag) and iron (Fe) nanoparticles to improve efficiency under visible light conditions [144]. Similarly, zinc oxide (ZnO)-based nanocomposites functionalized with graphene oxide have demonstrated superior performance in degrading anthraquinone dyes compared to traditional ZnO systems [145,146]. Additionally, the incorporation of magnetic nanoparticles in hybrid adsorption–photocatalysis processes has enabled easy separation and reusability, minimizing secondary waste generation [147]. Recent advancements have also explored the use of bioengineered microbial consortia for synergistic dye degradation. Studies have shown that mixed microbial cultures containing dye-degrading bacteria and fungi outperform single-species systems due to complementary enzymatic activities. For example, an engineered consortium of Pseudomonas aeruginosa and Aspergillus niger demonstrated a 99% degradation efficiency for Reactive Orange 16 within 24 h, compared to 75% when either species was used alone [148]. Similarly, genetic modifications in dye-degrading bacteria, such as Escherichia coli expressing laccase genes, have improved their ability to degrade a broader range of dyes [149,150]. Electrochemical methods are also gaining attention for dye degradation. Electro-Fenton oxidation, in combination with microbial treatment, has shown promising results in degrading recalcitrant dyes. In a study an electrochemical–Fenton hybrid process removed 96% of Reactive Yellow 145 within 90 min, significantly outperforming conventional Fenton treatment [151]. This approach highlights the potential of combining electrochemical and biological methods for enhanced dye degradation efficiency. The application of machine learning and artificial intelligence (AI) in optimizing dye degradation processes is an emerging trend. AI-driven models have been used to predict the optimal conditions for dye removal by integrating data from various physicochemical and biological treatment methods [131,140]. A study utilized machine learning algorithms to optimize a hybrid enzymatic–photocatalytic system, leading to a 20% improvement in dye removal efficiency compared to conventional optimization approaches. The complexity and persistence of textile dyes necessitate the adoption of integrated and hybrid treatment approaches. While microbial degradation, nanoparticle-based photocatalysis, and electrochemical methods have shown significant promise individually, their combination in hybrid systems offers a more robust and efficient solution for textile wastewater treatment. Future research should focus on further enhancing these systems through genetic engineering, advanced nanomaterials, and AI-driven process optimization to achieve sustainable and cost-effective dye degradation [103,152].
10. Future Outlook
The future of dye degradation using microorganisms is promising, driven by ongoing advancements in biotechnology, synthetic biology, and genetic engineering. Future research will likely focus on enhancing the capabilities of fungi, bacteria, yeasts, and algae to degrade a wider range of dyes more efficiently. Genetic modifications can increase the stability and activity of microbial enzymes involved in dye breakdown, making the process more robust under various environmental conditions [64]. For example, advances in CRISPR-based genome editing are allowing researchers to enhance the expression of laccases, peroxidases, and azoreductases in bacterial and fungal strains, significantly improving dye decolorization efficiency [153]. The development of engineered microbial consortia, where different species work synergistically, will also play a crucial role. These consortia can tackle complex dye molecules more effectively than single strains, offering higher decolorization and mineralization rates [63]. Moreover, integrating microbial systems with existing wastewater treatment infrastructure is crucial for ensuring large-scale practical applications. Recent studies have explored the immobilization of dye-degrading microbes on biofilm reactors, which not only improves their stability but also enhances degradation rates in continuous-flow treatment systems [76].
One of the major challenges in implementing microbial degradation at an industrial scale is process optimization for different environmental conditions. Future research will focus on adaptive evolution and synthetic biology approaches to develop robust microbial strains that can thrive in variable wastewater compositions, pH levels, and temperature fluctuations [61]. This includes the design of biosensors that allow the real-time monitoring of dye degradation and microbial activity, enabling the dynamic control of treatment [154]. Additionally, integrating microbial treatment with electrochemical bioreactors is emerging as a scalable and cost-effective alternative, reducing reliance on expensive catalysts and energy-intensive processes [60]. Advances in photocatalytic degradation are set to revolutionize dye treatment processes, particularly through the use of modified semiconductors like titanium dioxide (TiO2) [68]. Future research will focus on overcoming TiO2’s reliance on UV light by modifying its properties to enable visible light activation. This can be achieved by doping TiO2 with elements like nitrogen, sulfur, and graphene-based materials, which enhance its photocatalytic efficiency under natural sunlight [155]. The development of hybrid systems combining TiO2 with other semiconductors or catalysts will also be explored to improve degradation rates and reduce electron-hole recombination. Enhancing the surface area and mesoporosity of photocatalysts will further optimize dye adsorption and subsequent degradation [156]. Additionally, innovative reactor designs, such as fluidized bed reactors and light-enhanced membrane bioreactors, are being developed to improve photocatalytic treatment efficiency and scalability for large-scale wastewater applications [157]. The use of nanoparticles in dye degradation presents a promising future due to their unique properties, such as a high surface area, rapid adsorption, and ease of separation. Future research will focus on improving the synthesis and functionalization of nanoparticles to enhance their adsorption capacity and reusability [75]. Magnetic nanoparticles, in particular, will be developed further for their ability to be easily separated from treated water using an external magnetic field, minimizing waste and operational costs. Techniques like layer-by-layer (LbL) assembly and the creation of nanocomposite membranes will expand the applications of nanomaterials in dye degradation [158]. Additionally, the integration of nanoparticles with advanced oxidation processes (AOPs) will enhance the generation of reactive species, leading to more efficient degradation of complex dye molecules and other micropollutants [159]. Despite these technological advancements, the scalability and economic feasibility of dye degradation techniques remain key challenges [160]. One of the major barriers is the high cost associated with material synthesis, particularly in the production of advanced catalysts, nanoparticles, and engineered microbes [60]. To address this, research is shifting towards using low-cost, bio-derived materials, such as agricultural waste, biochar, and biopolymer-based nanocomposites, as sustainable alternatives for dye adsorption and catalytic degradation [113]. For example, lignin-derived nanoparticles and cellulose-based hydrogels have been shown to achieve comparable dye removal efficiencies to synthetic polymer membranes, offering an eco-friendly and cost-effective solution [161].
Moreover, reducing energy consumption in dye degradation is another crucial research focus. Traditional AOPs and photocatalytic processes often require high-energy UV irradiation or chemical oxidants, which increase operational costs. Future studies will explore energy-efficient strategies such as solar-driven photocatalysis, bioelectrochemical degradation, and microbial fuel cells, which can generate electricity while simultaneously breaking down dyes in wastewater [61]. In a study by [60], a microbial–electrochemical system coupled with a biofilm reactor achieved 90% dye degradation while producing bioelectricity, demonstrating the potential for energy self-sufficiency in wastewater treatment. Another approach to cost reduction involves resource recovery from dye-laden wastewater [20]. Emerging research is investigating ways to extract valuable byproducts, such as bioactive pigments, organic acids, and heavy metals, from treated wastewater streams. For example, the enzymatic degradation of azo dyes can yield aromatic amines that serve as precursors for pharmaceutical and dye industries [162]. Similarly, algae-based treatment systems can simultaneously remove dyes and generate biomass for biofuel production, providing an economic incentive for industries to adopt biological remediation strategies [163].
The integration of microbial, photocatalytic, and nanoparticle-based dye degradation methods offers a holistic approach to addressing wastewater treatment challenges. Future research will focus on combining these methods to leverage their individual strengths and achieve more comprehensive and efficient dye degradation. This interdisciplinary approach will involve optimizing process parameters, improving material stability, and ensuring environmental safety [113]. Collaborative efforts between researchers, industry, and regulatory bodies will be essential to scale these technologies for practical applications, ensuring they meet environmental standards and contribute to sustainable wastewater management. Advances in these areas will not only improve the effectiveness of dye degradation but also pave the way for innovative solutions in environmental remediation and pollution control [161]. Developing a combinatorial method for dye degradation involves challenges like scalability and sustainability of the process. These concerns have been addressed by different research groups through pilot-scale studies and techno-economic analyses. For example, a study by [164] demonstrated that a hybrid bioelectrochemical–oxidation system reduced dye treatment costs by 30% while achieving over 95% degradation efficiency at an industrial scale. Similarly, Zeng and his team [165] reported that co-fermentation strategies in microbial dye degradation can enhance the process yield while minimizing costs associated with enzyme production and nutrient supplementation. These findings emphasize the importance of techno-economic assessments in translating laboratory-scale innovations into commercially viable wastewater treatment solutions. Future research directions in dye degradation must prioritize emerging technologies that enhance efficiency, scalability, and economic feasibility. Advances in genetic engineering, nanomaterial synthesis, and hybrid treatment approaches offer significant potential for improving dye removal. However, addressing cost constraints through the development of low-cost materials, energy-efficient processes, and resource recovery strategies will be critical for large-scale implementation. By fostering interdisciplinary collaborations and integrating sustainable practices, researchers can pave the way for innovative, cost-effective, and environmentally friendly dye degradation technologies.
11. Conclusions
Integrating these diverse strategies demands a comprehensive understanding of their individual mechanisms and potential interactions. Combining microbial and enzymatic treatments with photocatalytic and nanoparticle approaches can lead to hybrid systems that maximize the strengths of each method while compensating for their weaknesses [75]. For instance, microbial consortia can initiate the breakdown of complex dye molecules, making them more willing to subsequent photocatalytic degradation. Similarly, nanoparticles can enhance the efficiency of microbial degradation by providing surfaces that support biofilm formation and increase microbial activity [138]. One promising area of research is the development of integrated bioreactor systems that combine multiple treatment processes in a single unit. These systems can be designed to sequentially apply microbial, photocatalytic, and nanoparticle-based treatments, ensuring comprehensive degradation of dyes [61]. Continuous monitoring and optimization of these bioreactors, aided by advancements in sensor technology and data analytics, can further enhance their performance and adaptability to varying wastewater compositions.
Moreover, the implementation of these advanced treatment technologies requires the careful consideration of economic and logistical factors. Cost-effective production and scalability of materials, such as photocatalysts and nanoparticles, are essential for widespread adoption [11]. Researchers must also address the potential environmental impacts of these materials, ensuring that they do not introduce new pollutants or hazards into the ecosystem [166]. Public policy and regulatory frameworks will play a pivotal role in promoting the adoption of sustainable dye degradation technologies. Governments and environmental agencies can incentivize the use of eco-friendly treatment methods through subsidies, grants, and stricter discharge regulations [75]. Collaboration with industries that produce dye-laden wastewater, such as textiles and pharmaceuticals, is crucial to develop tailored solutions that meet specific needs and operational constraints. Education and outreach efforts are equally important in fostering a culture of sustainability. Raising awareness about the environmental impacts of synthetic dyes and the benefits of advanced treatment technologies can drive consumer demand for more sustainable products and practices. Academic institutions, industry stakeholders, and environmental organizations should work together to develop training programs and resources that equip professionals with the knowledge and skills needed to implement and innovate in this field [161]. The path to effective dye degradation lies in a multifaceted approach that leverages the strengths of microbial, photocatalytic, and nanoparticle-based methods. By fostering interdisciplinary collaboration, optimizing technological processes, and promoting sustainable practices, we can address the environmental challenges posed by dye pollution and pave the way for a cleaner, more sustainable future [8].
Author Contributions
Conceptualization, A.A. and A.K.W.; methodology, C.C.; software, D.K.S.; validation, A.A., O.A. and A.W.W.; formal analysis, A.S.; investigation, S.M.; resources, R.S.; data curation, A.K.W. and R.S.; supervision, A.K.W.; project administration, R.S.; funding acquisition, S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, Z.; Feng, M.; Wang, Y.; Ling, X. Comparison of Treatment Performance and Microbial Community Evolution of Typical Dye Wastewater by Different Combined Processes. Ecotoxicol. Environ. Saf. 2024, 275, 116226. [Google Scholar] [CrossRef] [PubMed]
- Sonu, K.; Sogani, M.; Syed, Z.; Rajvanshi, J. Improved Degradation of Dye Wastewater and Enhanced Power Output in Microbial Fuel Cells with Chemically Treated Corncob Anodes. Biomass Convers. Biorefinery 2024, 14, 375–386. [Google Scholar] [CrossRef]
- George, G.; Ealias, A.M.; Saravanakumar, M.P. Advancements in Textile Dye Removal: A Critical Review of Layered Double Hydroxides and Clay Minerals as Efficient Adsorbents. Environ. Sci. Pollut. Res. 2024, 31, 12748–12779. [Google Scholar] [CrossRef] [PubMed]
- Hosseinnezhad, M.; Safapour, S. Sources, Chemistry, Classification, Challenges, and Prospects of Renewable Dyes and Pigments. Renew. Dye. Pigment. 2024, 1, 1–18. [Google Scholar]
- Jabar, J.M. Classification of Natural Dyes for Sustainable Exploitation. In Natural Dyes and Sustainability; Springer: Cham, Switzerland, 2024; Volume 1, pp. 153–191. [Google Scholar]
- Ikram, Z.; Azmat, E.; Perviaz, M. Degradation Efficiency of Organic Dyes on CQDs as Photocatalysts: A Review. ACS Omega 2024, 9, 10017–10029. [Google Scholar] [CrossRef]
- Tamburini, D. On the Reliability of Historic Books as Sources of Reference Samples of Early Synthetic Dyes–The Case of “The Coal Tar Colours of the Farbwerke Vorm. Meister, Lucius & Brüning, Höchst on the Main, Germany–A General Part”(1896). Dye. Pigment. 2024, 221, 111796. [Google Scholar]
- Sen, N.; Badiwal, A.; Singh, K.K.; Mukhopadhyay, S.; Shenoy, K.T. Optimization of Bromocresol Green Degradation Using Ozone Micro Bubbles: Response Surface Analysis and Techno-Commercial Aspects of a 75 kL/Day Scale-up Plant. Discov. Environ. 2024, 2, 48. [Google Scholar] [CrossRef]
- Gupta, S. A Review Study on Sustainable Dyes and Their Usage in the Fashion Industry. Int. J. Res. Anal. Rev. (IJRAR) 2024, 11, 63–72. [Google Scholar]
- Uğan, M.; Onac, C.; Kaya, A.; Köseoğlu, D.; Akdoğan, A. Removal of Reactive Red 195 Dye from Textile Industry Wastewater with Deep Eutectic Solvent-Based Green Extraction. J. Mol. Liq. 2024, 398, 124249. [Google Scholar] [CrossRef]
- Estévez, S.; Angelucci, D.M.; Moreira, M.T.; Tomei, M.C. Techno-Environmental and Economic Assessment of Color Removal Strategies from Textile Wastewater. Sci. Total Environ. 2024, 913, 169721. [Google Scholar] [CrossRef]
- Luo, X.; Jiang, L.; Zhao, R.; Wang, Y.; Xiao, X.; Ghazouani, S.; Yu, L.; Mai, Z.; Matsuyama, H.; Jin, P. Energy-Efficient Trehalose-Based Polyester Nanofiltration Membranes for Zero-Discharge Textile Wastewater Treatment. J. Hazard. Mater. 2024, 465, 133059. [Google Scholar] [CrossRef] [PubMed]
- Haridevamuthu, B.; Murugan, R.; Seenivasan, B.; Meenatchi, R.; Pachaiappan, R.; Almutairi, B.O.; Arokiyaraj, S.; Arockiaraj, J. Synthetic Azo-Dye, Tartrazine Induces Neurodevelopmental Toxicity via Mitochondria-Mediated Apoptosis in Zebrafish Embryos. J. Hazard. Mater. 2024, 461, 132524. [Google Scholar] [CrossRef] [PubMed]
- Kolya, H.; Kang, C.-W. Toxicity of Metal Oxides, Dyes, and Dissolved Organic Matter in Water: Implications for the Environment and Human Health. Toxics 2024, 12, 111. [Google Scholar] [CrossRef]
- Samianifard, S.M.; Kalaee, M.; Moradi, O.; Mahmoodi, N.M.; Zaarei, D. Novel Biocomposite (Starch/Metal–Organic Framework/Graphene Oxide): Synthesis, Characterization and Visible Light Assisted Dye Degradation. J. Photochem. Photobiol. A Chem. 2024, 450, 115417. [Google Scholar] [CrossRef]
- Swarna, S.; Govindarajan, V.U.; Anbalagan, A.; Christopher, D.; Muthuraman, M.S. Green Synthesis of Copper Oxide Nanoparticles Using Ziziphus Oenoplia Extract and Its Dye Degradation Properties. Biomass Convers. Biorefinery 2024, 1–12. [Google Scholar] [CrossRef]
- Ellafi, A.; Dali, A.; Mnif, S.; Ben Younes, S. Microbial Enzymatic Degradation, Spectral Analysis and Phytotoxicity Assessment of Congo Red Removal by Bacillus spp. Catal. Lett. 2023, 153, 3620–3633. [Google Scholar] [CrossRef]
- Pham, V.H.T.; Kim, J.; Chang, S.; Bang, D. Investigating Bio-Inspired Degradation of Toxic Dyes Using Potential Multi-Enzyme Producing Extremophiles. Microorganisms 2023, 11, 1273. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Vo, D.-V.N.; Jeevanantham, S.; Karishma, S.; Yaashikaa, P. A Review on Catalytic-Enzyme Degradation of Toxic Environmental Pollutants: Microbial Enzymes. J. Hazard. Mater. 2021, 419, 126451. [Google Scholar] [CrossRef]
- Wani, A.K.; Rahayu, F.; Ben Amor, I.; Quadir, M.; Murianingrum, M.; Parnidi, P.; Ayub, A.; Supriyadi, S.; Sakiroh, S.; Saefudin, S. Environmental Resilience through Artificial Intelligence: Innovations in Monitoring and Management. Environ. Sci. Pollut. Res. 2024, 31, 18379–18395. [Google Scholar] [CrossRef]
- Wani, A.K.; Chopra, C.; Singh, R.; Ahmad, S.; Américo-Pinheiro, J.H.P. Mining Microbial Tapestry Using High-Throughput Sequencing and In Silico Analysis of Trehalose Synthase (TreS) Derived from Hot Spring Metagenome. Biocatal. Agric. Biotechnol. 2023, 52, 102829. [Google Scholar] [CrossRef]
- Kour, D.; Khan, S.S.; Kour, H.; Kaur, T.; Devi, R.; Rai, P.K.; Judy, C.; McQuestion, C.; Bianchi, A.; Spells, S. Microbe-Mediated Bioremediation: Current Research and Future Challenges. J. Appl. Biol. Biotechnol. 2022, 10, 6–24. [Google Scholar] [CrossRef]
- Shoaib, M.; Ashar, A.; Bhutta, Z.A.; Muzammil, I.; Ali, M.; Kanwal, A. Biological Methods for Degradation of Textile Dyes from Textile Effluent. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 329–353. [Google Scholar]
- Vishani, D.B.; Shrivastav, A. Enzymatic Decolorization and Degradation of Azo Dyes. Dev. Wastewater Treat. Res. Process. 2022, 1, 419–432. [Google Scholar]
- Ikram, M.; Zahoor, M.; Naeem, M.; Islam, N.U.; Shah, A.B.; Shahzad, B. Bacterial Oxidoreductive Enzymes as Molecular Weapons for the Degradation and Metabolism of the Toxic Azo Dyes in Wastewater: A Review. Z. Für Phys. Chem. 2023, 237, 187–209. [Google Scholar] [CrossRef]
- Gomaa, H.; Emran, M.Y.; El-Gammal, M.A. Biodegradation of Azo Dye Pollutants Using Microorganisms. In Handbook of Biodegradable Materials; Springer: Cham, Switzerland, 2023; Volume 1, pp. 781–809. [Google Scholar]
- Khandare, S.D.; Teotia, N.; Kumar, M.; Diyora, P.; Chaudhary, D.R. Biodegradation and Decolorization of Trypan Blue Azo Dye by Marine Bacteria Vibrio Sp. JM-17. Biocatal. Agric. Biotechnol. 2023, 51, 102802. [Google Scholar] [CrossRef]
- Ali, E.; Amjad, I.; Rehman, A. Evaluation of Azo Dyes Degradation Potential of Fungal Strains and Their Role in Wastewater Treatment. Saudi J. Biol. Sci. 2023, 30, 103734. [Google Scholar] [CrossRef]
- Sun, S.; Liu, P.; Ullah, M. Efficient Azo Dye Biodecolorization System Using Lignin-Co-Cultured White-Rot Fungus. J. Fungi 2023, 9, 91. [Google Scholar] [CrossRef]
- Chatterjee, S.; Pandey, S. Degradation of Complex Textile Dyes by Some Leaf-Litter Dwelling Fungi. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2023, 93, 213–223. [Google Scholar] [CrossRef]
- Mary, J.E.; Krithika, T.; Kavitha, R. Biodegradation of Textile Dye by Ligninolytic Bacteria Isolated from Western Ghats. Int. J. Res. Rev. 2020, 7, 22–29. [Google Scholar]
- Kelewou, H.; Merzouki, M.; Lhassani, A. Biosorption of Textile Dyes Basic Yellow 2 (BY2) and Basic Green 4 (BG4) by the Live Yeast Saccharomyces Cerevisiae. J. Mater. Environ. Sci. 2014, 5, 633–640. [Google Scholar]
- Dhanavade, M.J.; Patil, P.J. Yeast and Fungal Mediated Degradation of Synthetic Dyes. In Current Developments in Bioengineering and Biotechnology; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1, pp. 371–409. [Google Scholar]
- Al-Tohamy, R.; Ali, S.S.; Xie, R.; Schagerl, M.; Khalil, M.A.; Sun, J. Decolorization of Reactive Azo Dye Using Novel Halotolerant Yeast Consortium HYC and Proposed Degradation Pathway. Ecotoxicol. Environ. Saf. 2023, 263, 115258. [Google Scholar] [CrossRef]
- Premarathna, K.; Lau, S.Y.; Chiong, T.; Show, P.-L.; Vithanage, M.; Lam, M.K. Greening up the Fight against Emerging Contaminants: Algae-Based Nanoparticles for Water Remediation. Clean Technol. Environ. Policy 2024, 1–18. [Google Scholar] [CrossRef]
- Panigrahi, S.; Priyadarshini, S.S.; Mishra, P.M.; Pradhan, N. Algal Biomass-Silver Nanoparticle Composite as a Heterogenous Catalyst for the Reduction of Congo Red. Water Air Soil Pollut. 2024, 235, 209. [Google Scholar] [CrossRef]
- Khandelwal, M.; Choudhary, S.; Kumawat, A.; Misra, K.P.; Rathore, D.S.; Khangarot, R.K. Asterarcys Quadricellulare Algae-Mediated Copper Oxide Nanoparticles as a Robust and Recyclable Catalyst for the Degradation of Noxious Dyes from Wastewater. RSC Adv. 2023, 13, 28179–28196. [Google Scholar] [CrossRef] [PubMed]
- El-Sheekh, M.M.; El Shafay, S.M.; El-Shanshoury, A.E.-R.R.; Hamouda, R.; Gharieb, D.Y.; Abou-El-Souod, G.W. Impact of Immobilized Algae and Its Consortium in Biodegradation of the Textile Dyes. Int. J. Phytoremediation 2023, 25, 687–696. [Google Scholar] [CrossRef]
- Yadav, M.; Yadav, H.S. Applications of Ligninolytic Enzymes to Pollutants, Wastewater, Dyes, Soil, Coal, Paper and Polymers. Environ. Chem. Lett. 2015, 13, 309–318. [Google Scholar] [CrossRef]
- Wen, J.; Gao, F.; Liu, H.; Wang, J.; Xiong, T.; Yi, H.; Zhou, Y.; Yu, Q.; Zhao, S.; Tang, X. Metallic Nanoparticles Synthesized by Algae: Synthetic Route, Action Mechanism, and the Environmental Catalytic Applications. J. Environ. Chem. Eng. 2023, 12, 111742. [Google Scholar] [CrossRef]
- Ali, S.S.; Al-Tohamy, R.; Koutra, E.; El-Naggar, A.H.; Kornaros, M.; Sun, J. Valorizing Lignin-like Dyes and Textile Dyeing Wastewater by a Newly Constructed Lipid-Producing and Lignin Modifying Oleaginous Yeast Consortium Valued for Biodiesel and Bioremediation. J. Hazard. Mater. 2021, 403, 123575. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Jose, P.A.; Ranjith, M.; Anandham, R.; Suganya, K.; Prabhakaran, J.; Thiyageshwari, S.; Johnson, J.; Gopal, N.O.; Kumutha, K. Decolourisation and Degradation of Azo Dyes by Mixed Fungal Culture Consisted of Dichotomomyces cejpii MRCH 1-2 and Phoma tropica MRCH 1-3. J. Environ. Chem. Eng. 2018, 6, 588–595. [Google Scholar] [CrossRef]
- Huang, J.; Pang, H.; Liu, Z.; Wang, X.; Zhang, C.; Zhang, W.; Liu, S.; He, W. Electrospinning Biohybrid Technology for Wastewater Treatment: Principle, Applications and Perspectives. Chem. Eng. J. 2024, 491, 151971. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Huang, J.; Ren, K.; Zhu, Y.; Yang, S. Alginate: Microbial Production, Functionalization, and Biomedical Applications. Int. J. Biol. Macromol. 2023, 242, 125048. [Google Scholar] [CrossRef]
- Barathi, S.; Aruljothi, K.; Karthik, C.; Padikasan, I.A.; Ashokkumar, V. Biofilm Mediated Decolorization and Degradation of Reactive Red 170 Dye by the Bacterial Consortium Isolated from the Dyeing Industry Wastewater Sediments. Chemosphere 2022, 286, 131914. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, D.; Hicham, E.A.; Naima, E.G. Biodegradation of Environmental Pollutants by Marine Yeasts. In Marine Organisms: A Solution to Environmental Pollution? Uses in Bioremediation and in Biorefinery; Springer: Cham, Switzerland, 2023; Volume 1, pp. 79–91. [Google Scholar]
- Shah, B.; Jain, K.; Jiyani, H.; Mohan, V.; Madamwar, D. Microaerophilic Symmetric Reductive Cleavage of Reactive Azo Dye—Remazole Brilliant Violet 5R by Consortium VIE6: Community Synergism. Appl. Biochem. Biotechnol. 2016, 180, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.; Cassoni, A.; Alves, S.; Moreira, P.; Pintado, M.; Castro, P. Removing Color While Lowering Toxicity: The Case for Decolorization of Textile Dyes and Simulated Effluents with Yeasts. Int. J. Environ. Sci. Technol. 2024, 21, 13–24. [Google Scholar] [CrossRef]
- Rana, S.; Handa, S.; Aggarwal, Y.; Puri, S.; Chatterjee, M. Role of Candida in the Bioremediation of Pollutants: A Review. Lett. Appl. Microbiol. 2023, 76, ovad103. [Google Scholar] [CrossRef]
- Anandita; Raees, K.; Shahadat, M.; Ali, S.W. Mechanistic Interaction of Microbe in Dye Degradation and the Role of Inherently Modified Organisms: A Review. Water Conserv. Sci. Eng. 2023, 8, 43. [Google Scholar] [CrossRef]
- Evangelista-Barreto, N.S.; Albuquerque, C.D.; Vieira, R.H.S.; Campos-Takaki, G.M. Cometabolic Decolorization of the Reactive Azo Dye Orange II by Geobacillus Stearothermophilus UCP 986. Text. Res. J. 2009, 79, 1266–1273. [Google Scholar] [CrossRef]
- Xiao, X.; Xu, C.-C.; Wu, Y.-M.; Cai, P.-J.; Li, W.-W.; Du, D.-L.; Yu, H.-Q. Biodecolorization of Naphthol Green B Dye by Shewanella Oneidensis MR-1 under Anaerobic Conditions. Bioresour. Technol. 2012, 110, 86–90. [Google Scholar] [CrossRef]
- Ajaz, M.; Rehman, A.; Khan, Z.; Nisar, M.A.; Hussain, S. Degradation of Azo Dyes by Alcaligenes aquatilis 3c and Its Potential Use in the Wastewater Treatment. Amb Express 2019, 9, 64. [Google Scholar] [CrossRef]
- Haque, M.M.; Hossen, M.N.; Rahman, A.; Roy, J.; Talukder, M.R.; Ahmed, M.; Ahiduzzaman, M.; Haque, M.A. Decolorization, Degradation and Detoxification of Mutagenic Dye Methyl Orange by Novel Biofilm Producing Plant Growth-Promoting Rhizobacteria. Chemosphere 2024, 346, 140568. [Google Scholar] [CrossRef]
- Dafale, N.; Wate, S.; Meshram, S.; Nandy, T. Kinetic Study Approach of Remazol Black-B Use for the Development of Two-Stage Anoxic–Oxic Reactor for Decolorization/Biodegradation of Azo Dyes by Activated Bacterial Consortium. J. Hazard. Mater. 2008, 159, 319–328. [Google Scholar] [CrossRef]
- Franciscon, E.; Grossman, M.J.; Paschoal, J.A.R.; Reyes, F.G.R.; Durrant, L.R. Decolorization and Biodegradation of Reactive Sulfonated Azo Dyes by a Newly Isolated Brevibacterium Sp. Strain VN-15. SpringerPlus 2012, 1, 37. [Google Scholar] [CrossRef]
- Meerbergen, K.; Willems, K.A.; Dewil, R.; Van Impe, J.; Appels, L.; Lievens, B. Isolation and Screening of Bacterial Isolates from Wastewater Treatment Plants to Decolorize Azo Dyes. J. Biosci. Bioeng. 2018, 125, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Paz, A.; Carballo, J.; Pérez, M.J.; Domínguez, J.M. Biological Treatment of Model Dyes and Textile Wastewaters. Chemosphere 2017, 181, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Nayak, J.K.; Maiti, A. Bacteria-Mediated Bio-Degradation of Reactive Azo Dyes Coupled with Bio-Energy Generation from Model Wastewater. Clean Technol. Environ. Policy 2020, 22, 651–667. [Google Scholar] [CrossRef]
- Zhong, J.; Wu, S.; Chen, W.-J.; Huang, Y.; Lei, Q.; Mishra, S.; Bhatt, P.; Chen, S. Current Insights into the Microbial Degradation of Nicosulfuron: Strains, Metabolic Pathways, and Molecular Mechanisms. Chemosphere 2023, 326, 138390. [Google Scholar] [CrossRef] [PubMed]
- Baffi, M.A.; de Azevedo, L.C.B.; Borges, M.F.; Bertini, S.B. Microbial Enzymes in Biodegradation of Organic Pollutants: Mechanisms and Applications. In Microbiome-Based Decontamination of Environmental Pollutants; Elsevier: Amsterdam, The Netherlands, 2024; pp. 213–242. [Google Scholar]
- Meng, Q.; Xu, Y.; Dai, L.; Ge, X.; Qiao, P. Regulation of fadR on the ROS Defense Mechanism in Shewanalla oneidensis. Biotechnol. Lett. 2024, 46, 691–698. [Google Scholar] [CrossRef]
- Singh, S.; Chaudhary, P.; Bhandari, G.; Jaiswal, D.K.; Upadhayay, V.K.; Kumar, A.; Saini, N.; Sharma, A. Role of Microbes in Dye Degradation. In Microbial Inoculants: Applications for Sustainable Agriculture; Springer: Singapore, 2024; Volume 1, pp. 349–373. [Google Scholar]
- Mohanty, S.S.; Kumar, A. Enhanced Degradation of Anthraquinone Dyes by Microbial Monoculture and Developed Consortium through the Production of Specific Enzymes. Sci. Rep. 2021, 11, 7678. [Google Scholar] [CrossRef]
- Morsi, R.; Bilal, M.; Iqbal, H.M.; Ashraf, S.S. Laccases and Peroxidases: The Smart, Greener and Futuristic Biocatalytic Tools to Mitigate Recalcitrant Emerging Pollutants. Sci. Total Environ. 2020, 714, 136572. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Okoh, A.I.; Nwodo, U.U. Aptitude of Oxidative Enzymes for Treatment of Wastewater Pollutants: A Laccase Perspective. Molecules 2019, 24, 2064. [Google Scholar] [CrossRef]
- Gałązka, A.; Jankiewicz, U.; Szczepkowski, A. Biochemical Characteristics of Laccases and Their Practical Application in the Removal of Xenobiotics from Water. Appl. Sci. 2023, 13, 4394. [Google Scholar] [CrossRef]
- Li, S.; Sun, K.; Latif, A.; Si, Y.; Gao, Y.; Huang, Q. Insights into the Applications of Extracellular Laccase-Aided Humification in Livestock Manure Composting. Environ. Sci. Technol. 2022, 56, 7412–7425. [Google Scholar] [CrossRef]
- Vaithyanathan, V.K.; Vaidyanathan, V.K.; Cabana, H. Laccase-Driven Transformation of High Priority Pesticides without Redox Mediators: Towards Bioremediation of Contaminated Wastewaters. Front. Bioeng. Biotechnol. 2022, 9, 770435. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Pathak, V.M.; Rajput, M. A Feasible Approach for Azo-Dye (Methyl Orange) Degradation by Textile Effluent Isolate Serratia Marcescens ED1 Strain for Water Sustainability: AST Identification, Degradation Optimization and Pathway Hypothesis. Heliyon 2024, 10, e32339. [Google Scholar] [CrossRef] [PubMed]
- Al-Sharabi, M.; Baiocco, D.; Lobel, B.T.; Cayre, O.J.; Zhang, Z.; Routh, A.F. Magnetic Zinc Oxide/Silica Microbeads for the Photocatalytic Degradation of Azo Dyes. Colloids Surf. A Physicochem. Eng. Asp. 2024, 695, 134169. [Google Scholar] [CrossRef]
- Lu, P.; Hu, X.; Chang, R.; Zhou, Y.; Bai, Y.; Zhou, Y.; Fu, G.; Zhang, Z. Diurnal-Independent, Visible-Light-Storing of Ag2O@ SrAl2O4: Eu2+, Dy3+ for the Round-the-Clock Decomposition of Ciprofloxacin. Sep. Purif. Technol. 2024, 330, 125274. [Google Scholar] [CrossRef]
- Butburee, T.; Kotchasarn, P.; Hirunsit, P.; Sun, Z.; Tang, Q.; Khemthong, P.; Sangkhun, W.; Thongsuwan, W.; Kumnorkaew, P.; Wang, H. New Understanding of Crystal Control and Facet Selectivity of Titanium Dioxide Ruling Photocatalytic Performance. J. Mater. Chem. A 2019, 7, 8156–8166. [Google Scholar] [CrossRef]
- Pan, J.; Liu, G.; Lu, G.Q.; Cheng, H. On the True Photoreactivity Order of {001}, {010}, and {101} Facets of Anatase TiO2 Crystals. Angew. Chem. Int. Ed. 2011, 50, 2133–2137. [Google Scholar] [CrossRef]
- Etafo, N.O.; Bamidele, M.O.; Bamisaye, A.; Alli, Y.A. Revolutionizing Photocatalysis: Unveiling Efficient Alternatives to Titanium (IV) Oxide and Zinc Oxide for Comprehensive Environmental Remediation. J. Water Process Eng. 2024, 62, 105369. [Google Scholar] [CrossRef]
- Li, R.; Li, T.; Zhou, Q. Impact of Titanium Dioxide (TiO2) Modification on Its Application to Pollution Treatment—A Review. Catalysts 2020, 10, 804. [Google Scholar] [CrossRef]
- Zauška, L.; Volavka, D.; Lisnichuk, M.; Zelenka, T.; Kinnertová, E.; Zelenková, G.; Bednarčík, J.; Zeleňák, V.; Sharma, A.; Nehra, S.P. Tuning the Photocatalytic Performance of Mesoporous Silica-Titanium Dioxide and Cobalt Titanate for Methylene Blue and Congo Red Adsorption/Photodegradation: Impact of Azo Dyes Concentration, Catalyst Mass, Wavelength, Reusability and Kinetic Properties. J. Photochem. Photobiol. A Chem. 2024, 451, 115522. [Google Scholar] [CrossRef]
- Kamble, G.S.; Natarajan, T.S.; Patil, S.S.; Thomas, M.; Chougale, R.K.; Sanadi, P.D.; Siddharth, U.S.; Ling, Y.-C. BiVO4 As a Sustainable and Emerging Photocatalyst: Synthesis Methodologies, Engineering Properties, and Its Volatile Organic Compounds Degradation Efficiency. Nanomaterials 2023, 13, 1528. [Google Scholar] [CrossRef]
- Ateia, M.; Alalm, M.G.; Awfa, D.; Johnson, M.S.; Yoshimura, C. Modeling the Degradation and Disinfection of Water Pollutants by Photocatalysts and Composites: A Critical Review. Sci. Total Environ. 2020, 698, 134197. [Google Scholar] [CrossRef]
- Badoni, A.; Prakash, J. Noble Metal Nanoparticles and Graphene Oxide Based Hybrid Nanostructures for Antibacterial Applications: Recent Advances, Synergistic Antibacterial Activities, and Mechanistic Approaches. Micro Nano Eng. 2024, 22, 100239. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Li, W.; Yang, Q.; Hou, Q.; Wei, L.; Liu, L.; Huang, F.; Ju, M. Enhancement of Photocatalytic Performance with the Use of Noble-Metal-Decorated TiO2 Nanocrystals as Highly Active Catalysts for Aerobic Oxidation under Visible-Light Irradiation. Appl. Catal. B Environ. 2017, 210, 352–367. [Google Scholar] [CrossRef]
- Guo, J.; Li, S.; Wang, J.; Wang, J. Dual-Recognition Immune-Co-Chemical ECL-Sensor Based on Ti, Mg@ N-CDs-Induced and Novel Signal-Sensing Units Poly (DVB-Co-PBA)-Reported for Alpha-Fetoprotein Detection. Sens. Actuators B Chem. 2021, 346, 130548. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, P.; Kumar, A.; Camargo, P.H.C.; Krishnan, V. Recent Advances in Plasmonic Photocatalysis Based on TiO2 and Noble Metal Nanoparticles for Energy Conversion, Environmental Remediation, and Organic Synthesis. Small 2022, 18, 2101638. [Google Scholar] [CrossRef]
- Wei, Z.; Janczarek, M.; Wang, K.; Zheng, S.; Kowalska, E. Morphology-Governed Performance of Plasmonic Photocatalysts. Catalysts 2020, 10, 1070. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Stathopoulos, V.N. Titanium Dioxide (TiO2)-Based Photocatalyst Materials Activity Enhancement for Contaminants of Emerging Concern (CECs) Degradation: In the Light of Modification Strategies. Chem. Eng. J. Adv. 2022, 10, 100262. [Google Scholar] [CrossRef]
- Marks, R.G.; Rockel, S.P.; Kerpen, K.; Somnitz, H.; Martin, P.R.; Jochmann, M.A.; Schmidt, T.C. Effects of pH-Dependent Speciation on the Photolytic Degradation Mechanism of Phosphonates. J. Photochem. Photobiol. A Chem. 2024, 448, 115327. [Google Scholar] [CrossRef]
- Ngo, A.C.R.; Tischler, D. Microbial Degradation of Azo Dyes: Approaches and Prospects for a Hazard-Free Conversion by Microorganisms. Int. J. Environ. Res. Public Health 2022, 19, 4740. [Google Scholar] [CrossRef]
- Albeladi, A.; Khan, Z.; Al-Thabaiti, S.A.; Patel, R.; Malik, M.A.; Mehta, S. Fe3O4-CdO Nanocomposite for Organic Dye Photocatalytic Degradation: Synthesis and Characterization. Catalysts 2024, 14, 71. [Google Scholar] [CrossRef]
- Al-nayili, A.; Khayoon, H.; Alshamsi, H.; Saady, N.C. A Novel Bimetallic (Au-Pd)-Decorated Reduced Graphene Oxide Nanocomposite Enhanced Rhodamine B Photocatalytic Degradation under Solar Irradiation. Mater. Today Sustain. 2023, 24, 100512. [Google Scholar] [CrossRef]
- Firmino, H.C.; Nascimento, E.P.; Araujo, R.N.; Loureiro, F.J.; Neves, G.A.; Morales, M.A.; Menezes, R.R. Nickel Ferrite/TiO2 Nanofibrous Composite: Enhanced Photocatalytic Dye Degradation Under Visible Light. Mater. Res. 2024, 27, e20230391. [Google Scholar] [CrossRef]
- Haleem, A.; Shafiq, A.; Chen, S.-Q.; Nazar, M. A Comprehensive Review on Adsorption, Photocatalytic and Chemical Degradation of Dyes and Nitro-Compounds over Different Kinds of Porous and Composite Materials. Molecules 2023, 28, 1081. [Google Scholar] [CrossRef]
- Yu, S.; Liu, Q.; Yang, W.; Han, K.; Wang, Z.; Zhu, H. Graphene–CeO2 Hybrid Support for Pt Nanoparticles as Potential Electrocatalyst for Direct Methanol Fuel Cells. Electrochim. Acta 2013, 94, 245–251. [Google Scholar] [CrossRef]
- Khodamorady, M.; Bahrami, K. Fe3O4@ BNPs@ ZnO–ZnS as a Novel, Reusable and Efficient Photocatalyst for Dye Removal from Synthetic and Textile Wastewaters. Heliyon 2023, 9, e16397. [Google Scholar] [CrossRef]
- Khalid, A.; Yasmin, N.; Kalsoom, A.; Abbas, Y.; Shahid, T.; Safdar, M.; Mirza, M. Investigation of Photocatalytic Properties of Bismuth Vanadium Based Oxyselenide for Textile Dye Removal. J. Dispers. Sci. Technol. 2023, 1–8. [Google Scholar] [CrossRef]
- Krishnan, V.; Joseph, C.G.; Taufiq-Yap, Y.H.; Teo, S.H.; Soloi, S.; Wid, N.; Abd Majid, M.H.; Farm, Y.Y.; Rodrigues, K.F. Sonophotocatalytic Degradation of Reactive Black 5 in Simulated Dye Wastewater Using ZnO and Activated Red Mud Sonophotocatalyst. Top. Catal. 2024, 67, 1194–1210. [Google Scholar] [CrossRef]
- Pang, X.; Zhang, H.; Qi, L.; Wang, Z.; Ma, G. Effectiveness of Photocatalysis of Fe78Si9B13/TiO2 Composites for Acid Orange 7 Degradation. J. Sol-Gel Sci. Technol. 2024, 110, 142–155. [Google Scholar] [CrossRef]
- Belikov, Y.A.; Snytnikova, O.A.; Sheven, D.G.; Fedunov, R.G.; Grivin, V.P.; Pozdnyakov, I.P. Laser Flash Photolysis and Quantum Chemical Studies of UV Degradation of Pharmaceutical Drug Chloramphenicol: Short-Lived Intermediates, Quantum Yields and Mechanism of Photolysis. Chemosphere 2024, 351, 141211. [Google Scholar] [CrossRef]
- Arunpandian, M.; Selvakumar, K.; Oh, T.H. Boosting Photocatalytic Performance of Cubic Co2SnO4-MnO2@ g-C3N5 Ternary Composites: Analysis of Morphology, Degradation Ability of Tetracycline Hydrochloride and Fragments Pathway Mechanism. Surf. Interfaces 2024, 51, 104717. [Google Scholar] [CrossRef]
- Mahmoodi, N.M. Zinc Ferrite Nanoparticle as a Magnetic Catalyst: Synthesis and Dye Degradation. Mater. Res. Bull. 2013, 48, 4255–4260. [Google Scholar] [CrossRef]
- Marimuthu, S.; Antonisamy, A.J.; Malayandi, S.; Rajendran, K.; Tsai, P.-C.; Pugazhendhi, A.; Ponnusamy, V.K. Silver Nanoparticles in Dye Effluent Treatment: A Review on Synthesis, Treatment Methods, Mechanisms, Photocatalytic Degradation, Toxic Effects and Mitigation of Toxicity. J. Photochem. Photobiol. B Biol. 2020, 205, 111823. [Google Scholar] [CrossRef]
- Anjali, K.; Raghunathan, R.; Devi, G.; Dutta, S. Photocatalytic Degradation of Methyl Red Using Seaweed Mediated Zinc Oxide Nanoparticles. Biocatal. Agric. Biotechnol. 2022, 43, 102384. [Google Scholar] [CrossRef]
- Rezaei, B.; Yari, P.; Sanders, S.M.; Wang, H.; Chugh, V.K.; Liang, S.; Mostufa, S.; Xu, K.; Wang, J.; Gómez-Pastora, J. Magnetic Nanoparticles: A Review on Synthesis, Characterization, Functionalization, and Biomedical Applications. Small 2024, 20, 2304848. [Google Scholar] [CrossRef]
- Wu, E.; Chen, H.; Tang, L.; Zeng, L.; Ji, H.; Zhu, M. Molecular Understanding on Ultraviolet Photolytic Degradation of Cyano Liquid Crystal Monomers. J. Hazard. Mater. 2024, 465, 133033. [Google Scholar] [CrossRef]
- Kim, J.; Kim, T.; Park, H.; Kim, M.-K.; Eom, S.; Choe, Y.; Choe, J.K.; Zoh, K.-D. Kinetics and Proposed Mechanisms of Hexafluoropropylene Oxide Dimer Acid (GenX) Degradation via Vacuum-UV (VUV) Photolysis and VUV/Sulfite Processes. J. Hazard. Mater. 2024, 463, 132864. [Google Scholar] [CrossRef]
- Jara, Y.S.; Mekiso, T.T.; Washe, A.P. Highly Efficient Catalytic Degradation of Organic Dyes Using Iron Nanoparticles Synthesized with Vernonia Amygdalina Leaf Extract. Sci. Rep. 2024, 14, 6997. [Google Scholar] [CrossRef]
- Moghaddas, S.M.T.H.; Elahi, B.; Javanbakht, V. Biosynthesis of Pure Zinc Oxide Nanoparticles Using Quince Seed Mucilage for Photocatalytic Dye Degradation. J. Alloys Compd. 2020, 821, 153519. [Google Scholar] [CrossRef]
- Mondal, A.; Adhikary, B.; Mukherjee, D. Room-Temperature Synthesis of Air Stable Cobalt Nanoparticles and Their Use as Catalyst for Methyl Orange Dye Degradation. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 248–257. [Google Scholar] [CrossRef]
- Bokare, A.D.; Chikate, R.C.; Rode, C.V.; Paknikar, K.M. Iron-Nickel Bimetallic Nanoparticles for Reductive Degradation of Azo Dye Orange G in Aqueous Solution. Appl. Catal. B Environ. 2008, 79, 270–278. [Google Scholar] [CrossRef]
- Gallegos-Cerda, S.D.; Hernández-Varela, J.D.; Pérez, J.J.C.; Huerta-Aguilar, C.A.; Victoriano, L.G.; Arredondo-Tamayo, B.; Hernández, O.R. Development of a Low-Cost Photocatalytic Aerogel Based on Cellulose, Carbon Nanotubes, and TiO2 Nanoparticles for the Degradation of Organic Dyes. Carbohydr. Polym. 2024, 324, 121476. [Google Scholar] [CrossRef] [PubMed]
- Jeevarathinam, M.; Asharani, I. Synthesis of CuO, ZnO Nanoparticles, and CuO-ZnO Nanocomposite for Enhanced Photocatalytic Degradation of Rhodamine B: A Comparative Study. Sci. Rep. 2024, 14, 9718. [Google Scholar] [CrossRef] [PubMed]
- Barciela, P.; Perez-Vazquez, A.; Prieto, M. Azo Dyes in the Food Industry: Features, Classification, Toxicity, Alternatives, and Regulation. Food Chem. Toxicol. 2023, 178, 113935. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, S.; Alkhanjaf, A.A.M.; Arora, N.K.; Saxena, B.; Umar, A.; Ibrahim, A.A.; Akhtar, M.S.; Mahajan, A.; Negi, S. Microbial Fuel Cells for Azo Dye Degradation: A Perspective Review. J. Ind. Eng. Chem. 2024, 142, 45–67. [Google Scholar] [CrossRef]
- Dey, C.; Nandi, M.; Goswami, M.M. pH Dependent Enhanced Synchronous Photocatalytic Removal of Cationic and Anionic Dyes by CoFe2O4 Magnetic Nanoparticles. J. Mol. Struct. 2023, 1277, 134859. [Google Scholar] [CrossRef]
- Cardito, A.; Carotenuto, M.; Sacco, O.; Albarano, L.; Vaiano, V.; Iannece, P.; Libralato, G.; Spica, V.R.; Lofrano, G. UV Light Assisted Degradation of Acid Orange Azo Dye by ZVI-ZnS and Effluent Toxicity Effects. Environ. Pollut. 2024, 343, 123226. [Google Scholar] [CrossRef]
- Singh, A.L.; Chaudhary, S.; Kumar, S.; Kumar, A.; Singh, A.; Yadav, A. Biodegradation of Reactive Yellow-145 Azo Dye Using Bacterial Consortium: A Deterministic Analysis Based on Degradable Metabolite, Phytotoxicity and Genotoxicity Study. Chemosphere 2022, 300, 134504. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Chandra, R.; Bharagava, R.N. Ecotoxicological and Health Concerns of Persistent Coloring Pollutants of Textile Industry Wastewater and Treatment Approaches for Environmental Safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Şengil, İ.A.; Özacar, M. The Decolorization of CI Reactive Black 5 in Aqueous Solution by Electrocoagulation Using Sacrificial Iron Electrodes. J. Hazard. Mater. 2009, 161, 1369–1376. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Allehyani, E.S.; Al-Harbi, S.A.; Hasan, Z.; Abomuti, M.A.; Rajor, H.K.; Oh, S. Investigation of Congo Red Toxicity towards Different Living Organisms: A Review. Processes 2023, 11, 807. [Google Scholar] [CrossRef]
- Dutta, S.; Adhikary, S.; Bhattacharya, S.; Roy, D.; Chatterjee, S.; Chakraborty, A.; Banerjee, D.; Ganguly, A.; Nanda, S.; Rajak, P. Contamination of Textile Dyes in Aquatic Environment: Adverse Impacts on Aquatic Ecosystem and Human Health, and Its Management Using Bioremediation. J. Environ. Manag. 2024, 353, 120103. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.L.; Picinini, J.; Silveira, M.D.; Camassola, M.; Visentim, A.P.; Salvador, M.; da Silva, J. Fluorosilicic Acid Induces DNA Damage and Oxidative Stress in Bone Marrow Mesenchymal Stem Cells. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2021, 861, 503297. [Google Scholar] [CrossRef]
- Chen, S.H.; Cheow, Y.L.; Ng, S.L.; Ting, A.S.Y. Removal of Triphenylmethane Dyes in Single-Dye and Dye-Metal Mixtures by Live and Dead Cells of Metal-Tolerant Penicillium simplicissimum. Sep. Sci. Technol. 2020, 55, 2410–2420. [Google Scholar] [CrossRef]
- Alexander, J.; Barregård, L.; Bignami, M.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R. Malachite Green in Food. Efsa J. 2016. [Google Scholar]
- Kumar, B.; Agrawal, K.; Verma, P. Microbial Electrochemical System: A Sustainable Approach for Mitigation of Toxic Dyes and Heavy Metals from Wastewater. J. Hazard. Toxic Radioact. Waste 2021, 25, 04020082. [Google Scholar] [CrossRef]
- Priya, P.S.; Nandhini, P.P.; Vaishnavi, S.; Pavithra, V.; Almutairi, M.H.; Almutairi, B.O.; Arokiyaraj, S.; Pachaiappan, R.; Arockiaraj, J. Rhodamine B, an Organic Environmental Pollutant Induces Reproductive Toxicity in Parental and Teratogenicity in F1 Generation in Vivo. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2024, 280, 109898. [Google Scholar] [CrossRef]
- Guan, Z.; Wang, Y.; Chen, W.; Li, Y.; Yue, W.; Cai, Z. Biodecolorization and Biodegradation of Sulfur Black by the Strain Aspergillus Sp. DS-28. Processes 2024, 12, 1818. [Google Scholar] [CrossRef]
- Ajima, M.N.O.; Pandey, P.K.; Kumar, K.; Poojary, N. Assessment of Mutagenic, Hematological and Oxidative Stress Biomarkers in Liver of Nile Tilapia, Oreochromis niloticus (Linnaeus, 1758) in Response to Sublethal Verapamil Exposure. Drug Chem. Toxicol. 2017, 40, 286–294. [Google Scholar] [CrossRef]
- Hoynes, G. Investigating the Metabolism of Tartrazine by the Human Gut Microbiome. Ph.D. Thesis, Kingston University, London, UK, 2021. [Google Scholar]
- Amchova, P.; Siska, F.; Ruda-Kucerova, J. Food Safety and Health Concerns of Synthetic Food Colors: An Update. Toxics 2024, 12, 466. [Google Scholar] [CrossRef]
- Kornblau, I.S.; El-Annan, J.F. Adverse Reactions to Fluorescein Angiography: A Comprehensive Review of the Literature. Surv. Ophthalmol. 2019, 64, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic Organic Dyes as Contaminants of the Aquatic Environment and Their Implications for Ecosystems: A Review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Sanjana, M.; Prajna, R.; Katti, U.S.; Kavitha, R.V. Bioremediation–the Recent Drift towards a Sustainable Environment. Environ. Sci. Adv. 2024, 3, 1097–1110. [Google Scholar]
- Pavithra, K.G.; Jaikumar, V. Removal of Colorants from Wastewater: A Review on Sources and Treatment Strategies. J. Ind. Eng. Chem. 2019, 75, 1–19. [Google Scholar] [CrossRef]
- El-Mas, S.M.; Hassaan, M.A.; El-Subruiti, G.M.; Eltaweil, A.S.; El Nemr, A. Microwave-Induced Degradation of Congo Red Dye in the Presence of 2D Ti3C2Tx MXene as a Catalyst. Sci. Rep. 2025, 15, 634. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, G.; Ahmad, T.; Mansoor, S.; Kaur, B. Enzyme Engineering: Current Trends and Future Perspectives. Food Rev. Int. 2021, 37, 121–154. [Google Scholar] [CrossRef]
- Miyashiro, C.S.; Mateus, G.A.P.; Dos Santos, T.R.T.; Paludo, M.P.; Bergamasco, R.; Fagundes-Klen, M.R. Synthesis and Performance Evaluation of a Magnetic Biocoagulant in the Removal of Reactive Black 5 Dye in Aqueous Medium. Mater. Sci. Eng. C 2021, 119, 111523. [Google Scholar] [CrossRef]
- Ferrentino, R.; Ceccato, R.; Marchetti, V.; Andreottola, G.; Fiori, L. Sewage Sludge Hydrochar: An Option for Removal of Methylene Blue from Wastewater. Appl. Sci. 2020, 10, 3445. [Google Scholar] [CrossRef]
- Islam, T.; Repon, M.R.; Islam, T.; Sarwar, Z.; Rahman, M.M. Impact of Textile Dyes on Health and Ecosystem: A Review of Structure, Causes, and Potential Solutions. Environ. Sci. Pollut. Res. 2023, 30, 9207–9242. [Google Scholar] [CrossRef]
- Aleksandrov, V. Nanoremediation: A New and Emerging Technology. In Phytoremediation and Biofortification; Apple Academic Press: Palm Bay, FL, USA, 2024; pp. 29–62. [Google Scholar]
- Solayman, H.M.; Hossen, M.A.; Abd Aziz, A.; Yahya, N.Y.; Leong, K.H.; Sim, L.C.; Monir, M.U.; Zoh, K.-D. Performance Evaluation of Dye Wastewater Treatment Technologies: A Review. J. Environ. Chem. Eng. 2023, 11, 109610. [Google Scholar] [CrossRef]
- Su, C.X.-H.; Low, L.W.; Teng, T.T.; Wong, Y.S. Combination and Hybridisation of Treatments in Dye Wastewater Treatment: A Review. J. Environ. Chem. Eng. 2016, 4, 3618–3631. [Google Scholar] [CrossRef]
- AlJaberi, F.Y.; Alardhi, S.M.; Ahmed, S.A.; Salman, A.D.; Juzsakova, T.; Cretescu, I.; Le, P.-C.; Chung, W.J.; Chang, S.W.; Nguyen, D.D. Can Electrocoagulation Technology Be Integrated with Wastewater Treatment Systems to Improve Treatment Efficiency? Environ. Res. 2022, 214, 113890. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.D.; Bassin, J.P.; Alves, T.L.M.; Sant’Anna, G.L.; Dezotti, M. Reactive Orange 16 Dye Degradation in Anaerobic and Aerobic MBBR Coupled with Ozonation: Addressing Pathways and Performance. Int. J. Environ. Sci. Technol. 2021, 18, 1991–2010. [Google Scholar] [CrossRef]
- Biswas, S. A Study on the Potential of Stenotrophomonas Koreensis and Bacillus Rigiliprofundi to Ameliorate the Toxicity of Industrial Dyes Malachite Green and Remazol Brilliant Blue R. Ph.D. Thesis, Jadavpur University, Kolkata, India, 2022. [Google Scholar]
- Ramesh, N.; Lai, C.W.; Johan, M.R.B.; Mousavi, S.M.; Badruddin, I.A.; Kumar, A.; Sharma, G.; Gapsari, F. Progress in Photocatalytic Degradation of Industrial Organic Dye by Utilising the Silver Doped Titanium Dioxide Nanocomposite. Heliyon 2024, 10, e40998. [Google Scholar] [CrossRef]
- Anil, K.; Surenjan, A. Green Synthesis, Characterisation, and Performance Evaluation of ZnO/TiO2 Nanocomposite for Cationic and Anionic Dye Removal from Aqueous Solutions under Solar Irradiation. Water Pract. Technol. 2024, 19, 824–838. [Google Scholar] [CrossRef]
- Folawewo, A.D.; Bala, M.D. Nanocomposite Zinc Oxide-Based Photocatalysts: Recent Developments in Their Use for the Treatment of Dye-Polluted Wastewater. Water 2022, 14, 3899. [Google Scholar] [CrossRef]
- Alamu, G.A.; Ayanlola, P.S.; Babalola, K.K.; Adedokun, O.; Sanusi, Y.K.; Fajinmi, G.R. Green Synthesis and Characterizations of Magnetic Iron Oxide Nanoparticles Using Moringa Oleifera Extract for Improved Performance in Dye-Sensitized Solar Cell. Chem. Phys. Impact 2024, 8, 100542. [Google Scholar] [CrossRef]
- Chakraborty, P.; Sarkar, S.; Das, P. Application of Potential Microbes in Bioremediation of Toxic Pollutants. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2024; pp. 171–185. [Google Scholar]
- Anegbe, B.; Ifijen, I.H. Recent Advances in the Application of Manganese Oxide Nanoparticles for Remediation of Soil Contaminated with Organic Pollutants. In TMS 2024 153rd Annual Meeting & Exhibition Supplemental Proceedings; The Minerals, Metals & Materials Society, Ed.; The Minerals, Metals & Materials Series; Springer Nature Switzerland: Cham, Switzerland, 2024; pp. 1358–1374. ISBN 978-3-031-50348-1. [Google Scholar]
- Kim, J.; Mayorga-Burrezo, P.; Song, S.-J.; Mayorga-Martinez, C.C.; Medina-Sánchez, M.; Pané, S.; Pumera, M. Advanced Materials for Micro/Nanorobotics. Chem. Soc. Rev. 2024, 53, 9190–9253. [Google Scholar] [CrossRef]
- Karabacakoğlu, B.; Karaduman, S. Reactive Yellow 145 Removal by Electro-Fenton with Fe–Carbon Fiber Electrode Pair: Optimization of Process Variables Based on Response Surface Methodology. Chem. Pap. 2024, 78, 1671–1685. [Google Scholar] [CrossRef]
- Adesibikan, A.A.; Emmanuel, S.S.; Olawoyin, C.O.; Ndungu, P. Cellulosic Metallic Nanocomposites for Photocatalytic Degradation of Persistent Dye Pollutants in Aquatic Bodies: A Pragmatic Review. J. Organomet. Chem. 2024, 1010, 123087. [Google Scholar] [CrossRef]
- Rathour, R.K.; Sharma, D.; Sharma, N.; Bhatt, A.K.; Singh, S.P. Engineered Microorganisms for Bioremediation. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 335–361. [Google Scholar]
- Coelho, G.D.; Silva, M.A.; de Melo Pinheiro, M.A.; Nadvorny, D.; Costa Amador, V.; Maia, R.T. In Silico and in Vitro Assays Suggests Congo Red Dye Degradation by a Lentinus Sp. Laccase Enzyme. J. Biomol. Struct. Dyn. 2024, 42, 3802–3813. [Google Scholar] [CrossRef] [PubMed]
- Anandan, S.; Ikuma, Y.; Niwa, K. An Overview of Semi-Conductor Photocatalysis: Modification of TiO2 Nanomaterials. Solid State Phenom. 2010, 162, 239–260. [Google Scholar]
- Peiris, S.; de Silva, H.B.; Ranasinghe, K.N.; Bandara, S.V.; Perera, I.R. Recent Development and Future Prospects of TiO2 Photocatalysis. J. Chin. Chem. Soc. 2021, 68, 738–769. [Google Scholar] [CrossRef]
- Pattanaik, P.; Sahoo, M. TiO2 Photocatalysis: Progress from Fundamentals to Modification Technology. Desalination Water Treat. 2014, 52, 6567–6590. [Google Scholar] [CrossRef]
- Bakar, B.; Birhanlı, E.; Ulu, A.; Boran, F.; Yeşilada, Ö.; Ateş, B. Immobilization of Trametes Trogii Laccase on Polyvinylpyrrolidone-Coated Magnetic Nanoparticles for Biocatalytic Degradation of Textile Dyes. Biocatal. Biotransformation 2024, 42, 194–211. [Google Scholar] [CrossRef]
- Kotwal, P.; Jasrotia, R.; Prakash, J.; Ahmed, J.; Verma, A.; Verma, R.; Kandwal, A.; Godara, S.K.; Kumari, S.; Maji, P.K. Magnetically Recoverable Sol-Gel Auto-Combustion Developed Ni1-xCuxDyyFe2-yO4 Magnetic Nanoparticles for Photocatalytic, Electrocatalytic, and Antibacterial Applications. Environ. Res. 2023, 231, 116103. [Google Scholar] [CrossRef]
- Wani, A.K.; Chopra, C.; Ansari, M.A.; Dar, M.A.; Américo-Pinheiro, J.H.P.; Singh, R. Characterization of Thermostable Carboxypeptidase from High-Altitude Hot Spring Metagenome. Int. J. Biol. Macromol. 2024, 276, 133974. [Google Scholar] [CrossRef]
- Aslam, S.; Subhan, F.; Liu, Z.; Yan, Z.; Ahmad, A.; Nazir, A.; Siddiqa, A.; Yaseen, M. Magnetic Fe3O4@ MIL-100 (Fe) Core-Shells Decorated with Gold Nanoparticles for Enhanced Catalytic Reduction of 4-Nitrophenol and Degradation of Azo Dye. Colloids Surf. A Physicochem. Eng. Asp. 2023, 660, 130904. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, X.; Wen, X.; Hou, C.; Chen, D.; Mu, Y.; Shen, J. Enhanced Azo Dye Reduction at Semiconductor-Microbe Interface: The Key Role of Semiconductor Band Structure. Water Res. 2024, 248, 120846. [Google Scholar] [CrossRef]
- Shafqat, M.; Mahmood, S.; Anjum, M.; Qadeer, S.; Mahmood, T.; Centritto, M.; Khalid, A. The Nexus of Phyto-Assisted Plant Growth-Promoting Bacterial Application for Bioremediation of Azo Dye. Int. J. Environ. Sci. Technol. 2024, 21, 5269–5284. [Google Scholar] [CrossRef]
- Bashir, Y.; Raj, R.; Ghangrekar, M.M.; Nema, A.K.; Das, S. Critical Assessment of Advanced Oxidation Processes and Bio-Electrochemical Integrated Systems for Removing Emerging Contaminants from Wastewater. RSC Sustain. 2023, 1, 1912–1931. [Google Scholar] [CrossRef]
- Zeng, W.; Yao, B.; Zhou, Y.; Yang, J.; Zhi, D. Combination of Electrochemical Advanced Oxidation and Biotreatment for Wastewater Treatment and Soil Remediation. J. Environ. Sci. 2024, 150, 36–53. [Google Scholar] [CrossRef]
- APHA–AWNA-WPCF. American Public Health Association Standard Methods for Examination of Water and Waste Water; APHA–AWNA-WPCF: New York, NY, USA, 1998; p. 1134. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).