Applied Time Series Analyses (2000–2017) of Vibrio vulnificus and Vibrio parahaemolyticus (Pathogenic and Non-Pathogenic Strains) in the Eastern Oyster, Crassostrea virginica
Abstract
1. Introduction
2. Materials and Methods
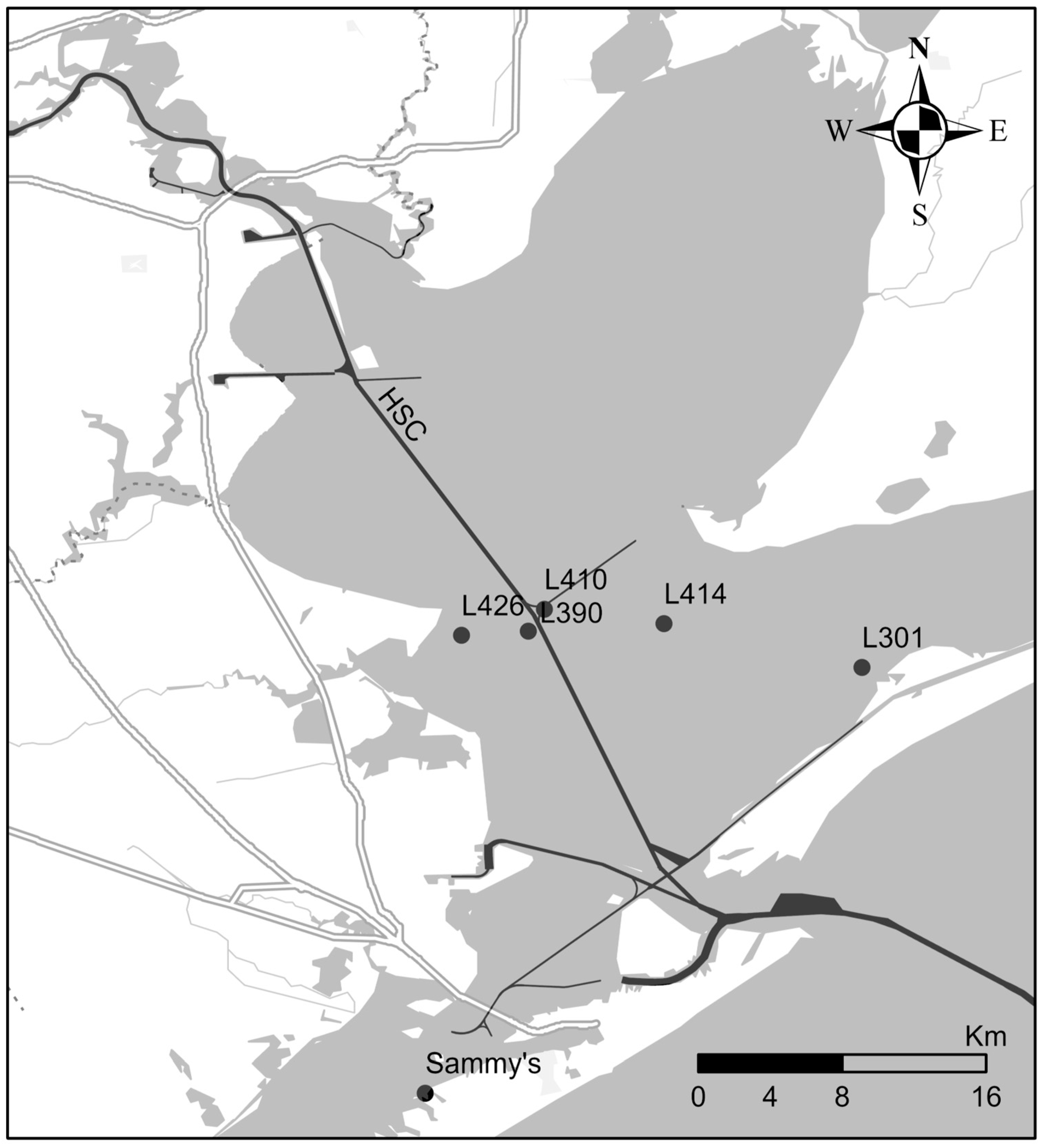
2.1. Sample Collection
2.2. Isolation and Enumeration of Vibrio spp.
2.3. Isolation and Enumeration of P. marinus
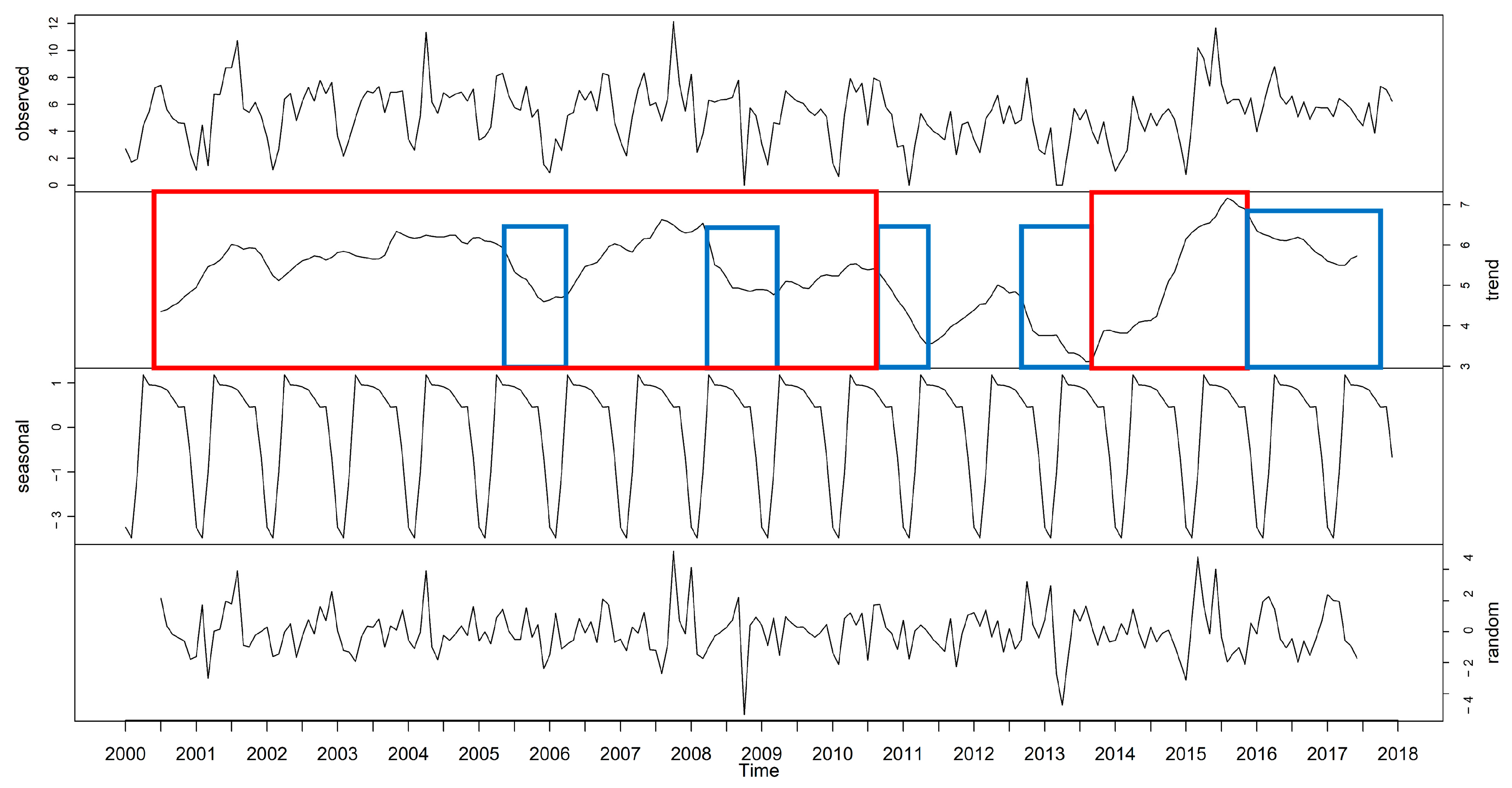
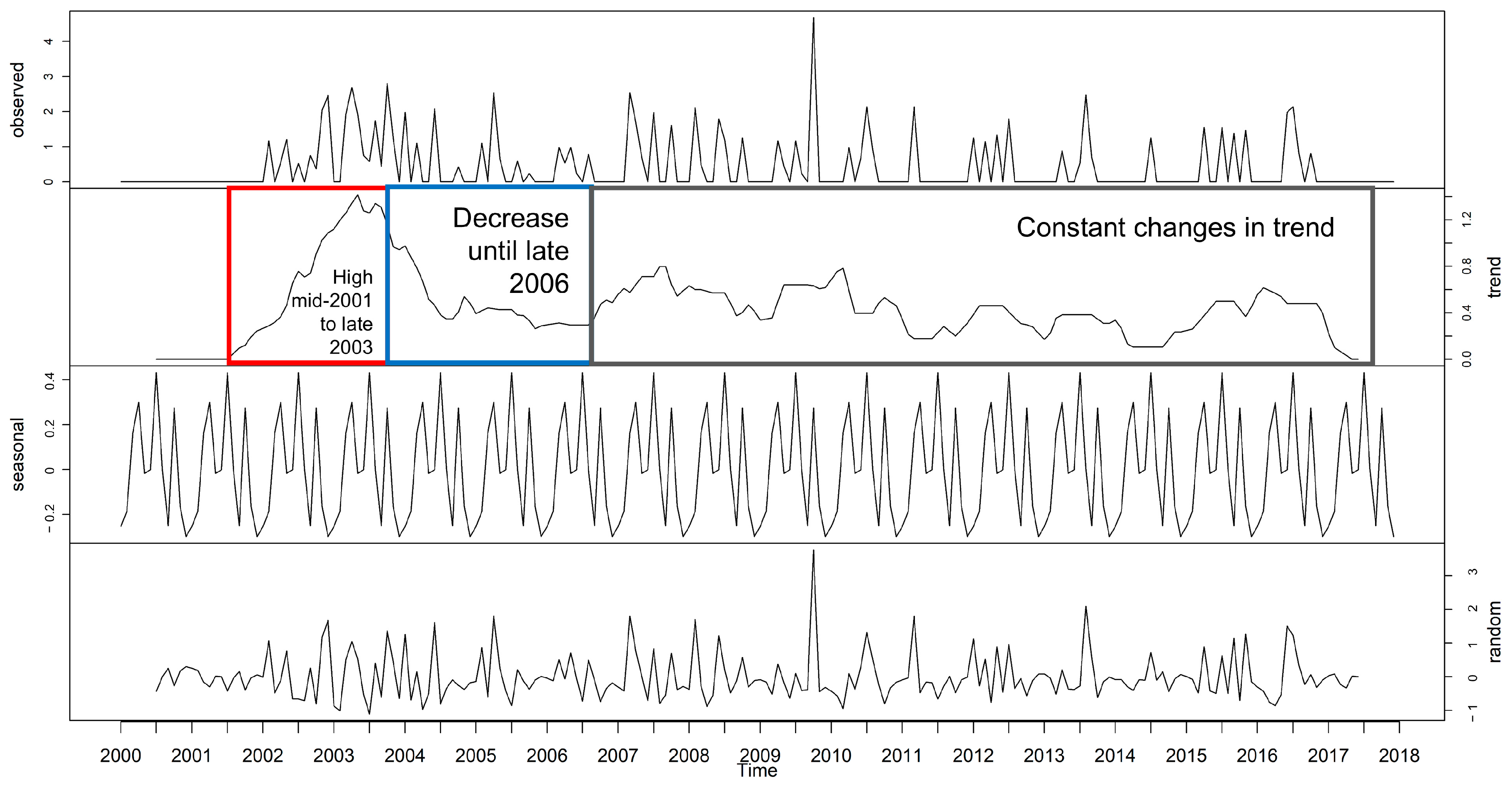
2.4. Decomposition of Time Series
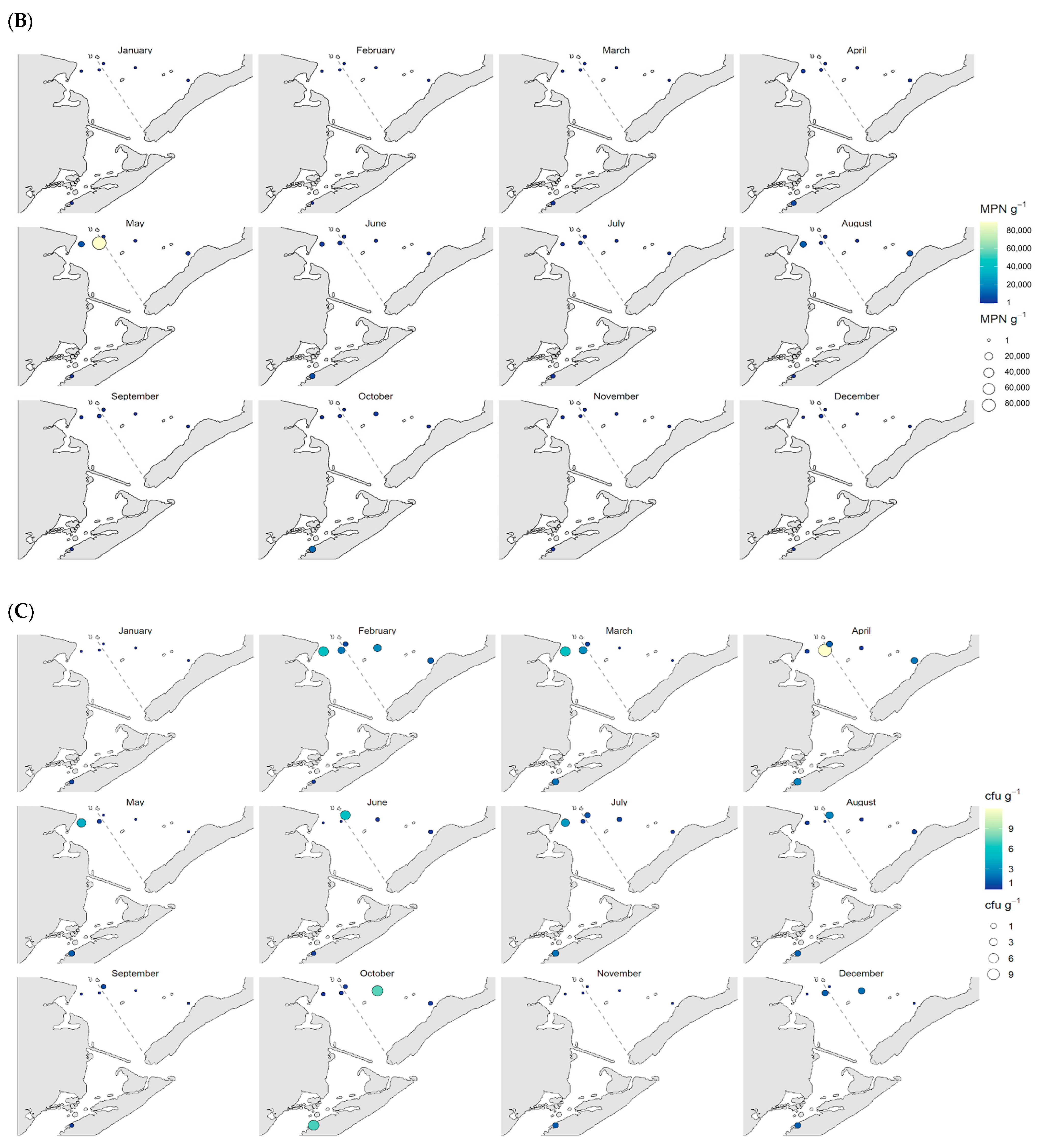
2.5. Spatial Distributions
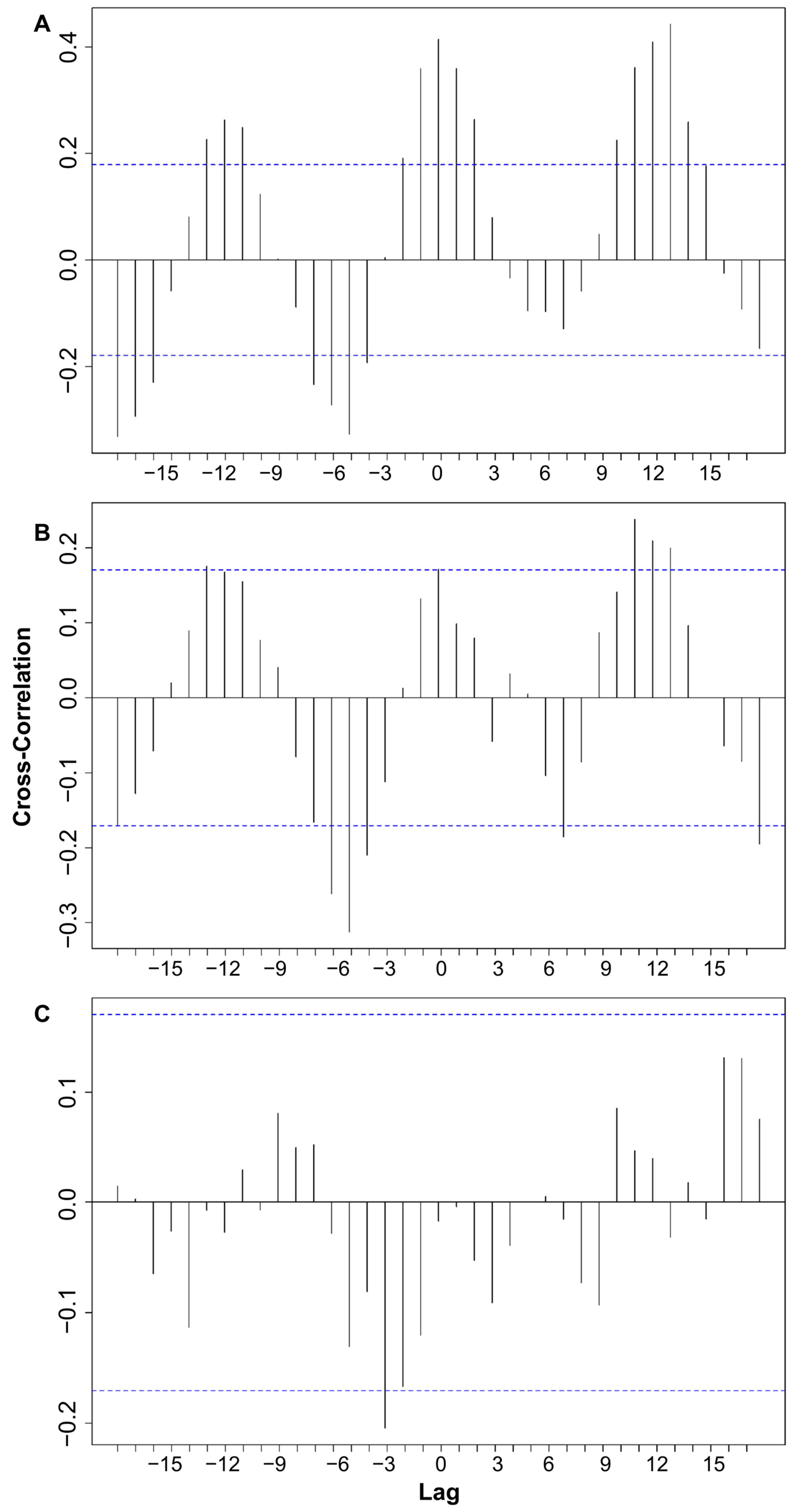
2.6. Cross-Correlations Between Time Series
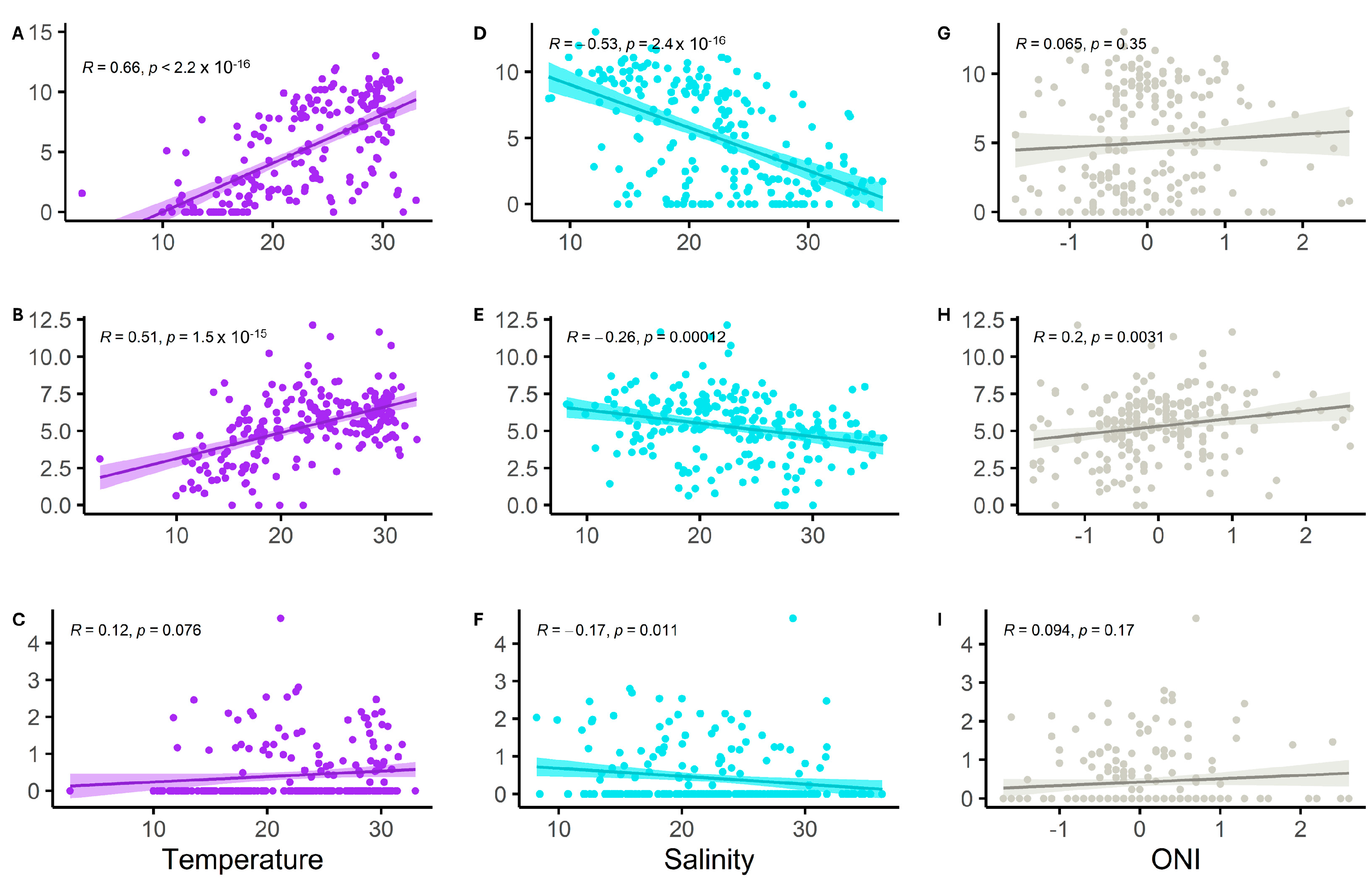
2.7. Environmental Effects on Vibrio spp.
3. Results
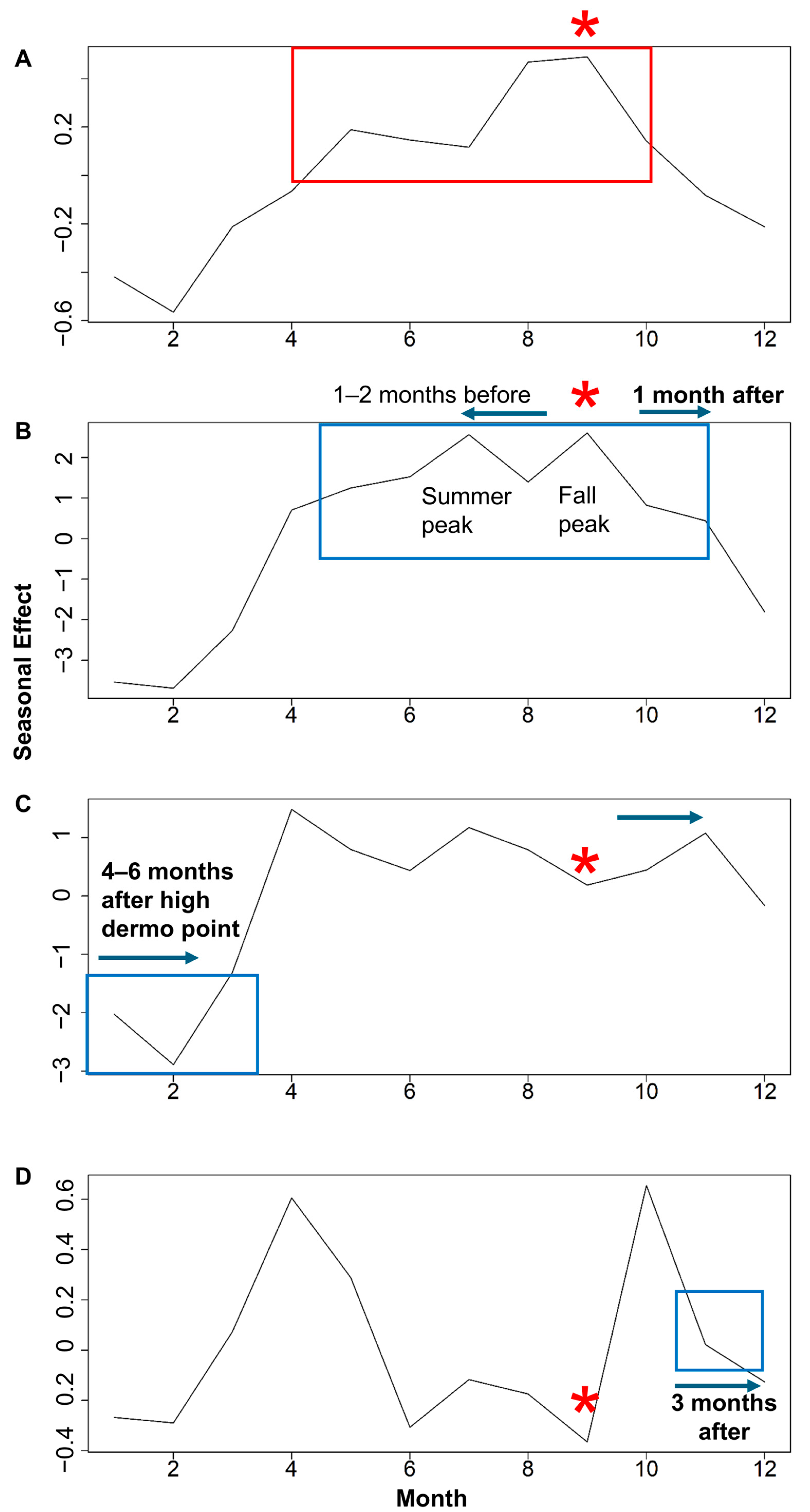
3.1. Time Series Decomposition: Trend, Seasonal, and Random Error
3.2. Spatial Distribution of Vibrio spp.
3.3. Cross-Correlations Between Time Series
3.4. Environmental Effect on Vibrio spp. Abundance
4. Discussion
4.1. Oysters and V. vulnificus
4.2. Oysters and V. parahaemolyticus
4.3. Vibrio spp. and P. marinus
4.4. Climate Change and Other Considerations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ceccarelli, D.; Hasan, N.A.; Hug, A.; Colwell, R.R. Distribution; and dynamics of epidemic; and pandemic Vibrio parahaemolyticus virulence factors. Front. Cell. Infect. Microbiol. 2013, 3, 97. [Google Scholar] [CrossRef]
- Iwamoto, M.; Ayers, T.; Mahon, B.E.; Swerdlow, D.L. Epidemiology of seafood-associated infections in the United States. Clin. Microbiol. Rev. 2010, 23, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, V.; Arshakm, K.; Korostynska, O.; Oliwa, K.; Adley, C. An Overview of food borne pathogen detection: In the perspective of biosensors. Biotechnol. Adv. 2010, 28, 232–254. [Google Scholar] [CrossRef]
- Letchumanan, V.; Chan, K.-G.; Lee, L.-H. Vibrio parahaemolyticus: A review on the pathogenesis; prevalence; and advance molecular identification techniques. Front. Microbiol. 2014, 5, 705. [Google Scholar] [CrossRef]
- Jones, J.L.; Lüdeke, C.H.M.; Bowers, J.C.; DeRosia-Banick, K.; Carey, D.H.; Hastbacke, W. Abundance of Vibrio cholerae, V. vulnificus, and V. parahaemolyticus in Oysters Crassostrea virginica and Clams Mercenaria mercenaria from Long Island Sound. Appl. Environ. Microbiol. 2014, 80, 7667–7672. [Google Scholar]
- Shapiro, R.; Altekruse, S.; Hutwagner, L.; Bishop, R.; Hammond, R.; Wilson, S.; Ray, B.; Thompson, S.; Tauxe, V.; Griffin, P. The role of Gulf Coast oysters harvested in warmer months in Vibrio vulnificus infections in the United States. J. Infect. Dis. 1998, 178, 752–759. [Google Scholar] [CrossRef]
- Archer, E.J.; Baker-Austin, C.; Osborn, T.J.; Jones, N.R.; Martínez-Urtaza, J.; Trinanes, J.; Oliver, J.D.; González, F.J.C.; Lake, I.R. Climate warming and increasing Vibrio vulnificus infections in North America. Sci. Rep. 2023, 13, 3893. [Google Scholar] [CrossRef]
- Daniels, N.A. Vibrio vulnificus oysters: Pearls and perils. Clin. Infect. Dis. 2011, 52, 788–792. [Google Scholar] [CrossRef]
- Strom, M.; Paranjpye, R.N.; Nilsson, W.B.; Turner, J.W.; Yanagida, G.K. Pathogen update: Vibrio species. In Woodhead Publishing Series in Food Science, Technology and Nutrition; Advances in Microbial Food Safety; Sofos, J., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 97–113. [Google Scholar] [CrossRef]
- Warner, E.B.; Oliver, J.D. Population structures of two genotypes of Vibrio vulnificus in oysters Crassostrea virginica and seawater. Appl. Environ. Microbiol. 2007, 741, 80–85. [Google Scholar]
- Froelich, B.; Oliver, J. The interactions of Vibrio vulnificus and the oyster Crassostrea virginica. Microb. Ecol. 2013, 65, 807–816. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Bad Bug Book: Foodborne Pathogenic Microorganisms and Natural Toxins, 2nd ed.; Food and Drug Administration: Washington, DC, USA, 2012; pp. 46–49. [Google Scholar]
- Velazquez-Roman, J.; Leon-Sicairos, N.; Flores-Villasenor, H.; Villafana-Rauda, S.; Canizalez-Roman, A. Association of pandemic Vibrio parahaemolyticus O3: K6 present in the coastal environment of Northwest Mexico with cases of recurrent diarrhea between 2004 and 2010. Appl. Environ. Microbiol. 2012, 78, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Qadri, F.; Chowdhury, N.R.; Takeda, Y.; Nair, G.B. Vibrio parahaemolyticus—Seafood Safety and Associations with Higher Organisms; Belkin, S., Colwell, R.R., Eds.; Oceans and Health: Pathogens in the Marine Environment; Springer: New York, NY, USA, 2005. [Google Scholar]
- Morris, J.; Black, R. Cholera & other vibrioses in the United States. N. Engl. J. Med. 1985, 312, 343–350. [Google Scholar] [PubMed]
- Daniels, N.A.; MacKinnon, L.; Bishop, R.; Altekruse, S.; Ray, B.; Hammond, R.M.; Thompson, S.; Wilson, S.; Bean, N.H.; Griffin, P.M.; et al. Vibrio parahaemolyticus infections in the United States, 1973–1998. J. Infect. Dis. 2000, 181, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Okuda, J.; Ishibashi, M.; Hayakawa, E.; Nishino, T.; Takeda, Y.; Mukhopadhyay, A.K.; Garg, S.; Bhattacharya, S.K.; Nair, G.B.; Nishibuchi, M. Emergence of a unique O3:K6 clone of Vibrio parahaemolyticus in Calcutta, India, and isolation of strains from the same clonal group from Southeast Asian travelers arriving in Japan. J. Clin. Microbiol. 1997, 35, 3150–3155. [Google Scholar] [CrossRef]
- Matsumoto, C.; Okuda, J.; Ishibashi, M.; Iwanaga, M.; Garg, P.; Ramamurthy, T.; Wong, H.C.; DePaola, A.; Kim, Y.B.; Albert, M.J.; et al. Pandemic spread of an O3:K6 clone of Vibrio parahaemolyticus and emergence of related strains evidenced by Arbitrarily Primed PCR & toxRS sequence analysis. J. Clin. Microbiol. 2000, 38, 578–585. [Google Scholar]
- Molenda, J.R.; Johnson, W.G.; Fishbein, M.; Wentz, B.; Mehlman, I.J.; Dadisman, T.A., Jr. Vibrio parahaemolyticus gastroenteritis in Maryland: Laboratory aspects. Appl. Microbiol. 1972, 24, 444–448. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Outbreak of Vibrio parahaemolyticus infections associated with eating raw oysters, Pacific Northwest, 1997. Morb. Mortal. Wkly. Rep. 1998, 47, 457–462. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Outbreak of Vibrio parahaemolyticus infection associated with eating raw oysters or clams harvested from Long Island Sound, Connecticut, New Jersey, and New York, 1998. Morb. Mortal. Wkly. Rep. 1999, 48, 48–51. [Google Scholar]
- DePaola, A.; Jones, J.L.; Woods, J.; Burkhardt, W.; Calci, K.R., III; Krantz, J.A.; Bowers, J.C.; Kasturi, K.; Byars, R.H.; Jacobs, E.; et al. Bacterial and viral pathogens in live oysters: 2007 United States market survey. Appl. Environ. Microbiol. 2010, 76, 2754–2768. [Google Scholar] [CrossRef]
- Kaneko, T.; Colwell, R.R. Ecology of Vibrio parahaemolyticus in Chesapeake Bay. J. Bacteriol. 1973, 113, 24–32. [Google Scholar] [CrossRef]
- Sarkar, B.L.; Nair, G.B.; Banerjee, A.K.; Pal, S.C. Seasonal distribution of Vibrio parahaemolyticus in freshwater environs and in association with freshwater fishes in Calcutta. Appl. Environ. Microbiol. 1985, 49, 132–136. [Google Scholar] [PubMed]
- Davis, J.W.; Sizemore, R.K. Incidence of Vibrio species associated with blue crabs Callinectes sapidus collected from Galveston Bay, Texas. Appl. Environ. Microbiol. 1982, 43, 1092–1097. [Google Scholar] [PubMed]
- White, M.E.; Powell, E.N.; Wilson, E.A.; Ray, S.M. The Spatial Distribution of Perkinsus Marinus, a Protozoan Parasite, in Relation to its Oyster Host Crassostrea Virginica and an Ectoparasitic Gastropod Boonea Impressa. J. Mar. Biol. Assoc. UK 1989, 693, 703–717. [Google Scholar] [CrossRef]
- Powell, E.N.; Klinck, J.M.; Guo, X.; Hofmann, E.E.; Ford, S.E.; Bushek, D. Can oysters Crassostrea virginica develop resistance to dermo disease in the field: The impediment posed by climate cycles. J. Mar. Res. 2012, 702–703, 309–355. [Google Scholar]
- Ray, S.M. A culture technique for diagnosis of infections with Dermocystidium marinum Mackin, Owen, and Collier in oysters. Science 1952, 116, 306–361. [Google Scholar]
- Craig, A.; Powell, E.N.; Fay, R.R.; Brooks, J.M. Distribution of Perkinsus marinus in gulf coast oyster populations. Estuaries 1989, 12, 82–91. [Google Scholar] [CrossRef]
- Silvy, E.; Gelwick, F.; Silvy, N. Factors affecting Dermo disease Perkinsus marinus in eastern oysters Crassostrea virginica in Galveston Bay, Texas. J. Environ. Sci. Eng. 2020, A9, 227–245. [Google Scholar] [CrossRef]
- Gaona-Hernández, A.; Jurgens, L.J.; Pollack, J.B.; Du, J.; Jensen, C.C.; Quigg, A. Impact of Perkinsus marinus on oyster health: A survey after a regional data gap. J. Shellfish Res. 2025; In review. [Google Scholar]
- Tall, B.D.; La Peyre, J.F.; Bier, J.W.; Miliotis, M.D.; Hanes, D.E.; Kothary, M.H.; Shah, D.B.; Faisal, M. Perkinsus marinus extracellular protease modulates survival of Vibrio vulnificus in Eastern oyster Crassostrea virginica hemocytes. Appl. Environ. Microbiol. 1999, 659, 4261–4263. [Google Scholar] [CrossRef]
- Walther, B.D.; Bachand, P.T.; Hinson, A.; O’Donnell, C.A.; Turner, J.W. Investigating reconstructed inflows and pathogen infection patterns between low-relief and high-relief subtropical oyster reefs. Exp. Results 2020, 1, e23. [Google Scholar] [CrossRef]
- Bienlien, L.M.; Audemard, C.; Reece, K.S.; Carnegie, R.B. Impact of parasitism on levels of human-pathogenic Vibrio species in eastern oysters. J. Appl. Microbiol. 2022, 1322, 760–771. [Google Scholar]
- Stanley, J.G.; Sessers, M.A. Species profiles: Life histories of environmental requirements of coastal fishes and invertebrates Gulf of Mexico—American oyster. U.S. Fish. Wildl. Serv. Biol. Rep. 1986, 11, 25. [Google Scholar]
- Eastern Oyster Biological Review Team (EOBRT). Status Review of the Eastern Oyster Crassostrea virginica; Report to the National Marine Fisheries Service; Northeast Regional Office: Gloucester, UK, 2007. [Google Scholar]
- Vanoy, R.W.; Tamplin, M.L.; Schwarz, J.R. Ecology of Vibrio vulnificus in Galveston Bay oysters, suspended particulate matter; sediment and seawater: Detection by monoclonal anti body immunoassay-most probable number procedures. J. Ind. Microbiol. Biot. 1992, 93, 219–223. [Google Scholar] [CrossRef]
- Montagna, P.A.; Douglas, A.R. Freshwater Inflows to Texas Bays and Estuaries. A Regional-Scale Review, Synthesis, and Recommendations; Springer Nature: Cham, Switzerland, 2025; 411p, Available online: https://library.oapen.org/handle/20.500.12657/96985 (accessed on 5 January 2025).
- Kaysner, C.A.; DePaola, A.; Jones, J.; Vibrio. Bacteriological Analytical Manual Online. 2004. Available online: https://www.fda.gov/food/laboratory-methods-food/bam-chapter-9-vibrio (accessed on 28 June 2024).
- Tamplin, M.L.; Martin, A.L.; Ruple, A.D.; Cook, D.W.; Kaspar, C.W. Enzyme immunoassay for identification of Vibrio vulnificus in seawater, sediment, and oysters. Appl. Environ. Microbiol. 1991, 574, 1235–1240. [Google Scholar]
- Wright, A.C.; Miceli, G.A.; Landry, W.L.; Christy, J.B.; Watkins, W.D.; Morris, J.G., Jr. Rapid identification of Vibrio vulnificus on nonselective media with an alkaline phosphatase-labeled oligonucleotide probe. Appl. Environ. Microbiol. 1993, 592, 541–546. [Google Scholar]
- McCarthy, S.A.; DePaola, A.; Cook, D.W.; Kaysner, C.A.; Hill, W.E. Evaluation of alkaline phosphatase-and digoxigenin-labelled probes for detection of the thermolabile hemolysin tlh gene of Vibrio parahaemolyticus. Lett. Appl. Microbiol. 1999, 281, 66–70. [Google Scholar]
- McCarthy, S.A.; DePaola, A.; Kaysner, C.A.; Hill, W.E.; Cook, D.W. Evaluation of nonisotopic DNA hybridization methods for detection of the tdh gene of Vibrio parahaemolyticus. J. Food Prot. 2000, 6312, 1660–1664. [Google Scholar]
- Mackin, J.G. Oyster diseases caused by Dermocystidium marinum and other microorganisms in Louisiana. Publ. Inst. Mar. Sci. Univ. Tex. 1962, 7, 132–229. [Google Scholar]
- Benavides, I.F.; Santacruz, M.; Romero-Leiton, J.P.; Barreto, C.; Selvaraj, J.J. Assessing methods for multiple imputation of systematic missing data in marine fisheries time series with a new validation algorithm. Aquac. Fish. 2023, 8, 587–599. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation 776 for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Posit Team. RStudio: Integrated Development Environment for R. Posit Software; PBC, 756; Posit Team: Boston, MA, USA, 2023; Available online: http://www.posit.co/ (accessed on 30 May 2024).
- Trapletti, A.; Hornik, K. tseries: Time Series Analysis and Computational Finance. R Package Version 0.10-58. 2024. Available online: https://CRAN.R-project.org/package=tseries (accessed on 30 May 2024).
- Zeileis, A.; Grothendieck, G. zoo: S3 Infrastructure for Regular and Irregular Time Series. J. Stat. Softw. 2005, 14, 1–27. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Pebesma, E.; Bivand, R. Classes and methods for spatial data in R. R News 2005, 5, 9–13. Available online: https://CRAN.R-project.org/doc/Rnews/ (accessed on 30 May 2024).
- Encomio, V.G.; Stickler, S.M.; Allen, S.K.; Chu, F.L. Performance of “natural dermo-resistant” oyster stocks—Survival, disease, growth, condition and energy reserves. J. Shellfish Res. 2005, 241, 143–155. [Google Scholar]
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. R Package Version 0.6.0. 2023. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 28 June 2024).
- Raszl, S.M.; Froelich, B.A.; Vieira1, C.R.W.; Blackwood, A.D.; Noble, R.T. Vibrio parahaemolyticus and Vibrio vulnificus in South America: Water, seafood and human infections. J. Appl. Microbiol. 2016, 121, 1201–1222. [Google Scholar] [CrossRef]
- Oliver, J.D.; Warner, R.A.; Cleland, D.R. Distribution of Vibrio vulnificus and other lactose-fermenting vibrios in the marine environment. Appl. Environ. Microbiol. 1983, 453, 985–998. [Google Scholar]
- Lin, M.; Payne, D.A.; Schwarz, J.R. Intraspecific diversity of Vibrio vulnificus in Galveston Bay water and oysters as determined by randomly amplified polymorphic DNA PCR. Appl. Environ. Microbiol. 2003, 696, 3170–3175. [Google Scholar]
- Johnson, C.N.; Flowers, A.R.; Noriea, N.F., III; Zimmerman, A.M.; Bowers, J.C.; DePaola, A.; Grimes, D.J. Relationships between environmental factors and pathogenic vibrios in the Northern Gulf of Mexico. Appl. Environ. Microbiol. 2010, 76, 7076–7084. [Google Scholar] [CrossRef]
- Parvathi, A.; Kumar, H.S.; Karunasagar, I.; Karunasagar, I. Detection and enumeration of Vibrio vulnificus in oysters from two estuaries along the southwest coast of India, using molecular methods. Appl. Environ. Microbiol. 2004, 7011, 6909–6913. [Google Scholar] [CrossRef]
- Motes, M.L.; DePaola, A.; Cook, D.W.; Veazey, J.E.; Hunsucker, J.C.; Garthright, W.E.; Blodgett, R.J.; Chirtel, S.J. Influence of water temperature and salinity on Vibrio vulnificus in Northern Gulf and Atlantic coast oysters Crassostrea virginica. Appl. Environ. Microbiol. 1998, 644, 1459–1465. [Google Scholar]
- Odeyemi, O.A. Incidence and prevalence of Vibrio parahaemolyticus in seafood: A systematic review and meta-analysis. SpringerPlus 2016, 5, 464. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, J.; Li, H.; Tan, S.; Chen, Y.; Yu, H. Prevalence; Antibiotic Susceptibility and Diversity of Vibrio parahaemolyticus Isolates in Seafood from South China. Front. Microbiol. 2017, 8, 2566. [Google Scholar] [CrossRef]
- Cabrera-Garcia, M.E.; Vazquez-Salinas, C.; Quinones-Ramirez, E.I. Serologic and molecular characterization of Vibrio parahaemolyticus strains isolated from seawater and fish products of the Gulf of Mexico. Appl. Environ. Microbiol. 2004, 70, 6401–6406. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Hara-Kudo, Y.; Krantz, J.A.; Benner, R.A., Jr.; Smith, A.B.; Dambaugh, T.R.; Bowers, J.C.; DePaola, A. Comparison of molecular detection methods for Vibrio parahaemolyticus and Vibrio vulnificus. Food Microbiol. 2012, 30, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Randa, M.A.; Polz, M.F.; Lim, E. Effects of temperature and salinity on Vibrio vulnificus population dynamics as assessed by quantitative PCR. Appl. Environ. Microbiol. 2004, 709, 5469–5476. [Google Scholar] [CrossRef]
- Hofmann, E.E.; Powell, E.N.; Klinck, J.M.; Saunders, G. Modelling diseased oyster populations I. Perkinsus marinus infections in oysters. J. Shellfish Res. 1995, 14, 121–151. [Google Scholar]
- Beseres Pollack, J.; Palmer, T.A.; Williams, A.E. Medium-term monitoring reveals effects of El Niño Southern Oscillation climate variability on local salinity and faunal dynamics on a restored oyster reef. PLoS ONE 2021, 16, e0255931. [Google Scholar] [CrossRef]
- Camelo, J.; Mayo, T.L.; Gutmann, E.D. Projected Climate Change Impacts on Hurricane Storm Surge Inundation in the Coastal United States. Front. Built Environ. 2020, 6, 588049. [Google Scholar] [CrossRef]
- Fasullo, J.T.; Otto-Bliesner, B.L.; Stevenson, S. ENSO’s changing influence on temperature; precipitation; and wildfire in a warming climate. Geophys. Res. Lett. 2018, 45, 9216–9225. [Google Scholar] [CrossRef]
- Neokye, E.O.; Wang, X.; Thakur, K.K.; Quijón, P.A.; Nawaz, R.A. Climate change impacts on oyster aquaculture—Part II: Impact assessment and adaptation measures. Environ. Res. 2024, 259, 119535. [Google Scholar] [CrossRef]
- Okon, E.M.; Birikorang, H.N.; Munir, M.B.; Kari, Z.A.; Téllez-Isaías, G.; Khalifa, N.E.; Abdelnour, S.A.; Eissa, M.E.H.; Al-Farga, A.; Dighiesh, H.S.; et al. A Global Analysis of Climate Change and the Impacts on Oyster Diseases. Sustainability 2023, 15, 12775. [Google Scholar] [CrossRef]
- Walker, J.R.; Bente, D.A.; Burch, M.T.; Cerqueira, F.M.; Ren, P.; Labonté, J.M. Molecular assessment of oyster microbiomes and viromes reveals their potential as pathogen and ecological sentinels. One Health 2025, 20, 100973. [Google Scholar] [CrossRef]
- Palmer, T.A.; Ueling, P.; Pollack, J.B. Using oyster tissue toxicity as an indicator of disturbed environments. Int. J. Environ. Sci. Technol. 2015, 12, 2111–2116. [Google Scholar]
- Quigg, A.; Windham, R.; Zhang, Z.; Fleur Swartz, R.; Anis, A.; Ray, S. Swan Lake: Pilot Study Site Utilizing Processed Industrial Wastewater to Manage Salinity in Lower Galveston Bay; Final Report of the Coastal Coordination Council Pursuant to National Oceanic and Atmospheric Administration Award No. NA08NOS4190458; Texas A&M University: College Station, TX, USA, 2010; p. 82. Available online: https://www.glo.texas.gov/coastal/coastal-projects/swan-lake-pilot-study-site-utilizing-processed-industrial-wastewater (accessed on 2 August 2024).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quigg, A.; Gaona-Hernández, A.; Hochman, M.S.; Ray, S.M.; Schwarz, J.R. Applied Time Series Analyses (2000–2017) of Vibrio vulnificus and Vibrio parahaemolyticus (Pathogenic and Non-Pathogenic Strains) in the Eastern Oyster, Crassostrea virginica. Bacteria 2025, 4, 17. https://doi.org/10.3390/bacteria4020017
Quigg A, Gaona-Hernández A, Hochman MS, Ray SM, Schwarz JR. Applied Time Series Analyses (2000–2017) of Vibrio vulnificus and Vibrio parahaemolyticus (Pathogenic and Non-Pathogenic Strains) in the Eastern Oyster, Crassostrea virginica. Bacteria. 2025; 4(2):17. https://doi.org/10.3390/bacteria4020017
Chicago/Turabian StyleQuigg, Antonietta, Aurora Gaona-Hernández, Mona S. Hochman, Sammy M. Ray, and John R. Schwarz. 2025. "Applied Time Series Analyses (2000–2017) of Vibrio vulnificus and Vibrio parahaemolyticus (Pathogenic and Non-Pathogenic Strains) in the Eastern Oyster, Crassostrea virginica" Bacteria 4, no. 2: 17. https://doi.org/10.3390/bacteria4020017
APA StyleQuigg, A., Gaona-Hernández, A., Hochman, M. S., Ray, S. M., & Schwarz, J. R. (2025). Applied Time Series Analyses (2000–2017) of Vibrio vulnificus and Vibrio parahaemolyticus (Pathogenic and Non-Pathogenic Strains) in the Eastern Oyster, Crassostrea virginica. Bacteria, 4(2), 17. https://doi.org/10.3390/bacteria4020017





